Sleep Disorders in Neuromuscular Diseases: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
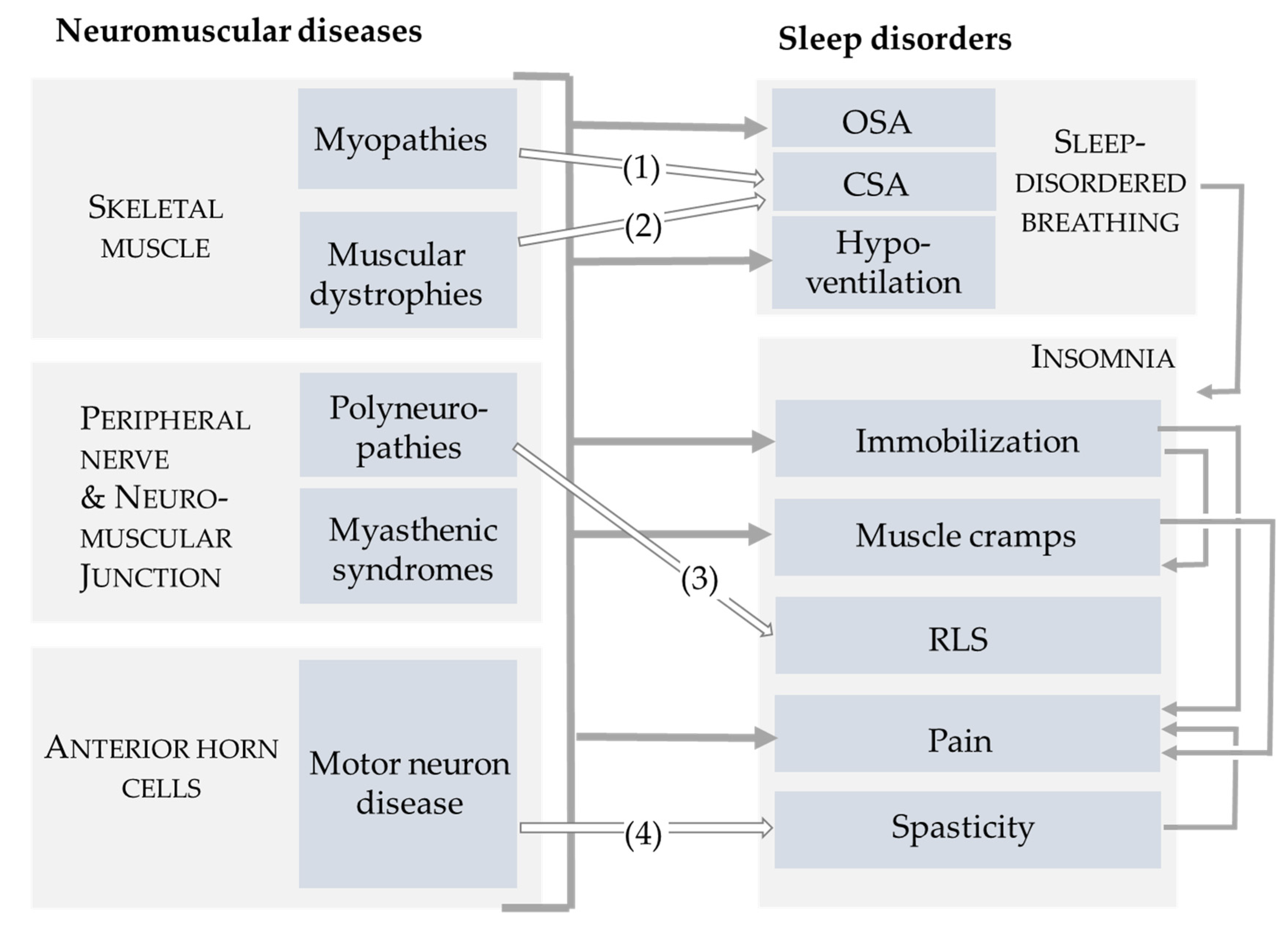
3. Burden of Symptoms
4. Muscle Cramps, Spasticity, and Pain
5. Restless Legs Syndrome and Periodic Limb Movements in Sleep
6. Sleep-Disordered Breathing
6.1. Obstructive Sleep Apnea
6.2. Central Sleep Apnea
6.3. Nocturnal Hypoventilation
7. Noninvasive Ventilation
8. Weakness of Cough
9. Important Aspects in Selected NMDs
9.1. Amyotrophic Lateral Sclerosis
9.2. Myotonic Dystrophy Type 1
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASM | American Academy of Sleep Medicine |
| AHI | Apnea hypopnea index |
| ALS | Amyotrophic lateral sclerosis |
| BMD | Becker muscular dystrophy |
| CIDP | Chronic inflammatory demyelinating polyneuropathy |
| CMT | Charcot–Marie–Tooth disease |
| CPAP | Continuous positive airway pressure |
| CSA | Central sleep apnea |
| CSA-CSR | Central sleep apnea with Cheyne–Stokes respiration |
| CSF | Cerebrospinal fluid |
| CO2 | Carbon dioxide |
| DM1 | Myotonic dystrophy type 1 |
| DMD | Duchenne muscular dystrophy |
| EDS | Excessive daytime sleepiness |
| FVC | Forced vital capacity |
| FSHD | Facio-scapulo-humeral muscular dystrophy |
| HSP | Hereditary spastic paraplegia |
| LEMS | Lambert–Eaton myasthenic syndrome |
| LGMD | Limb–girdle muscular dystrophies |
| MIP | Maximum inspiratory pressure |
| N3 | Slow-wave sleep |
| NH | Nocturnal hypoventilation |
| NIV | Noninvasive ventilation |
| NMD | Neuromuscular disorders |
| NPPV | Noninvasive positive pressure ventilation |
| OSA | Obstructive sleep apnea |
| PCF | Peak cough flow |
| pCO2 | Carbon dioxide tension |
| ptcCO2 | Transcutaneous carbon dioxide tension |
| PLM | Periodic leg movements |
| PSG | Polysomnography |
| PVA | Patient–ventilator asynchrony |
| REM | Rapid eye movement |
| RLS | Restless legs syndrome |
| SDB | Sleep-disordered breathing |
| SMA | Spinal muscular atrophy |
| SNIP | Sniff nasal inspiratory pressure |
References
- Anonymous Rare Diseases. Available online: https://ec.europa.eu/health/non_communicable_diseases/rare_diseases_en (accessed on 16 June 2023).
