The Parasomnias and Sleep Related Movement Disorders—A Look Back at Six Decades of Scientific Studies
Abstract
1. The Personnel and Technical Advances
- A large number of channels (N = 15) on the two available Alvar EEG machines. Most other centers at the time were using only 8 or 10 channels.
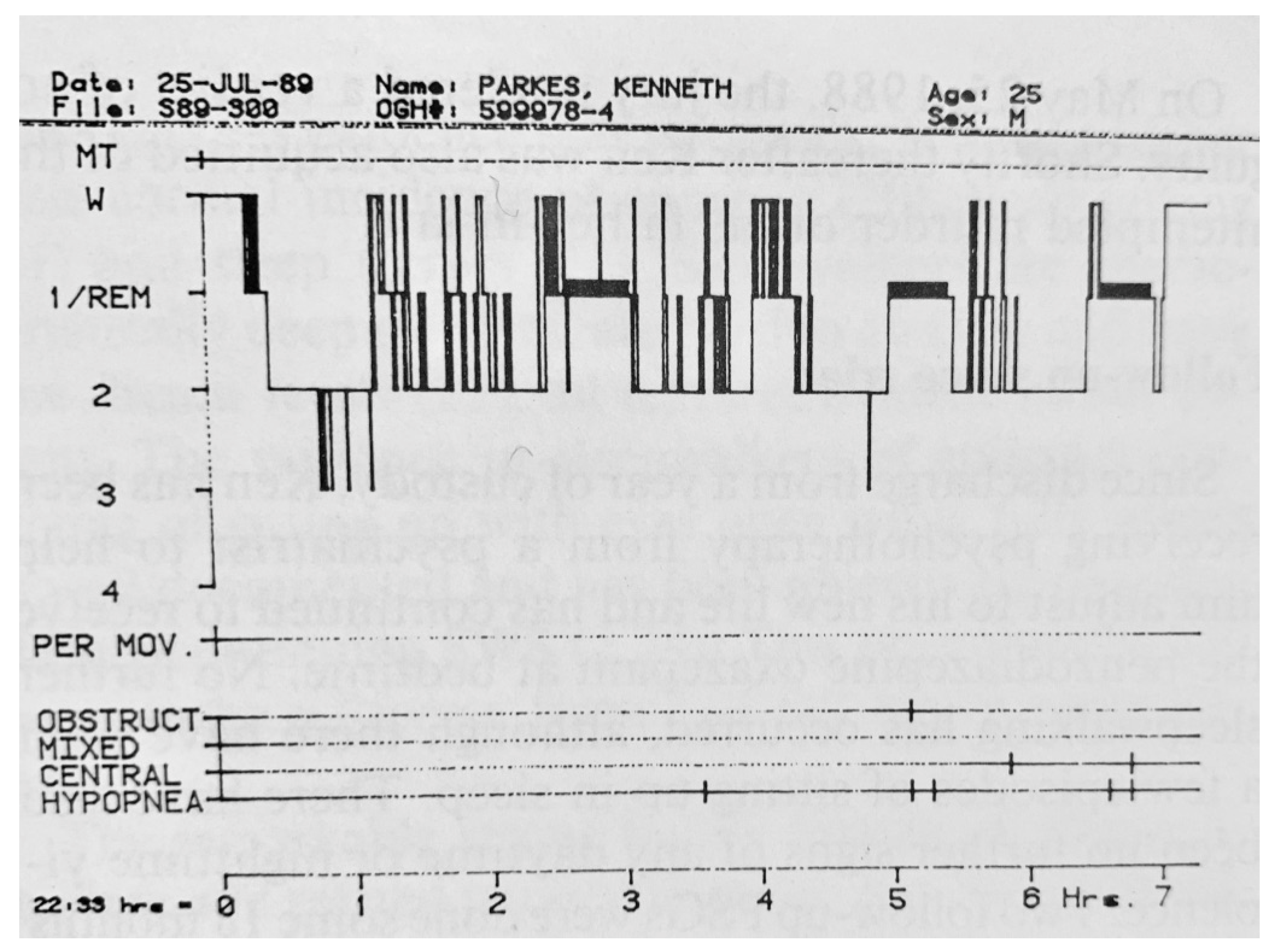
- Multi-channel EEG, EOG (horizontal and vertical), submental EMG, peripheral limb EMGs, ECG (rate and variability), respiration (thoracic and abdominal to detect obstructive and central apneas and hypopneas), electrodermogram (skin resistance or skin potentials), body movements by actigraphy, and the pipigram for the study of enuresis.
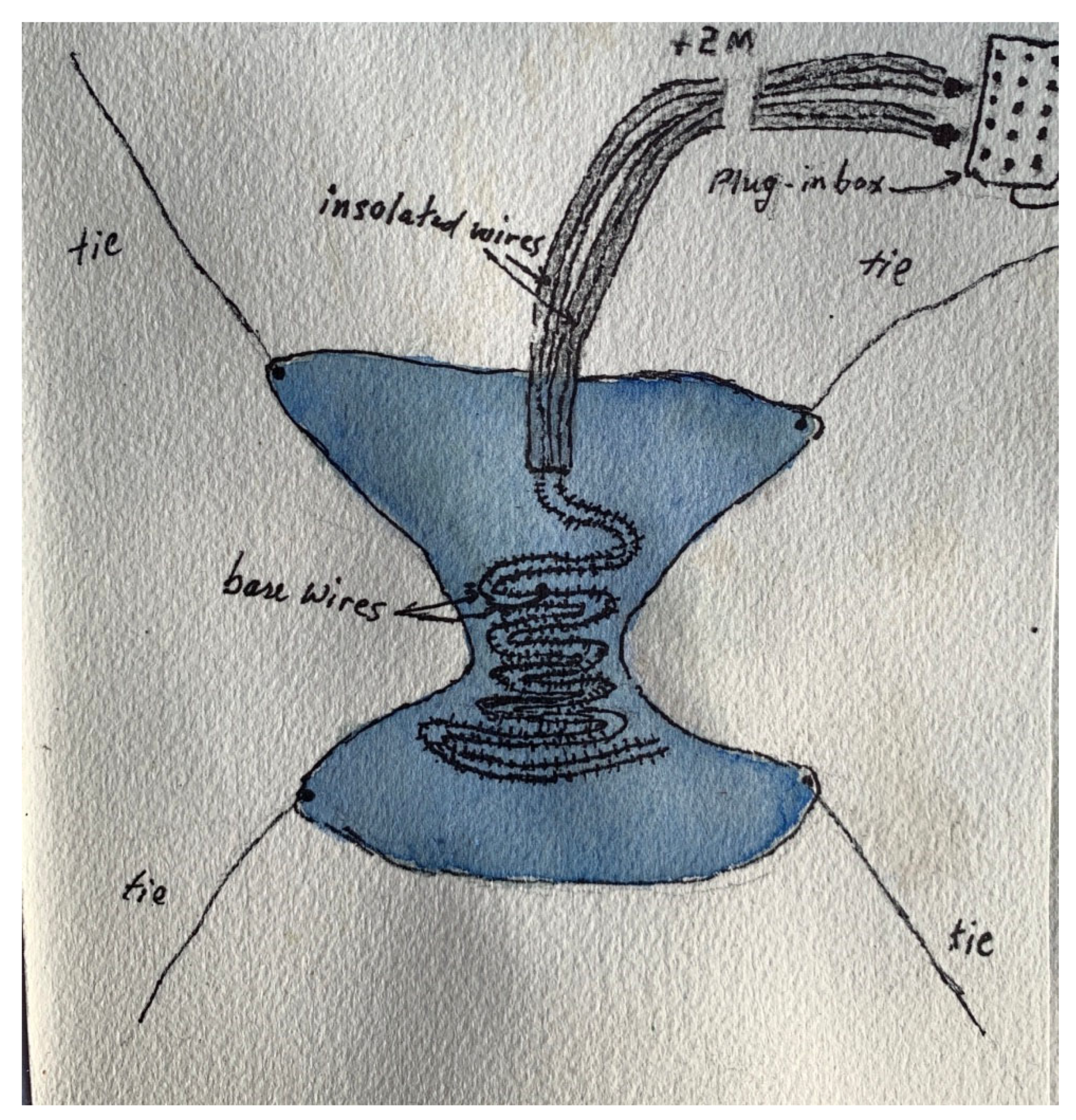
- The use of very long wire electrodes which permitted sleepwalkers to get out of bed, walk a short distance, and return to bed.
- Telemetry—A 4 channel Alvar system (montage: Cz-Oz and CZ-earlobe) and EOG (horizontal only). This was sufficient to differentiate and analyze NREM sleep, REM sleep and wakefulness. If the number of channels was reduced to 3, we could hear FM music on the unused channel!
- Cinematography—there were large wall fixtures for cameras to film parasomnia events. The system required very bright floodlights in order to have high quality images.
- A new system to classify different stages of somnolence, especially at sleep onset that was created by Professor Gastaut. It had substages 1A1, 1A2 and 1A3.
