Sleep and Stroke-Related Delirium: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria for Systematic Review
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Collection Process
2.5. Outcomes
2.6. Quality and Certainty of Evidence Assessment
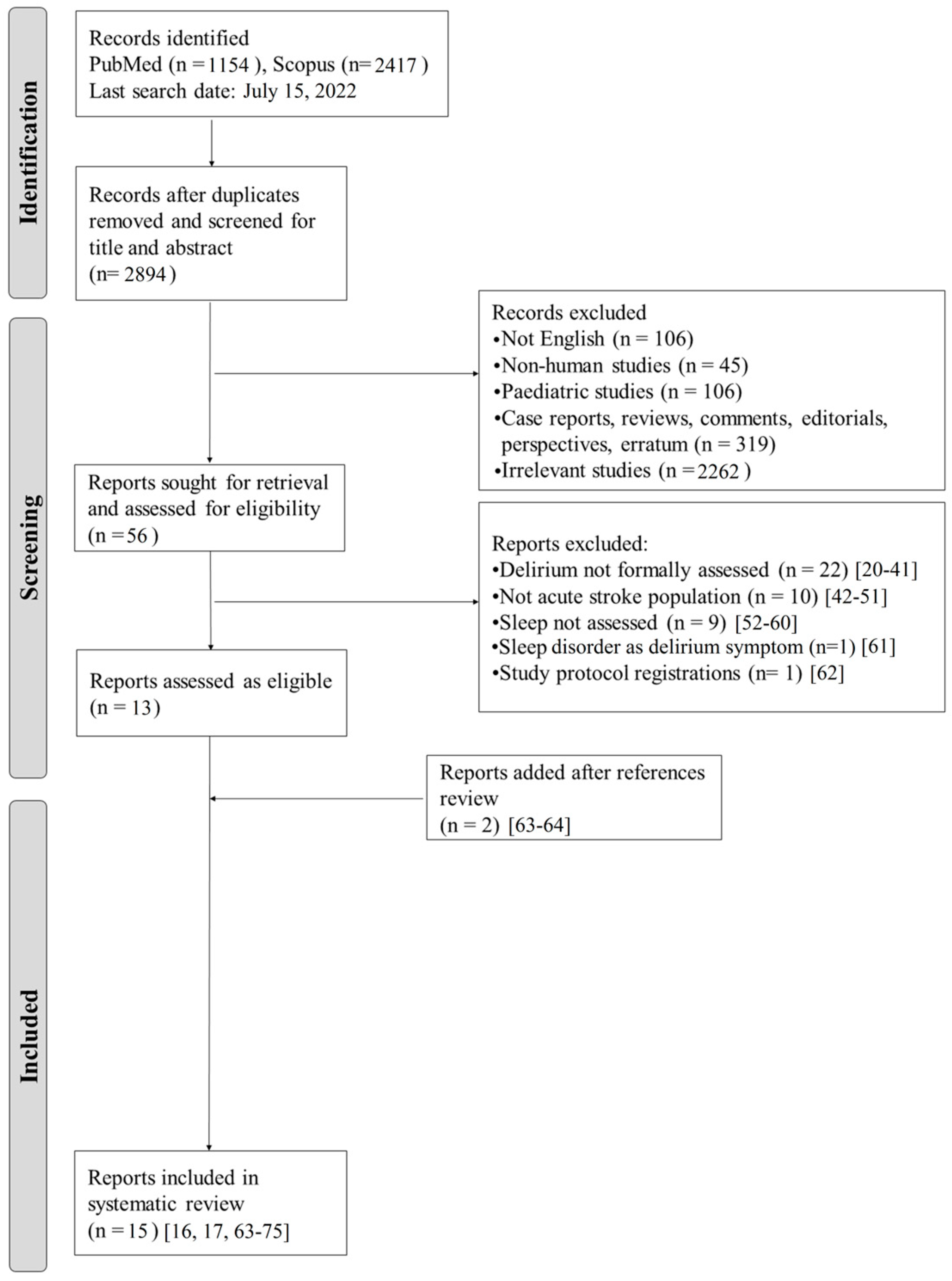
3. Results
3.1. Study Selection
3.2. Study Characteristics
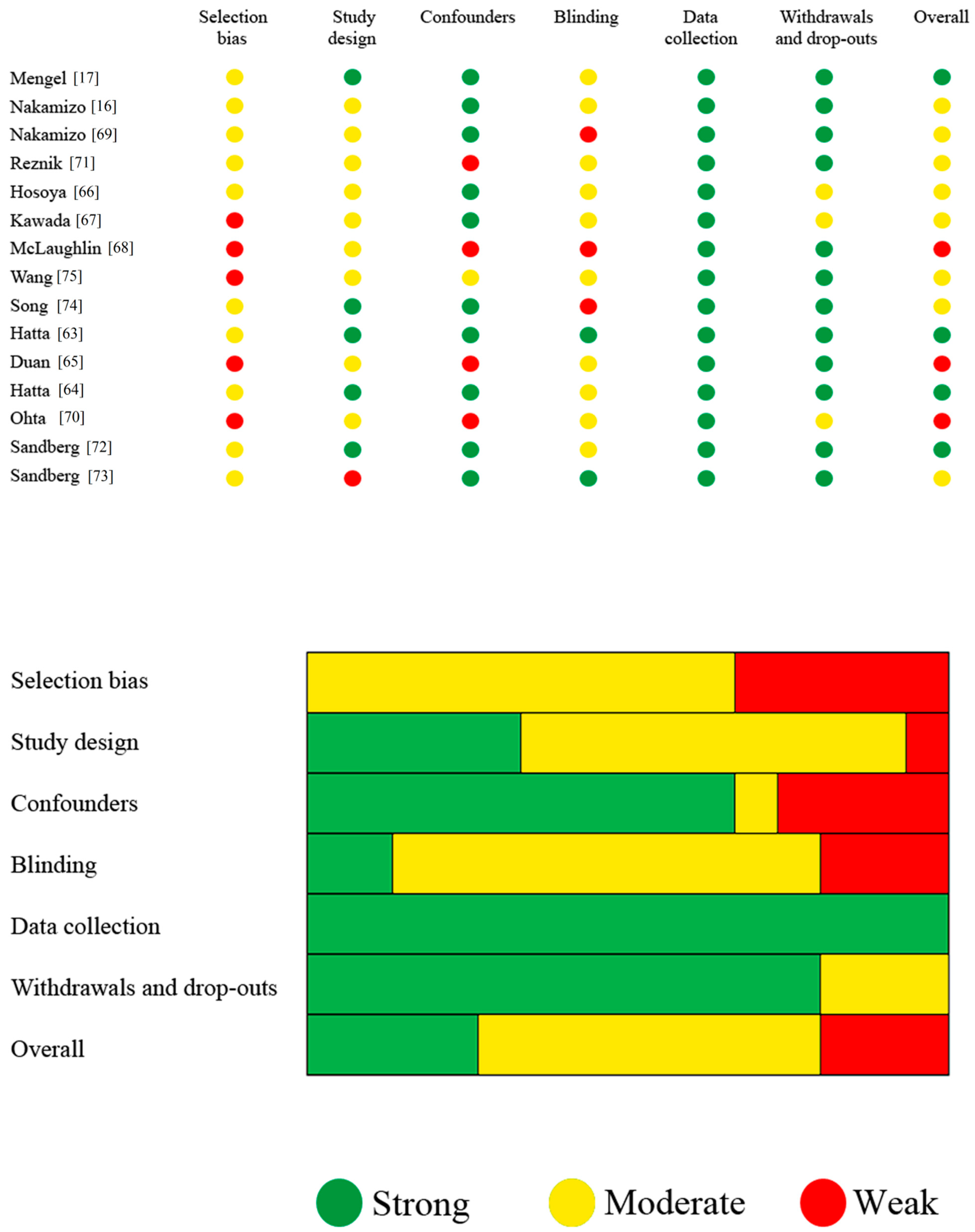
3.3. Study Quality Assessment
3.4. Outcome Measures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAM-ICU | Cognitive Assessment Method-Intensive Care Unit |
| DOS | Delirium Observation Screening |
| DRS-R98 | Delirium Rating Scale-Revised 98 |
| DSM-IV | Diagnostic and Statistical Manual of Mental Disorders IV Edition |
| DSM-V | Diagnostic and Statistical Manual of Mental Disorders V Edition |
| EPHPP | Effective Public Healthcare Practice Project |
| GABAR-A | Gamma aminobutyric acid receptor agonist |
| ICDSC | Intensive Care Delirium Screening Checklist |
| ICU | Intensive Care Unit |
| nCPAP | nasal Continuous Positive Airway Pressure |
| NICU | Neuro Intensive Care Unit |
| OBS | Organic Brain Syndrome Scale |
| PICO | Population, Intervention, Comparison, Outcome |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| RASS | Richmond Agitation Sedation Scale |
| SDB | Sleep-disordered breathing |
| SU | Stroke unit |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Shaw, R.C.; Walker, G.; Elliott, E.; Quinn, T.J. Occurrence Rate of Delirium in Acute Stroke Settings: Systematic Review and Meta-Analysis. Stroke 2019, 50, 3028–3036. [Google Scholar] [CrossRef]
- Pasińska, P.; Wilk, A.; Kowalska, K.; Szyper-Maciejowska, A.; Klimkowicz-Mrowiec, A. The long-term prognosis of patients with delirium in the acute phase of stroke: PRospective Observational POLIsh Study (PROPOLIS). J. Neurol. 2019, 266, 2710–2717. [Google Scholar] [CrossRef]
- Rollo, E.; Brunetti, V.; Scala, I.; Callea, A.; Marotta, J.; Vollono, C.; Frisullo, G.; Broccolini, A.; Calabresi, P.; Della Marca, G. Impact of delirium on the outcome of stroke: A prospective, observational, cohort study. J. Neurol. 2022, in press. [Google Scholar] [CrossRef]
- Oldenbeuving, A.W.; de Kort, P.L.M.; Jansen, B.P.W.; Algra, A.; Kappelle, L.J.; Roks, G. Delirium in the acute phase after stroke: Incidence, risk factors, and outcome. Neurology 2011, 76, 993–999. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Telias, I.; Wilcox, M.E. Sleep and Circadian Rhythm in Critical Illness. Crit. Care. 2019, 23, 82. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.W.; Wang, L.; Lydic, R.; Baghdoyan, H.A.; Shi, X.Y.; Zhang, H. Promoting sleep and circadian health may prevent postoperative delirium: A systematic review and meta-analysis of randomized clinical trials. Sleep Med. Rev. 2019, 48, 101207. [Google Scholar] [CrossRef]
- Weinhouse, G.L.; Schwab, R.J.; Watson, P.L.; Patil, N.; Vaccaro, B.; Pandharipande, P.; Ely, E.W. Bench-to-bedside review: Delirium in ICU patients—Importance of sleep deprivation. Crit. Care 2009, 13, 234. [Google Scholar] [CrossRef]
- Brunetti, V.; Rollo, E.; Broccolini, A.; Frisullo, G.; Scala, I.; Della Marca, G. Sleep and Stroke: Opening Our Eyes to Current Knowledge of a Key Relationship. Curr. Neurol. Neurosci. Rep. 2022, 22, 767–779. [Google Scholar] [CrossRef]