- Deenen, J.C.; Horlings, C.G.; Verschuuren, J.J.; Verbeek, A.L.; van Engelen, B.G. The Epidemiology of Neuromuscular Disorders: A Comprehensive Overview of the Literature. J. Neuromuscul. Dis. 2015, 2, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Raheja, D.; Stephens, H.E.; Lehman, E.; Walsh, S.; Yang, C.; Simmons, Z. Patient-Reported Problematic Symptoms in an ALS Treatment Trial. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Günther, R.; Richter, N.; Sauerbier, A.; Chaudhuri, K.R.; Martinez-Martin, P.; Storch, A.; Hermann, A. Non-Motor Symptoms in Patients Suffering from Motor Neuron Diseases. Front. Neurol. 2016, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Boentert, M.; Dziewas, R.; Heidbreder, A.; Happe, S.; Kleffner, I.; Evers, S.; Young, P. Fatigue, Reduced Sleep Quality and Restless Legs Syndrome in Charcot-Marie-Tooth Disease: A Web-Based Survey. J. Neurol. 2010, 257, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Abad, M.; Buczyner, J.R.; Venza, B.R.; Scharf, S.M.; Kwan, J.Y.; Lubinski, B.; Russell, J.W. Poor Sleep Quality in Patients With Amyotrophic Lateral Sclerosis at the Time of Diagnosis. J. Clin. Neuromuscul. Dis. 2018, 20, 60–68. [Google Scholar] [CrossRef]
- Laberge, L.; Begin, P.; Montplaisir, J.; Mathieu, J. Sleep Complaints in Patients with Myotonic Dystrophy. J. Sleep Res. 2004, 13, 95–100. [Google Scholar] [CrossRef]
- Bourke, S.C.; Tomlinson, M.; Williams, T.L.; Bullock, R.E.; Shaw, P.J.; Gibson, G.J. Effects of Non-Invasive Ventilation on Survival and Quality of Life in Patients with Amyotrophic Lateral Sclerosis: A Randomised Controlled Trial. Lancet Neurol. 2006, 5, 140–147. [Google Scholar] [CrossRef]
- Bourke, S.C.; Bullock, R.E.; Williams, T.L.; Shaw, P.J.; Gibson, G.J. Noninvasive Ventilation in ALS: Indications and Effect on Quality of Life. Neurology 2003, 61, 171–177. [Google Scholar] [CrossRef]
- Lyall, R.A.; Donaldson, N.; Fleming, T.; Wood, C.; Newsom-Davis, I.; Polkey, M.I.; Leigh, P.N.; Moxham, J. A Prospective Study of Quality of Life in ALS Patients Treated with Noninvasive Ventilation. Neurology 2001, 57, 153–156. [Google Scholar] [CrossRef]
- Vitacca, M.; Montini, A.; Lunetta, C.; Banfi, P.; Bertella, E.; De Mattia, E.; Lizio, A.; Volpato, E.; Lax, A.; Morini, R.; et al. Impact of an Early Respiratory Care Program with NIV Adaptation in Patients with ALS. Eur. J. Neurol. 2017. [Google Scholar] [CrossRef]
- Fermin, A.M.; Afzal, U.; Culebras, A. Sleep in Neuromuscular Diseases. Sleep Med. Clin. 2016, 11, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.I.; Ansevin, C.; Siciliano, G. The Role of Sleep in Neuromuscular Disorders. Front. Neurol. 2023, 14, 1195302. [Google Scholar] [CrossRef]
- Yang, S.; Miglis, M.G.; Jaradeh, S.; Muppidi, S. Myasthenia Symptom Burden, Fatigue, and Sleep: Are They Related? J. Clin. Neuromuscul. Dis. 2021, 22, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Morse, C.I.; Onambele-Pearson, G.; Edwards, B.; Wong, S.C.; Jacques, M.F. Objective and Subjective Measures of Sleep in Men with Muscular Dystrophy. PLoS ONE 2022, 17, e0274970. [Google Scholar] [CrossRef] [PubMed]
- Della Marca, G.; Frusciante, R.; Dittoni, S.; Vollono, C.; Buccarella, C.; Iannaccone, E.; Rossi, M.; Scarano, E.; Pirronti, T.; Cianfoni, A.; et al. Sleep Disordered Breathing in Facioscapulohumeral Muscular Dystrophy. J. Neurol. Sci. 2009, 285, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Laberge, L.; Maltais, A.; Auclair, J.; Mathieu, J.; Gagnon, C. Evolution of Sleep Complaints in Myotonic Dystrophy Type 1: A 9-Year Longitudinal Study. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2023, 1–3. [Google Scholar] [CrossRef]
- Boentert, M.; Karabul, N.; Wenninger, S.; Stubbe-Drager, B.; Mengel, E.; Schoser, B.; Young, P. Sleep-Related Symptoms and Sleep-Disordered Breathing in Adult Pompe Disease. Eur. J. Neurol. 2015, 22, 369–376.e27. [Google Scholar] [CrossRef]
- Neu, D.; Mairesse, O.; Hoffmann, G.; Valsamis, J.B.; Verbanck, P.; Linkowski, P.; Le Bon, O. Do “sleepy” and “Tired” Go Together? Rasch Analysis of the Relationships between Sleepiness, Fatigue and Nonrestorative Sleep Complaints in a Nonclinical Population Sample. Neuroepidemiology 2010, 35, 1–11. [Google Scholar] [CrossRef]
- Theadom, A.; Rodrigues, M.; Ranta, A.; Poke, G.; Love, D.; Jones, K.; Ao, B.T.; Hammond-Tooke, G.; Parmar, P.; O’Grady, G.; et al. Impact and Predictors of Quality of Life in Adults Diagnosed with a Genetic Muscle Disorder: A Nationwide Population-Based Study. Qual. Life Res. 2022, 31, 1657–1666. [Google Scholar] [CrossRef]
- Boentert, M.; Brenscheidt, I.; Glatz, C.; Young, P. Effects of Non-Invasive Ventilation on Objective Sleep and Nocturnal Respiration in Patients with Amyotrophic Lateral Sclerosis. J. Neurol. 2015, 262, 2073–2082. [Google Scholar] [CrossRef]
- Crescimanno, G.; Misuraca, A.; Purrazzella, G.; Greco, F.; Marrone, O. Subjective Sleep Quality in Stable Neuromuscular Patients under Non-Invasive Ventilation. Sleep Med. 2014, 15, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Crescimanno, G.; Greco, F.; D’Alia, R.; Messina, L.; Marrone, O. Quality of Life in Long Term Ventilated Adult Patients with Duchenne Muscular Dystrophy. Neuromuscul. Disord. NMD 2019, 29, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Atkeson, A.D.; RoyChoudhury, A.; Harrington-Moroney, G.; Shah, B.; Mitsumoto, H.; Basner, R.C. Patient-Ventilator Asynchrony with Nocturnal Noninvasive Ventilation in ALS. Neurology 2011, 77, 549–555. [Google Scholar] [CrossRef]
- Zizzi, C.E.; Luebbe, E.; Mongiovi, P.; Hunter, M.; Dilek, N.; Garland, C.; Ciafaloni, E.; Zaidman, C.M.; Kissel, J.T.; McDermott, M.P.; et al. The Spinal Muscular Atrophy Health Index: A Novel Outcome for Measuring How a Patient Feels and Functions. Muscle Nerve 2021, 63, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Costa, J.F.; Branas-Pampillón, M.; Medina-Cantillo, J.; Povedano, M.; Pitarch-Castellano, I.; López-Lobato, M.; Fernández-Ramos, J.A.; Lafuente-Hidalgo, M.; Rojas-García, R.; Caballero-Caballero, J.M.; et al. Validation of a Set of Instruments to Assess Patient- and Caregiver-Oriented Measurements in Spinal Muscular Atrophy: Results of the SMA-TOOL Study. Neurol. Ther. 2023, 12, 89–105. [Google Scholar] [CrossRef]
- Bourke, S.C.; McColl, E.; Shaw, P.J.; Gibson, G.J. Validation of Quality of Life Instruments in ALS. Amyotroph. Lateral Scler. Mot. Neuron. Disord. 2004, 5, 55–60. [Google Scholar] [CrossRef]
- Brizzi, K.T.; Bridges, J.F.P.; Yersak, J.; Balas, C.; Thakur, N.; Galvin, M.; Hardiman, O.; Heatwole, C.; Ravits, J.; Simmons, Z.; et al. Understanding the Needs of People with ALS: A National Survey of Patients and Caregivers. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 355–363. [Google Scholar] [CrossRef]
- Maksymowicz-Śliwińska, A.; Lulé, D.; NieporĘcki, K.; Ciećwierska, K.; Ludolph, A.C.; Kuźma-Kozakiewicz, M. The Quality of Life and Depression in Primary Caregivers of Patients with Amyotrophic Lateral Sclerosis Is Affected by Patient-Related and Culture-Specific Conditions. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 317–326. [Google Scholar] [CrossRef]
- Schoser, B.; Laforêt, P.; Kruijshaar, M.E.; Toscano, A.; van Doorn, P.A.; van der Ploeg, A.T.; European POmpe Consortium (EPOC). Minutes of the European pompe consortium (EPOC) meeting march 27 to 28, 2015, Munich, Germany. Acta Myol. 2015, 34, 141–143. [Google Scholar]
- Löscher, W.N.; Huemer, M.; Stulnig, T.M.; Simschitz, P.; Iglseder, S.; Eggers, C.; Moser, H.; Möslinger, D.; Freilinger, M.; Lagler, F.; et al. Pompe Disease in Austria: Clinical, Genetic and Epidemiological Aspects. J. Neurol. 2018, 265, 159–164. [Google Scholar] [CrossRef]
- Troha Gergeli, A.; Neubauer, D.; Golli, T.; Butenko, T.; Loboda, T.; Maver, A.; Osredkar, D. Prevalence and Genetic Subtypes of Congenital Myasthenic Syndromes in the Pediatric Population of Slovenia. Eur. J. Paediatr. Neurol. 2020, 26, 34–38. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Third Edition, Text Revision (ICSD-3-TR). 2023. Available online: https://aasm.org/clinical-resources/international-classification-sleep-disorders/ (accessed on 15 August 2023).
