Six-Month Brain Health Outcomes in the Geriatric Population After Mild Traumatic Brain Injury: A Prospective Neuroimaging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Acute Clinical Assessment and Management
2.3. Neuroimaging and Injury Classification
- Uncomplicated mTBI: no acute intracranial pathology visible on CT.
- Complicated mTBI: presence of acute traumatic intracranial lesions on CT.
2.4. Sociodemographic and Clinical Data Collection
2.5. Outcome Measures at Six Months
2.6. Statistical Analysis
3. Results
3.1. Cohort Characteristics and Overall Outcomes
3.2. Predictors of Outcome in Older Adults
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| mTBI | Mild traumatic brain injury |
| CT | Computed tomography |
| GCS | Glasgow Coma Scale |
| GOSE | Glasgow Outcome Scale–Extended |
| SF-12 | 12-Item Short Form Health Survey |
| RPQ | Rivermead Post-Concussion Symptoms Questionnaire |
| PCL-5 | PTSD Checklist for DSM-5 |
| PTSD | Post-traumatic stress disorder |
| DSM-5 | Diagnostic and Statistical Manual for Mental Disorders |
| PHQ-9 | Patient Health Questionnaire-9 |
| TUG | Timed Up and Go test |
References
- Corriero, A.; Fornaciari, A.; Terrazzino, S.; Zangari, R.; Izzi, A.; Peluso, L.; Savi, M.; Faso, C.; Cavallini, L.; Polato, M.; et al. The impact of age and intensity of treatment on the outcome of traumatic brain injury. Front. Neurol. 2024, 15, 1471209. [Google Scholar] [CrossRef]
- Mao, A.; Su, J.; Ren, M.; Chen, S.; Zhang, H. Risk prediction models for falls in hospitalized older patients: A systematic review and meta-analysis. BMC Geriatr. 2025, 25, 29. [Google Scholar] [CrossRef] [PubMed]
- Gürü, S.; Özensoy, H.S.; Ertürk, N.; Örün, S.; Ceyhan, M.A. Analyses of the characteristics and prognosis of elderly patients visiting a high-capacity Turkish emergency department due to an occupational accident: A cross-sectional study. BMC Geriatr. 2025, 25, 610. [Google Scholar] [CrossRef] [PubMed]
- Von Steinbüechel, N.; Hahm, S.; Muehlan, H.; Arango-Lasprilla, J.C.; Bockhop, F.; Čović, A.; Schmidt, S.; Steyerberg, E.W.; Maas, A.I.R.; Menon, D.; et al. Impact of Sociodemographic, Premorbid, and Injury-Related Factors on Patient-Reported Outcome Trajectories after Traumatic Brain Injury (TBI). J. Clin. Med. 2023, 12, 2246. [Google Scholar] [CrossRef] [PubMed]
- Perumpalath, N.; Jineesh, T.; Rajendran, V.R.; Devarajan, E.; Rajan, P.; Subramnian, G.; Juvaina, P.; Pillai, S.S. Traumatic Intra Cranial Injury—Computed Tomography, per Operative and Post Mortem Find Ings: A Prognostic Correlation. J. Evol. Med. Dent. Sci. 2015, 4, 11043–11058. [Google Scholar] [CrossRef]
- Jha, D.K.; Chauhan, R.N. Diagnosis and Management of Computed Tomography in Head Injury. Int. J. Med. Biomed. Stud. 2019, 3, 284–289. [Google Scholar] [CrossRef]
- Lo, T.W.; Chan, G.H. Understanding the life experiences of elderly in social isolation from the social systems perspective: Using Hong Kong as an illustrating example. Front. Psychiatry 2023, 14, 1114135. [Google Scholar] [CrossRef]
- Söderman, M.; Bondesson, A.; Pettersson, T.; Gustafsson, L. “Intensive-Home-Rehabilitation” Intervention for Older Persons: A Follow-Up Study of Team Members’ Perceptions. J. Multidiscip. Health 2023, 16, 2207–2216. [Google Scholar] [CrossRef]
- Erwander, K.; Agvall, B.; Ivarsson, K. The role of vital signs in predicting mortality risk in elderly patients visiting the emergency department. BMC Emerg. Med. 2025, 25, 144. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.H.; Pyun, S.B. Comparison of Functional Outcomes Between Elderly and Young. 2024. Available online: https://journals.lww.com/topicsingeriatricrehabilitation/abstract/2019/04000/comparison_of_functional_outcomes_between_elderly.10.aspx (accessed on 22 August 2025).