- Jaramillo, V.; Jendoubi, J.; Maric, A.; Mensen, A.; Heyse, N.C.; Eberhard-Moscicka, A.K.; Wiest, R.; Bassetti, C.L.A.; Huber, R. Thalamic Influence on Slow Wave Slope Renormalization during Sleep. Ann. Neurol. 2021, 90, 821–833. [Google Scholar] [CrossRef]
- Gottlieb, E.; Landau, E.; Baxter, H.; Werden, E.; Howard, M.E.; Brodtmann, A. The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: A systematic review. Sleep Med. Rev. 2019, 45, 54–69. [Google Scholar] [CrossRef]
- Johnson, K.G.; Johnson, D.C. Frequency of Sleep Apnea in Stroke and TIA Patients: A Meta-analysis. J. Clin. Sleep Med. 2010, 6, 131–137. [Google Scholar] [CrossRef]
- Hasan, F.; Gordon, C.; Wu, D.; Huang, H.C.; Yuliana, L.T.; Susatia, B.; Marta, O.F.D.; Chiu, H.Y. Dynamic Prevalence of Sleep Disorders Following Stroke or Transient Ischemic Attack: Systematic Review and Meta-Analysis. Stroke 2021, 52, 655–663. [Google Scholar] [CrossRef]
- Bassetti, C.L.; Hermann, D.M. Sleep and stroke. Handb. Clin. Neurol. 2011, 99, 1051–1072. [Google Scholar]
- Nakamizo, T.; Kanda, T.; Kudo, Y.; Sugawara, E.; Hashimoto, E.; Okazaki, A.; Usuda, M.; Nagai, T.; Hara, H.; Johkura, K. Effects of uncomfortable care and histamine H2-antagonists on delirium in acute stroke: A propensity score analysis. J. Neurol. Sci. 2020, 420, 117251. [Google Scholar] [CrossRef]
- Mengel, A.; Zurloh, J.; Boßelmann, C.; Brendel, B.; Stadler, V.; Sartor-Pfeiffer, J.; Meisel, A.; Fleischmann, R.; Ziemann, U.; Poli, S.; et al. Delirium REduction after administration of melatonin in acute ischemic stroke (DREAMS): A propensity score–matched analysis. Eur. J. Neurol. 2021, 28, 1958–1966. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Thomas, B.H.; Ciliska, D.; Dobbins, M.; Micucci, S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef]
- Aaronson, J.A.; van Bennekom, C.A.; Hofman, W.F.; van Bezeij, T.; Aardweg, J.G.v.D.; Groet, E.; Kylstra, W.A.; Schmand, B. Obstructive Sleep Apnea is Related to Impaired Cognitive and Functional Status after Stroke. Sleep 2015, 38, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Alverzo, J.P. Predictors of disorientation among brain injury and stroke patients during rehabilitation. Rehabil. Nurs. 2005, 30, 230–238. [Google Scholar] [CrossRef]
- Bakken, L.N.; Kim, H.S.; Finset, A.; Lerdal, A. Stroke patients’ functions in personal activities of daily living in relation to sleep and socio-demographic and clinical variables in the acute phase after first-time stroke and at six months of follow-up. J. Clin. Nurs. 2012, 21, 1886–1895. [Google Scholar] [CrossRef]
- Sangiorgi, G.B.; Barbagallo, M.; Giordano, M.; Meli, M.; Panzarasa, R. alpha-Glycerophosphocholine in the mental recovery of cerebral ischemic attacks. An Italian multicenter clinical trial. Ann. N. Y. Acad. Sci. 1994, 717, 253–269. [Google Scholar] [CrossRef]
- Buijck, B.I.; Zuidema, S.U.; Spruit-van Eijk, M.; Geurts, A.C.; Koopmans, R.T. Neuropsychiatric symptoms in geriatric patients admitted to skilled nursing facilities in nursing homes for rehabilitation after stroke: A longitudinal multicenter study. Int. J. Geriatr. Psychiatry 2012, 27, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Pinedo, F.; Hernández-Pérez, J.M.; Zurdo, M.; Rodríguez-Fúnez, B.; Hernández-Bayo, J.M.; García-Fernández, C.; Cueli-Rincón, B.; Castro-Posada, J.A. Influence of premorbid psychopathology and lesion location on affective and behavioral disorders after ischemic stroke. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zhang, S.; Li, X.; Shi, L. Dexmedetomidine is superior to midazolam for sedation and cerebral protection in postoperative hypertensive intracerebral hemorrhage patients: A retrospective study. J. Int. Med. Res. 2020, 48, 300060520957554. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L.; Elder, J.; Schiff, N.D.; Victor, J.D.; Goldfine, A.M. Post-Stroke Apathy and Hypersomnia Lead to Worse Outcomes from Acute Rehabilitation. Transl. Stroke Res. 2014, 5, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Siccoli, M.; Brugger, P.; Wachter, K.; Mathis, J.; Achermann, P.; Bassetti, C.L. Evolution of Neurological, Neuropsychological and Sleep-Wake Disturbances after Paramedian Thalamic Stroke. Stroke 2008, 39, 62–68. [Google Scholar] [CrossRef]
- Jacquin, A.; Binquet, C.; Rouaud, O.; Graule-Petot, A.; Daubail, B.; Osseby, G.-V.; Bonithon-Kopp, C.; Giroud, M.; Béjot, Y. Post-Stroke Cognitive Impairment: High Prevalence and Determining Factors in a Cohort of Mild Stroke. J. Alzheimers Dis. 2014, 40, 1029–1038. [Google Scholar] [CrossRef]
- Johnson, M.L.; Roberts, M.D.; Ross, A.R.; Witten, C.M. Methylphenidate in stroke patients with depression. Am. J. Phys. Med. Rehabil. 1992, 71, 239–241. [Google Scholar] [CrossRef]
- Li, J.; You, S.-J.; Xu, Y.-N.; Yuan, W.; Shen, Y.; Huang, J.-Y.; Xiong, K.-P.; Liu, C.-F. Cognitive impairment and sleep disturbances after minor ischemic stroke. Sleep Breath. 2018, 23, 455–462. [Google Scholar] [CrossRef]
- Ma, G.; Sun, L.; Qie, Z.; He, J.; Cui, F. Factors Associated with Benzodiazepines Prolonged-Term Use in Post-Stroke Subjective Sleep Disturbance: A Single-Centre Retrospective Study from China. Drug Des. Dev. Ther. 2021, 15, 2469–2481. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.R.A.; Muir, R.T.; Black, S.E.; Gao, F.; Swartz, R.H.; Murray, B.J.; Boulos, M.I. Subcortical Brain Involvement Is Associated with Impaired Performance on the Psychomotor Vigilance Task after Minor Stroke. Neurorehabilit. Neural Repair 2018, 32, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Tobisawa, M.; Sasaki, M.; Suzuki, A. Outstanding Symptoms of Poststroke Depression during the Acute Phase of Stroke. PLoS ONE 2016, 11, e0163038. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Stolwyk, R.J.; New, P.W.; Anderson, C. Sleep disturbance and deficits of sustained attention following stroke. J. Clin. Exp. Neuropsychol. 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.M.; Bayley, M.; Green, R.; Murray, B.J.; Bradley, T.D. Influence of Continuous Positive Airway Pressure on Outcomes of Rehabilitation in Stroke Patients with Obstructive Sleep Apnea. Stroke 2011, 42, 1062–1067. [Google Scholar] [CrossRef]
- Siccoli, M.M.; Rölli-Baumeler, N.; Achermann, P.; Bassetti, C.L. Correlation between sleep and cognitive functions after hemispheric ischaemic stroke. Eur. J. Neurol. 2008, 15, 565–572. [Google Scholar] [CrossRef]
- Trewin, V.F.; Lawrence, C.J.; Veitch, G.B.A. An investigation of the association of benzodiazepines and other hypnotics with the incidence of falls in the elderly. J. Clin. Pharm. Ther. 1992, 17, 129–133. [Google Scholar] [CrossRef]