- Rajabally, Y.A.; Delmont, E.; Hiew, F.L.; Aubé-Nathier, A.-C.; Grapperon, A.-M.; Cassereau, J.; Attarian, S. Prevalence, Correlates and Impact of Pain and Cramps in Anti-MAG Neuropathy: A Multicentre European Study. Eur. J. Neurol. 2018, 25, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Stephens, H.E.; Joyce, N.C.; Oskarsson, B. National Study of Muscle Cramps in ALS in the USA. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 32–36. [Google Scholar] [CrossRef]
- Blyton, F.; Chuter, V.; Walter, K.E.; Burns, J. Non-Drug Therapies for Lower Limb Muscle Cramps. Cochrane Database Syst. Rev. 2012, 1, CD008496. [Google Scholar] [CrossRef]
- El-Tawil, S.; Al Musa, T.; Valli, H.; Lunn, M.P.T.; Brassington, R.; El-Tawil, T.; Weber, M. Quinine for Muscle Cramps. Cochrane Database Syst. Rev. 2015, CD005044. [Google Scholar] [CrossRef] [PubMed]
- Fardet, L.; Nazareth, I.; Petersen, I. Association Between Long-Term Quinine Exposure and All-Cause Mortality. JAMA 2017, 317, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.D.; Macklin, E.A.; Simmons, Z.; Knox, A.S.; Greenblatt, D.J.; Atassi, N.; Graves, M.; Parziale, N.; Salameh, J.S.; Quinn, C.; et al. A Randomized Trial of Mexiletine in ALS. Neurology 2016, 86, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Kerstens, H.C.J.W.; Van Lith, B.J.H.; Nijkrake, M.J.; De Swart, B.J.M.; Van den Bemd, L.A.C.; Smeets, R.J.E.M.; Klemens, F.; Van de Warrenburg, B.P.C.; Van der Wees, P.J.; Geurts, A.C.H. Healthcare Needs, Expectations, Utilization, and Experienced Treatment Effects in Patients with Hereditary Spastic Paraplegia: A Web-Based Survey in the Netherlands. Orphanet J. Rare Dis. 2021, 16, 283. [Google Scholar] [CrossRef]
- Chou, R.; Peterson, K.; Helfand, M. Comparative Efficacy and Safety of Skeletal Muscle Relaxants for Spasticity and Musculoskeletal Conditions: A Systematic Review. J. Pain Symptom Manag. 2004, 28, 140–175. [Google Scholar] [CrossRef]
- Lapeyre, E.; Kuks, J.B.M.; Meijler, W.J. Spasticity: Revisiting the Role and the Individual Value of Several Pharmacological Treatments. NeuroRehabilitation 2010, 27, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Bhai, S.F.; Brown, A.; Gajewski, B.; Kimminau, K.S.; Waitman, L.R.; Pasnoor, M.; Barohn, R.J. PAIN-CONTRoLS Study Team A Secondary Analysis of PAIN-CONTRoLS: Pain’s Impact on Sleep, Fatigue, and Activities of Daily Living. Muscle Nerve 2022, 66, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.; Eriksson, M.E.V.; Gribble, L.; Gouni, R.; Johnsen, S.; Coppini, D.V.; Kerr, D. Randomized, Placebo-Controlled Comparison of Amitriptyline, Duloxetine, and Pregabalin in Patients with Chronic Diabetic Peripheral Neuropathic Pain: Impact on Pain, Polysomnographic Sleep, Daytime Functioning, and Quality of Life. Diabetes Care 2012, 35, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, C.; Dueñas, M.; Barrera, C.; Moratalla, G.; Failde, I. Sleep Characteristics in Diabetic Patients Depending on the Occurrence of Neuropathic Pain and Related Factors. Int. J. Environ. Res. Public Health 2020, 17, 8125. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Mora, G.; Lauria, G. Pain in Amyotrophic Lateral Sclerosis. Lancet Neurol. 2017, 16, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Hurwitz, N.; Radakovic, R.; Boyce, E.; Peryer, G. Prevalence of Pain in Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 449–458. [Google Scholar] [CrossRef]
- Abresch, R.T.; Carter, G.T.; Jensen, M.P.; Kilmer, D.D. Assessment of Pain and Health-Related Quality of Life in Slowly Progressive Neuromuscular Disease. Am. J. Hosp. Palliat. Care 2002, 19, 39–48. [Google Scholar] [CrossRef]
- Kelly, C.R.; Saw, J.-L.; Thapa, P.; Mandrekar, J.; Naddaf, E. Systemic Manifestations and Symptom Burden of Facioscapulohumeral Muscular Dystrophy in a Referral Cohort. Muscle Nerve 2022, 65, 415–421. [Google Scholar] [CrossRef]
- Stokholm, R.N.; Handberg, C.; Knudsen, L.F. Prevalence of Chronic Pain in a National Cohort of Patients with Limb-Girdle Muscular Dystrophy: A Cross-Sectional Study. Disabil. Rehabil. 2022, 44, 7802–7810. [Google Scholar] [CrossRef]
- Kim, A.; Park, M.; Shin, H.-I. Pain Characteristics among Individuals with Duchenne Muscular Dystrophy According to Their Clinical Stage. BMC Musculoskelet. Disord. 2022, 23, 536. [Google Scholar] [CrossRef]
- van Vliet, J.; Tieleman, A.A.; Verrips, A.; Timmerman, H.; van Dongen, R.T.M.; van Engelen, B.G.M.; Wilder-Smith, O.H.G. Qualitative and Quantitative Aspects of Pain in Patients With Myotonic Dystrophy Type 2. J. Pain 2018, 19, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Ribiere, C.; Bernardin, M.; Sacconi, S.; Delmont, E.; Fournier-Mehouas, M.; Rauscent, H.; Benchortane, M.; Staccini, P.; Lantéri-Minet, M.; Desnuelle, C. Pain Assessment in Charcot-Marie-Tooth (CMT) Disease. Ann. Phys. Rehabil. Med. 2012, 55, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Nader, A.; Pirvulescu, I.; Pynadath, A.; Rahavard, B.B.; Candido, K.D. Circadian Pain Patterns in Human Pain Conditions—A Systematic Review. Pain Pract. Off. J. World Inst. Pain 2023, 23, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal Tunnel Syndrome: Clinical Features, Diagnosis, and Management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef]
- Attal, N.; Cruccu, G.; Baron, R.; Haanpää, M.; Hansson, P.; Jensen, T.S.; Nurmikko, T. European Federation of Neurological Societies EFNS Guidelines on the Pharmacological Treatment of Neuropathic Pain: 2010 Revision. Eur. J. Neurol. 2010, 17, 1113-e88. [Google Scholar] [CrossRef]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-Based Medicines for Chronic Neuropathic Pain in Adults. Cochrane Database Syst. Rev. 2018, 3, CD012182. [Google Scholar] [CrossRef]
