The Role of Neurorehabilitation in Post-COVID-19 Syndrome
Abstract
1. Introduction
2. Neurological Manifestations of Post-COVID-19 Syndrome
2.1. Fatigue
2.2. Cognitive Symptoms
2.3. Other Frequent Symptoms
3. Treatment of Post-COVID-19 Neurological Symptoms
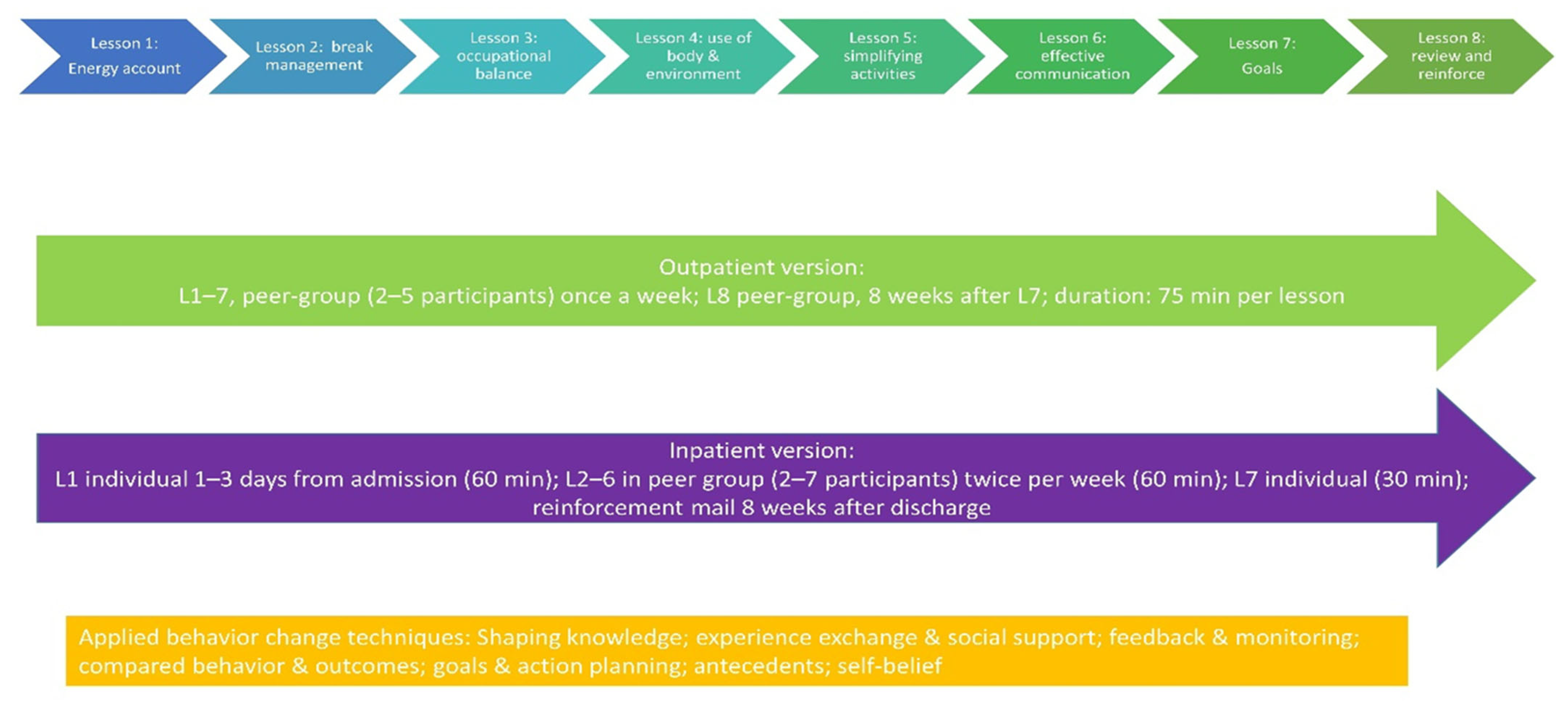
3.1. Occupational Therapy
3.2. Physiotherapy
3.2.1. Graduated Exercise Therapy
3.2.2. Pacing
3.3. Heart Rate Monitoring
- -
- (220 − age) × 0.55 = guideline (in beats per minute (bpm));
- -
- Resting heart rate as a measure for activity management. Resting heart rate can be determined by having the person lie flat in bed and calculating the average resting heart rate over 7 days. The benchmark is now set as 15 bpm above the resting heart rate.