- van Almenkerk, S.; Depla, M.F.; Smalbrugge, M.; Eefsting, J.A.; Hertogh, C.M. Institutionalized Stroke Patients: Status of Functioning of an Under Researched Population. J. Am. Med. Dir. Assoc. 2012, 13, 634–639. [Google Scholar] [CrossRef]
- Matsuzono, K.; Mashiko, T.; Ozawa, T.; Miura, K.; Suzuki, M.; Furuya, K.; Ozawa, M.; Anan, Y.; Shimazaki, H.; Koide, R.; et al. Management Effort for Delirium and Insomnia in Patients with Acute Ischemic Stroke (MEDIAS) Study. Psychiatry Clin. Neurosci. 2020, 74, 279–280. [Google Scholar] [CrossRef]
- Küçükdeveci, A.A.; Tennant, A.; Hardo, P.; Chamberlain, M.A. Sleep problems in stroke patients: Relationship with shoulder pain. Clin. Rehabil. 1996, 10, 166–172. [Google Scholar] [CrossRef]
- Bohlken, J.; Kostev, K. Prevalence and risk factors for delirium diagnosis in patients followed in general practices in Germany. Int. Psychogeriatry 2018, 30, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Allen, H.; Tomenson, B.; Duignan, D.; Byrne, J. Bright light therapy for agitation in dementia: A randomized controlled trial. Int. Psychogeriatry 2009, 21, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Corona, A.; Praga, F.; Minari, C.; Giannotti, C.; Castelli, A.; Raimondi, F. A reorientation strategy for reducing delirium in the critically ill. Results of an interventional study. Minerva Anestesiol. 2012, 78, 1026–1033. [Google Scholar]
- Conn, D.K.; Goldman, Z. Pattern of Use of Antidepressants in Long-Term Care Facilities for the Elderly. J. Geriatr. Psychiatry Neurol. 1992, 5, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Cui, L.; Xu, Y.; Pradeep, P.; Li, G. The risk factors of the permanent pacemaker implantation in patients with postoperative delirium. Int. J. Cardiol. 2015, 179, 214–216. [Google Scholar] [CrossRef]
- Dai, H.; Liu, X.; Feng, D.; Li, R.; Shen, H. Evidence-based nursing combined with cognitive function training can reduce the incidence of delirium in ICU patients and improve their cognitive function. Am. J. Transl. Res. 2021, 13, 3262–3269. [Google Scholar]
- Douven, E.; Köhler, S.; Schievink, S.H.; van Oostenbrugge, R.J.; Staals, J.; Verhey, F.R.; Aalten, P. Temporal Associations between Fatigue, Depression, and Apathy after Stroke: Results of the Cognition and Affect after Stroke, a Prospective Evaluation of Risks Study. Cerebrovasc. Dis. 2017, 44, 330–337. [Google Scholar] [CrossRef]
- Foroughan, M.; Delbari, A.; Said, S.E.; AkbariKamrani, A.A.; Rashedi, V.; Zandi, T. Risk factors and clinical aspects of delirium in elderly hospitalized patients in Iran. Aging Clin. Exp. Res. 2016, 28, 313–319. [Google Scholar] [CrossRef]
- Harris, Y.; Gorelick, P.B.; Cohen, D.; Dollear, W.; Forman, H.; Freels, S. Psychiatric symptoms in dementia associated with stroke: A case-control analysis among predominantly African-American patients. J. Natl. Med. Assoc. 1994, 86, 697–702. [Google Scholar]
- Kim, H.; Chung, S.; Joo, Y.H.; Lee, J.S. The major risk factors for delirium in a clinical setting. Neuropsychiatr. Dis. Treat. 2016, 12, 1787–1793. [Google Scholar]
- Limpawattana, P.; Panitchote, A.; Tangvoraphonkchai, K.; Suebsoh, N.; Eamma, W.; Chanthonglarng, B.; Tiamkao, S. Delirium in critical care: A study of incidence, prevalence, and associated factors in the tertiary care hospital of older Thai adults. Aging Ment. Health 2016, 20, 74–80. [Google Scholar] [CrossRef]