- Derry, S.; Rice, A.S.; Cole, P.; Tan, T.; Moore, R.A. Topical Capsaicin (High Concentration) for Chronic Neuropathic Pain in Adults. Cochrane Database Syst. Rev. 2017, 1, CD007393. [Google Scholar] [CrossRef]
- Akyuz, G.; Kenis, O. Physical Therapy Modalities and Rehabilitation Techniques in the Management of Neuropathic Pain. Am. J. Phys. Med. Rehabil. 2014, 93, 253–259. [Google Scholar] [CrossRef]
- Didriksen, M.; Rigas, A.S.; Allen, R.P.; Burchell, B.J.; Di Angelantonio, E.; Nielsen, M.H.; Jennum, P.; Werge, T.; Erikstrup, C.; Pedersen, O.B.; et al. Prevalence of Restless Legs Syndrome and Associated Factors in an Otherwise Healthy Population: Results from the Danish Blood Donor Study. Sleep Med. 2017, 36, 55–61. [Google Scholar] [CrossRef]
- Boentert, M.; Knop, K.; Schuhmacher, C.; Gess, B.; Okegwo, A.; Young, P. Sleep Disorders in Charcot-Marie-Tooth Disease Type 1. J. Neurol. Neurosurg. Psychiatry 2014, 85, 319–325. [Google Scholar] [CrossRef]
- Gemignani, F.; Marbini, A.; Di Giovanni, G.; Salih, S.; Terzano, M.G. Charcot-Marie-Tooth Disease Type 2 with Restless Legs Syndrome. Neurology 1999, 52, 1064–1066. [Google Scholar] [CrossRef]
- Teodoro, T.; Viana, P.; Abreu, D.; Conceição, I.; Peralta, R.; Ferreira, J.J. A Peripheral Pathway to Restless Legs Syndrome? Clues from Familial Amyloid Polyneuropathy. Park. Relat. Disord. 2015, 21, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Hattan, E.; Chalk, C.; Postuma, R.B. Is There a Higher Risk of Restless Legs Syndrome in Peripheral Neuropathy? Neurology 2009, 72, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Gemignani, F.; Brindani, F.; Vitetta, F.; Marbini, A.; Calzetti, S. Restless Legs Syndrome in Diabetic Neuropathy: A Frequent Manifestation of Small Fiber Neuropathy. J. Peripher. Nerv. Syst. 2007, 12, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Gemignani, F.; Marbini, A.; Di Giovanni, G.; Salih, S.; Margarito, F.P.; Pavesi, G.; Terzano, M.G. Cryoglobulinaemic Neuropathy Manifesting with Restless Legs Syndrome. J. Neurol. Sci. 1997, 152, 218–223. [Google Scholar] [CrossRef]
- Ning, P.; Mu, X.; Yang, X.; Li, T.; Xu, Y. Prevalence of Restless Legs Syndrome in People with Diabetes Mellitus: A Pooling Analysis of Observational Studies. eClinicalMedicine 2022, 46, 101357. [Google Scholar] [CrossRef]
- Lam, E.M.; Shepard, P.W.; St Louis, E.K.; Dueffert, L.G.; Slocumb, N.; McCarter, S.J.; Silber, M.H.; Boeve, B.F.; Olson, E.J.; Somers, V.K.; et al. Restless Legs Syndrome and Daytime Sleepiness Are Prominent in Myotonic Dystrophy Type 2. Neurology 2013, 81, 157–164. [Google Scholar] [CrossRef]
- Liu, S.; Shen, D.; Tai, H.; Su, N.; Ding, Q.; Fu, H.; Zhang, K.; Wang, Z.; Liu, M.; Huang, Y.; et al. Restless Legs Syndrome in Chinese Patients With Sporadic Amyotrophic Lateral Sclerosis. Front. Neurol. 2018, 9, 735. [Google Scholar] [CrossRef]
- Winkelman, J.W.; Armstrong, M.J.; Allen, R.P.; Chaudhuri, K.R.; Ondo, W.; Trenkwalder, C.; Zee, P.C.; Gronseth, G.S.; Gloss, D.; Zesiewicz, T. Practice Guideline Summary: Treatment of Restless Legs Syndrome in Adults: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2016, 87, 2585–2593. [Google Scholar] [CrossRef]
- Romigi, A.; Izzi, F.; Pisani, V.; Placidi, F.; Pisani, L.R.; Marciani, M.G.; Corte, F.; Panico, M.B.; Torelli, F.; Uasone, E.; et al. Sleep Disorders in Adult-Onset Myotonic Dystrophy Type 1: A Controlled Polysomnographic Study. Eur. J. Neurol. 2011, 18, 1139–1145. [Google Scholar] [CrossRef]
- Lo Coco, D.; Puligheddu, M.; Mattaliano, P.; Congiu, P.; Borghero, G.; Fantini, M.L.; La Bella, V.; Ferri, R. REM Sleep Behavior Disorder and Periodic Leg Movements during Sleep in ALS. Acta Neurol. Scand. 2017, 135, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Langenbruch, L.; Perez-Mengual, S.; Glatz, C.; Young, P.; Boentert, M. Disorders of Sleep in Spinal and Bulbar Muscular Atrophy (Kennedy’s Disease). Sleep Breath. 2020, 25, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine Task Force. Sleep-Related Breathing Disorders in Adults: Recommendations for Syndrome Definition and Measurement Techniques in Clinical Research. Sleep 1999, 22, 667–689. [Google Scholar] [CrossRef]
- Horner, R.L. Pathophysiology of Obstructive Sleep Apnea. J. Cardiopulm. Rehabil. Prev. 2008, 28, 289–298. [Google Scholar] [CrossRef]
- Friedman, M.; Hamilton, C.; Samuelson, C.G.; Lundgren, M.E.; Pott, T. Diagnostic Value of the Friedman Tongue Position and Mallampati Classification for Obstructive Sleep Apnea: A Meta-Analysis. Otolaryngol.—Head Neck Surg. 2013, 148, 540–547. [Google Scholar] [CrossRef]
- Nozoe, K.T.; Moreira, G.A.; Tolino, J.R.C.; Pradella-Hallinan, M.; Tufik, S.; Andersen, M.L. The Sleep Characteristics in Symptomatic Patients with Duchenne Muscular Dystrophy. Sleep Breath. Schlaf Atm. 2015, 19, 1051–1056. [Google Scholar] [CrossRef]
- Bianchi, M.L.; Losurdo, A.; Di Blasi, C.; Santoro, M.; Masciullo, M.; Conte, G.; Valenza, V.; Damiani, A.; Della Marca, G.; Silvestri, G. Prevalence and Clinical Correlates of Sleep Disordered Breathing in Myotonic Dystrophy Types 1 and 2. Sleep Breath. 2014, 18, 579–589. [Google Scholar] [CrossRef]
- Punjabi, N.M. The Epidemiology of Adult Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef]
- Nicolle, M.W.; Rask, S.; Koopman, W.J.; George, C.F.P.; Adams, J.; Wiebe, S. Sleep Apnea in Patients with Myasthenia Gravis. Neurology 2006, 67, 140–142. [Google Scholar] [CrossRef]
- Boentert, M.; Glatz, C.; Helmle, C.; Okegwo, A.; Young, P. Prevalence of Sleep Apnoea and Capnographic Detection of Nocturnal Hypoventilation in Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 89, 418–424. [Google Scholar] [CrossRef]
