Diagnostic and Therapeutic Approaches in Neurorehabilitation after Traumatic Brain Injury and Disorders of Consciousness
Abstract
1. Introduction
2. Epidemiology of TBI and Burden of Disease
3. Network-Based Mechanisms and Their Dysfunction on Circuitry and Cellular Levels after TBI
4. Challenges in the Clinical Diagnosis of TBI Patients with DoC
5. Neuroimaging and Neurophysiological Approaches to Improve Diagnosis of Different States of Consciousness
5.1. Electrophysiology
5.2. FDG-PET
5.3. Functional MRI
5.4. Fluid Biomarkers
6. Neurorehabilitation of TBI Patients—Established Treatment Options and New Approaches
6.1. Pharmacologic Therapies
6.2. Transcranial Electrical Stimulation
6.3. Transcranial Magnetic Stimulation
6.4. Vagus Nerve Stimulation
6.5. Sensory Therapies
6.6. Motor Therapy
6.7. Psychoeducational Therapeutic Approaches in Post-TBI Fatigue
7. Practice Guidelines in Neurorehabilitation
- should receive multi-professional neurological rehabilitation treatment (level of evidence B);
- should receive a treatment attempt with amantadine in increasing doses up to 400 mg daily taken orally to improve the state of consciousness (level of evidence B);
- should be verticalized on a tilt table (level of evidence B);
- should undergo multisensory stimulation to improve their reactivity to the environment. Especially auditory stimuli with a high degree of emotional and autobiographic reference should be applied in the context of music therapy (level of evidence B);
- should undergo a therapy attempt with tDCS over the dorsolateral prefrontal cortex (DLPFC) with a duration of 20 min/day over at least 5 days in addition to conventional therapies (level of evidence B).
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- Andelic, N.; Røe, C.; Tenovuo, O.; Azouvi, P.; Dawes, H.; Majdan, M.; Ranta, J.; Howe, E.I.; Wiegers, E.J.; Tverdal, C.; et al. Unmet Rehabilitation Needs after Traumatic Brain Injury across Europe: Results from the CENTER-TBI Study. J. Clin. Med. 2021, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Andelic, N.; Ye, J.; Tornas, S.; Roe, C.; Lu, J.; Bautz-Holter, E.; Moger, T.; Sigurdardottir, S.; Schanke, A.-K.; Aas, E. Cost-Effectiveness Analysis of an Early-Initiated, Continuous Chain of Rehabilitation after Severe Traumatic Brain Injury. J. Neurotrauma 2014, 31, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Cogné, M.; Tenovuo, O.; Røe, C.; Andelic, N.; Majdan, M.; Ranta, J.; Ylen, P.; Dawes, H.; Azouvi, P.; et al. Predictors of Access to Rehabilitation in the Year Following Traumatic Brain Injury: A European Prospective and Multicenter Study. Neurorehabilit. Neural Repair 2020, 34, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, M.P.; Marwitz, J.H.; Harrison-Felix, C. Thirty years of national institute on disability, independent living and rehabilitation research traumatic brain injury model system centers research—An update. J. Head Trauma Rehabil. 2018, 33, 363. [Google Scholar] [CrossRef]
- Ponsford, J.; Harrison-Felix, C.; Ketchum, J.M.; Spitz, G.; Miller, A.C.; Corrigan, J.D. Outcomes 1 and 2 Years After Moderate to Severe Traumatic Brain Injury: An International Comparative Study. Arch. Phys. Med. Rehabil. 2021, 102, 371–377. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Mushkudiani, N.; Perel, P.; Butcher, I.; Lu, J.; McHugh, G.S.; Murray, G.D.; Marmarou, A.; Roberts, I.; Habbema, J.D.F.; et al. Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics. PLoS Med. 2008, 5, e165. [Google Scholar] [CrossRef]
- Collaborators, M.C.T. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ 2008, 336, 425–429. [Google Scholar] [CrossRef]
- Helmrich, I.R.A.R.; van Klaveren, D.; Dijkland, S.A.; Lingsma, H.F.; Polinder, S.; Wilson, L.; von Steinbuechel, N.; van der Naalt, J.; Maas, A.I.R.; Steyerberg, E.W.; et al. Development of prognostic models for Health-Related Quality of Life following traumatic brain injury. Qual. Life Res. 2021, 31, 451–471. [Google Scholar] [CrossRef]
- Stein, M.B.; Jain, S.; Giacino, J.T.; Levin, H.; Dikmen, S.; Nelson, L.D.; Vassar, M.J.; Okonkwo, D.O.; Diaz-Arrastia, R.; Robertson, C.S.; et al. Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury: A TRACK-TBI study. JAMA Psychiatry 2019, 76, 249–258. [Google Scholar] [CrossRef]
- Jacobs, B.; Beems, T.; Stulemeijer, M.; van Vugt, A.B.; van der Vliet, T.M.; Borm, G.F.; Vos, P.E.; Pérez, D.A.; Gallardo, A.J.L.; Agramonte, J.A.D.; et al. Outcome Prediction in Mild Traumatic Brain Injury: Age and Clinical Variables Are Stronger Predictors than CT Abnormalities. J. Neurotrauma 2010, 27, 655–668. [Google Scholar] [CrossRef]
- Lingsma, H.F.; Yue, J.K.; Maas, A.I.; Steyerberg, E.W.; Manley, G.T.; Cooper, S.R.; Dams-O’Connor, K.; Gordon, W.A.; Menon, D.K.; Mukherjee, P.; et al. Outcome Prediction after Mild and Complicated Mild Traumatic Brain Injury: External Validation of Existing Models and Identification of New Predictors Using the TRACK-TBI Pilot Study. J. Neurotrauma 2015, 32, 83–94. [Google Scholar] [CrossRef]
- Howe, E.I.; Zeldovich, M.; Andelic, N.; von Steinbuechel, N.; Fure, S.C.R.; Borgen, I.M.H.; Forslund, M.V.; Hellstrøm, T.; Søberg, H.L.; Sveen, U.; et al. Rehabilitation and outcomes after complicated vs uncomplicated mild TBI: Results from the CENTER-TBI study. BMC Health Serv. Res. 2022, 22, 1536. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Wiegers, E.; Sewalt, C.; Buki, A.; Citerio, G.; De Keyser, V.; Ercole, A.; Kunzmann, K.; Lanyon, L.; Lecky, F.; et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: A European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019, 18, 923–934. [Google Scholar] [CrossRef]
- Yue, J.K.; Yuh, E.L.; Korley, F.K.; Winkler, E.A.; Sun, X.; Puffer, R.C.; Deng, H.; Choy, W.; Chandra, A.; Taylor, S.R.; et al. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: A prospective multicentre study. Lancet Neurol. 2019, 18, 953–961. [Google Scholar] [CrossRef]