- Mirski, M.A.; Lewin, J.J., 3rd; LeDroux, S.; Thompson, C.; Murakami, P.; Zink, E.K.; Griswold, M. Cognitive improvement during continuous sedation in critically ill, awake and responsive patients: The Acute Neurological ICU Sedation Trial (ANIST). Intensive Care Med. 2010, 36, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Korupolu, R. Rehabilitation Quality Improvement in an Intensive Care Unit Setting: Implementation of a Quality Improvement Model. Top. Stroke Rehabil. 2010, 17, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Owusu, K.A.; Kurczewski, L.; Armahizer, M.J.; Zichichi, A.; Maciel, C.B.; Heavner, M.S. DEXmedetomidine compared to PROpofol in NEurocritical Care [DEXPRONE]: A multicenter retrospective evaluation of clinical utility and safety. J. Crit. Care 2020, 60, 79–83. [Google Scholar] [CrossRef]
- Rollo, E.; Callea, A.; Brunetti, V.; Vollono, C.; Marotta, J.; Della Marca, G. Physical restraint precipitates delirium in stroke patients. J. Neurol. Sci. 2021, 421, 117290. [Google Scholar] [CrossRef]
- Su, C.-C.; Yang, Y.-H.K.; Lai, E.C.-C.; Hsieh, C.-Y.; Cheng, C.-L.; Chen, C.-H.; Lin, H.-J.; Sung, S.-F.; Chen, Y.-W. Comparative safety of antipsychotic medications in elderly stroke survivors: A nationwide claim data and stroke registry linkage cohort study. J. Psychiatr. Res. 2021, 139, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Inada, T.; Ichida, M.; Ozaki, N. Risk factors for inducing violence in patients with delirium. Brain Behav. 2021, 11, e2276. [Google Scholar] [CrossRef]
- Tutuarima, J.; van der Meulen, J.; de Haan, R.; van Straten, A.; Limburg, M. Risk Factors for Falls of Hospitalized Stroke Patients. Stroke 1997, 28, 297–301. [Google Scholar] [CrossRef]
- Weiss, B.; Paul, N.; Spies, C.D.; Ullrich, D.; Ansorge, I.; Salih, F.; Wolf, S.; Luetz, A. Influence of Patient-Specific Covariates on Test Validity of Two Delirium Screening Instruments in Neurocritical Care Patients (DEMON-ICU). Neurocrit. Care 2021, 36, 452–462. [Google Scholar] [CrossRef]
- Nydahl, P.; Baumgarte, F.; Berg, D.; Bergjan, M.; Borzikowsky, C.; Franke, C.; Green, D.; Hannig, A.; Hansen, H.C.; Hauss, A.; et al. Delirium on stroke units: A prospective, multicentric quality-improvement project. J. Neurol. 2022, 269, 3735–3744. [Google Scholar] [CrossRef]
- Azimaraghi, O.; Hammer, M.; Santer, P.; Platzbecker, K.; Althoff, F.C.; Patrocinio, M.; Grabitz, S.D.; Wongtangman, K.; Rumyantsev, S.; Xu, X.; et al. Study protocol for a randomised controlled trial evaluating the effects of the orexin receptor antagonist suvorexant on sleep architecture and delirium in the intensive care unit. BMJ Open 2020, 10, e038474. [Google Scholar] [CrossRef] [PubMed]
- Hatta, K.; Kishi, Y.; Wada, K.; Takeuchi, T.; Ito, S.; Kurata, A.; Murakami, K.; Sugita, M.; Usui, C.; Nakamura, H. Preventive Effects of Suvorexant on Delirium: A Randomized Placebo-Controlled Trial. J. Clin. Psychiatry 2017, 78, e970–e979. [Google Scholar] [CrossRef] [PubMed]