- Pincherle, A.; Patruno, V.; Raimondi, P.; Moretti, S.; Dominese, A.; Martinelli-Boneschi, F.; Pasanisi, M.B.; Canioni, E.; Salerno, F.; Deleo, F.; et al. Sleep Breathing Disorders in 40 Italian Patients with Myotonic Dystrophy Type 1. Neuromuscul. Disord. NMD 2012, 22, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Spiesshoefer, J.; Runte, M.; Heidbreder, A.; Dreher, M.; Young, P.; Brix, T.; Boentert, M. Sleep-Disordered Breathing and Effects of Non-Invasive Ventilation on Objective Sleep and Nocturnal Respiration in Patients with Myotonic Dystrophy Type I. Neuromuscul. Disord. 2019, 29, 302–309. [Google Scholar] [CrossRef]
- Runte, M.; Spiesshoefer, J.; Heidbreder, A.; Dreher, M.; Young, P.; Brix, T.; Boentert, M. Sleep-Related Breathing Disorders in Facioscapulohumeral Dystrophy. Sleep Breath. 2019, 23, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Sakinci, O.; Ersoy, B.; Sezer, R.G.; Yilmaz, H. Assessment of Sleep-Related Breathing Disorders in Patients with Duchenne Muscular Dystrophy. J. Clin. Med. Res. 2012, 4, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef]
- Ramezani, R.J.; Stacpoole, P.W. Sleep Disorders Associated with Primary Mitochondrial Diseases. J. Clin. Sleep Med. 2014, 10, 1233–1239. [Google Scholar] [CrossRef]
- Randerath, W.; Verbraecken, J.; Andreas, S.; Arzt, M.; Bloch, K.E.; Brack, T.; Buyse, B.; De Backer, W.; Eckert, D.J.; Grote, L.; et al. Definition, Discrimination, Diagnosis and Treatment of Central Breathing Disturbances during Sleep. Eur. Respir. J. 2017, 49, 1600959. [Google Scholar] [CrossRef]
- Coniglio, A.C.; Mentz, R.J. Sleep Breathing Disorders in Heart Failure. Heart Fail. Clin. 2020, 16, 45–51. [Google Scholar] [CrossRef]
- Arbustini, E.; Di Toro, A.; Giuliani, L.; Favalli, V.; Narula, N.; Grasso, M. Cardiac Phenotypes in Hereditary Muscle Disorders: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2485–2506. [Google Scholar] [CrossRef]
- Voigt, J.; Emani, S.; Gupta, S.; Germany, R.; Khayat, R. Meta-Analysis Comparing Outcomes of Therapies for Patients With Central Sleep Apnea and Heart Failure With Reduced Ejection Fraction. Am. J. Cardiol. 2020, 127, 73–83. [Google Scholar] [CrossRef]
- Schwarz, E.I.; Scherff, F.; Haile, S.R.; Steier, J.; Kohler, M. Effect of Treatment of Central Sleep Apnea/Cheyne-Stokes Respiration on Left Ventricular Ejection Fraction in Heart Failure: A Network Meta-Analysis. J. Clin. Sleep Med. 2019, 15, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Osanai, S.; Akiba, Y.; Nakano, H.; Matsumoto, H.; Yahara, O.; Onodera, S. Charcot-Marie-Tooth Disease with Diaphragmatic Weakness. Intern. Med. Tokyo Jpn. 1992, 31, 1267–1270. [Google Scholar] [CrossRef][Green Version]
- McEnery, T.; Walsh, R.; Burke, C.; McGowan, A.; Faul, J.; Cormican, L. Phrenic Nerve Palsy Secondary to Parsonage-Turner Syndrome: A Diagnosis Commonly Overlooked. Lung 2017, 195, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Berger KI, R.D.M. Pathophysiology of Hypoventilation during Sleep. Sleep Med. Clin. 2014, 9, 289–300. [Google Scholar] [CrossRef]
- Ogna, A.; Quera Salva, M.A.; Prigent, H.; Mroue, G.; Vaugier, I.; Annane, D.; Lofaso, F.; Orlikowski, D. Nocturnal Hypoventilation in Neuromuscular Disease: Prevalence According to Different Definitions Issued from the Literature. Sleep Breath. 2016, 20, 575–581. [Google Scholar] [CrossRef]
- Georges, M.; Nguyen-Baranoff, D.; Griffon, L.; Foignot, C.; Bonniaud, P.; Camus, P.; Pepin, J.L.; Rabec, C. Usefulness of Transcutaneous PCO2 to Assess Nocturnal Hypoventilation in Restrictive Lung Disorders. Respirology 2016, 21, 1300–1306. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Khan, A.; Frazer-Green, L.; Amin, R.; Wolfe, L.; Faulkner, G.; Casey, K.; Sharma, G.; Selim, B.; Zielinski, D.; Aboussouan, L.S.; et al. Respiratory Management of Patients With Neuromuscular Weakness: An American College of Chest Physicians Clinical Practice Guideline and Expert Panel Report. Chest 2023, 164, 394–413. [Google Scholar] [CrossRef]
- Windisch, W.; Dreher, M.; Geiseler, J.; Siemon, K.; Brambring, J.; Dellweg, D.; Grolle, B.; Hirschfeld, S.; Kohnlein, T.; Mellies, U.; et al. Guidelines for Non-Invasive and Invasive Home Mechanical Ventilation for Treatment of Chronic Respiratory Failure—Update 2017. Pneumologie 2017, 71, 722–795. [Google Scholar] [CrossRef]
- Ambrosino, N.; Carpenè, N.; Gherardi, M. Chronic Respiratory Care for Neuromuscular Diseases in Adults. Eur. Respir. J. 2009, 34, 444–451. [Google Scholar] [CrossRef]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dubé, B.-P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS Statement on Respiratory Muscle Testing at Rest and during Exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef] [PubMed]
- Fromageot, C.; Lofaso, F.; Annane, D.; Falaize, L.; Lejaille, M.; Clair, B.; Gajdos, P.; Raphaël, J.C. Supine Fall in Lung Volumes in the Assessment of Diaphragmatic Weakness in Neuromuscular Disorders. Arch. Phy.s Med. Rehabil. 2001, 82, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Spiesshoefer, J.; Lutter, R.; Kabitz, H.-J.; Henke, C.; Herkenrath, S.; Randerath, W.; Young, P.; Dreher, M.; Görlich, D.; Boentert, M. Respiratory Muscle Function Tests and Diaphragm Ultrasound Predict Nocturnal Hypoventilation in Slowly Progressive Myopathies. Front. Neurol. 2021, 12, 731865. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Dräger, B.; Boentert, M. Validation of the Single Breath Count Test for Assessment of Inspiratory Muscle Strength in Healthy Subjects and People with Neuromuscular Disorders. J. Neuromuscul. Dis. 2023, 10, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Spiesshoefer, J.; Henke, C.; Herkenrath, S.; Brix, T.; Randerath, W.; Young, P.; Boentert, M. Transdiapragmatic Pressure and Contractile Properties of the Diaphragm Following Magnetic Stimulation. Respir. Physiol. Neurobiol. 2019, 266, 47–53. [Google Scholar] [CrossRef]