- Marešová, P.; Krejcar, O.; Maskuriy, R.; Bakar, N.A.A.; Selamat, A.; Truhlářová, Z.; Horak, J.; Joukl, M.; Vítkova, L. Challenges and opportunity in mobility among older adults—Key determinant identification. BMC Geriatr. 2023, 23, 447. [Google Scholar] [CrossRef]
- Cossio-Bolaños, M.; Vidal-Espinoza, R.; Villar-Cifuentes, I.; de Campos, L.F.C.C.; de Lázari, M.S.R.; Urra-Albornoz, C.; Sulla-Torres, J.; Gomez-Campos, R. Functional fitness benchmark values for older adults: A systematic review. Front. Public Health 2024, 12, 1335311. [Google Scholar] [CrossRef]
- Barrett, C.A.; Goetting, M.G.; Lyerla, R.; Fogarty, K.J. Subsequent Emergency Department Visits in Geriatric Mild Traumatic Brain Injury: Relationship with Fall, Payor, and Discharge Outcome. Healthcare 2025, 13, 1236. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, Y.; Lee, J.; Kim, S.; Kim, Y.; Kim, W.Y. Incidence and outcomes of delayed intracranial hemorrhage: A population-based cohort study. Sci. Rep. 2024, 14, 19502. [Google Scholar] [CrossRef]
- Sergeyenko, Y.; Andreae, M.E.; Segal, M. Diagnosis and Management of Mild Traumatic Brain Injury (mTBI): A Comprehensive, Patient-centered Approach. Curr. Pain Headache Rep. 2025, 29, 19. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Corderman, S.; Berlinberg, E.J.; Schoenthaler, A.; Horwitz, L.I. Assessment of Patient Education Delivered at Time of Hospital Discharge. JAMA Intern. Med. 2023, 183, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Austad, K.; Lee, J.H.; Lanney, H.; Rapoport, V.O.; Wornhoff, R.; McDaniel, K.L.; Li-Garrison, L.; Jack, B.W. Evaluating the quality and equity of patient hospital discharge instructions. BMC Health Serv. Res. 2025, 25, 291. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Mandado, O.; Periáñez, J.A. An effective psychological intervention in reducing internalized stigma and improving recovery outcomes in people with severe mental illness. Psychiatry Res. 2020, 295, 113635. [Google Scholar] [CrossRef]
- Salas, C.E.; Rojas-Líbano, D.; de Castro, O.A.; Cruces, R.; Evans, J.J.; Radovic, D.; Arévalo-Romero, C.; Torres, J.; Aliaga, Á. Social isolation after acquired brain injury: Exploring the relationship between network size, functional support, loneliness and mental health. Neuropsychol. Rehabil. 2021, 32, 2294–2318. [Google Scholar] [CrossRef]