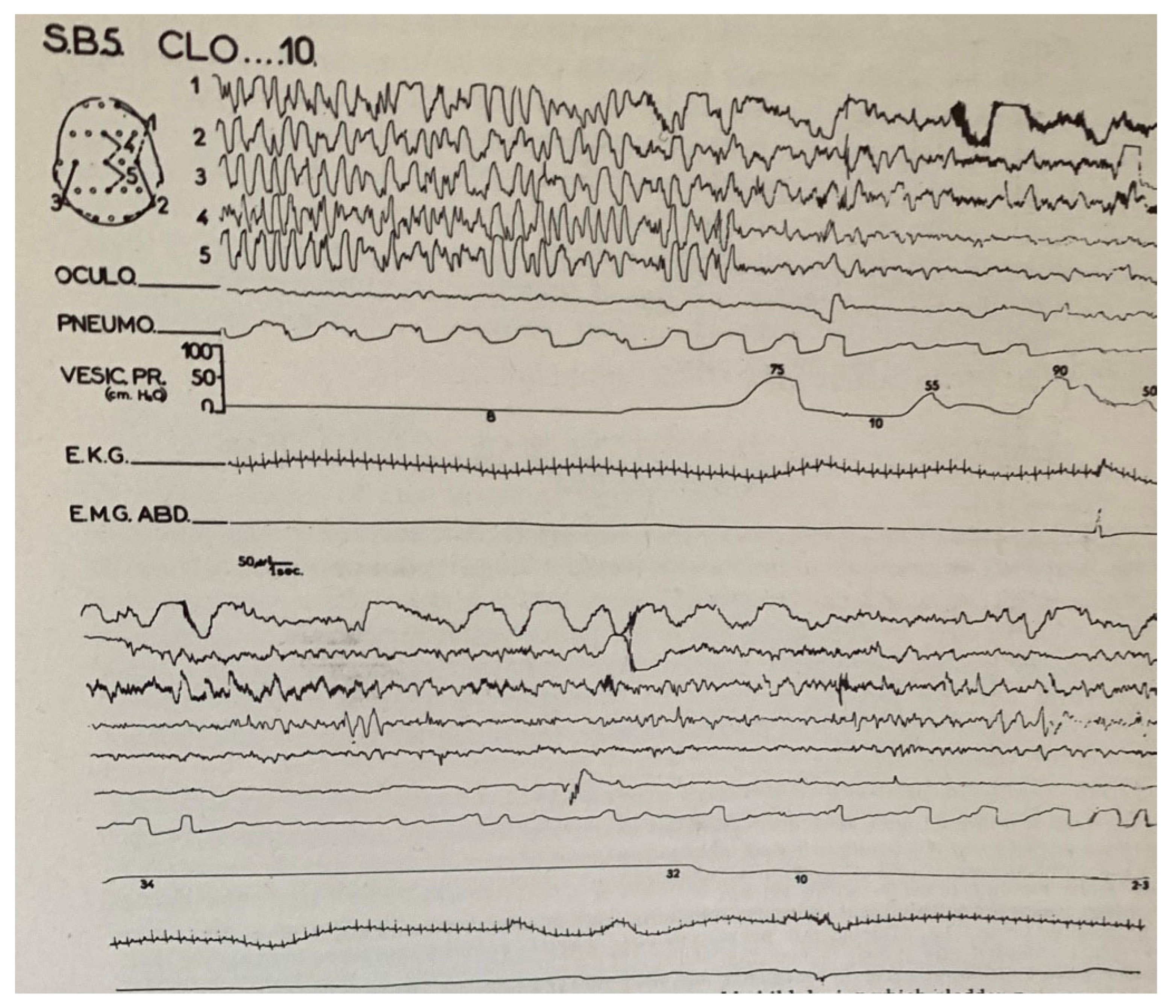
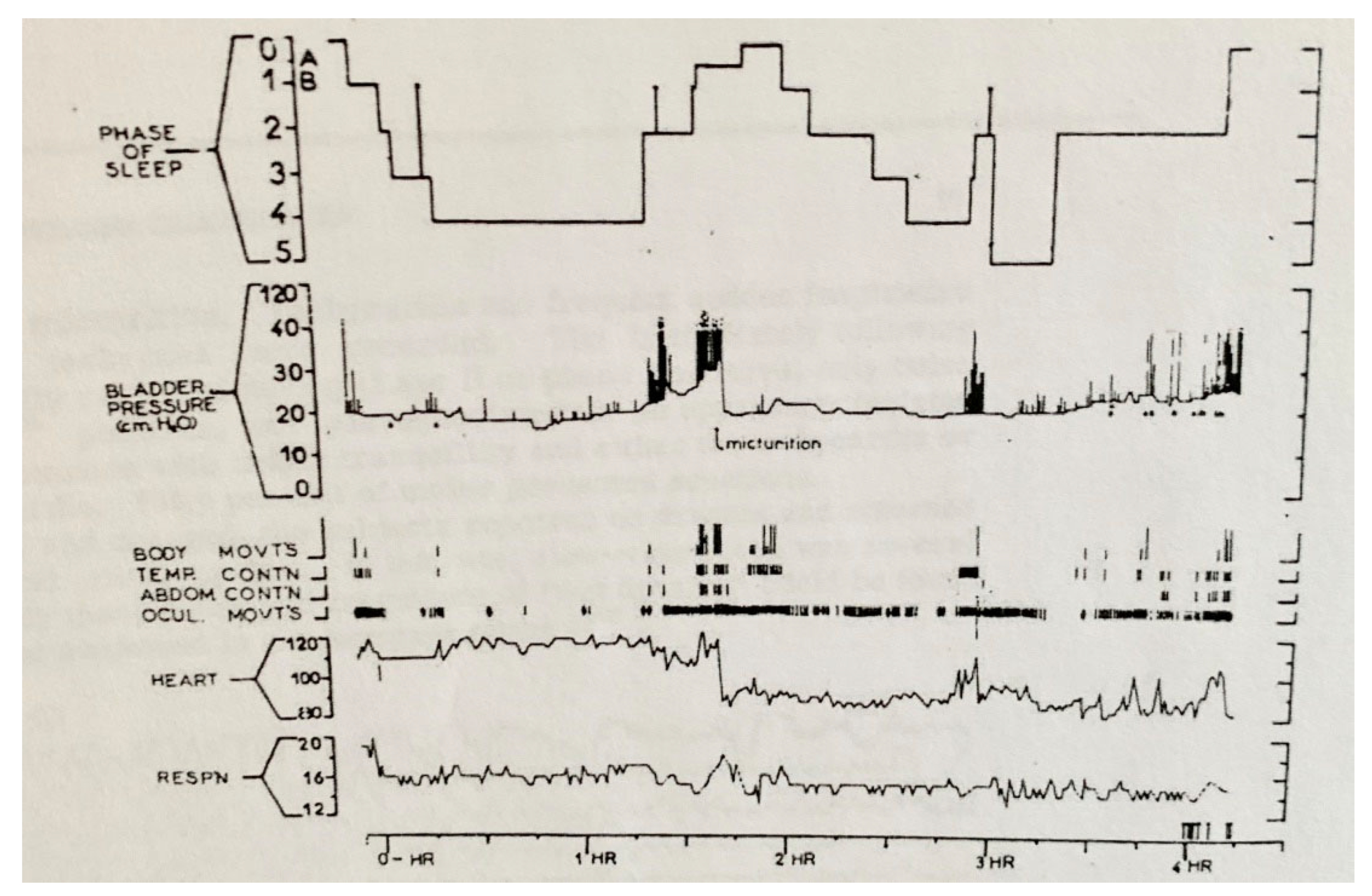
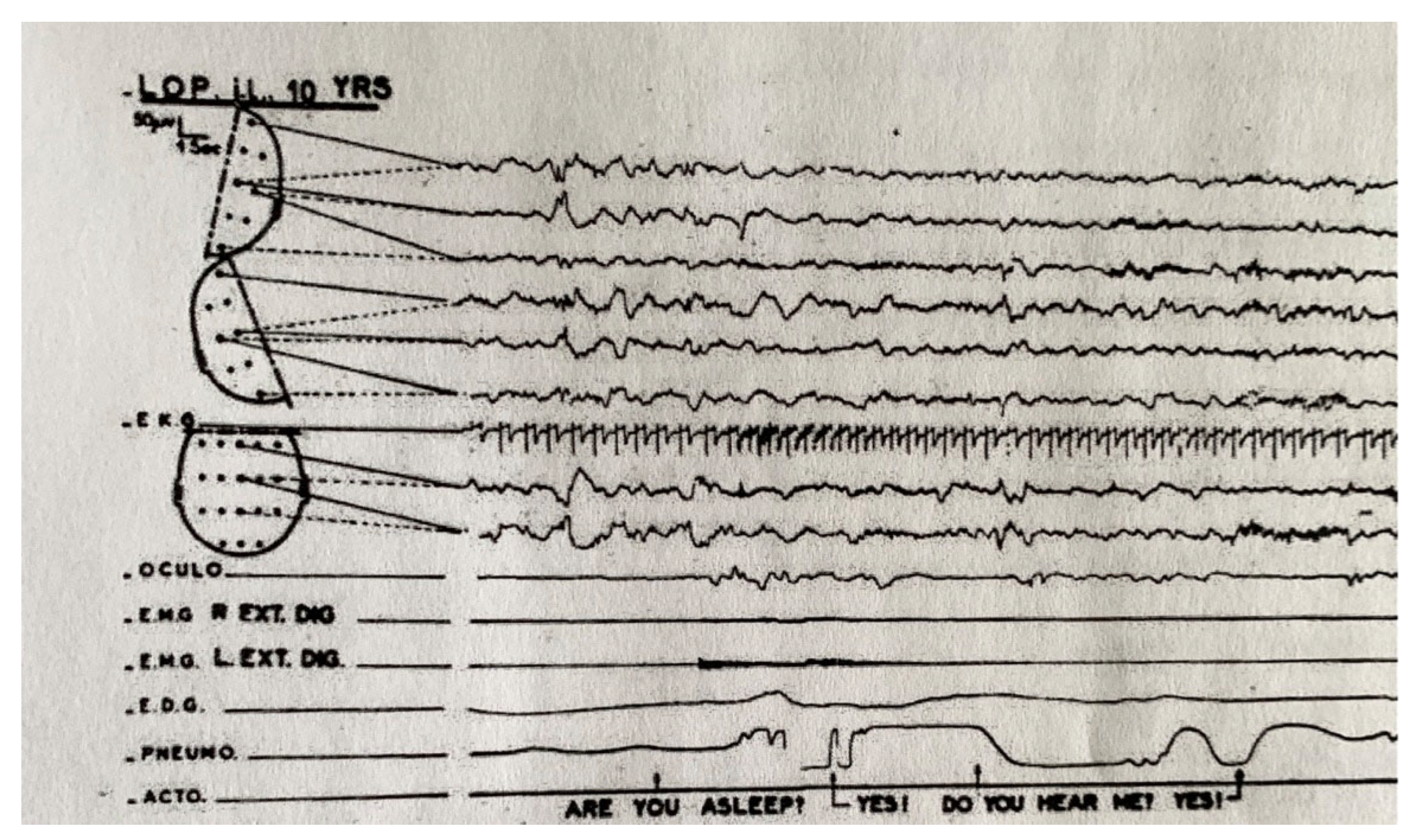
2. Enuresis in Children
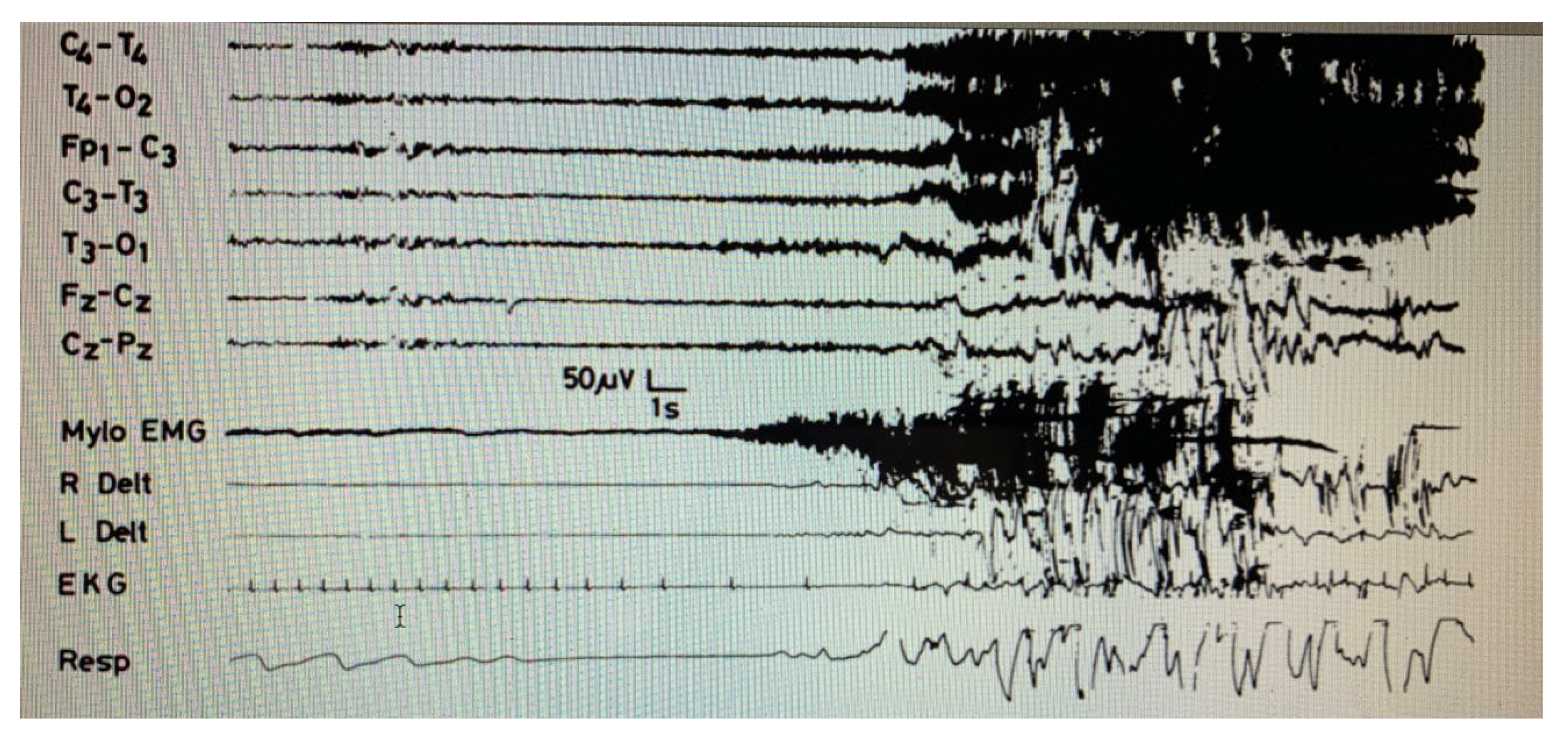
3. Sleepwalking
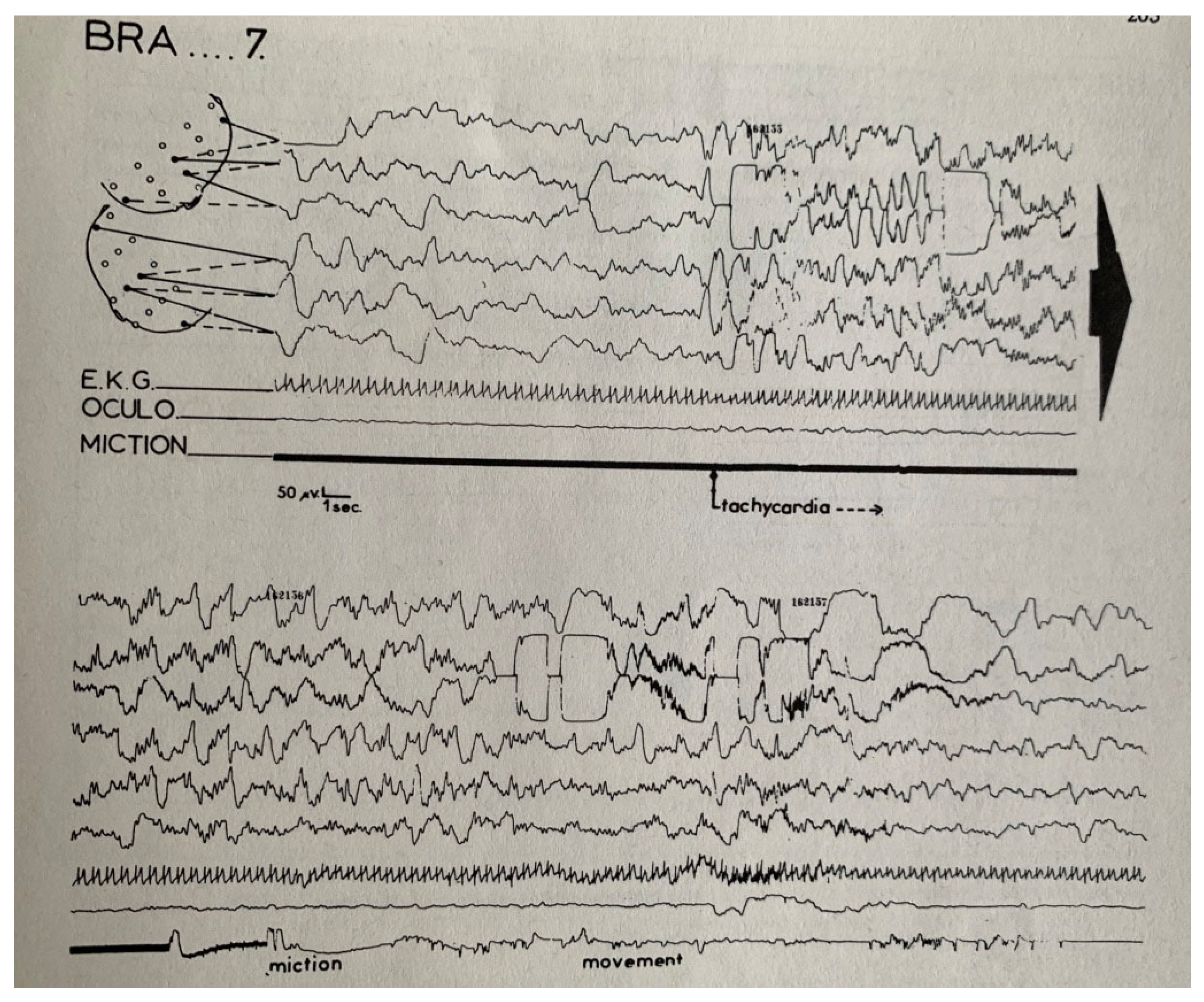
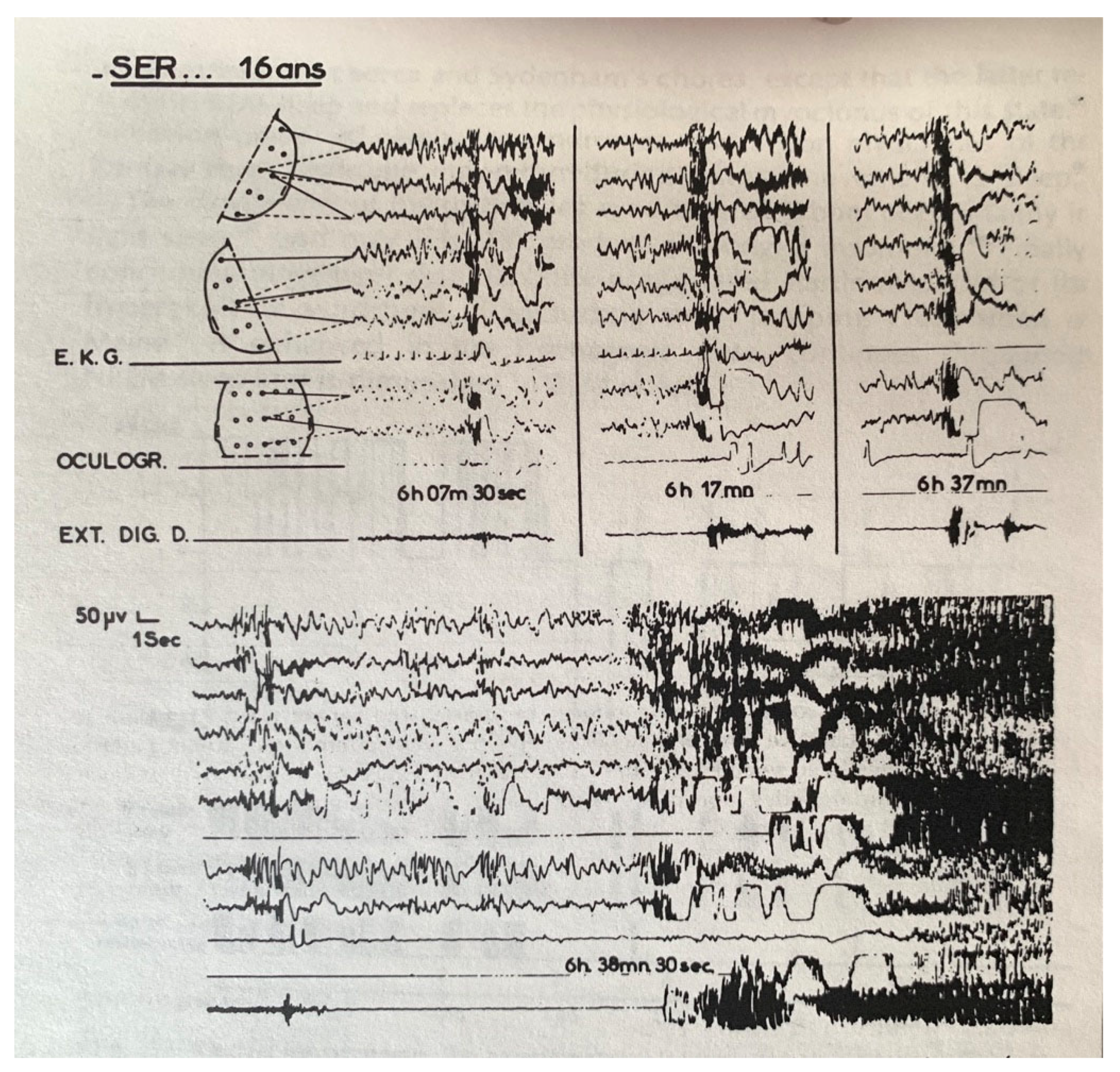
4. Sleep Terrors (Night Terrors, Pavor Nocturnus, Incubus Attacks)
5. Confusional Arousals
6. Development of the Concept of “Disorders of Arousal”
7. Aggression and Sleep
8. Nocturnal Anxiety Attacks
9. Vivid Hypnagogic and Hypnopompic Hallucinations
10. REM Nightmares (REM Sleep Terrifying Dreams)
11. REM Sleep Behavior Disorder (RBD)
12. Nocturnal Sleep Paralysis
13. Nocturnal Paroxysmal Dystonia
14. Sleep Talking (Somniloquy)
15. Sleep Starts (Hypnic Jerks)
16. Jactatio Capitis Nocturna
17. Bruxism
18. Sexsomnia
19. Epileptic Seizures
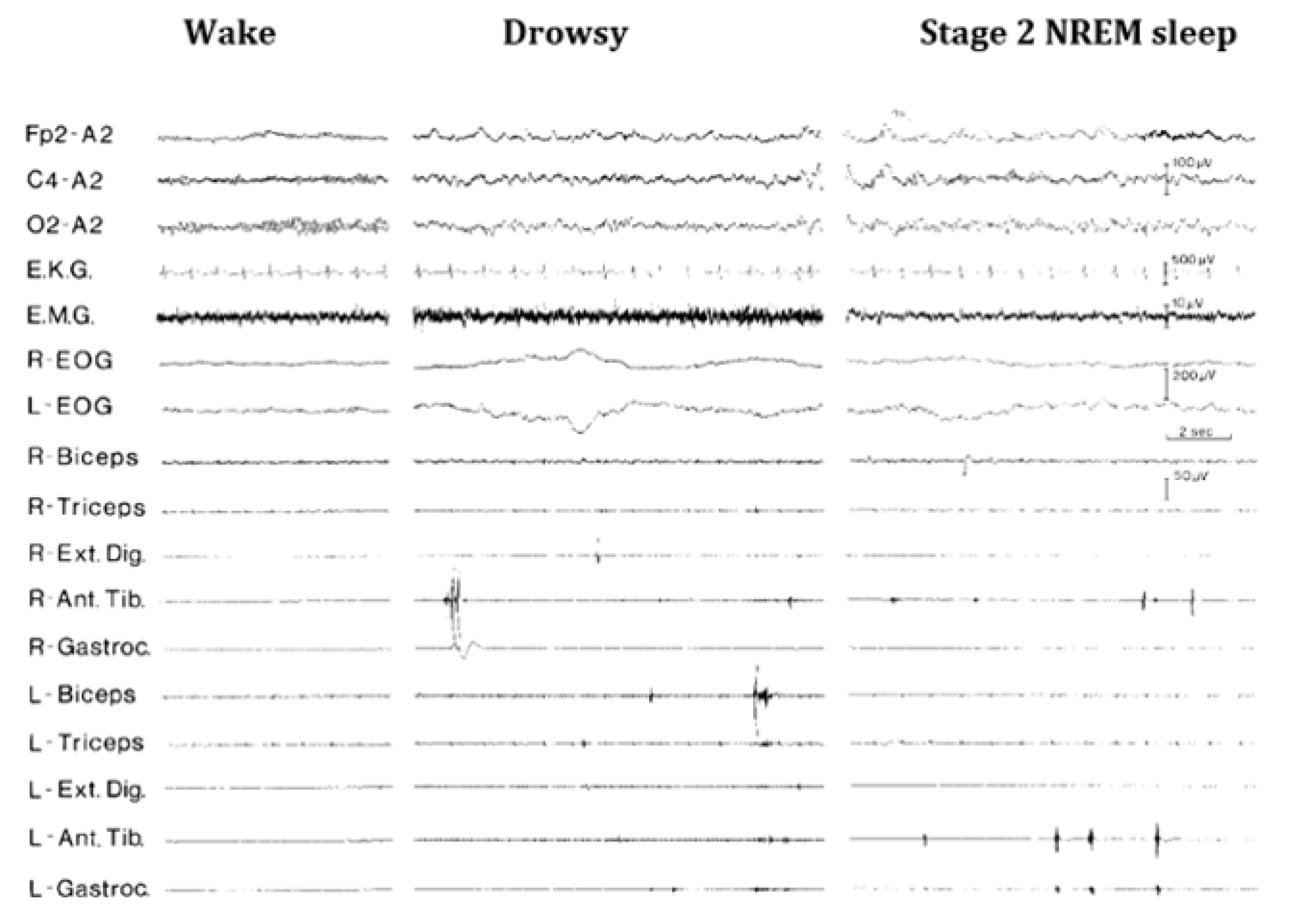
20. Periodic Limb Movement Disorder (PLMs)
21. Hypnagogic Foot Tremor and Alternating Leg Activation
22. Propriospinal Myoclonus
23. Restless Leg Syndrome (RLS)
24. Exploding Head Syndrome
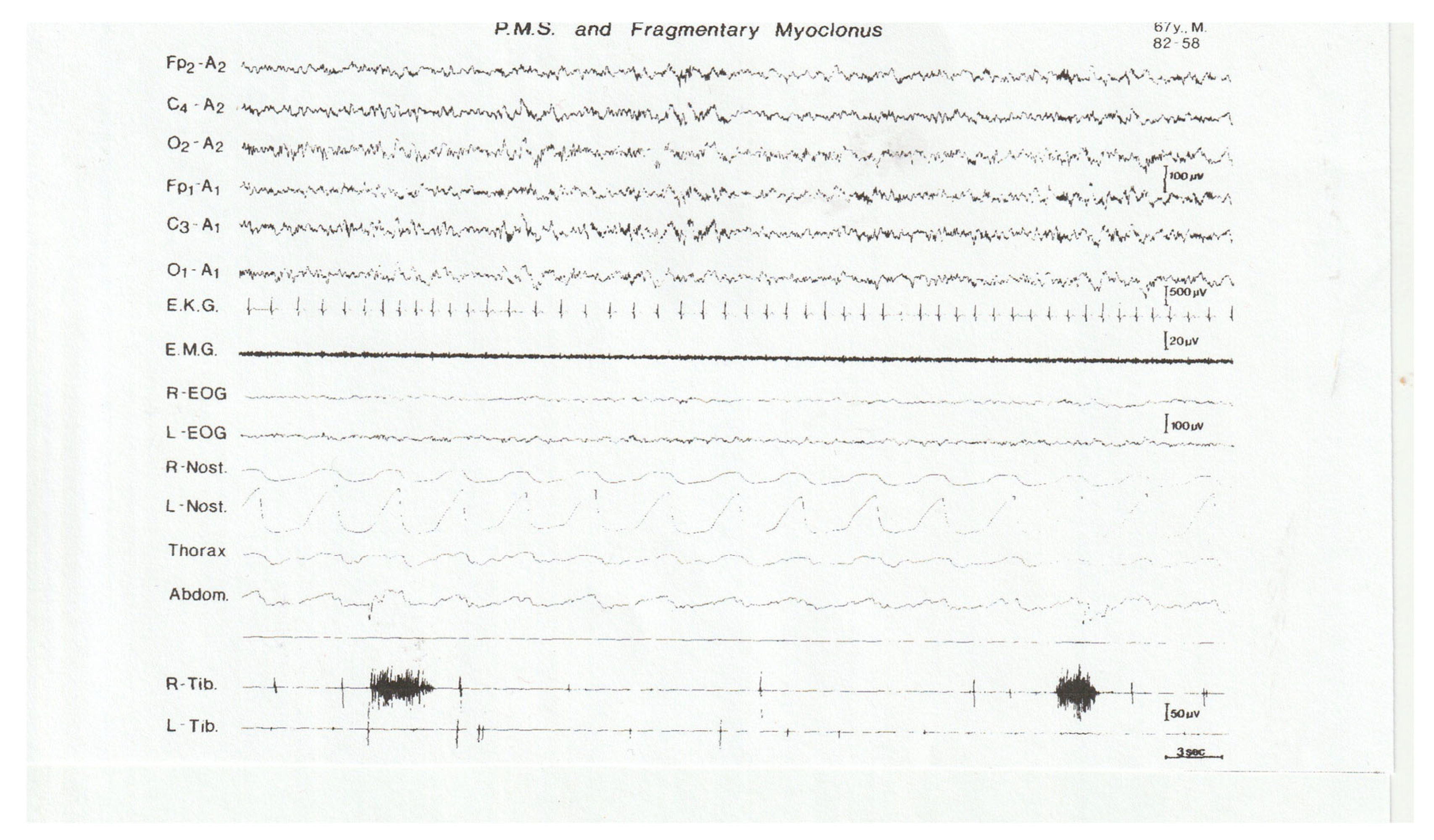
25. Excessive Fragmentary Myoclonus (EFM)
26. Sleep-Related Leg Cramps
27. The Future
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batini, C.; Fressy, J.; Gastaut, H. A propos du sommeil chez le sujet normal. Rev. Neurol. 1962, 106, 216–218. [Google Scholar]
- Batini, C.; Fressy, J.; Gastaut, H. Étude du reflexe cutané-plantaire au cours des différentes phases du sommeil. Rev. Neurol. 1963, 108, 172. [Google Scholar]
- Fressy, J. Apport de la Polygraphie Dans L’etude Polygraphique de la Phase Dite Des Mouvements Oculaires au Cours du Sommeil Chez L’homme. Thèse de Troisième Cycle; Université de Marseille: Marseille, France, 1963; 102p. [Google Scholar]
- Orwell, G. Such Were the Joys; Harcourt: New York, NY, USA, 1945. [Google Scholar]
- Ditman, K.S.; Blinn, K.A. Sleep levels in enuresis. Amer. J. Psychiatr. 1955, 111, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Saint-Laurent, J.; Batini, C.; Broughton, R. A polygraphic study of nocturnal enuresis in the epileptic child. Electroenceph. Clin. Neurophysiol. 1963, 15, 904. [Google Scholar]
- Broughton, R.; Gastaut, H. Polygraphic studies of enuresis nocturna. Electroenceph. Clin. Neurophysiol. 1964, 16, 625. [Google Scholar]
- Broughton, R.; Gastaut, H. Further polygraphic sleep studies of enuresis nocturna (intravesicular pressure). Electroenceph. Clin. Neurophysiol. 1964, 16, 626. [Google Scholar]
- Gastaut, H.; Broughton, R. A clinical and polygraphic study of episodic phenomena during sleep. The Sakel Academic Lecture. Recent Adv. Biol. Psychiatr. 1965, 7, 197–223. [Google Scholar]