- Fayssoil, A.; Nguyen, L.S.; Stojkovic, T.; Prigent, H.; Carlier, R.; Amthor, H.; Bergounioux, J.; Zini, J.; Damez-Fontaine, S.; Wahbi, K.; et al. Determinants of Diaphragm Inspiratory Motion, Diaphragm Thickening, and Its Performance for Predicting Respiratory Restrictive Pattern in Duchenne Muscular Dystrophy. Muscle Nerve 2022, 65, 89–95. [Google Scholar] [CrossRef]
- Harlaar, L.; Ciet, P.; van Tulder, G.; Brusse, E.; Timmermans, R.G.M.; Janssen, W.G.M.; de Bruijne, M.; van der Ploeg, A.T.; Tiddens, H.A.W.M.; van Doorn, P.A.; et al. Diaphragmatic Dysfunction in Neuromuscular Disease, an MRI Study. Neuromuscul. Disord. NMD 2022, 32, 15–24. [Google Scholar] [CrossRef]
- Kushida, C.A.; Littner, M.R.; Morgenthaler, T.; Alessi, C.A.; Bailey, D.; Coleman, J.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice Parameters for the Indications for Polysomnography and Related Procedures: An Update for 2005. Sleep 2005, 28, 499–521. [Google Scholar] [CrossRef]
- Storre, J.H.; Magnet, F.S.; Dreher, M.; Windisch, W. Transcutaneous Monitoring as a Replacement for Arterial PCO(2) Monitoring during Nocturnal Non-Invasive Ventilation. Respir. Med. 2011, 105, 143–150. [Google Scholar] [CrossRef]
- Palot, A.; Nguyên, X.-L.; Launois, S.; Prigent, A.; Graml, A.; Aversenq, E.; Koltes, C.; Recart, D.; Lavergne, F. Effect of Switching from Continuous to Bilevel Positive Airway Pressure on Sleep Quality in Patients with Obstructive Sleep Apnea: The Prospective POP IN VAuto Study. J. Thorac. Dis. 2023, 15, 918–927. [Google Scholar] [CrossRef]
- Andersen, P.M.; Abrahams, S.; Borasio, G.D.; de Carvalho, M.; Chio, A.; Van Damme, P.; Hardiman, O.; Kollewe, K.; Morrison, K.E.; Petri, S.; et al. EFNS Guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS)--Revised Report of an EFNS Task Force. Eur. J. Neurol. 2012, 19, 360–375. [Google Scholar] [CrossRef]
- Berry, R.B.; Chediak, A.; Brown, L.K.; Finder, J.; Gozal, D.; Iber, C.; Kushida, C.A.; Morgenthaler, T.; Rowley, J.A.; Davidson-Ward, S.L.; et al. Best Clinical Practices for the Sleep Center Adjustment of Noninvasive Positive Pressure Ventilation (NPPV) in Stable Chronic Alveolar Hypoventilation Syndromes. J. Clin. Sleep Med. 2010, 6, 491–509. [Google Scholar] [PubMed]
- Recommendations|Motor Neurone Disease: Assessment and Management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/NG42/chapter/recommendations#non-invasive-ventilation (accessed on 17 January 2021).
- Kleopa, K.A.; Sherman, M.; Neal, B.; Romano, G.J.; Heiman-Patterson, T. Bipap Improves Survival and Rate of Pulmonary Function Decline in Patients with ALS. J. Neurol. Sci. 1999, 164, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Carratu, P.; Spicuzza, L.; Cassano, A.; Maniscalco, M.; Gadaleta, F.; Lacedonia, D.; Scoditti, C.; Boniello, E.; Di Maria, G.; Resta, O. Early Treatment with Noninvasive Positive Pressure Ventilation Prolongs Survival in Amyotrophic Lateral Sclerosis Patients with Nocturnal Respiratory Insufficiency. Orphanet J. Rare Dis. 2009, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Boussaïd, G.; Prigent, H.; Laforet, P.; Raphaël, J.-C.; Annane, D.; Orlikowski, D.; Lofaso, F. Effect and Impact of Mechanical Ventilation in Myotonic Dystrophy Type 1: A Prospective Cohort Study. Thorax 2018, 73, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Miura, T.; Ishikawa, Y.; Aoyagi, T.; Ogata, H.; Hamada, S.; Minami, R. Duchenne Muscular Dystrophy: Survival by Cardio-Respiratory Interventions. Neuromuscul. Disord. 2011, 21, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Gregoretti, C.; Ottonello, G.; Chiarini Testa, M.B.; Mastella, C.; Ravà, L.; Bignamini, E.; Veljkovic, A.; Cutrera, R. Survival of Patients with Spinal Muscular Atrophy Type 1. Pediatrics 2013, 131, e1509–e1514. [Google Scholar] [CrossRef]
- Annane, D.; Orlikowski, D.; Chevret, S. Nocturnal Mechanical Ventilation for Chronic Hypoventilation in Patients with Neuromuscular and Chest Wall Disorders. Cochrane Database Syst. Rev. 2014, CD001941. [Google Scholar] [CrossRef]
- Vrijsen, B.; Buyse, B.; Belge, C.; Robberecht, W.; Van Damme, P.; Decramer, M.; Testelmans, D. Noninvasive Ventilation Improves Sleep in Amyotrophic Lateral Sclerosis: A Prospective Polysomnographic Study. J. Clin. Sleep Med. 2015, 11, 559–566. [Google Scholar] [CrossRef]
- Boentert, M.; Drager, B.; Glatz, C.; Young, P. Sleep-Disordered Breathing and Effects of Noninvasive Ventilation in Patients with Late-Onset Pompe Disease. J. Clin. Sleep Med. 2016, 12, 1623–1632. [Google Scholar] [CrossRef]
- Crescimanno, G.; Canino, M.; Marrone, O. Asynchronies and Sleep Disruption in Neuromuscular Patients under Home Noninvasive Ventilation. Respir. Med. 2012, 106, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, B.; Buyse, B.; Belge, C.; Vanpee, G.; Van Damme, P.; Testelmans, D. Randomized Cross-over Trial of Ventilator Modes during Non-Invasive Ventilation Titration in Amyotrophic Lateral Sclerosis. Respirology 2017, 22, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Panyarath, P.; Adam, V.; Kimoff, R.J.; Kaminska, M. Alveolar Ventilation-Targeted Versus Spontaneous/Timed Mode for Home Noninvasive Ventilation in Amyotrophic Lateral Sclerosis. Respir. Care 2022, 67, 1109–1120. [Google Scholar] [CrossRef]