3.4. Treatment of Neuropathic Pain
3.5. Treatment of Headaches
3.6. Treatment of Sleep Disorders
- (1)
- Do not drink caffeinated beverages (coffee, black tea, or cola) after lunch;
- (2)
- Avoid alcohol to a large extent and do not use it as a sleeping pill under any circumstances;
- (3)
- No heavy meals in the evening;
- (4)
- Regular physical activity;
- (5)
- Gradually reduce mental and physical exertion before going to bed;
- (6)
- Introduce a personal bedtime ritual;
- (7)
- Create a pleasant atmosphere in the bedroom (quiet and darkened);
- (8)
- Do not look at an alarm clock or wristwatch during the night.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.P.; Gaughan, C. Technical Article: Updated Estimates of the Prevalence of Post-Acute Symptoms among People with Coronavirus (COVID-19) in the UK: 26 April 2020 to 1 August 2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymtomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021 (accessed on 26 November 2022).
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Jacobson, K.B.; Rao, M.; Bonilla, H.; Subramanian, A.; Hack, I.; Madrigal, M.; Singh, U.; Jagannathan, P.; Grant, P. Patients with Uncomplicated Coronavirus Disease 2019 (COVID-19) Have Long-Term Persistent Symptoms and Functional Impairment Similar to Patients with Severe COVID-19: A Cautionary Tale During a Global Pandemic. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e826–e829. [Google Scholar] [CrossRef]
- Diem, L.; Fregolente-Gomes, L.; Warncke, J.D.; Hammer, H.; Friedli, C.; Kamber, N.; Jung, S.; Bigi, S.; Funke-Chambour, M.; Chan, A.; et al. Fatigue in Post-COVID-19 Syndrome: Clinical Phenomenology, Comorbidities and Association with Initial Course of COVID-19. J. Central Nerv. Syst. Dis. 2022, 14, 1–7. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Engl, M.; Romanello, R.; Nardone, R.; Bonini, I.; Koch, G.; Saltuari, L.; Quartarone, A.; et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J. Neurol. Sci. 2021, 420, 117271. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention: Post-Exertional Malaise (PEM). Available online: https://www.cdc.gov/me-cfs/healthcare-providers/clinical-care-patients-mecfs/treating-most-disruptive-symptoms.html (accessed on 26 November 2022).
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS ONE 2018, 13, e0197811. [Google Scholar] [CrossRef] [PubMed]
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic Fatigue and Postexertional Malaise in People Living with Long COVID: An Observational Study. Phys. Ther. 2022, 102, pzac005. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Ohanian, D.; Brown, A.; Sunnquist, M.; McManimen, S.; Klebek, L.; Fox, P.; Sorenson, M. Differentiating Multiple Sclerosis from Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. Insights Biomed. 2017, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Empfehlungen für die Versicherungsmedizinische Abklärung in der Schweiz bei Post-Covid-19-Erkrankung-Arbeitsgruppe Post-COVID-19 Versicherungsmedizin. Available online: https://www.swiss-insurance-medicine.ch/storage/app/media/Downloads/Dokumente/covid-19/220317_Post-Covid-19-Erkrankung_Empfehlung.pdf (accessed on 26 November 2022).