- Amyot, F.; Arciniegas, D.B.; Brazaitis, M.P.; Curley, K.C.; Diaz-Arrastia, R.; Gandjbakhche, A.; Herscovitch, P.; Hinds, S.R., 2rd; Manley, G.T.; Pacifico, A.; et al. A Review of the Effectiveness of Neuroimaging Modalities for the Detection of Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1693–1721. [Google Scholar] [CrossRef]
- Sandsmark, D.K.; Bashir, A.; Wellington, C.L.; Diaz-Arrastia, R. Cerebral Microvascular Injury: A Potentially Treatable Endophenotype of Traumatic Brain Injury-Induced Neurodegeneration. Neuron 2019, 103, 367–379. [Google Scholar] [CrossRef]
- Jolly, A.E.; Bălăeţ, M.; Azor, A.; Friedland, D.; Sandrone, S.; Graham, N.S.N.; Zimmerman, K.; Sharp, D.J. Detecting axonal injury in individual patients after traumatic brain injury. Brain 2021, 144, 92–113. [Google Scholar] [CrossRef]
- Palacios, E.M.; Yuh, E.L.; Chang, Y.-S.; Yue, J.K.; Schnyer, D.M.; Okonkwo, D.O.; Valadka, A.B.; Gordon, W.A.; Maas, A.I.R.; Vassar, M.; et al. Resting-State Functional Connectivity Alterations Associated with Six-Month Outcomes in Mild Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1546–1557. [Google Scholar] [CrossRef]
- O’donnell, A.; Pauli, R.; Banellis, L.; Sokoliuk, R.; Hayton, T.; Sturman, S.; Veenith, T.; Yakoub, K.M.; Belli, A.; Chennu, S.; et al. The prognostic value of resting-state EEG in acute post-traumatic unresponsive states. Brain Commun. 2021, 3, fcab017. [Google Scholar] [CrossRef]
- Mondello, S.; Sorinola, A.; Czeiter, E.; Vámos, Z.; Amrein, K.; Synnot, A.; Donoghue, E.; Sándor, J.; Wang, K.; Diaz-Arrastia, R.; et al. Blood-Based Protein Biomarkers for the Management of Traumatic Brain Injuries in Adults Presenting to Emergency Departments with Mild Brain Injury: A Living Systematic Review and Meta-Analysis. J. Neurotrauma 2021, 38, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.C.; Mejia, L.L.P.; Robertson, C. A Precision Medicine Agenda in Traumatic Brain Injury. Front. Pharmacol. 2022, 13, 713100. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Majdan, M.; Plancikova, D.; Brazinova, A.; Rusnak, M.; Nieboer, D.; Feigin, V.L.; Maas, A. Epidemiology of traumatic brain injuries in Europe: A cross-sectional analysis. Lancet Public Health 2016, 1, e76–e83. [Google Scholar] [CrossRef]
- Dams-O’Connor, K.; Cuthbert, J.P.; Whyte, J.; Corrigan, J.D.; Faul, M.; Harrison-Felix, C. Traumatic Brain Injury among Older Adults at Level I and II Trauma Centers. J. Neurotrauma 2013, 30, 2001–2013. [Google Scholar] [CrossRef]
- Gardner, R.C.; Dams-O’Connor, K.; Morrissey, M.R.; Manley, G.T. Geriatric Traumatic Brain Injury: Epidemiology, Outcomes, Knowledge Gaps, and Future Directions. J. Neurotrauma 2018, 35, 889–906. [Google Scholar] [CrossRef]
- Dams-O’Connor, K.; Ketchum, J.M.; Cuthbert, J.P.; Corrigan, J.D.; Hammond, F.M.; Haarbauer-Krupa, J.; Kowalski, R.G.; Miller, A.C. Functional Outcome Trajectories Following Inpatient Rehabilitation for TBI in the United States: A NIDILRR TBIMS and CDC Interagency Collaboration. J. Head Trauma Rehabil. 2020, 35, 127. [Google Scholar] [CrossRef]
- Majdan, M.; Melichova, J.; Plancikova, D.; Sivco, P.; Maas, A.I.R.; Feigin, V.L.; Polinder, S.; Haagsma, J.A. Burden of Traumatic Brain Injuries in Children and Adolescents in Europe: Hospital Discharges, Deaths and Years of Life Lost. Children 2022, 9, 105. [Google Scholar] [CrossRef]
- van Ierssel, J.; Osmond, M.; Hamid, J.; Sampson, M.; Zemek, R. What is the risk of recurrent concussion in children and adolescents aged 5–18 years? A systematic review and meta-analysis. Br. J. Sports Med. 2021, 55, 663–669. [Google Scholar] [CrossRef]
- Ledoux, A.-A.; Webster, R.J.; Clarke, A.E.; Fell, D.B.; Knight, B.D.; Gardner, W.; Cloutier, P.; Gray, C.; Tuna, M.; Zemek, R. Risk of Mental Health Problems in Children and Youths Following Concussion. JAMA Netw. Open 2022, 5, e221235. [Google Scholar] [CrossRef]
- Majdan, M.; Plancikova, D.; Maas, A.; Polinder, S.; Feigin, V.; Theadom, A.; Rusnak, M.; Brazinova, A.; Haagsma, J. Years of life lost due to traumatic brain injury in Europe: A cross-sectional analysis of 16 countries. PLoS Med. 2017, 14, e1002331. [Google Scholar] [CrossRef]
- Jochems, D.; van Wessem, K.J.P.; Houwert, R.M.; Brouwers, H.B.; Dankbaar, J.W.; van Es, M.A.; Geurts, M.; Slooter, A.J.C.; Leenen, L.P.H. Outcome in Patients with Isolated Moderate to Severe Traumatic Brain Injury. Crit. Care Res. Pract. 2018, 2018, 3769418. [Google Scholar] [CrossRef]
- Turgeon, A.F.; Lauzier, F.; Simard, J.-F.; Scales, D.C.; Burns, K.E.; Moore, L.; Zygun, D.A.; Bernard, F.; Meade, M.O.; Dung, T.C.; et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: A Canadian multicentre cohort study. Can. Med. Assoc. J. 2011, 183, 1581–1588. [Google Scholar] [CrossRef]
- Robertsen, A.; Førde, R.; Skaga, N.O.; Helseth, E. Treatment-limiting decisions in patients with severe traumatic brain injury in a Norwegian regional trauma center. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 44. [Google Scholar] [CrossRef]
- Verkade, M.A.; Epker, J.L.; Nieuwenhoff, M.D.; Bakker, J.; Kompanje, E.J.O. Withdrawal of Life-Sustaining Treatment in a Mixed Intensive Care Unit: Most Common in Patients with Catastropic Brain Injury. Neurocritical Care 2012, 16, 130–135. [Google Scholar] [CrossRef]
- Graham, D.I.; McIntosh, T.K.; Maxwell, W.L.; Nicoll, J.A.R. Recent advances in neurotrauma. J. Neuropathol. Exp. Neurol. 2000, 59, 641–651. [Google Scholar] [CrossRef]
- Gurdjian, E.S. Re-evaluation of the biomechanics of blunt impact injury of the head. Surg. Gynecol. Obstet. 1975, 140, 845–850. [Google Scholar]
- Bigler, E.D. Anterior and middle cranial fossa in traumatic brain injury: Relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology 2007, 21, 515–531. [Google Scholar] [CrossRef]