- Ramsay, M.; Mandal, S.; Suh, E.-S.; Steier, J.; Douiri, A.; Murphy, P.B.; Polkey, M.; Simonds, A.; Hart, N. Parasternal Electromyography to Determine the Relationship between Patient-Ventilator Asynchrony and Nocturnal Gas Exchange during Home Mechanical Ventilation Set-Up. Thorax 2015, 70, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Crescimanno, G.; Marrone, O.; Vianello, A. Efficacy and Comfort of Volume-Guaranteed Pressure Support in Patients with Chronic Ventilatory Failure of Neuromuscular Origin. Respirology 2011, 16, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Aarrestad, S.; Qvarfort, M.; Kleiven, A.L.; Tollefsen, E.; Skjønsberg, O.H.; Janssens, J.-P. Sleep Related Respiratory Events during Non-Invasive Ventilation of Patients with Chronic Hypoventilation. Respir. Med. 2017, 132, 210–216. [Google Scholar] [CrossRef]
- Schellhas, V.; Glatz, C.; Beecken, I.; Okegwo, A.; Heidbreder, A.; Young, P.; Boentert, M. Upper Airway Obstruction Induced by Non-Invasive Ventilation Using an Oronasal Interface. Sleep Breath. 2018, 22, 781–788. [Google Scholar] [CrossRef]
- Georges, M.; Attali, V.; Golmard, J.L.; Morelot-Panzini, C.; Crevier-Buchman, L.; Collet, J.M.; Tintignac, A.; Morawiec, E.; Trosini-Desert, V.; Salachas, F.; et al. Reduced Survival in Patients with ALS with Upper Airway Obstructive Events on Non-Invasive Ventilation. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1045–1050. [Google Scholar] [CrossRef]
- Ogna, A.; Nardi, J.; Prigent, H.; Quera Salva, M.A.; Chaffaut, C.; Lamothe, L.; Chevret, S.; Annane, D.; Orlikowski, D.; Lofaso, F. Prognostic Value of Initial Assessment of Residual Hypoventilation Using Nocturnal Capnography in Mechanically Ventilated Neuromuscular Patients: A 5-Year Follow-up Study. Front. Med. Lausanne 2016, 3, 40. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Bermejo, J.; Morelot-Panzini, C.; Arnol, N.; Meininger, V.; Kraoua, S.; Salachas, F.; Similowski, T. Prognostic Value of Efficiently Correcting Nocturnal Desaturations after One Month of Non-Invasive Ventilation in Amyotrophic Lateral Sclerosis: A Retrospective Monocentre Observational Cohort Study. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 373–379. [Google Scholar] [CrossRef]
- Chatwin, M.; Simonds, A.K. Long-Term Mechanical Insufflation-Exsufflation Cough Assistance in Neuromuscular Disease: Patterns of Use and Lessons for Application. Respir. Care 2020, 65, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Stehling, F.; Bouikidis, A.; Schara, U.; Mellies, U. Mechanical Insufflation/Exsufflation Improves Vital Capacity in Neuromuscular Disorders. Chron. Respir. Dis. 2015, 12, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Spataro, R.; Lo Re, M.; Piccoli, T.; Piccoli, F.; La Bella, V. Causes and Place of Death in Italian Patients with Amyotrophic Lateral Sclerosis. Acta Neurol. Scand. 2010, 122, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Barthlen, G.M.; Lange, D.J. Unexpectedly Severe Sleep and Respiratory Pathology in Patients with Amyotrophic Lateral Sclerosis. Eur. J. Neurol. 2000, 7, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F.; Henderson, R.D.; Morrison, S.C.; Brown, M.; Hutchinson, N.; Douglas, J.A.; Robinson, P.J.; McCombe, P.A. Use of Respiratory Function Tests to Predict Survival in Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. 2010, 11, 194–202. [Google Scholar] [CrossRef]
- Pinto, S.; Pinto, A.; de Carvalho, M. Phrenic Nerve Studies Predict Survival in Amyotrophic Lateral Sclerosis. Clin. Neurophysiol. 2012, 123, 2454–2459. [Google Scholar] [CrossRef]
- Polkey, M.I.; Lyall, R.A.; Yang, K.; Johnson, E.; Leigh, P.N.; Moxham, J. Respiratory Muscle Strength as a Predictive Biomarker for Survival in Amyotrophic Lateral Sclerosis. Am. J. Respir. Crit. Care Med. 2017, 195, 86–95. [Google Scholar] [CrossRef]
- Bote, S.M.; Martinez, N.P.; Amarilla, C.E.; Ugalde, P.F.; Gonzalez-Bermejo, J.; Collado, N.F.; Gamez, B.J. Overnight Pulse Oximetry to Determine Prognostic Factors in Subjects With Amyotrophic Lateral Sclerosis. Respir. Care 2020, 65, 1128–1134. [Google Scholar] [CrossRef]
- Capozzo, R.; Quaranta, V.N.; Pellegrini, F.; Fontana, A.; Copetti, M.; Carratù, P.; Panza, F.; Cassano, A.; Falcone, V.A.; Tortelli, R.; et al. Sniff Nasal Inspiratory Pressure as a Prognostic Factor of Tracheostomy or Death in Amyotrophic Lateral Sclerosis. J. Neurol. 2015, 262, 593–603. [Google Scholar] [CrossRef]
- Atalaia, A.; De Carvalho, M.; Evangelista, T.; Pinto, A. Sleep Characteristics of Amyotrophic Lateral Sclerosis in Patients with Preserved Diaphragmatic Function. Amyotroph. Lateral Scler. 2007, 8, 101–105. [Google Scholar] [CrossRef]
- Congiu, P.; Mariani, S.; Milioli, G.; Parrino, L.; Tamburrino, L.; Borghero, G.; Defazio, G.; Pereira, B.; Fantini, M.L.; Puligheddu, M. Sleep Cardiac Dysautonomia and EEG Oscillations in Amyotrophic Lateral Sclerosis. Sleep 2019, 42, zsz164. [Google Scholar] [CrossRef] [PubMed]
- Puligheddu, M.; Congiu, P.; Aricò, D.; Rundo, F.; Borghero, G.; Marrosu, F.; Fantini, M.L.; Ferri, R. Isolated Rapid Eye Movement Sleep without Atonia in Amyotrophic Lateral Sclerosis. Sleep Med. 2016, 26, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, F.G.; Schelhaas, H.J.; Lammers, G.J.; Verbeek, M.M.; Overeem, S. CSF Hypocretin-1 Levels Are Normal in Patients with Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. 2009, 10, 487–489. [Google Scholar] [CrossRef]
- Meola, G. Clinical Aspects, Molecular Pathomechanisms and Management of Myotonic Dystrophies. Acta Myol. 2013, 32, 154–165. [Google Scholar]
- Summ, O.; Mathys, C.; Grimm, T.; Groß, M. Central Bradypnea and Ataxic Breathing in Myotonic Dystrophy Type 1—A Clinical Case Report. J. Neuromuscul. Dis. 2023, 10, 465–471. [Google Scholar] [CrossRef]