- Biagianti, B.; Di Liberto, A.; Nicolo Edoardo, A.; Lisi, I.; Nobilia, L.; de Ferrabonc, G.D.; Zanier, E.R.; Stocchetti, N.; Brambilla, P. Cognitive Assessment in SARS-CoV-2 Patients: A Systematic Review. Front. Aging Neurosci. 2022, 14, 909661. [Google Scholar] [CrossRef]
- Lynch, S.; Ferrando, S.J.; Dornbush, R.; Shahar, S.; Smiley, A.; Klepacz, L. Screening for brain fog: Is the montreal cognitive assessment an effective screening tool for neurocognitive complaints post-COVID-19? Gen. Hosp. Psychiatry 2022, 78, 80–86. [Google Scholar] [CrossRef]
- Diem, L.; Schwarzwald, A.; Friedli, C.; Hammer, H.; Gomes-Fregolente, L.; Warncke, J.; Weber, L.; Kamber, N.; Chan, A.; Bassetti, C.; et al. Multidimensional phenotyping of the post-COVID-19 syndrome: A Swiss survey study. CNS Neurosci. Ther. 2022, 28, 1953–1963. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Stainsby, B.; Howitt, S.; Porr, J. Neuromusculoskeletal disorders following SARS: A case series. J. Can. Chiropr. Assoc. 2011, 55, 32–39. [Google Scholar]
- El Sayed, S.; Gomaa, S.; Shokry, D.; Kabil, A.; Eissa, A. Sleep in post-COVID-19 recovery period and its impact on different domains of quality of life. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 172. [Google Scholar] [CrossRef]
- Schwarzl, G.; Hayden, M.; Limbach, M.; Schultz, K. The prevalence of Obstructive Sleep Apnea (OSA) in patients recovering from COVID-19. ERJ Open Res. 2021, 7, 24. [Google Scholar] [CrossRef]
- Kavi, L. Postural tachycardia syndrome and long COVID: An update. Br. J. Gen. Pract. 2022, 72, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Stahlberg, M.; Reistam, U.; Fedorowski, A.; Villacorta, H.; Horiuchi, Y.; Bax, J.; Pitt, B.; Matskeplishvili, S.; Luscher, T.F.; Weichert, I.; et al. Post-COVID-19 Tachycardia Syndrome: A Distinct Phenotype of Post-Acute COVID-19 Syndrome. Am. J. Med. 2021, 134, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Moggio, L.; Marotta, N.; Agostini, F.; Tasselli, A.; Drago Ferrante, V.; Curci, C.; Calafiore, D.; Ferraro, F.; Bernetti, A.; et al. Impact of Rehabilitation on Fatigue in Post-COVID-19 Patients: A Systematic Review and Meta-Analysis. Appl. Sci. 2022, 12, 8593. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Koczulla, A.R.; Ankermann, T.; Behrends, U.; Berlit, P.; Böing, S.; Brinkmann, F.; Franke, C.; Glöckl, R.; Gogoll, C.; Hummel, T.; et al. S1-Leitlinie Post-COVID/Long-COVID. Pneumologie 2022, 76, 855–907. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Myalgic Encephalomyelitis (or Encephalopathy)/Chronic Fatigue Syndrome: Diagnosis and Management—In Development [GID-NG10091]. Available online: https://www.nice.org.uk/guidance/indevelopment/gid-ng10091 (accessed on 26 November 2022).