- Hatta, K.; Kishi, Y.; Wada, K.; Takeuchi, T.; Ito, S.; Kurata, A.; Murakami, K.; Sugita, M.; Usui, C.; Nakamura, H. Preventive effects of ramelteon on delirium: A randomized placebo-controlled trial. JAMA Psychiatry 2014, 71, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, C.-Y.; Qiu, B.; Ma, G.-Y. Naloxone Treatment for Poststroke Agitated Delirium in Hospitalized Older Adults: A Pilot Study. J. Am. Geriatr. Soc. 2016, 64, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, R.; Sato, Y.; Ishida, E.; Shibamoto, H.; Hino, S.; Yokote, H.; Kamata, T. Association between Delirium and Prehospitalization Medication in Poststroke Patients. J. Stroke Cerebrovasc. Dis. 2018, 27, 1914–1920. [Google Scholar] [CrossRef]
- Kawada, K.; Ohta, T.; Tanaka, K.; Miyamura, M.; Tanaka, S. Addition of Suvorexant to Ramelteon Therapy for Improved Sleep Quality with Reduced Delirium Risk in Acute Stroke Patients. J. Stroke Cerebrovasc. Dis. 2019, 28, 142–148. [Google Scholar] [CrossRef]
- McLaughlin, D.C.; Hartjes, T.M.; Freeman, W.D. Sleep Deprivation in Neurointensive Care Unit Patients from Serial Neurological Checks: How Much Is Too Much? J. Neurosci. Nurs. 2018, 50, 205–210. [Google Scholar] [CrossRef]
- Nakamizo, T.; Kanda, T.; Kudo, Y.; Sugawara, E.; Hashimoto, E.; Okazaki, A.; Usuda, M.; Nagai, T.; Hara, H.; Johkura, K. Development of a clinical score, PANDA, to predict delirium in stroke care unit. J. Neurol. Sci. 2020, 415, 116956. [Google Scholar] [CrossRef]
- Ohta, T.; Murao, K.; Miyake, K.; Takemoto, K. Melatonin Receptor Agonists for Treating Delirium in Elderly Patients with Acute Stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, 1107–1110. [Google Scholar] [CrossRef]
- Reznik, M.E.; Drake, J.; Margolis, S.A.; Moody, S.; Murray, K.; Costa, S.; Mahta, A.; Wendell, L.C.; Thompson, B.B.; Rao, S.S.; et al. Deconstructing Poststroke Delirium in a Prospective Cohort of Patients with Intracerebral Hemorrhage*. Crit. Care Med. 2020, 48, 111–118. [Google Scholar] [CrossRef]
- Sandberg, O.; Franklin, K.; Bucht, G.; Eriksson, S.; Gustafson, Y. Nasal continuous positive airway pressure in stroke patients with sleep apnoea: A randomized treatment study. Eur. Respir. J. 2001, 18, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, O.; Franklin, K.A.; Bucht, G.; Gustafson, Y. Sleep Apnea, Delirium, Depressed Mood, Cognition, and ADL Ability after Stroke. J. Am. Geriatr. Soc. 2001, 49, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, M.; Jung, D. The Effects of Delirium Prevention Guidelines on Elderly Stroke Patients. Clin. Nurs. Res. 2018, 27, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, Y.; Wang, N.; Chen, W.; Bao, Y.; Qin, Q.; Xiao, Q.; Li, S. Risk factors for the incidence of delirium in cerebrovascular patients in a Neurosurgery Intensive Care Unit: A prospective study. J. Clin. Nurs. 2018, 27, 407–415. [Google Scholar] [CrossRef]
- Thomas, M.; Sing, H.; Belenky, G.; Holcomb, H.; Mayberg, H.; Dannals, R.; Wagner, H., Jr.; Thorne, D.; Popp, K.; Rowland, L.; et al. Neural basis of alertness cognitive performance impairments during sleepiness, I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J. Sleep Res. 2000, 9, 335–352. [Google Scholar] [CrossRef]
- Daou, M.; Telias, I.; Younes, M.; Brochard, L.; Wilcox, M.E. Abnormal Sleep, Circadian Rhythm Disruption, and Delirium in the ICU: Are They Related? Front. Neurol. 2020, 11, 549908. [Google Scholar] [CrossRef]
- Terzaghi, M.; Sartori, I.; Rustioni, V.; Manni, R. Sleep disorders and acute nocturnal delirium in the elderly: A comorbidity not to be overlooked. Eur. J. Intern. Med. 2014, 25, 350–355. [Google Scholar] [CrossRef]