- Hawkins, D.R.; Scott, J.; Thresher, G. Sleep Patterns in Enuretic Children; Association for the Psychophysiological Study of Sleep: Washington, DC, USA, 1965. [Google Scholar]
- Ritvo, E.R.; Ornitz, W.M.; Gottlieb, F. Arousal and non-arousal enuretic events. Am. J. Psychiatr. 1969, 126, 77–84. [Google Scholar] [CrossRef]
- Gambi, D.; Pinto, F.; Torriolio, M.G. Night Sleep in Enuretic Children. In Sleep 1974; Levin, P., Koella, W.P., Eds.; Karger: Basel, Switzerland, 1975; pp. 92–97. [Google Scholar]
- Kales, A.; Kales, J.D.; Jacobson, A.; Humphrey, F.J.; Soldatos, C.R. Effects of Imipramine on Enuretic Frequency and Sleep Stages. Pediatrics 1977, 60, 431–436. [Google Scholar] [CrossRef]
- Mikkelsen, E.J.; Rapoport, J.L.; Gruenau, S. Sleep patterns in childhood enuresis. In Sleep Research; Chase, M.H., Mitler, M.M., Walter, P.I., Eds.; Brain Information Service/Brain Research Institute: Los Angeles, CA, USA, 1978; p. 242. [Google Scholar]
- Muellner, S.R. Development of urinary control in children: A new concept in cause, prevention and treatment of primary en-uresis. J. Am. Med. Assoc. 1960, 172, 1256–1261. [Google Scholar] [CrossRef]
- Di Perri, R.; Meduri, M. L’enuresi notturna uteriori elementi in tema di diagnostic strumentale. Riv. Neurol. 1972, 27, 22–27. [Google Scholar]
- Di Perri, R.; Meduri, M. A polygraphic approach to the study of enuresis nocturna. In Sleep 1974; Levin, P., Koella, W.P., Eds.; Karger: Basel, Switzerland, 1975; pp. 413–416. [Google Scholar]
- Bradley, W.E. Electroencephalography and Bladder Innervation. J. Urol. 1977, 118, 412–414. [Google Scholar] [CrossRef]
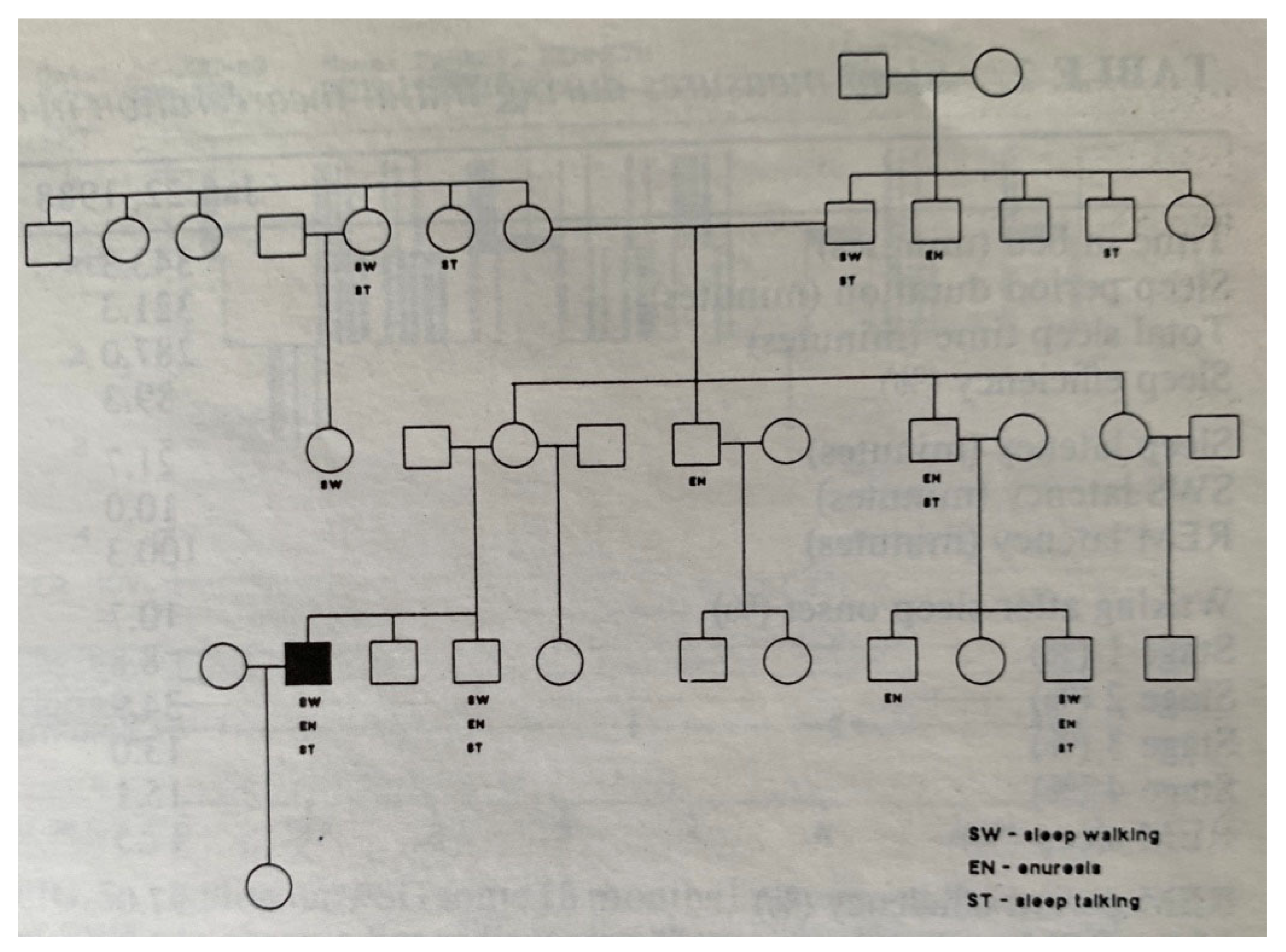
- Bakwin, H. Enuresis in twins. Am. J. Dis. Child. 1971, 121, 222–225. [Google Scholar] [CrossRef] [PubMed]
- von Gontard, A.; Heron, J.; Joinson, C. Family history of nocturnal enuresis and urinary incontinence: Results from a large epidemiological study. J. Urol. 2011, 185, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Petit, D.; Pennestri, M.-H.; Paquet, J. Childhood sleepwalking and sleep terrors: A longitudinal study of prevalence and familial aggregation. JAMA Pediatr. 2015, 169, 653–658. [Google Scholar] [CrossRef]
- Tassinari, C.A.; Rubboli, G.; Gardella, E. Central pattern generators for a common semiology in frontal-limbic seizures and parasomnias: A neuroethologic approach. Neurol. Sci. 2006, 3, 225–232. [Google Scholar]
- Gastaut, H.; Batini, C.; Broughton, R. Études électroencéphalographiques des phénomènes épisodiques non-épileptiques au cours du sommeil. In Le Sommeil Normal et Pathologique; Fischgold, H., Ed.; Masson: Paris, France, 1965; pp. 215–236. [Google Scholar]