- Hersche, R.; Weise, A.; Michel, G.; Kesselring, J.; Barbero, M.; Kool, J. Development and Preliminary Evaluation of a 3-Week Inpatient Energy Management Education Program for People with Multiple Sclerosis-Related Fatigue. Int. J. MS Care 2019, 21, 265–274. [Google Scholar] [CrossRef]
- Hersche, R.; Weise, A.; Michel, G.; Kesselring, J.; Bella, S.D.; Barbero, M.; Kool, J. Three-week inpatient energy management education (IEME) for persons with multiple sclerosis-related fatigue: Feasibility of a randomized clinical trial. Mult. Scler. Relat. Disord. 2019, 35, 26–33. [Google Scholar] [CrossRef]
- Patt, N.; Kool, J.; Hersche, R.; Oberste, M.; Walzik, D.; Joisten, N.; Caminada, D.; Ferrara, F.; Gonzenbach, R.; Nigg, C.R.; et al. High-intensity interval training and energy management education, compared with moderate continuous training and progressive muscle relaxation, for improving health-related quality of life in persons with multiple sclerosis: Study protocol of a randomized controlled superiority trial with six months’ follow-up. BMC Neurol. 2021, 21, 65. [Google Scholar] [CrossRef]
- Hersche, R.; Weise, A. Occupational Therapy-Based Energy Management Education in People with Post-COVID-19 Condition-Related Fatigue: Results from a Focus Group Discussion. Occup. Ther. Int. 2022, 2022, 4590154. [Google Scholar] [CrossRef]
- Abrahams, H.J.G.; Gielissen, M.F.M.; Donders, R.R.T.; Goedendorp, M.M.; van der Wouw, A.J.; Verhagen, C.; Knoop, H. The efficacy of Internet-based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: A randomized controlled trial. Cancer 2017, 123, 3825–3834. [Google Scholar] [CrossRef]
- Janse, A.; Worm-Smeitink, M.; Bleijenberg, G.; Donders, R.; Knoop, H. Efficacy of web-based cognitive-behavioural therapy for chronic fatigue syndrome: Randomised controlled trial. Br. J. Psychiatry 2018, 212, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Menting, J.; Tack, C.J.; van Bon, A.C.; Jansen, H.J.; van den Bergh, J.P.; Mol, M.; Goedendorp, M.M.; Donders, R.; Knoop, H. Web-based cognitive behavioural therapy blended with face-to-face sessions for chronic fatigue in type 1 diabetes: A multicentre randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Kuut, T.A.; Muller, F.; Aldenkamp, A.; Assmann-Schuilwerve, E.; Braamse, A.; Geerlings, S.E.; Gibney, K.B.; Kanaan, R.A.A.; Nieuwkerk, P.; Olde Hartman, T.C.; et al. A randomised controlled trial testing the efficacy of Fit after COVID, a cognitive behavioural therapy targeting severe post-infectious fatigue following COVID-19 (ReCOVer): Study protocol. Trials 2021, 22, 867. [Google Scholar] [CrossRef] [PubMed]
- Flottorp, S.A.; Brurberg, K.G.; Fink, P.; Knoop, H.; Wyller, V.B.B. New NICE guideline on chronic fatigue syndrome: More ideology than science? Lancet 2022, 399, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. NICE backtracks on graded exercise therapy and CBT in draft revision to CFS guidance. BMJ 2020, 371, m4356. [Google Scholar] [CrossRef] [PubMed]
- White, P.D.; Etherington, J. Adverse outcomes in trials of graded exercise therapy for adult patients with chronic fatigue syndrome. J. Psychosom. Res. 2021, 147, 110533. [Google Scholar] [CrossRef]
- Abonie, U.S.; Hoekstra, F.; Seves, B.L.; Woude, L.; Dekker, R.; Hettinga, F.J. Associations between Activity Pacing, Fatigue, and Physical Activity in Adults with Multiple Sclerosis: A Cross Sectional Study. J. Funct. Morphol. Kinesiol. 2020, 5, 43. [Google Scholar] [CrossRef]
- Abonie, U.S.; Sandercock, G.R.H.; Heesterbeek, M.; Hettinga, F.J. Effects of activity pacing in patients with chronic conditions associated with fatigue complaints: A meta-analysis. Disabil. Rehabil. 2020, 42, 613–622. [Google Scholar] [CrossRef]
- Goudsmit, E.M.; Nijs, J.; Jason, L.A.; Wallman, K.E. Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: A consensus document. Disabil. Rehabil. 2012, 34, 1140–1147. [Google Scholar] [CrossRef]
- Antcliff, D.; Keenan, A.M.; Keeley, P.; Woby, S.; McGowan, L. Survey of activity pacing across healthcare professionals informs a new activity pacing framework for chronic pain/fatigue. Musculoskelet. Care 2019, 17, 335–345. [Google Scholar] [CrossRef]
- van Campen, C.L.M.; Rowe, P.C.; Visser, F.C. Heart Rate Thresholds to Limit Activity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients (Pacing): Comparison of Heart Rate Formulae and Measurements of the Heart Rate at the Lactic Acidosis Threshold during Cardiopulmonary Exercise Testing. Adv. Phys. Educ. 2020, 10, 138–154. [Google Scholar] [CrossRef]
- Altea Long COVID Network. Available online: https://www.altea-network.com/ (accessed on 26 November 2022).