- Blumbergs, P.; Scott, G.; Manavis, J.; Wainwright, H.; Simpson, D.; McLean, A. Stalning af amyloid percursor protein to study axonal damage in mild head Injury. Lancet 1994, 344, 1055–1056. [Google Scholar] [CrossRef]
- Sharp, D.J.; Scott, G.; Leech, R. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 2014, 10, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Doyle, D.; Ford, I.; Gennarelli, T.A.; Graham, D.I.; Mclellan, D.R. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology 1989, 15, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Meaney, D.F.; Shull, W.H. Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 2003, 18, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.E.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G.; et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011, 70, 374–383. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef]
- Adams, J.H.; Graham, D.I.; Jennett, B. The neuropathology of the vegetative state after an acute brain insult. Brain 2000, 123, 1327–1338. [Google Scholar] [CrossRef]
- Kinnunen, K.M.; Greenwood, R.; Powell, J.H.; Leech, R.; Hawkins, P.C.; Bonnelle, V.; Patel, M.C.; Counsell, S.J.; Sharp, D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011, 134, 449–463. [Google Scholar] [CrossRef]
- Mesulam, M.-M. From sensation to cognition. Brain J. Neurol. 1998, 121, 1013–1052. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Beckmann, C.F.; DeLuca, M.; Devlin, J.T.; Smith, S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1001–1013. [Google Scholar] [CrossRef]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Greicius, M.D.; Supekar, K.; Menon, V.; Dougherty, R.F. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cereb. Cortex 2008, 19, 72–78. [Google Scholar] [CrossRef]
- Bonnelle, V.; Leech, R.; Kinnunen, K.M.; Ham, T.E.; Beckmann, C.F.; De Boissezon, X.; Greenwood, R.J.; Sharp, D.J. Default Mode Network Connectivity Predicts Sustained Attention Deficits after Traumatic Brain Injury. J. Neurosci. 2011, 31, 13442–13451. [Google Scholar] [CrossRef]
- Bonnelle, V.; Ham, T.E.; Leech, R.; Kinnunen, K.M.; Mehta, M.A.; Greenwood, R.J.; Sharp, D.J. Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. USA 2012, 109, 4690–4695. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Sharp, D.; Bonnelle, V.; De Boissezon, X.; Beckmann, C.F.; James, S.G.; Patel, M.C.; Mehta, M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. USA 2010, 107, 6106–6111. [Google Scholar] [CrossRef]
- Smith, D.H.; Johnson, V.E.; Stewart, W. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat. Rev. Neurol. 2013, 9, 211–221. [Google Scholar] [CrossRef]
- Lye, T.C.; Shores, E.A. Traumatic brain injury as a risk factor for Alzheimer’s disease: A review. Neuropsychol. Rev. 2000, 10, 115–129. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Fisher, A.M.; Tagge, C.A.; Zhang, X.L.; Velisek, L.; Sullivan, J.A.; Upreti, C.; Kracht, J.M.; Ericsson, M.; Wojnarowicz, M.W.; et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012, 4, ra134–ra160. [Google Scholar]
- Sayed, N.; Culver, C.; Dams-O’Connor, K.; Hammond, F.; Diaz-Arrastia, R.; Guetta, G.; Hahn-Ketter, A.E.; Fedor, A.; Li, W.; Risacher, S.L.; et al. Clinical Phenotype of Dementia after Traumatic Brain Injury. J. Neurotrauma 2013, 30, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Stein, T.D.; Nowinski, C.J.; Stern, R.A.; Daneshvar, D.H.; Alvarez, V.E.; Lee, H.-S.; Hall, G.; Wojtowicz, S.M.; Baugh, C.M.; et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013, 136, 43–64. [Google Scholar] [CrossRef] [PubMed]
- de Calignon, A.; Polydoro, M.; Suárez-Calvet, M.; William, C.; Adamowicz, D.H.; Kopeikina, K.J.; Pitstick, R.; Sahara, N.; Ashe, K.H.; Carlson, G.A.; et al. Propagation of Tau Pathology in a Model of Early Alzheimer’s Disease. Neuron 2012, 73, 685–697. [Google Scholar] [CrossRef]
- Seeley, W.W.; Crawford, R.K.; Zhou, J.; Miller, B.L.; Greicius, M.D. Neurodegenerative Diseases Target Large-Scale Human Brain Networks. Neuron 2009, 62, 42–52. [Google Scholar] [CrossRef]
- Shitaka, Y.; Tran, H.T.; Bennett, R.E.; Sanchez, L.; Levy, M.A.; Dikranian, K.; Brody, D.L. Repetitive Closed-Skull Traumatic Brain Injury in Mice Causes Persistent Multifocal Axonal Injury and Microglial Reactivity. J. Neuropathol. Exp. Neurol. 2011, 70, 551–567. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef]
- Gentleman, S. Microglia in protein aggregation disorders: Friend or foe? Neuropathol. Appl. Neurobiol. 2013, 39, 45–50. [Google Scholar] [CrossRef]
- Holmin, S.; Mathiesen, T. Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport 1999, 10, 1889–1891. [Google Scholar] [CrossRef]
- Marmarou, A.; Lu, J.; Butcher, I.; McHugh, G.S.; Murray, G.D.; Steyerberg, E.W.; Mushkudiani, N.A.; Choi, S.; Maas, A.I. Prognostic Value of the Glasgow Coma Scale and Pupil Reactivity in Traumatic Brain Injury Assessed Pre-Hospital and on Enrollment: An IMPACT Analysis. J. Neurotrauma 2007, 24, 270–280. [Google Scholar] [CrossRef]
- Teasdale, G.; Murray, G.; Parker, L.; Jennett, B. Prognostic Factors in the First Week of Acute Head Injuries. Implication for Treatment. In Proceedings of the 6th European Congress of Neurosurgery, Paris, France, 15–20 July 1979; Springer: Berlin/Heidelberg, Germany, 1979; pp. 13–16. [Google Scholar]
- Jennett, B. Defining brain damage after head injury. J. R. Coll. Physicians Lond. 1979, 13, 197. [Google Scholar]
- Bhatty, G.B.; Kapoor, N. The Glasgow Coma Scale: A mathematical critique. Acta Neurochir. 1993, 120, 132–135. [Google Scholar] [CrossRef]
- Reith, F.C.; Lingsma, H.F.; Gabbe, B.J.; Lecky, F.E.; Roberts, I.; Maas, A.I. Differential effects of the Glasgow Coma Scale Score and its Components: An analysis of 54,069 patients with traumatic brain injury. Injury 2017, 48, 1932–1943. [Google Scholar] [CrossRef]