- Austin, C.A.M.; Yi, J.B.; Lin, F.-C.; Pandharipande, P.M.; Ely, E.W.; Busby-Whitehead, J.; Carson, S.S. The Association of Selective Serotonin Reuptake Inhibitors with Delirium in Critically Ill Adults: A Secondary Analysis of the Bringing to Light the Risk Factors and Incidence of Neuropsychologic Dysfunction in ICU Survivors ICU Study. Crit. Care Explor. 2022, 4, e0740. [Google Scholar] [CrossRef]
- Watson, P.L.; Ceriana, P.; Fanfulla, F. Delirium: Is sleep important? Best Pract. Res. Clin. Anaesthesiol. 2012, 26, 355–366. [Google Scholar] [CrossRef]
| First Author (Year) | Study Design | Age (Years) | Pt | Ctl | Setting | Outcome Measures | Sleep Measures | Sleep Interventions | Stroke Type | Delirium Incidence | Delirium Measures | Sleep Factors Associated with Delirium |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mengel (2021) [17] | Int | Melatonin: 64 ± 13 Controls: 73 ± 15 | 300 (164) † | 273 (164) † | SU | DLR: prevalence, risk factors, characteristics, time to onset, and duration | None | Melatonin 2 mg nightly | Ischemic | Melatonin: 42/164 Controls: 60/164 | RASS, ICDSC | Melatonin prevents DLR (OR 0.597, CI 0.372–0.958) |
| Nakamizo (2021) [16] | Int | Total: 75 (64–83) DLR+: 84 (72–89) DLR−: 74 (64–83) | 83 | 304 | SU | Incidence of DLR within 5 days from admission | History of insomnia and nocturnal lifestyle | Frequent night-time care | Ischemic and hemorrhagic | Frequent night-time care: 20/83 Controls: 22/304 | ICDSC | Frequent night-time care (OR 2.1, CI 1.2–3.7) |
| Nakamizo (2020) [69] | Obs | Total: 75 (64–83) DLR+: 84 (72–89) DLR−: 74 (64–83) | 387 | 0 | SU | Prediction model for DLR | Nocturnal lifestyle, insomnia, and frequent night-time care | Frequent night-time care | Ischemic and hemorrhagic | 42/387 | ICDSC | Insomnia OR 2.5 (CI 1.1–5.4) Nocturnal lifestyle OR 5.5 (CI 1.7–16.4) Frequent night-time care OR 4.1 (CI 2.0–8.3) |
| Reznik (2019) [71] | Obs | Total: 68 ± 18 DLR+: 70 ± 19 DLR−: 65 ± 18 | 60 | 0 | NICU, SU | DLR: incidence and features | Sleep–wake disturbance | Not performed | Hemorrhagic | 34/60 | CAM-ICU, ICDSC, DSM-V | Sleep–wake cycle disturbances are increased in DLR (p < 0.001) |
| Hosoya (2018) [66] | Obs | DLR+: 75 ± 1 DLR−: 69 ± 1 | 269 | 0 | SU | DLR incidence | Somnolence and prior sleep medications | Not performed | Ischemic, hemorrhagic, and SAH | 97/269 | ICDSC | Post-stroke somnolence (p < 0.001), medications before stroke (p = 0.032) |
| Kawada (2018) [67] | Int | Suvorexant: 79 (72–84) GABAR-A: 76 (67–84) | 232 | 0 | Not specified | Sleep improvement, DLR incidence, and length of hospitalization | Subjective sleep, nursing observations, and hospital records | Ramelteon + suvorexant vs. Ramelteon + GABAR-A | Ischemic and hemorrhagic | Suvorexant: 9/128 GABAR-A: 32/104 | CAM-ICU, RASS | Suvorexant prevents DLR (OR 0.19, CI 0.09–0.44) |
| McLaughlin (2018) [68] | Obs | 54 (34–88) | 20 | 0 | NICU | DLR incidence and neurological deterioration | Sleep deprivation | Hourly neurological examination | Ischemic, hemorrhagic, and SAH (16/20) | 15/20 | CAM-ICU | Hourly neurological examinations favors DLR * |
| Wang (2018) [75] | Obs | DLR+: 56 (45–64) DLR−: 57 (46–65) | 128 | 0 | NICU | DLR: incidence and risk factors | Sleep deprivation | Not performed | Not specified | 27/64 | CAM-ICU | Sleep deprivation OR 8.03 (CI 1.04–62.17) |