- Workwell Foundation. ME/CFS Activity Management with a Heart Rate Monitor. Available online: https://workwellfoundation.org/wp-content/uploads/2021/03/HRM-Factsheet.pdf (accessed on 26 November 2022).
- Diagnose und Nicht Interventionelle Therapie Neuropathischer Schmerzen. Available online: https://register.awmf.org/assets/guidelines/030-114l_S2k_Diagnose-nicht-interventionelle-Therapie-neuropathischer-Schmerzen_2022-06.pdf (accessed on 26 November 2022).
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Keller, A.; Müller, B.; Wöhlbier, H.G.; Kropp, P. Progressive muscle relaxation according to Jacobson for migraine prophylaxis: Clinical effectiveness and mode of action. Schmerz 2018, 32, 250–258. [Google Scholar] [CrossRef]
- Do, T.P.; Remmers, A.; Schytz, H.W.; Schankin, C.; Nelson, S.E.; Obermann, M.; Hansen, J.M.; Sinclair, A.J.; Gantenbein, A.R.; Schoonman, G.G. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology 2019, 92, 134–144. [Google Scholar] [CrossRef]
- Therapie des Episodischen und Chronischen Kopfschmerzes vom Spannungstyp und Anderer Chronischer Täglicher Kopfschmerzen. Available online: https://dgn.org/leitlinie/201 (accessed on 26 November 2022).
- Becker, W.J.; Findlay, T.; Moga, C.; Scott, N.A.; Harstall, C.; Taenzer, P. Guideline for primary care management of headache in adults. Can. Fam. Physician 2015, 61, 670–679. [Google Scholar] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders ICHD-3, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Goncalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Gehrman, P.; Perlis, M.; Umscheid, C.A. Comparative effectiveness of cognitive behavioral therapy for insomnia: A systematic review. BMC Fam. Pract. 2012, 13, 40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diem, L.; Höfle, O.; Fregolente, L.; Hoepner, R. The Role of Neurorehabilitation in Post-COVID-19 Syndrome. Clin. Transl. Neurosci. 2023, 7, 13. https://doi.org/10.3390/ctn7020013
Diem L, Höfle O, Fregolente L, Hoepner R. The Role of Neurorehabilitation in Post-COVID-19 Syndrome. Clinical and Translational Neuroscience. 2023; 7(2):13. https://doi.org/10.3390/ctn7020013
Chicago/Turabian StyleDiem, Lara, Oliver Höfle, Livia Fregolente, and Robert Hoepner. 2023. "The Role of Neurorehabilitation in Post-COVID-19 Syndrome" Clinical and Translational Neuroscience 7, no. 2: 13. https://doi.org/10.3390/ctn7020013
APA StyleDiem, L., Höfle, O., Fregolente, L., & Hoepner, R. (2023). The Role of Neurorehabilitation in Post-COVID-19 Syndrome. Clinical and Translational Neuroscience, 7(2), 13. https://doi.org/10.3390/ctn7020013