- Jacobson, A.; Lehmann, D.; Kales, A. EEG recordings during somnambulistic sitting and walking. Science 1965, 148, 975–977. [Google Scholar] [CrossRef]
- Broughton, R. Sleep disorders: Disorders of arousal? Enuresis, sleepwalking, sleep terrors and confusional states of arousal are not in “dreaming sleep”. Science 1968, 159, 1070–1078. [Google Scholar] [CrossRef]
- Gastaut, H.; Broughton, R. Paroxysmal events and certain phases of sleep. Percept. Mot. Ski. 1963, 109, 333–334. [Google Scholar]
- Montplaisir, J.; Petit, D.; Pilon, M. Does sleepwalking impair daytime vigilance? J. Clin. Sleep Med. 2011, 7, 219. [Google Scholar] [CrossRef]
- Kales, J.D.; Kales, A.; Soldatos, C.R.; Chamberlin, K.; Martin, E.D. Sleepwalking and night terrors related to febrile illness. Am. J. Psychiatry 1979, 136, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Broughton, R. Confusional sleep disorders: Interrelationship with memory consolidation and retrieval in sleep. In Hinks Memorial Lectures: A Triune Concept of the Brain and Behaviour; Maclean, P., Boag, P.J., Campbell, D., Eds.; University of Toronto Press: Toronto, ON, Canada, 1973; pp. 115–127. [Google Scholar]
- Bassetti, C.; Weder, B. SPECT during sleepwalking. Lancet 2000, 356, 484–485. [Google Scholar] [CrossRef]
- Yellowless, D. Homicide by a somnambulist. J. Ment. Sci. 1878, 24, 451–458. [Google Scholar] [CrossRef]
- Broughton, R.J.; Shimizu, T. Sleep, and violence: A medical and forensic challenge. Sleep 1995, 18, 727–730. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broughton, R.; Cartwright, R.; Doucette, D.; Edmeads, J.; Edwardh, M.; Ervin, F.; Orchard, B.; Hill, R.; Turrell, G.; Billings, R. Homicidal Somnambulism: A Case Report. Sleep 1994, 17. [Google Scholar] [CrossRef]
- Pedley, T.A.; Guilleminault, C. Episodic nocturnal wanderings responsive to anticonvulsant drug therapy. Ann. Neurol. 1977, 2, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zadra, A.; Pilon, M.; Montplaisir, J. Polysomnographic diagnosis of sleepwalking: Effects of sleep deprivation. Ann. Neurol. 2008, 63, 513–519. [Google Scholar] [CrossRef]
- Desjardins, P.M.; Carrier, J.; Lina, J.-M.; Fortin, B.M.; Gosselin, N.; Montplaisir, J.; Zadra, A. EEG Functional Connectivity Prior to Sleepwalking: Evidence of Interplay Between Sleep and Wakefulness. Sleep 2017, 40. [Google Scholar] [CrossRef]
- Gaudreau, H.; Joncas, S.; Zadra, A.; Montplaisir, J. Dynamics of slow-wave activity during the NREM sleep of sleepwalkers and control subjects. Sleep 2000, 23, 755–760. [Google Scholar] [CrossRef]