| Song (2017) [74] | Int | Treated: 75 ± 6 Control: 74 ± 7 | 54 | 54 | SU | DLR incidence and severity, stroke impact, and length of hospitalization | History of sleep disorder | Multicomponent intervention | Not specified | Treated: 4/54 Controls: 13/54 | DOS | Multicomponent intervention prevents DLR (p = 0.017) |
| Hatta (2017) [63] | Int | Suvorexant: 79 ± 7 Placebo: 78 ± 6 | 36 | 36 | ICU, acute wards | DLR incidence and severity and serum biomarkers predictor of DLR | Subjective sleep and staff observations | Suvorexant 15 mg/nightly for 3 days | Stroke prevalence: 7/36 placebo group and 7/36 suvorexant group | Suvorexant: 0/36 Placebo: 6/36 | DSM-V, DRS-R98 | Suvorexant prevents DLR (p = 0.025) |
| Duan (2016) [65] | Int | 81 (64–94) | 18 | 0 | Not specified | DLR severity | Sleep–wake cycle disturbance | Naloxone (1.2–4.0 mg) once or twice daily | Ischemic | 18/18 | DRS-R98 | Naloxone improves DLR severity * |
| Hatta (2014) [64] | Int | Ramelteon: 78 ± 7 Placebo: 78 ± 7 | 33 | 34 | ICU, acute wards | DLR incidence and severity | Subjective sleep and staff observations | Ramelteon 8 mg/nightly for 7 days | Stroke prevalence: 9/34 placebo group and 12/33 ramelteon group | Ramelteon: 1/33 Placebo: 11/34 | DSM-IV, DRS-R98 | Ramelteon prevents DLR (p = 0.003) |
| Ohta (2013) [70] | Int | Ramelteon: 76 ± 6 Sedatives: 77 ± 6 | 7 | 21 | Not specified | DLR severity | Insomnia | Ramelteon vs. other sedatives | Ischemic, hemorrhagic, and subarachnoid hemorrhage | 28/28 | CAM-ICU, RASS | Ramelteon improves RASS (p = 0.013) |
| Sandberg (2001) [72] | Int | CPAP: 78 ± 6 Controls: 77 ± 8 | 31 | 28 | Geriatric Stroke Rehab | Depressive symptoms, ADL, cognitive functions, and DLR incidence | Cardiorespiratory polygraphy | nCPAP for 4 weeks | Ischemic and hemorrhagic | Baseline: nCPAP—22/31 and Control—24/28 Day 28: nCPAP—15% and Control—19% | DSM-IV | nCPAP not effective (p = 0.881) |
| Sandberg (2001) [73] | Obs | Total: 77 ± 8 | 133 | 0 | Geriatric Stroke Rehab | Depressive symptoms, ADL, cognitive functions, and DLR incidence | Cardiorespiratory polygraphy | Not performed | Ischemic and hemorrhagic | Sleep apnea: 58/78 Not Sleep apnea: 30/55 | DSM-IV, OBS | Sleep apnea (p = 0.018) and apnea-related desaturation (OR 12.1, CI 3.0–48.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunetti, V.; Rollo, E.; Scala, I.; Marotta, J.; Callea, A.; Imperatori, C.; Della Marca, G. Sleep and Stroke-Related Delirium: A Systematic Review. Clin. Transl. Neurosci. 2023, 7, 22. https://doi.org/10.3390/ctn7030022
Brunetti V, Rollo E, Scala I, Marotta J, Callea A, Imperatori C, Della Marca G. Sleep and Stroke-Related Delirium: A Systematic Review. Clinical and Translational Neuroscience. 2023; 7(3):22. https://doi.org/10.3390/ctn7030022
Chicago/Turabian StyleBrunetti, Valerio, Eleonora Rollo, Irene Scala, Jessica Marotta, Antonio Callea, Claudio Imperatori, and Giacomo Della Marca. 2023. "Sleep and Stroke-Related Delirium: A Systematic Review" Clinical and Translational Neuroscience 7, no. 3: 22. https://doi.org/10.3390/ctn7030022
APA StyleBrunetti, V., Rollo, E., Scala, I., Marotta, J., Callea, A., Imperatori, C., & Della Marca, G. (2023). Sleep and Stroke-Related Delirium: A Systematic Review. Clinical and Translational Neuroscience, 7(3), 22. https://doi.org/10.3390/ctn7030022