- Teoh, L.S.G.; Gowardman, J.R.; Larsen, P.D.; Green, R.; Galletly, D. Glasgow Coma Scale: Variation in mortality among permutations of specific total scores. Intensiv. Care Med. 2000, 26, 157–161. [Google Scholar] [CrossRef]
- McCrea, M.A.; Giacino, J.T.; Barber, J.; Temkin, N.R.; Nelson, L.D.; Levin, H.S.; Dikmen, S.; Stein, M.; Bodien, Y.G.; Boase, K.; et al. Functional Outcomes over the First Year after Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study. JAMA Neurol. 2021, 78, 982–992. [Google Scholar] [CrossRef]
- Nelson, L.D.; Temkin, N.R.; Dikmen, S.; Barber, J.; Giacino, J.T.; Yuh, E.; Levin, H.S.; McCrea, M.A.; Stein, M.B.; Mukherjee, P.; et al. Recovery after mild traumatic brain injury in patients presenting to US level I trauma centers: A transforming research and clinical knowledge in traumatic brain injury (TRACK-TBI) study. JAMA Neurol. 2019, 76, 1049–1059. [Google Scholar] [CrossRef]
- Nelson, L.D.; Ranson, J.; Ferguson, A.R.; Okonkwo, D.O.; Valadka, A.B.; Manley, G.T.; McCrea, M.A.; The TRACK-TBI Investigators; Riemann, L.; Mikolic, A.; et al. Validating Multi-Dimensional Outcome Assessment Using the Traumatic Brain Injury Common Data Elements: An Analysis of the TRACK-TBI Pilot Study Sample. J. Neurotrauma 2017, 34, 3158–3172. [Google Scholar] [CrossRef]
- Kondziella, D.; Friberg, C.K.; Frokjaer, V.G.; Fabricius, M.; Møller, K. Preserved consciousness in vegetative and minimal conscious states: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 485–492. [Google Scholar] [CrossRef]
- Claassen, J.; Doyle, K.; Matory, A.; Couch, C.; Burger, K.M.; Velazquez, A.; Okonkwo, J.U.; King, J.-R.; Park, S.; Agarwal, S.; et al. Detection of Brain Activation in Unresponsive Patients with Acute Brain Injury. N. Engl. J. Med. 2019, 380, 2497–2505. [Google Scholar] [CrossRef]
- Schiff, N.D. Cognitive Motor Dissociation Following Severe Brain Injuries. JAMA Neurol. 2015, 72, 1413–1415. [Google Scholar] [CrossRef]
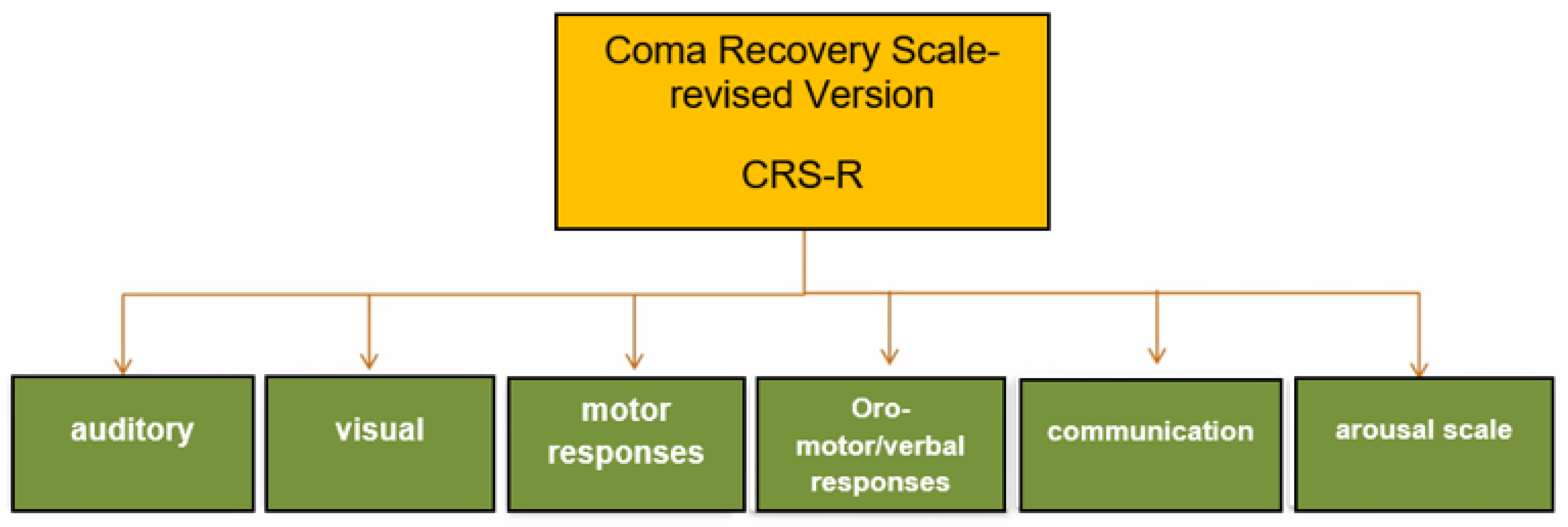
- Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; Kalmar, K.; Pape, T.L.-B.; Zafonte, R.; et al. Assessment Scales for Disorders of Consciousness: Evidence-Based Recommendations for Clinical Practice and Research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; DiPasquale, M.C.; Vaccaro, M. Assessment of command-following in minimally conscious brain injured patients. Arch. Phys. Med. Rehabil. 1999, 80, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Fins, J.J.; Laureys, S.; Schiff, N.D. Disorders of consciousness after acquired brain injury: The state of the science. Nat. Rev. Neurol. 2014, 10, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Boltzmann, M.; Schmidt, S.B.; Gutenbrunner, C.; Krauss, J.K.; Stangel, M.; Höglinger, G.U.; Wallesch, C.-W.; Rollnik, J.D. The influence of the CRS-R score on functional outcome in patients with severe brain injury receiving early rehabilitation. BMC Neurol. 2021, 21, 44. [Google Scholar] [CrossRef]
- Schnakers, C.; Bauer, C.; Formisano, R.; Noé, E.; Llorens, R.; Lejeune, N.; Farisco, M.; Teixeira, L.; Morrissey, A.-M.; De Marco, S.; et al. What names for covert awareness? A systematic review. Front. Hum. Neurosci. 2022, 16, 971315. [Google Scholar] [CrossRef]
- Kampfl, A.; Schmutzhard, E.; Franz, G.; Pfausler, B.; Haring, H.-P.; Ulmer, H.; Felber, S.; Golaszewski, S.; Aichner, F. Prediction of recovery from post-traumatic vegetative state with cerebral magnetic-resonance imaging. Lancet 1998, 351, 1763–1767. [Google Scholar] [CrossRef]
- Galanaud, D.; Perlbarg, V.; Gupta, R.; Stevens, R.D.; Sanchez, P.; Tollard, E.; De Champfleur, N.M.; Dinkel, J.; Faivre, S.; Stot-Ares, G.; et al. Assessment of White Matter Injury and Outcome in Severe Brain Trauma. A Prospective Multicenter Cohort. Surv. Anesthesiol. 2012, 117, 1300–1310. [Google Scholar] [CrossRef]
- Luyt, C.-E.; Galanaud, D.; Perlbarg, V.; Vanhaudenhuyse, A.; Stevens, R.D.; Gupta, R.; Besancenot, H.; Krainik, A.; Audibert, G.; Combes, A.; et al. Diffusion tensor imaging to predict long-term outcome after cardiac arrest: A bicentric pilot study. J. Am. Soc. Anesthesiol. 2012, 117, 1311–1321. [Google Scholar] [CrossRef]
- Voss, H.U.; Uluç, A.M.; Dyke, J.P.; Watts, R.; Kobylarz, E.J.; McCandliss, B.D.; Heier, L.A.; Beattie, B.J.; Hamacher, K.A.; Vallabhajosula, S.; et al. Possible axonal regrowth in late recovery from the minimally conscious state. J. Clin. Investig. 2006, 116, 2005–2011. [Google Scholar] [CrossRef]
- Bruno, M.-A.; Gosseries, O.; Ledoux, D.; Hustinx, R.; Laureys, S. Assessment of consciousness with electrophysiological and neurological imaging techniques. Curr. Opin. Crit. Care 2011, 17, 146–151. [Google Scholar] [CrossRef]
- Sandroni, C.; Cavallaro, F.; Callaway, C.W.; D’arrigo, S.; Sanna, T.; Kuiper, M.A.; Biancone, M.; Della Marca, G.; Farcomeni, A.; Nolan, J.P. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: A systematic review and meta-analysis. Part 2: Patients treated with therapeutic hypothermia. Resuscitation 2013, 84, 1324–1338. [Google Scholar] [CrossRef]
- Engemann, D.A.; Raimondo, F.; King, J.-R.; Rohaut, B.; Louppe, G.; Faugeras, F.; Annen, J.; Cassol, H.; Gosseries, O.; Fernandez-Slezak, D.; et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain 2018, 141, 3179–3192. [Google Scholar] [CrossRef]
- Chennu, S.; Annen, J.; Wannez, S.; Thibaut, A.; Chatelle, C.; Cassol, H.; Martens, G.; Schnakers, C.; Gosseries, O.; Menon, D.; et al. Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain 2017, 140, 2120–2132. [Google Scholar] [CrossRef]
- Bagnato, S.; Boccagni, C.; Sant’angelo, A.; Prestandrea, C.; Mazzilli, R.; Galardi, G. EEG predictors of outcome in patients with disorders of consciousness admitted for intensive rehabilitation. Clin. Neurophysiol. 2015, 126, 959–966. [Google Scholar] [CrossRef]