- Fisher, K.; Seidler, Z.E.; King, K.; Oliffe, J.L.; Rice, S. Men’s anxiety: A systematic review. J. Affect. Disord. 2021, 295, 688–702. [Google Scholar] [CrossRef]
- Herreen, D.; Rice, S.; Zajac, I. Brief assessment of male depression in clinical care: Validation of the Male Depression Risk Scale short form in a cross-sectional study of Australian men. BMJ Open 2022, 12, e053650. [Google Scholar] [CrossRef]
- Palmer, R.M.; Smith, B.J.; Kite, J.; Phongsavan, P. The socio-ecological determinants of help-seeking practices and healthcare access among young men: A systematic review. Health Promot. Int. 2024, 39, daae024. [Google Scholar] [CrossRef]
- Wågberg, S.; Stålnacke, B.-M.; Magnusson, B.M. Gender and Age Differences in Outcomes after Mild Traumatic Brain Injury. J. Clin. Med. 2023, 12, 4883. [Google Scholar] [CrossRef]
- Mitchell, A.; Elmasry, Y.E.T.; Poelgeest, E.P.; van Welsh, T. Anticoagulant use in older persons at risk for falls: Therapeutic dilemmas—A clinical review. Eur. Geriatr. Med. 2023, 14, 683–696. [Google Scholar] [CrossRef]
- Lim, J.Y.; Jee, Y.; Choi, S.G.; Torbati, S.; Berdahl, C.T.; Lee, S.H. Redefining Trauma Triage for Elderly Adults: Development of Age-Specific Guidelines for Improved Patient Outcomes Based on a Machine-Learning Algorithm. Medicina 2025, 61, 784. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.J.; McCormick, W.C.; Kagan, S.H. Traumatic Brain Injury in Older Adults: Epidemiology, Outcomes, and Future Implications. J. Am. Geriatr. Soc. 2006, 54, 1590–1595. [Google Scholar] [CrossRef]
- Tyler, C.M.; Dini, M.E.; Perrin, P.B. Group-Based Patterns of Life Satisfaction and Functional Independence over the 10 Years after Traumatic Brain Injury in Older Adults: A Model Systems Study. Int. J. Environ. Res. Public Health 2023, 20, 5643. [Google Scholar] [CrossRef]
- Coffeng, S.M.; Abdulle, A.E.; van der Horn, H.J.; de Koning, M.E.; ter Maaten, J.C.; Spikman, J.M.; van der Naalt, J. Good Health-Related Quality of Life in Older Patients One Year after mTBI despite Incomplete Recovery: An Indication of the Disability Paradox? J. Clin. Med. 2024, 13, 2655. [Google Scholar] [CrossRef]

| Variable | Value |
|---|---|
| Mean age (years) | 72.1 ± 6.8 (range 65–89) |
| Sex (male/female) | 53.8/46.2% |
| Mechanism of injury | Fall 68%; Traffic 15%; Other 17% |
| CT findings | Normal: 54%; Lesion present: 46% |