- Lecendreux, M.; Bassetti, C.L.; Dauvilliers, Y.; Mayer, G.; Neidhart, E.; Tafti, M. HLA and genetic susceptibility to sleepwalking. Mol. Psychiatry 2003, 8, 114–117. [Google Scholar] [CrossRef]
- Jones, E. On the Nightmare; Hogarth: London, UK, 1949. [Google Scholar]
- Gastaut, H.; Batini, C.; Broughton, R. Étude électroencéphalographique des manifestations paroxystiques non-épileptiques au cours du sommeil nocturne. Rev. Neurol. 1964, 110, 309. [Google Scholar]
- Fisher, C.; Byrne, J.; Edwards, A. A psychophysiological study of nightmares. J. Psychoanal. Assoc. 1970, 18, 747–782. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Kahn, E.; Edwards, A. A psychological study of nightmares and night terrors: Physiological aspects of the stage 4 sleep terror. J. Nerv. Ment. Dis. 1973, 157, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Kahn, E.; Edwards, A. A psychophysiological study of nightmares and night terrors: The suppression of stage 4-night terrors with diazepam. Arch. Gen. Psychiatr. 1973, 28, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, C.; Mancia, D.; Bernardina, B.; Gastaut, H. Pavor nocturnus of non-epileptic nature in epileptic children. Electroencephalogr. Clin. Neurophysiol. 1972, 33, 603–607. [Google Scholar] [CrossRef]
- Gastaut, H.; Dongier, M.; Broughton, R. Electroencephalographic and clinical study of diurnal and nocturnal anxiety attacks. Electroenceph. Clin. Neurophysiol. 1964, 17, 475. [Google Scholar]
- Hartmann, E. Dreams and Nightmares: The Origin and Meaning of Dreams; Perseus Publishing: New York, NY, USA, 2000; p. 328. [Google Scholar]
- Hartmann, E.; Russ, D.; Van Der Kolk, B.; Falke, R.; Oldfield, M. A preliminary study of the personality of the nightmare sufferer: Relationship to schizophrenia and creativity? Am. J. Psychiatry 1981, 138, 794–797. [Google Scholar] [CrossRef]
- Boydon, A.D.; Pott, M.; Starks, P.T. An evolutionary perspective on sleep terrors. Evol. Med. Public Health 2018, 20, 100–105. [Google Scholar] [CrossRef]
- Marc, C. De la Folie; Ballière: Paris, France, 1840; pp. 658–668. [Google Scholar]
- von Gudden, H. Die physiologische und pathologische Schlaftrunkenheit. Arch. Für Psychiatr. 1905, 40, 989. [Google Scholar] [CrossRef][Green Version]
- Ohayon, M.; Mahowald, M.W.; Leger, D. Are confusional arousals pathological? Neurology 2014, 83, 834–841. [Google Scholar] [CrossRef]
- Roth, B.; Nevsimalova, S.; Rechtschaffen, A. Hypersomnia with “Sleep Drunkenness”. Arch. Gen. Psychiatry 1972, 26, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Feltin, M.; Brougthon, R. Differential effects of arousal from slow wave versus REM sleep. Psychophysiology 1968, 5, 231. [Google Scholar]