- Yu, H.; Laberge, L.; Jaussent, I.; Bayard, S.; Scholtz, S.; Raoul, M.; Pages, M.; Dauvilliers, Y. Daytime Sleepiness and REM Sleep Characteristics in Myotonic Dystrophy: A Case-Control Study. Sleep 2011, 34, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, E.; Carnicelli, L.; Crapanzano, D.; Maestri, M.; Simoncini, C.; Baldanzi, S.; Falorni, M.; Garbarino, S.; Mancuso, M.; Bonuccelli, U.; et al. Disruption of Sleep-Wake Continuum in Myotonic Dystrophy Type 1: Beyond Conventional Sleep Staging. Neuromuscul. Disord. NMD 2018, 28, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Laberge, L.; Begin, P.; Dauvilliers, Y.; Beaudry, M.; Laforte, M.; Jean, S.; Mathieu, J. A Polysomnographic Study of Daytime Sleepiness in Myotonic Dystrophy Type 1. J. Neurol. Neurosurg. Psychiatry 2009, 80, 642–646. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, J.E.; Lin, L.; Iranzo, A.; Genis, D.; Martí, M.J.; Santamaria, J.; Mignot, E. Decreased Hypocretin-1 (Orexin-A) Levels in the Cerebrospinal Fluid of Patients with Myotonic Dystrophy and Excessive Daytime Sleepiness. Sleep 2003, 26, 287–290. [Google Scholar] [CrossRef]
- Ciafaloni, E.; Mignot, E.; Sansone, V.; Hilbert, J.E.; Lin, L.; Lin, X.; Liu, L.C.; Pigeon, W.R.; Perlis, M.L.; Thornton, C.A. The Hypocretin Neurotransmission System in Myotonic Dystrophy Type 1. Neurology 2008, 70, 226–230. [Google Scholar] [CrossRef]
- Liguori, C.; Spanetta, M.; Fernandes, M.; Placidi, F.; Massa, R.; Romigi, A.; Izzi, F.; Mauro, L.; Greco, G.; Frezza, E.; et al. The Actigraphic Documentation of Circadian Sleep-Wake Rhythm Dysregulation in Myotonic Dystrophy Type 1. Sleep Med. 2021, 88, 134–139. [Google Scholar] [CrossRef]
- Winblad, S.; Samuelsson, L.; Lindberg, C.; Meola, G. Cognition in Myotonic Dystrophy Type 1: A 5-Year Follow-up Study. Eur. J. Neurol. 2016, 23, 1471–1476. [Google Scholar] [CrossRef]
- Peric, S.; Bjelica, B.; Bozovic, I.; Pesovic, J.; Paunic, T.; Banovic, M.; Brkusanin, M.; Aleksic, K.; Basta, I.; Pavicevic, D.S.; et al. Fatigue in Myotonic Dystrophy Type 1: A Seven-Year Prospective Study. Acta Myol. 2019, 38, 239–244. [Google Scholar] [PubMed]
- Wintzen, A.R.; Lammers, G.J.; van Dijk, J.G. Does Modafinil Enhance Activity of Patients with Myotonic Dystrophy: A Double-Blind Placebo-Controlled Crossover Study. J. Neurol. 2007, 254, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Orlikowski, D.; Chevret, S.; Quera-Salva, M.A.; Laforêt, P.; Lofaso, F.; Verschueren, A.; Pouget, J.; Eymard, B.; Annane, D. Modafinil for the Treatment of Hypersomnia Associated with Myotonic Muscular Dystrophy in Adults: A Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled, 4-Week Trial. Clin. Ther. 2009, 31, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
| Etiology | Anatomical Origin | ||
|---|---|---|---|
| Skeletal Muscle | Peripheral Nerve and Neuromuscular Junction | Anterior Horn Cells | |
| Inflammatory | Poly-/Dermatomyositis, Necrotizing Myositis, Sporadic Inclusion Body Myositis | Guillain–Barré Syndrome, Chronic Inflammatory Demyelinating Neuropathy, Multifocal Motor Neuropathy, Systemic vasculitis; NMJ; Ocular myasthenia, Myasthenia gravis | Vasculitis |
| Infectious | Streptococci, Trichinosis, HIV, Influenza A/B, Epstein-Barr Virus | Lyme Disease, Leprosy | Lyme Disease, Leprosy |
| Toxic | Ethanol, Statins, Fibrates, Steroids, Critical Illness Myopathy | Ethanol, Chemotherapeutics, Critical Illness Neuropathy, Renal Failure, Liver Disease, Chloroquine, D-penicillamine, Gentamicin, Quinidine, Botulinum Toxin | |
| Metabolic | Vitamin D Deficiency, Hypokalemia, Hyperkalemia | Vitamin deficiencies (B1, B6, B12, Folic acid) | - |
| Endocrine | Hypothyroidism, Hyperparathyroidism, Hypercortisolism | Hypothyroidism, Diabetes Mellitus | Diabetic Radiculopathy or Plexopathy |
| Degenerative | Inclusion Body Myositis (Sporadic) | Idiopathic Polyneuropathy | Amyotrophic Lateral Sclerosis |
| Hereditary | Muscular dystrophies, Congenital Myopathies, Metabolic Myopathies, Mitochondrial Myopathies, Myotonic myopathies, Hereditary Inclusion Body Myositis | Charcot–Marie–Tooth Disease, Fabry’s Disease, Transthyretin Familial Amyloid Neuropathy; NMJ; Congenital Myasthenic Syndromes | Spinal Muscular Atrophy, Bulbar and Spinal Muscular Atrophy (Kennedy’s Disease), Amyotrophic Lateral Sclerosis (Familial) |
| Idiopathic | |||
| Condition | Prevalence (Range or Estimate) | |
|---|---|---|
| Muscle | DMD | 0.70–4.70 |
| BMD | 0.07–3.65 | |
| Myotonic dystrophies | 7.1–26.5 | |
| FSHD | 2.03–6.8 | |
| LGMD | 0.81–6.9 | |
| Pompe disease | 0.28–0.35 | |
| Polymyositis | 3.45–9.7 | |
| Dermatomyositis | 1.97–21.42 | |
| Peripheral nerve and NMJ | MG | 5.35–35.0 |
| LEMS | 0.23–0.40 | |
| CMS | 2.22 (per 100,000 children) | |
| CMT | 3.10–82.30 | |
| CIDP | 0.67–8.90 | |
| Anterior horn cells | ALS | 1.07–11.31 |
| SMA | 1.30–3.20 |
| Condition | Prevalence | |
|---|---|---|
| MG | 36% | [81] |
| CMT1 | 38% | [62] |
| ALS | 45.6% | [82] |
| SBMA | 61% | [74] |
| DM1 | 41–60% | [79,83,84] |
| FSHD | 55%; 25% | [16,85] |
| DMD | 16.6%; 18% | [78,86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boentert, M. Sleep Disorders in Neuromuscular Diseases: A Narrative Review. Clin. Transl. Neurosci. 2023, 7, 23. https://doi.org/10.3390/ctn7030023
Boentert M. Sleep Disorders in Neuromuscular Diseases: A Narrative Review. Clinical and Translational Neuroscience. 2023; 7(3):23. https://doi.org/10.3390/ctn7030023
Chicago/Turabian StyleBoentert, Matthias. 2023. "Sleep Disorders in Neuromuscular Diseases: A Narrative Review" Clinical and Translational Neuroscience 7, no. 3: 23. https://doi.org/10.3390/ctn7030023
APA StyleBoentert, M. (2023). Sleep Disorders in Neuromuscular Diseases: A Narrative Review. Clinical and Translational Neuroscience, 7(3), 23. https://doi.org/10.3390/ctn7030023