| Types of CT lesions | Subdural hematoma: 20; Contusion: 15; SAH: 6; Epidural: 2 |
| Anticoagulant use | 4% (n = 4) |
| Functional independence (GOSE ≥ 5) | 94.9% |
| Complete recovery (GOSE = 8) | 43% |
| Return to pre-injury activities | 74% |
| Predictor | Associated Outcome(s) |
|---|---|
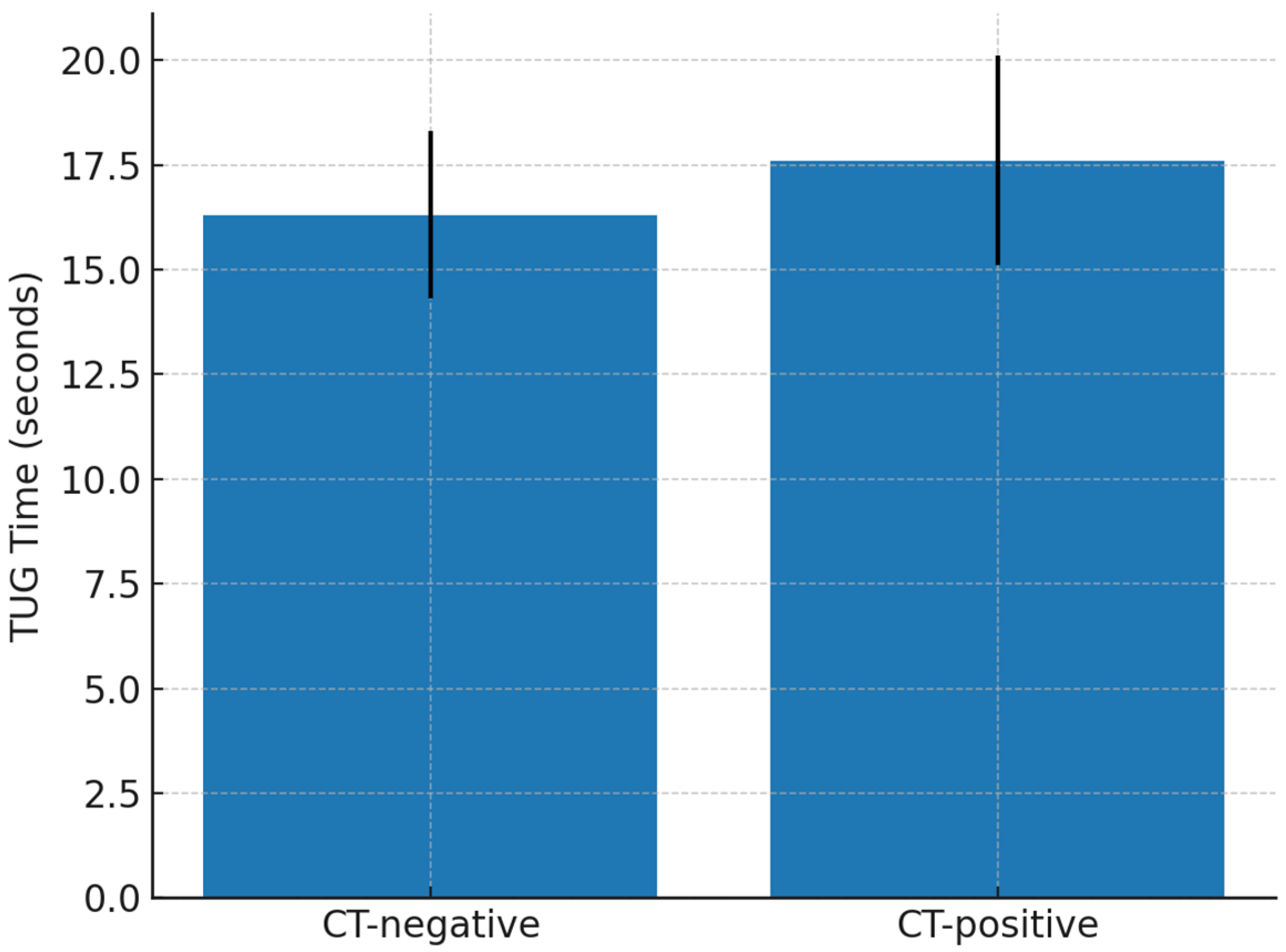
| CT lesion (complicated mTBI) | Worse function and mobility: GOSE 6.9 vs. 7.4 (p = 0.025); TUG 17.6 vs. 16.3 s (p = 0.012). No effect on psychological outcomes. |
| Advanced age (within ≥65) | Small but significant decline in GOSE (β ≈ −0.012, p = 0.026); slower TUG. |
| Lower education | Markedly worse recovery: GOSE 6.65 vs. 7.18 (p = 0.001); SF-12 PCS 55.4 vs. 79.9 (p < 0.001); slower TUG (18.96 vs. 16.47 s, p < 0.001). |
| Female sex | Trend toward higher PTSD, depression, and RPQ scores; lower physical scores. |
| Social isolation | Lower SF-12 MCS, higher depression risk. |
| Anticoagulation (delayed reversal) | Major complications, lowest GOSE (5). Prompt reversal → outcomes similar to non-anticoagulated. |
| Severe acute headache | Independent predictor of poorer outcome: lower GOSE, reduced SF-12 PCS (p < 0.01). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvat, I.; Golubović, J.; Djilvesi, D.; Jelača, B.; Vuleković, P. Six-Month Brain Health Outcomes in the Geriatric Population After Mild Traumatic Brain Injury: A Prospective Neuroimaging Study. Clin. Transl. Neurosci. 2025, 9, 40. https://doi.org/10.3390/ctn9030040
Horvat I, Golubović J, Djilvesi D, Jelača B, Vuleković P. Six-Month Brain Health Outcomes in the Geriatric Population After Mild Traumatic Brain Injury: A Prospective Neuroimaging Study. Clinical and Translational Neuroscience. 2025; 9(3):40. https://doi.org/10.3390/ctn9030040
Chicago/Turabian StyleHorvat, Igor, Jagoš Golubović, Djula Djilvesi, Bojan Jelača, and Petar Vuleković. 2025. "Six-Month Brain Health Outcomes in the Geriatric Population After Mild Traumatic Brain Injury: A Prospective Neuroimaging Study" Clinical and Translational Neuroscience 9, no. 3: 40. https://doi.org/10.3390/ctn9030040
APA StyleHorvat, I., Golubović, J., Djilvesi, D., Jelača, B., & Vuleković, P. (2025). Six-Month Brain Health Outcomes in the Geriatric Population After Mild Traumatic Brain Injury: A Prospective Neuroimaging Study. Clinical and Translational Neuroscience, 9(3), 40. https://doi.org/10.3390/ctn9030040