- Broughton, R.; Shimizu, T. Dangerous behaviours by night. In Forensic Aspects of Sleep; Shapiro, C., Smith, A.M., Eds.; John Wiley & Sons: London, UK; New York, NY, USA, 1997; pp. 65–83. [Google Scholar]
- Broughton, R.; Billings, R.; Cartwright, R. Homicidal somnambulism: A case report. Sleep 1994, 1, 253–264. [Google Scholar]
- Schenck, C.H.; Bundlie, S.R.; Ettinger, M.C. Chronic behavior disorders of human REM sleep: A new category of parasomnia. Sleep 1986, 9, 293–308. [Google Scholar] [CrossRef]
- Jouvet, M.; Delorme, F. Locus coeruleus et sommeil paradoxal. CR Soc. Biol. 1965, 159, 895–899. [Google Scholar]
- Snyder, S. Isolated sleep paralysis after time-zone changes (jetlag). Chronobiologica 1983, 10, 377–379. [Google Scholar]
- Everett, H.C. Sleep paralysis in medical students. J. Nerv. Ment. Dis. 1963, 136, 283–287. [Google Scholar] [CrossRef]
- Penn, N.E.; Kripke, D.F.; Scharff, J. Sleep Paralysis Among Medical Students. J. Psychol. 1981, 107, 247–252. [Google Scholar] [CrossRef]
- Roth, B.; Bruhova, S.; Berkova, L. Familial sleep paralysis. Schweiz. Arch. Neurol. Neurochir. Psychiatr. 1968, 102, 321–330. [Google Scholar]
- Nan’no, H.; Hiskikawa, Y.; Koida, H. A neurophysiological study of sleep paralysis in narcoleptic patients. Electroenceph. Clin. Neurophysiol. 1970, 28, 382–390. [Google Scholar] [CrossRef]
- Dahlitz, M.; Parkes, J.D. Sleep paralysis. Lancet 1993, 341, 406–407. [Google Scholar] [CrossRef]
- Lugaresi, E.; Cirignotta, F. Hypnogenic paroxysmal dystonia: Epileptic seizure or new syndrome? Sleep 1981, 4, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lugaresi, E.; Cirignotta, F.; Montagna, P. Nocturnal paroxysmal dystonia. J. Neurol. Neurosurg. Psychiatry 1986, 49, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Oswald, I. Sudden Bodily Jerks on Falling Asleep. Brain 1959, 82, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Broughton, R.J. Some important underemphasized aspects of sleep onset. In Sleep Onset Mechanisms; Ogilvie, R., Harsh, J., Eds.; American Psychological Association: Arlington, VA, USA, 1994; pp. 19–36. [Google Scholar]
- Silvestri, R.; Walters, A.S. Rhythmic movements in sleep disorders and epileptic seizures during sleep. Sleep Sci. Pract. 2020, 4, 5. [Google Scholar] [CrossRef]
- Tassinari, C.A.; Gardella, E.; Cantalupo, G.; Rubboli, G. Relationship of Central Pattern Generators with Parasomnias and Sleep-Related Epileptic Seizures. Sleep Med. Clin. 2012, 7, 125–134. [Google Scholar] [CrossRef]
- Reding, G.R.; Zepelin, H.; Robinson, J.E. Nocturnal tooth-grinding: All-night psychophysiological studies. J. Dent. Res. 1968, 47, 787–797. [Google Scholar] [CrossRef]