- Estraneo, A.; Fiorenza, S.; Magliacano, A.; Formisano, R.; Mattia, D.; Grippo, A.; Romoli, A.M.; Angelakis, E.; Cassol, H.; Thibaut, A.; et al. Multicenter prospective study on predictors of short-term outcome in disorders of consciousness. Neurology 2020, 95, e1488–e1499. [Google Scholar] [CrossRef]
- Laureys, S.; Goldman, S.; Phillips, C.; Van Bogaert, P.; Aerts, J.; Luxen, A.; Franck, G.; Maquet, P. Impaired Effective Cortical Connectivity in Vegetative State: Preliminary Investigation Using PET. Neuroimage 1999, 9, 377–382. [Google Scholar] [CrossRef]
- Laureys, S.; Lemaire, C.; Maquet, P.; Phillips, C.; Franck, G. Cerebral metabolism during vegetative state and after recovery to consciousness. J. Neurol. Neurosurg. Psychiatry 1999, 67, 121–122. [Google Scholar] [CrossRef]
- Phillips, C.L.; Bruno, M.-A.; Maquet, P.; Boly, M.; Noirhomme, Q.; Schnakers, C.; Vanhaudenhuyse, A.; Bonjean, M.; Hustinx, R.; Moonen, G.; et al. “Relevance vector machine” consciousness classifier applied to cerebral metabolism of vegetative and locked-in patients. Neuroimage 2011, 56, 797–808. [Google Scholar] [CrossRef]
- Corrigan, J.D.; Smith-Knapp, K.; Granger, C.V. Validity of the functional independence measure for persons with traumatic brain injury. Arch. Phys. Med. Rehabil. 1997, 78, 828–834. [Google Scholar] [CrossRef]
- Demertzi, A.; Soddu, A.; Laureys, S. Consciousness supporting networks. Curr. Opin. Neurobiol. 2013, 23, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Boly, M.; Phillips, C.; Balteau, E.; Schnakers, C.; Degueldre, C.; Moonen, G.; Luxen, A.; Peigneux, P.; Faymonville, M.-E.; Maquet, P.; et al. Consciousness and cerebral baseline activity fluctuations. Hum. Brain Mapp. 2008, 29, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Demertzi, A.; Vanhaudenhuyse, A.; Brédart, S.; Heine, L.; di Perri, C.; Laureys, S. Looking for the Self in Pathological Unconsciousness. Front. Hum. Neurosci. 2013, 7, 538. [Google Scholar] [CrossRef] [PubMed]
- Boly, M.; Tshibanda, L.; Vanhaudenhuyse, A.; Noirhomme, Q.; Schnakers, C.; Ledoux, D.; Boveroux, P.; Garweg, C.; Lambermont, B.; Phillips, C.; et al. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum. Brain Mapp. 2009, 30, 2393–2400. [Google Scholar] [CrossRef]
- Vanhaudenhuyse, A.; Noirhomme, Q.; Tshibanda, L.J.-F.; Bruno, M.-A.; Boveroux, P.; Schnakers, C.; Soddu, A.; Perlbarg, V.; Ledoux, D.; Brichant, J.-F.; et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2010, 133, 161–171. [Google Scholar] [CrossRef]
- Cauda, F.; Micon, B.M.; Sacco, K.; Duca, S.; D’Agata, F.; Geminiani, G.; Canavero, S. Disrupted intrinsic functional connectivity in the vegetative state. J. Neurol. Neurosurg. Psychiatry 2009, 80, 429–431. [Google Scholar] [CrossRef]
- Di, H.; Boly, M.; Weng, X.; Ledoux, D.; Laureys, S. Neuroimaging activation studies in the vegetative state: Predictors of recovery? Clin. Med. 2008, 8, 502. [Google Scholar] [CrossRef]
- Edlow, B.L.; Giacino, J.T.; Wu, O. Functional MRI and outcome in traumatic coma. Curr. Neurol. Neurosci. Rep. 2013, 13, 375. [Google Scholar] [CrossRef]
- Graham, N.S.N.; Zimmerman, K.A.; Moro, F.; Heslegrave, A.; Maillard, S.A.; Bernini, A.; Miroz, J.-P.; Donat, C.K.; Lopez, M.Y.; Bourke, N.; et al. Axonal marker neurofilament light predicts long-term outcomes and progressive neurodegeneration after traumatic brain injury. Sci. Transl. Med. 2021, 13, eabg9922. [Google Scholar] [CrossRef]
- Czeiter, E.; Amrein, K.; Gravesteijn, B.Y.; Lecky, F.; Menon, D.K.; Mondello, S.; Newcombe, V.F.; Richter, S.; Steyerberg, E.W.; Vyvere, T.V.; et al. Blood biomarkers on admission in acute traumatic brain injury: Relations to severity, CT findings and care path in the CENTER-TBI study. Ebiomedicine 2020, 56, 102785. [Google Scholar] [CrossRef]
- Helmrich, I.R.A.R.; Czeiter, E.; Amrein, K.; Büki, A.; Lingsma, H.F.; Menon, D.K.; Mondello, S.; Steyerberg, E.W.; von Steinbüchel, N.; Wang, K.K.W.; et al. Incremental prognostic value of acute serum biomarkers for functional outcome after traumatic brain injury (CENTER-TBI): An observational cohort study. Lancet Neurol. 2022, 21, 792–802. [Google Scholar] [CrossRef]
- Okonkwo, D.O.; Puffer, R.C.; Puccio, A.M.; Yuh, E.L.; Yue, J.K.; Diaz-Arrastia, R.; Korley, F.K.; Wang, K.K.W.; Sun, X.; Taylor, S.R.; et al. Point-of-Care Platform Blood Biomarker Testing of Glial Fibrillary Acidic Protein versus S100 Calcium-Binding Protein B for Prediction of Traumatic Brain Injuries: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury Study. J. Neurotrauma 2020, 37, 2460–2467. [Google Scholar] [CrossRef]
- Wang, K.K.; Kobeissy, F.H.; Shakkour, Z.; Tyndall, J.A. Thorough overview of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein as tandem biomarkers recently cleared by US Food and Drug Administration for the evaluation of intracranial injuries among patients with traumatic brain injury. Acute Med. Surg. 2021, 8, e622. [Google Scholar] [CrossRef]
- Papa, L.; Silvestri, S.; Brophy, G.M.; Giordano, P.; Falk, J.L.; Braga, C.F.; Tan, C.N.; Ameli, N.J.; Demery, J.A.; Dixit, N.K.; et al. GFAP Out-Performs S100β in Detecting Traumatic Intracranial Lesions on Computed Tomography in Trauma Patients with Mild Traumatic Brain Injury and Those with Extracranial Lesions. J. Neurotrauma 2014, 31, 1815–1822. [Google Scholar] [CrossRef]
- Mondello, S.; Jeromin, A.; Buki, A.; Bullock, R.; Czeiter, E.; Kovacs, N.; Barzo, P.; Hayes, R.L.; Wang, K.K.W.; Yang, Z.; et al. Glial Neuronal Ratio: A Novel Index for Differentiating Injury Type in Patients with Severe Traumatic Brain Injury. J. Neurotrauma 2012, 29, 1096–1104. [Google Scholar] [CrossRef]
- Mondello, S.; Papa, L.; Buki, A.; Bullock, M.R.; Czeiter, E.; Tortella, F.C.; Wang, K.K.; Hayes, R.L. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: A case control study. Crit. Care 2011, 15, R156. [Google Scholar] [CrossRef]
- Whitehouse, D.P.; Monteiro, M.; Czeiter, E.; Vyvere, T.V.; Valerio, F.; Ye, Z.; Amrein, K.; Kamnitsas, K.; Xu, H.; Yang, Z.; et al. Relationship of admission blood proteomic biomarkers levels to lesion type and lesion burden in traumatic brain injury: A CENTER-TBI study. Ebiomedicine 2022, 75, 103777. [Google Scholar] [CrossRef] [PubMed]
- Korley, F.K.; Jain, S.; Sun, X.; Puccio, A.M.; Yue, J.K.; Gardner, R.C.; Wang, K.K.W.; Okonkwo, D.O.; Yuh, E.L.; Mukherjee, P.; et al. Prognostic value of day-of-injury plasma GFAP and UCH-L1 concentrations for predicting functional recovery after traumatic brain injury in patients from the US TRACK-TBI cohort: An observational cohort study. Lancet Neurol. 2022, 21, 803–813. [Google Scholar] [CrossRef]
- Seabury, S.A.; Gaudette, É.; Goldman, D.P.; Markowitz, A.J.; Brooks, J.; McCrea, M.A.; Okonkwo, D.O.; Manley, G.T.; The TRACK-TBI Investigators. Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: Results from the TRACK-TBI study. JAMA Netw. Open 2018, 1, e180210. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; et al. Author response: Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2019, 92, 1699–1709. [Google Scholar] [CrossRef]
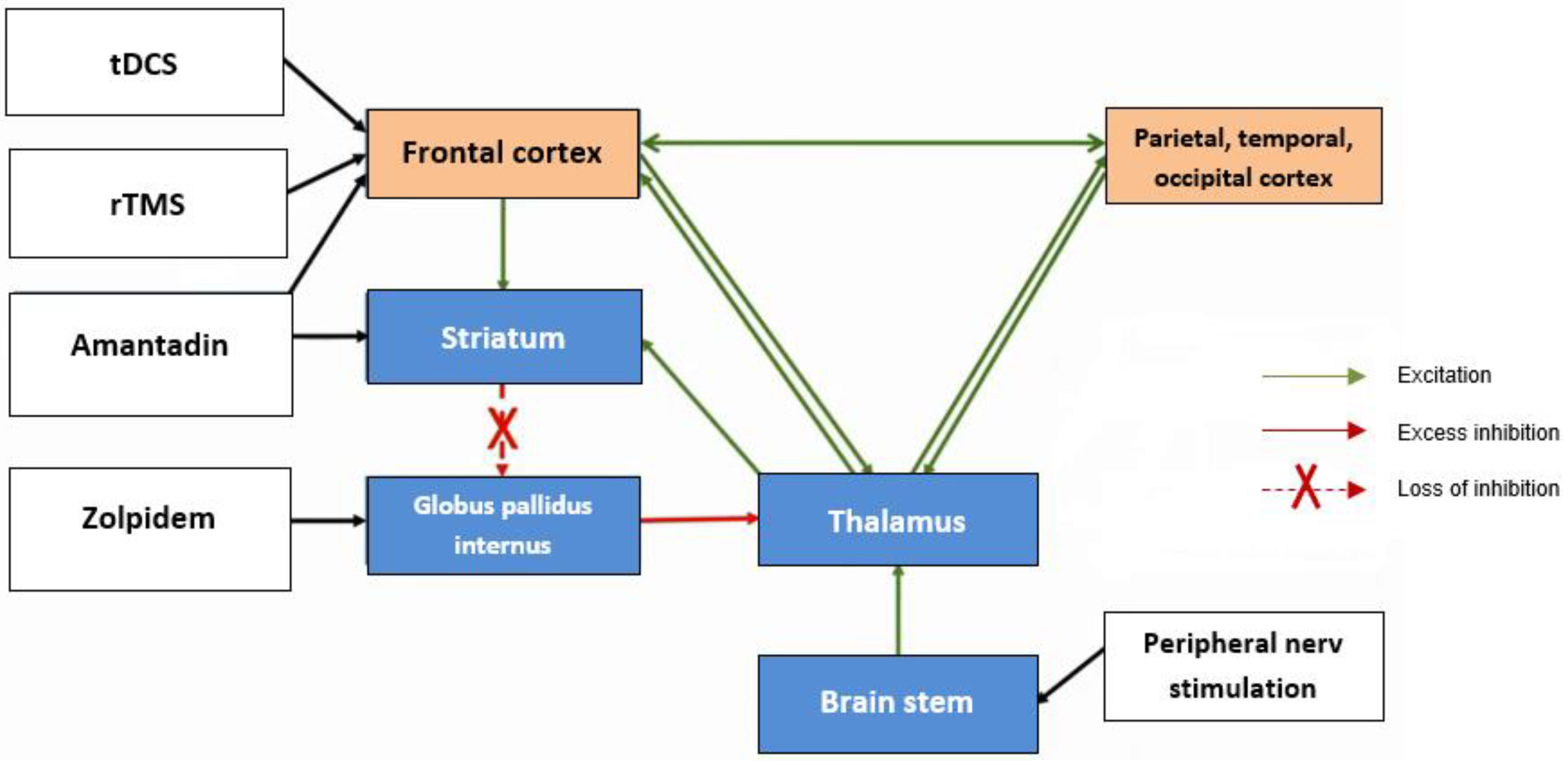
- Giacino, J.T.; Whyte, J.; Bagiella, E.; Kalmar, K.; Childs, N.; Khademi, A.; Eifert, B.; Long, D.; Katz, D.I.; Cho, S.; et al. Placebo-Controlled Trial of Amantadine for Severe Traumatic Brain Injury. N. Engl. J. Med. 2012, 366, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Eban-Rothschild, A.; Rothschild, G.; Giardino, W.J.; Jones, J.R.; de Lecea, L. VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat. Neurosci. 2016, 19, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Fridman, E.A.; Beattie, B.J.; Broft, A.; Laureys, S.; Schiff, N.D. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc. Natl. Acad. Sci. USA 2014, 111, 6473–6478. [Google Scholar] [CrossRef]
- Matsuda, W.; Komatsu, Y.; Yanaka, K.; Matsumura, A. Levodopa treatment for patients in persistent vegetative or minimally conscious states. Neuropsychol. Rehabil. 2005, 15, 414–427. [Google Scholar] [CrossRef]
- Ugoya, S.O.; Akinyemi, R.O. The Place of l-Dopa/Carbidopa in Persistent Vegetative State. Clin. Neuropharmacol. 2010, 33, 279–284. [Google Scholar] [CrossRef]
- Tucker, C.; Sandhu, K. The Effectiveness of Zolpidem for the Treatment of Disorders of Consciousness. Neurocritical Care 2016, 24, 488–493. [Google Scholar] [CrossRef]
- Sutton, J.A.; Clauss, R.P. A review of the evidence of zolpidem efficacy in neurological disability after brain damage due to stroke, trauma and hypoxia: A justification of further clinical trials. Brain Inj. 2017, 31, 1019–1027. [Google Scholar] [CrossRef]
- Williams, S.T.; Conte, M.M.; Goldfine, A.M.; Noirhomme, Q.; Gosseries, O.; Thonnard, M.; Beattie, B.; Hersh, J.; Katz, D.I.; Victor, J.D.; et al. Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severe brain injury. eLife 2013, 2, e01157. [Google Scholar] [CrossRef]
- Whyte, J.; Rajan, R.; Rosenbaum, A.; Katz, D.; Kalmar, K.; Seel, R.; Greenwald, B.; Zafonte, R.; Demarest, D.; Brunner, R.; et al. Zolpidem and restoration of consciousness. Am. J. Phys. Med. Rehabil. 2014, 93, 101–113. [Google Scholar] [CrossRef]
- Thonnard, M.; Gosseries, O.; Demertzi, A.; Lugo, Z.; Vanhaudenhuyse, A.; Bruno, M.-A.; Chatelle, C.; Thibaut, A.; Charland-Verville, V.; Habbal, D.; et al. Effect of zolpidem in chronic disorders of consciousness: A prospective open-label study. Funct. Neurol. 2013, 28, 259. [Google Scholar]
- Lin, P.-H.; Kuo, L.-T.; Luh, H.-T. The Roles of Neurotrophins in Traumatic Brain Injury. Life 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Schoch, K.M.; Madathil, S.K.; Saatman, K.E. Genetic Manipulation of Cell Death and Neuroplasticity Pathways in Traumatic Brain Injury. Neurotherapeutics 2012, 9, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.G.; Hoffman, S.W. Concepts of CNS Plasticity in the Context of Brain Damage and Repair. J. Head Trauma Rehabil. 2003, 18, 317–341. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-S.; Zhang, G.; Liebl, D.J.; Kernie, S.G. Traumatic Brain Injury-Induced Hippocampal Neurogenesis Requires Activation of Early Nestin-Expressing Progenitors. J. Neurosci. 2008, 28, 12901–12912. [Google Scholar] [CrossRef]
- Madathil, S.K.; Saatman, K.E. IGF-1/IGF-R Signaling in Traumatic Brain Injury: Impact on Cell Survival, Neurogenesis, and Behavioral Outcome; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Skinner, S.J.; Geaney, M.S.; Rush, R.; Rogers, M.-L.; Emerich, D.F.; Thanos, C.G.; Vasconcellos, A.V.; Tan, P.L.; Elliott, R.B. Choroid plexus transplants in the treatment of brain diseases. Xenotransplantation 2006, 13, 284–288. [Google Scholar] [CrossRef]
- Johanson, C.; Stopa, E.; Baird, A.; Sharma, H. Traumatic brain injury and recovery mechanisms: Peptide modulation of periventricular neurogenic regions by the choroid plexus–CSF nexus. J. Neural Transm. 2011, 118, 115–133. [Google Scholar] [CrossRef]
- Skinner, S.J.M.; Geaney, M.S.; Lin, H.; Muzina, M.; Anal, A.K.; Elliott, R.B.; Tan, P.L.J. Encapsulated living choroid plexus cells: Potential long-term treatments for central nervous system disease and trauma. J. Neural Eng. 2009, 6, 065001. [Google Scholar] [CrossRef]
- Pedraza, C.E.; Podlesniy, P.; Vidal, N.; Arévalo, J.C.; Lee, R.; Hempstead, B.; Ferrer, I.; Iglesias, M.; Espinet, C. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Am. J. Pathol. 2005, 166, 533–543. [Google Scholar] [CrossRef]
- Harrington, A.W.; Leiner, B.; Blechschmitt, C.; Arevalo, J.C.; Lee, R.; Mörl, K.; Meyer, M.; Hempstead, B.L.; Yoon, S.O.; Giehl, K.M. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc. Natl. Acad. Sci. USA 2004, 101, 6226–6230. [Google Scholar] [CrossRef]
- Chiaretti, A.; Antonelli, A.; Riccardi, R.; Genovese, O.; Pezzotti, P.; Di Rocco, C.; Tortorolo, L.; Piedimonte, G. Nerve growth factor expression correlates with severity and outcome of traumatic brain injury in children. Eur. J. Paediatr. Neurol. 2008, 12, 195–204. [Google Scholar] [CrossRef]