- Glaros, A.G.; Rao, S.M. Bruxism a critical review. Psychol. Bull. 1977, 84, 767–781. [Google Scholar] [CrossRef]
- Richmond, G.; Rugh, J.D.; Dolfi, R.; Wasilewsky, J.W. Survey of bruxism in an institutionalized mentally retarded population. Am. J. Ment. Defic. 1984, 88. [Google Scholar]
- Ware, J.C.; Rugh, J. Destructive bruxism: Sleep stage relationship. Sleep 1988, 11, 172–181. [Google Scholar] [CrossRef][Green Version]
- Meletti, S.; Cantalupo, G.; Volpi, L.; Rubboli, G.; Magaudda, A.; Tassinari, C.A. Rhythmic teeth grinding induced by temporal lobe seizures. Neurology 2004, 62, 2306–2309. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.J.; Khoury, S.; Abe, S.; Yamaguchi, T.; Raphael, K. Bruxism physiology and pathology: An overview for clinicians. J. Oral Rehabilitation 2008, 35, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Mayer, P.; Heinzer, R.; Lavigne, G. Sleep bruxism in respiratory medicine practice. Chest 2016, 149, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.; Tralanovic, N.N.; Fedoroff, J.P. Sexsomnia—A new parasomnia? Can. J. Psychiatry 2003, 48, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.L.; Poyares, D. Sexsomnia: Abnormal sexual behavior during sleep. Brain Res. Rev. 2007, 56, 271–282. [Google Scholar] [CrossRef]
- Rodriguez, H.A.; Iranzo, A.; Giaf, C. Sexsomnia: Parasomnia associated with sexual behaviour during sleep. Neurologia 2014, 29, 146–152. [Google Scholar]
- Ingravallo, F.; Poli, F.; Gilmore, E.V.; Pizza, F.; Vignatelli, L.; Schenck, C.H.; Plazzi, G. Sleep-Related Violence and Sexual Behavior in Sleep: A Systematic Review of Medical-Legal Case Reports. J. Clin. Sleep Med. 2014, 10, 927–935. [Google Scholar] [CrossRef]
- Schenck, C.H. Update on sexsomnia, sleep related sexual seizures, and forensic implications. NeuroQuantology 2015, 13, 519–541. [Google Scholar] [CrossRef]
- Soca, R.; Kennan, J.; Schenck, C.H. Parasomnia overlap disorder with sexual behaviors during sleep in a patient with obstructive sleep apnea. J. Clin. Sleep Med. 2016, 12, 1189–1191. [Google Scholar] [CrossRef]
- Sayin, U.; Schenck, C.H. Neuroanatomy and neurochemistry of sexual desire, pleasure, love and orgasm. Sexus 2019, 4, 907–946. [Google Scholar]
- Sterman, B.; Shouse, M.N.; Passouant, P. Sleep and Epilepsy; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Degen, R.; Rodin, E.A. Epilepsy, Sleep and Sleep Deprivation; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Janz, D. The grand mal epilepsies and the sleeping-waking cycle. Epilepsia 1962, 3, 69–109. [Google Scholar] [CrossRef] [PubMed]
- Gastaut, H.; Roger, J.; Ouachi, S. An electro-clinical study of seizures of tonic expression. Epilepsia 1963, 3, 69–109. [Google Scholar] [CrossRef] [PubMed]