- Munoz, M.J.; Kumar, R.G.; Oh, B.-M.; Conley, Y.P.; Wang, Z.; Failla, M.D.; Wagner, A.K. Cerebrospinal Fluid Cortisol Mediates Brain-Derived Neurotrophic Factor Relationships to Mortality after Severe TBI: A Prospective Cohort Study. Front. Mol. Neurosci. 2017, 10, 44. [Google Scholar] [CrossRef]
- Bliźniewska-Kowalska, K.; Łukasik, M.; Gałecki, P. Cerebrolysin—Mechanism of action and application in psychiatry and neurology. Pharmacother. Psychiatry Neurol. 2019, 35, 9–23. [Google Scholar] [CrossRef]
- König, P.; Waanders, R.; Witzmann, A.; Lanner, G.; Haffner, Z.; Haninec, P.; Gmeinbauer, R.; Zimmermann-Meinzingen, S. Cerebrolysin in traumatic brain injury—A pilot study of a neurotrophic and neurogenic agent in the treatment of acute traumatic brain injury. J. Neurol. Neurochir. Psychiatr. 2006, 7, 12–20. [Google Scholar]
- Chen, C.-C.; Wei, S.-T.; Tsaia, S.-C.; Chen, X.-X.; Cho, D.-Y. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: Double-blind, placebo-controlled, randomized study. Br. J. Neurosurg. 2013, 27, 803–807. [Google Scholar] [CrossRef]
- Muresanu, D.F.; Ciurea, A.V.; Gorgan, R.M.; Gheorghita, E.; Florian, S.I.; Stan, H.; Blaga, A.; Ianovici, N.; Iencean, S.M.; Turliuc, D.; et al. A retrospective, multi-center cohort study evaluating the severity-related effects of cerebrolysin treatment on clinical outcomes in traumatic brain injury. CNS Neurol. Disord. Drug Targets 2015, 14, 587–599. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef]
- Cavinato, M.; Genna, C.; Formaggio, E.; Gregorio, C.; Storti, S.F.; Manganotti, P.; Casanova, E.; Piperno, R.; Piccione, F. Behavioural and electrophysiological effects of tDCS to prefrontal cortex in patients with disorders of consciousness. Clin. Neurophysiol. 2019, 130, 231–238. [Google Scholar] [CrossRef]
- Ali, M.M.; Sellers, K.K.; Fröhlich, F. Transcranial Alternating Current Stimulation Modulates Large-Scale Cortical Network Activity by Network Resonance. J. Neurosci. 2013, 33, 11262–11275. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Schneider, T.R.; Rach, S.; Trautmann-Lengsfeld, S.A.; Engel, A.K.; Herrmann, C.S. Entrainment of Brain Oscillations by Transcranial Alternating Current Stimulation. Curr. Biol. 2014, 24, 333–339. [Google Scholar] [CrossRef]
- Thibaut, A.; Bruno, M.-A.; LeDoux, D.; Demertzi, A.; Laureys, S. tDCS in patients with disorders of consciousness: Sham-controlled randomized double-blind study. Neurology 2014, 82, 1112–1118. [Google Scholar] [CrossRef]
- Martens, G.; Lejeune, N.; O’Brien, A.T.; Fregni, F.; Martial, C.; Wannez, S.; Laureys, S.; Thibaut, A. Randomized controlled trial of home-based 4-week tDCS in chronic minimally conscious state. Brain Stimul. 2018, 11, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Müri, R.M.; Cazzoli, D.; Nef, T.; Mosimann, U.P.; Hopfner, S.; Nyffeler, T. Non-Invasive Brain Stimulation in Neglect Rehabilitation: An Update. Front. Hum. Neurosci. 2013, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Cincotta, M.; Giovannelli, F.; Chiaramonti, R.; Bianco, G.; Godone, M.; Battista, D.; Cardinali, C.; Borgheresi, A.; Sighinolfi, A.; D’Avanzo, A.M.; et al. No effects of 20 Hz-rTMS of the primary motor cortex in vegetative state: A randomised, sham-controlled study. Cortex 2015, 71, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gao, J.; Pan, S.; Meng, F.; Pan, G.; Li, J.; Luo, B. Effects of High-Frequency Repetitive Transcranial Magnetic Stimulation on Cerebral Hemodynamics in Patients with Disorders of Consciousness: A Sham-Controlled Study. Eur. Neurol. 2016, 76, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Russo, M.; Leo, A.; Bramanti, P.; Quartarone, A.; Calabrò, R.S. A single session of repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex in patients with unresponsive wakefulness syndrome: Preliminary results. Neurorehabilit. Neural Repair 2015, 29, 603–613. [Google Scholar] [CrossRef]
- Casali, A.G.; Gosseries, O.; Rosanova, M.; Boly, M.; Sarasso, S.; Casali, K.R.; Casarotto, S.; Bruno, M.-A.; Laureys, S.; Tononi, G.; et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013, 5, 198ra105. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Li, C.; Li, X.; He, J.; Bai, Y. Transcranial magnetic stimulation-evoked connectivity reveals modulation effects of repetitive transcranial magnetic stimulation on patients with disorders of consciousness. Neuroreport 2019, 30, 1307–1315. [Google Scholar] [CrossRef]
- Mensen, A.; Bodart, O.; Thibaut, A.; Wannez, S.; Annen, J.; Laureys, S.; Gosseries, O. Decreased Evoked Slow-Activity after tDCS in Disorders of Consciousness. Front. Syst. Neurosci. 2020, 14, 62. [Google Scholar] [CrossRef]
- Blethyn, K.L.; Hughes, S.W.; Crunelli, V. Evidence for electrical synapses between neurons of the nucleus reticularis thalami in the adult brain in vitro. Thalamus Relat. Syst. 2008, 4, 13–20. [Google Scholar] [CrossRef]
- Hindriks, R.; van Putten, M.J. Thalamo-cortical mechanisms underlying changes in amplitude and frequency of human alpha oscillations. Neuroimage 2013, 70, 150–163. [Google Scholar] [CrossRef]
- Cooper, J.B.; Jane, J.A.; Alves, W.M.; Cooper, E.B. Right median nerve electrical stimulation to hasten awakening from coma. Brain Inj. 1999, 13, 261–267. [Google Scholar] [CrossRef]
- Peri, C.V.; Shaffrey, M.E.; Farace, E.; Cooper, E.; Alves, W.M.; Cooper, J.B.; Young, J.S.; Jane, J.A. Pilot study of electrical stimulation on median nerve in comatose severe brain injured patients: 3-month outcome. Brain Inj. 2001, 15, 903–910. [Google Scholar] [CrossRef]
- Lei, J.; Wang, L.; Gao, G.; Cooper, E.; Jiang, J.; Hånell, A.; Greer, J.E.; Jacobs, K.M.; Guilfoyle, M.R.; Carpenter, K.L.; et al. Right Median Nerve Electrical Stimulation for Acute Traumatic Coma Patients. J. Neurotrauma 2015, 32, 1584–1589. [Google Scholar] [CrossRef]
- Edlow, B.L.; Sanz, L.R.D.; Polizzotto, L.; Pouratian, N.; Rolston, J.D.; Snider, S.B.; Thibaut, A.; Stevens, R.D.; Gosseries, O.; Akbari, Y.; et al. Therapies to Restore Consciousness in Patients with Severe Brain Injuries: A Gap Analysis and Future Directions. Neurocritical Care 2021, 35, 68–85. [Google Scholar] [CrossRef]
- Iriki, A.; Corazzol, M.; Lio, G.; Lefevre, A.; Deiana, G.; Tell, L.; André-Obadia, N.; Bourdillon, P.; Guenot, M.; Desmurget, M.; et al. Faculty Opinions recommendation of Restoring consciousness with vagus nerve stimulation. Curr. Biol. 2017, 27, R994–R996. [Google Scholar] [CrossRef]
- Hakon, J.; Moghiseh, M.; Poulsen, I.; Msc, C.M.; Hansen, C.P.; Sabers, A. Transcutaneous Vagus Nerve Stimulation in Patients with Severe Traumatic Brain Injury: A Feasibility Trial. Neuromodul. Technol. Neural Interface 2020, 23, 859–864. [Google Scholar] [CrossRef]
- Noé, E.; Ferri, J.; Colomer, C.; Moliner, B.; O’Valle, M.; Ugart, P.; Rodriguez, C.; Llorens, R. Feasibility, safety and efficacy of transauricular vagus nerve stimulation in a cohort of patients with disorders of consciousness. Brain Stimul. 2020, 13, 427–429. [Google Scholar] [CrossRef]
- Giacino, J.T. Sensory stimulation: Theoretical perspectives and the evidence for effectiveness. NeuroRehabilitation 1996, 6, 69–78. [Google Scholar] [CrossRef]
- O’kelly, J.; James, L.; Palaniappan, R.; Taborin, J.; Fachner, J.; Magee, W.L. Neurophysiological and Behavioral Responses to Music Therapy in Vegetative and Minimally Conscious States. Front. Hum. Neurosci. 2013, 7, 884. [Google Scholar] [CrossRef]
- Perrin, F.; Castro, M.; Tillmann, B.; Luauté, J. Promoting the use of personally relevant stimuli for investigating patients with disorders of consciousness. Front. Psychol. 2015, 6, 1102. [Google Scholar] [CrossRef]
- Boltzmann, M.; Schmidt, S.B.; Gutenbrunner, C.; Krauss, J.K.; Stangel, M.; Höglinger, G.U.; Wallesch, C.-W.; Münte, T.F.; Rollnik, J.D. Auditory Stimulation Modulates Resting-State Functional Connectivity in Unresponsive Wakefulness Syndrome Patients. Front. Neurosci. 2021, 15, 554194. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Hu, N.; Wang, T. Music Interventions for Disorders of Consciousness: A Systematic Review and Meta-analysis. J. Neurosci. Nurs. 2020, 52, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Frazzitta, G.; Zivi, I.; Valsecchi, R.; Bonini, S.; Maffia, S.; Molatore, K.; Sebastianelli, L.; Zarucchi, A.; Matteri, D.; Ercoli, G.; et al. Effectiveness of a very early stepping verticalization protocol in severe acquired brain injured patients: A randomized pilot study in ICU. PLoS ONE 2016, 11, e0158030. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.B.; Ashman, T.; Bushnik, T.; Cai, X.; Farrell-Carnahan, L.; Gumber, S.; Hart, T.; Rosenthal, J.; Dijkers, M. Systematic review of interventions for fatigue after traumatic brain injury: A NIDRR traumatic brain injury model systems study. J. Head Trauma Rehabil. 2014, 29, 490–497. [Google Scholar] [CrossRef]
- Winward, C.; Sackley, C.; Metha, Z.; Rothwell, P.M. A Population-Based Study of the Prevalence of Fatigue after Transient Ischemic Attack and Minor Stroke. Stroke 2009, 40, 757–761. [Google Scholar] [CrossRef]
- Ali, A.M.; Morfin, J.B.; Mills, J.A.; Pasipanodya, E.C.; Maas, Y.J.M.; Huang, E.; Dirlikov, B.M.; Englander, J.; Zedlitz, A. Fatigue after Traumatic Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2022, 37, E249–E257. [Google Scholar] [CrossRef]
- Johansson, B.; Bjuhr, H.; Rönnbäck, L. Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Inj. 2012, 26, 1621–1628. [Google Scholar] [CrossRef]
- Edlow, B.L.; Claassen, J.; Schiff, N.D.; Greer, D.M. Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nat. Rev. Neurol. 2021, 17, 135–156. [Google Scholar] [CrossRef]
- Schnakers, C.; Monti, M.M. Disorders of consciousness after severe brain injury: Therapeutic options. Curr. Opin. Neurol. 2017, 30, 573–579. [Google Scholar] [CrossRef]
- Thibaut, A.; Schiff, N.; Giacino, J.; Laureys, S.; Gosseries, O. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. 2019, 18, 600–614. [Google Scholar] [CrossRef]
- Andreas Bender, S.B.; Bodechtel, U.; Eifert, B.; Elsner, B.; Feddersen, B.; Freivogel, S.; Hoffmann, B.; Hucke, B.; Huge, V.; HundGeorgiadis, M.; et al. S3-LL Neurologische Rehabilitation bei Koma und schwerer Bewusstseinsstörung im Erwachsenenalter. In Leitlinien für die Neurorehabilitation, 1st ed.; Deutsche Gesellschaft für Neurorehabilitation e.v. (Dgnr) (Hrsgb.): Rheinbach, Germany, 2022. [Google Scholar]
- Kondziella, D.; Bender, A.; Diserens, K.; van Erp, W.; Estraneo, A.; Formisano, R.; Laureys, S.; Naccache, L.; Ozturk, S.; Rohaut, B.; et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 2020, 27, 741–756. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippert, J.; Guggisberg, A.G. Diagnostic and Therapeutic Approaches in Neurorehabilitation after Traumatic Brain Injury and Disorders of Consciousness. Clin. Transl. Neurosci. 2023, 7, 21. https://doi.org/10.3390/ctn7030021
Lippert J, Guggisberg AG. Diagnostic and Therapeutic Approaches in Neurorehabilitation after Traumatic Brain Injury and Disorders of Consciousness. Clinical and Translational Neuroscience. 2023; 7(3):21. https://doi.org/10.3390/ctn7030021
Chicago/Turabian StyleLippert, Julian, and Adrian G. Guggisberg. 2023. "Diagnostic and Therapeutic Approaches in Neurorehabilitation after Traumatic Brain Injury and Disorders of Consciousness" Clinical and Translational Neuroscience 7, no. 3: 21. https://doi.org/10.3390/ctn7030021
APA StyleLippert, J., & Guggisberg, A. G. (2023). Diagnostic and Therapeutic Approaches in Neurorehabilitation after Traumatic Brain Injury and Disorders of Consciousness. Clinical and Translational Neuroscience, 7(3), 21. https://doi.org/10.3390/ctn7030021