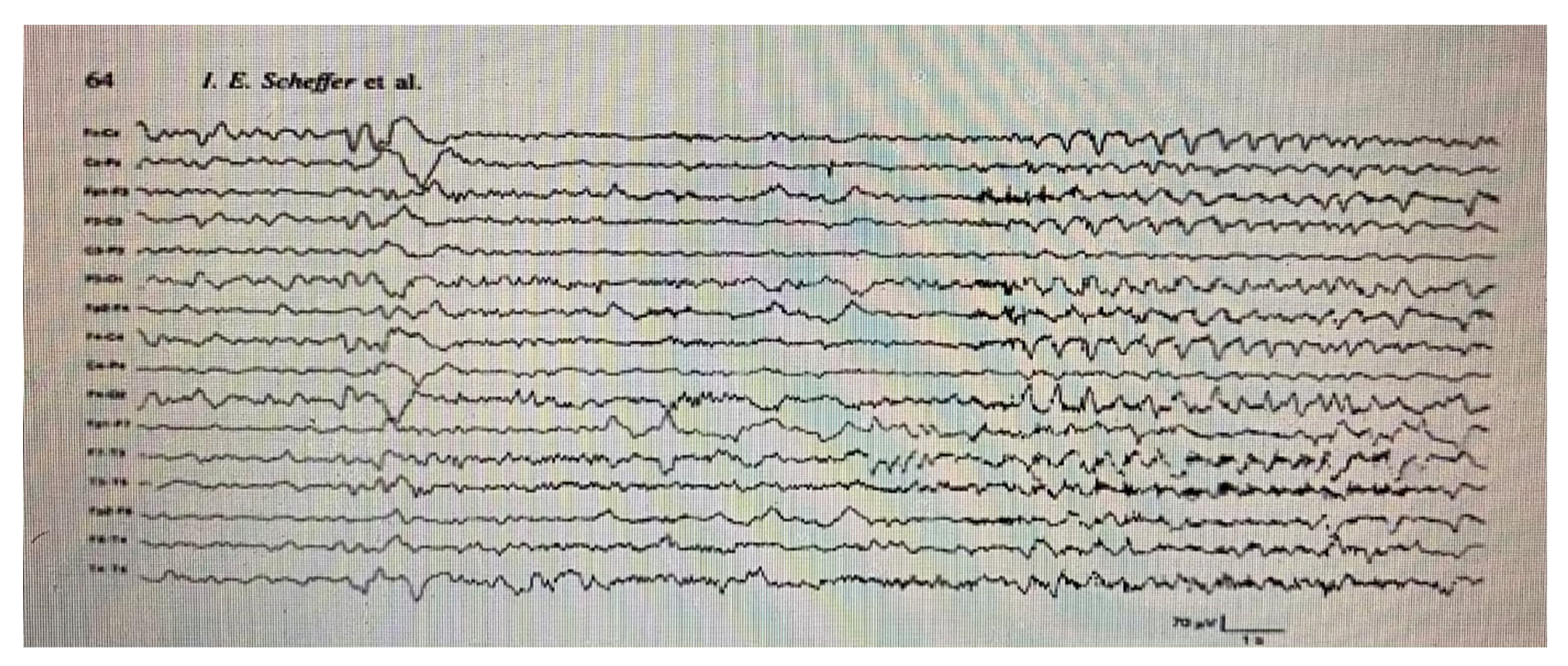
- Scheffer, I.E.; Bhatia, K.P.; Lopes-Cendes, I.; Fish, D.R.; Marsden, C.D.; Andermann, E.; Andermann, F.; Desbiens, R.; Keene, D.; Cendes, F.; et al. Autosomal dominant nocturnal frontal lobe epilepsy. Brain 1995, 118, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Bhatia, K.P.; Lopes-Cendes, I. Autosomal dominant frontal epilepsy misdiagnosed as sleep disorder. Lancet 1994, 343, 515–517. [Google Scholar] [CrossRef]
- Phillips, H.; Scheffer, I.; Crossland, K.; Bhatia, K.; Fish, D.; Marsden, C.; Howell, S.; Stephenson, J.; Tolmie, J.; Plazzi, G.; et al. Autosomal Dominant Nocturnal Frontal-Lobe Epilepsy: Genetic Heterogeneity and Evidence for a Second Locus at 15q24. Am. J. Hum. Genet. 1998, 63, 1108–1116. [Google Scholar] [CrossRef]
- Provini, F.; Plazzi, G.; Tinuper, P.; Vandi, S.; Lugaresi, E.; Montagna, P. Nocturnal frontal lobe epilepsy: A clinical and polygraphic overview of 100 consecutive cases. Brain 1999, 122, 1017–1031. [Google Scholar] [CrossRef]
- Sharpless, B.A.; Zimmerman, J.A. Exploding Head Syndrome. In Unusual and Rare Disorders: A Handbook for Clinical Practice and Research; Sharpless, B.A., Ed.; Oxford University Press: Oxford, UK, 2016; pp. 39–52. [Google Scholar] [CrossRef]
- Sachs, C.; Svanborg, E. The exploding head syndrome: Polysomnographic recordings and therapeutic suggestions. Sleep 1991, 14, 263–266. [Google Scholar] [CrossRef]
- Kallweit, U.; Khatami, K.; Bassetti, C.L. Exploding head syndrome—More than “snapping of the brain”. Sleep Med. 2008, 9, 589. [Google Scholar] [CrossRef]
- Broughton, R.; Tolentino, M.A.; Krelina, M. Excessive fragmentary myoclonus in NREM sleep: A report of 38 cases. Electroencephalogr. Clin. Neurophysiol. 1985, 61, 123–133. [Google Scholar] [CrossRef]
- Lins, O.; Castonguay, M.; Dunham, W.; Nevsimalova, S.; Broughton, R. Excessive Fragmentary Myoclonus: Time of Night and Sleep Stage Distributions. Can. J. Neurol. Sci. 1993, 20, 142–146. [Google Scholar] [CrossRef][Green Version]
- Sobreira-Neto, M.A.; Pena-Pereira, M.A.; Tavares, E.S. Excessive fragmentary myoclonus in patients with Parkinson’s disease: Prevalence and clinic-polysomnographic profile. Sleep Breath 2015, 19, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Nepozitek, J.; Sonka, K. Excessive fragmentary myoclonus: What do we know? Prague Med. Rep. 2017, 118, 5–13. [Google Scholar] [CrossRef] [PubMed][Green Version]

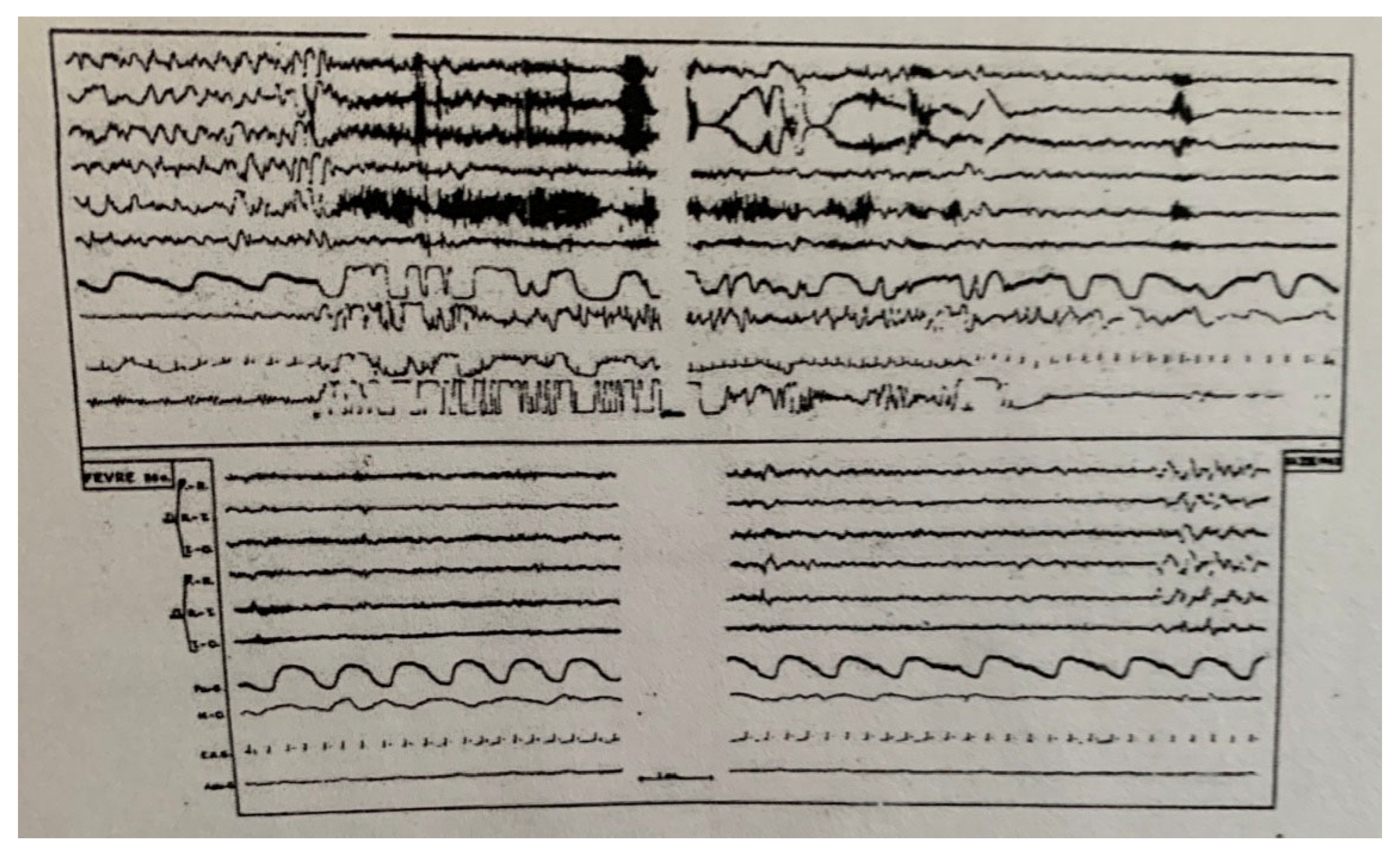
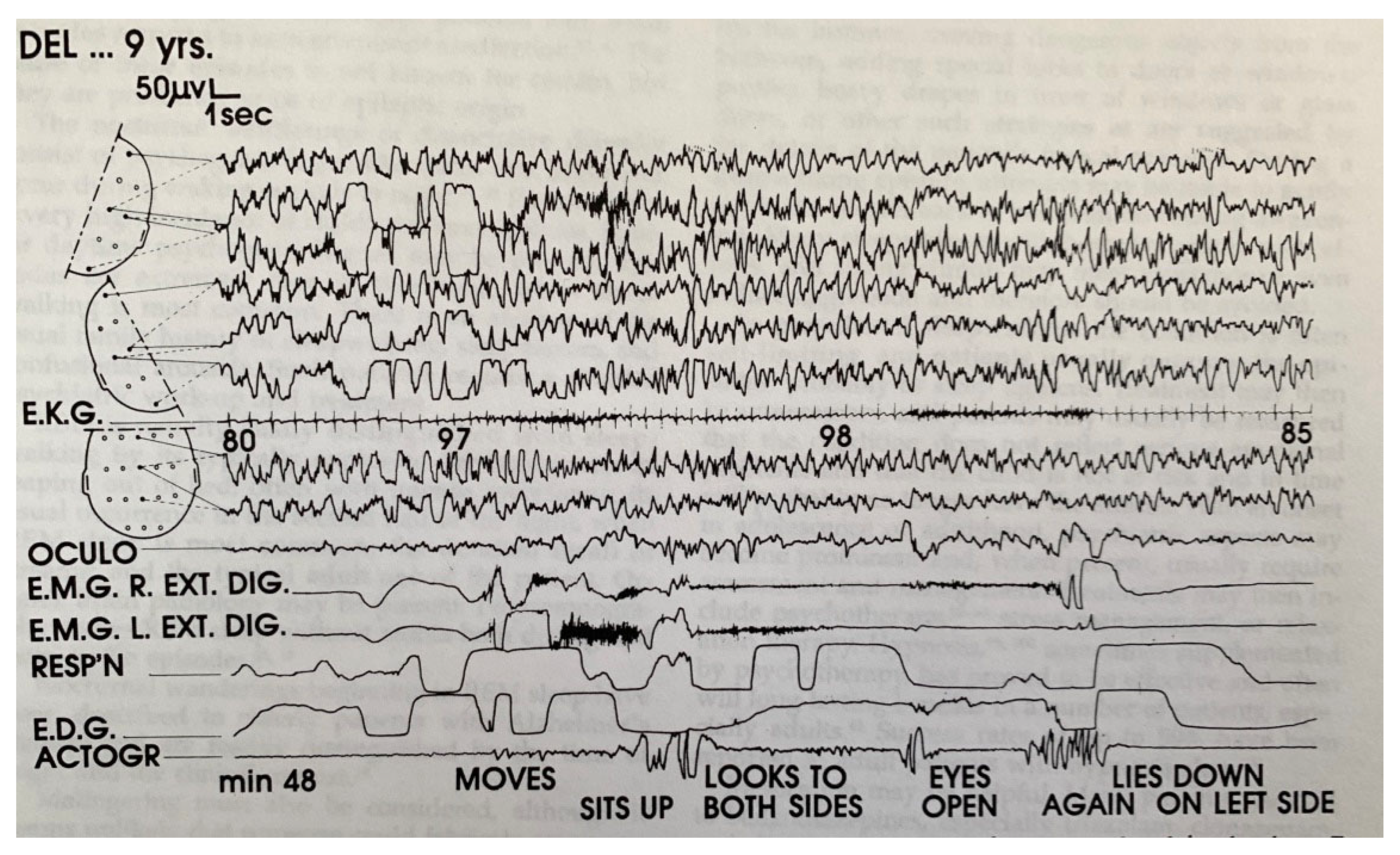
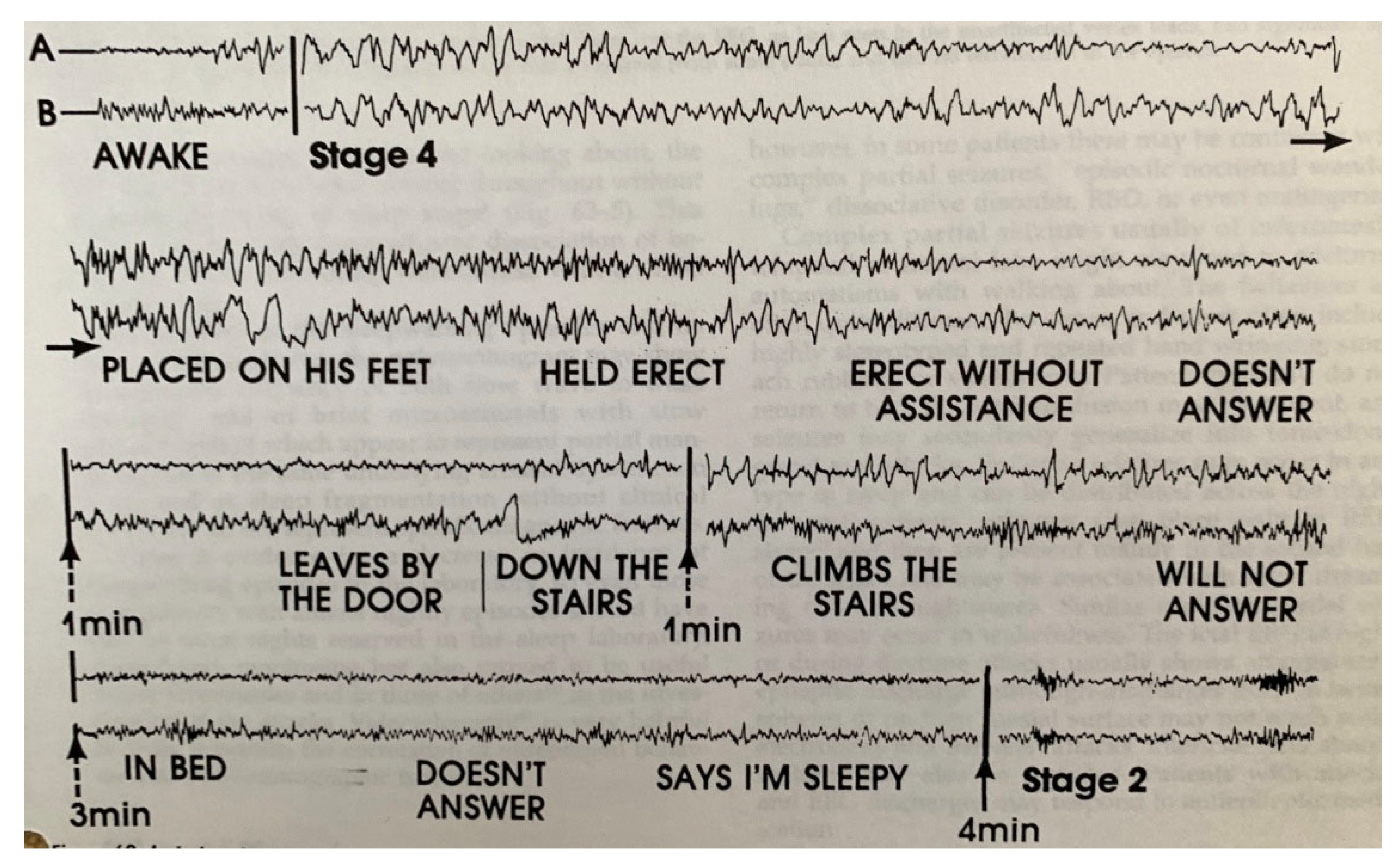
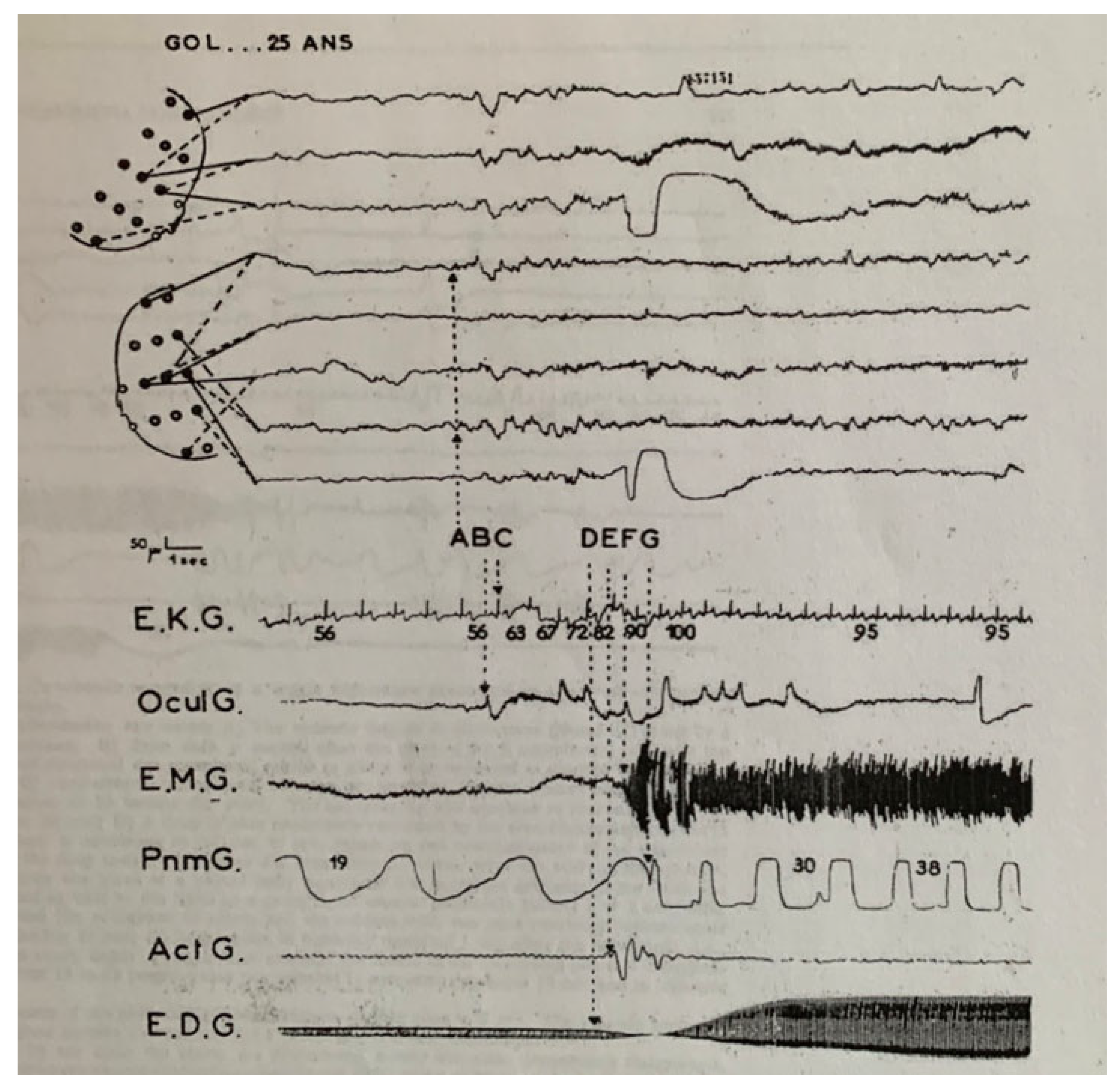
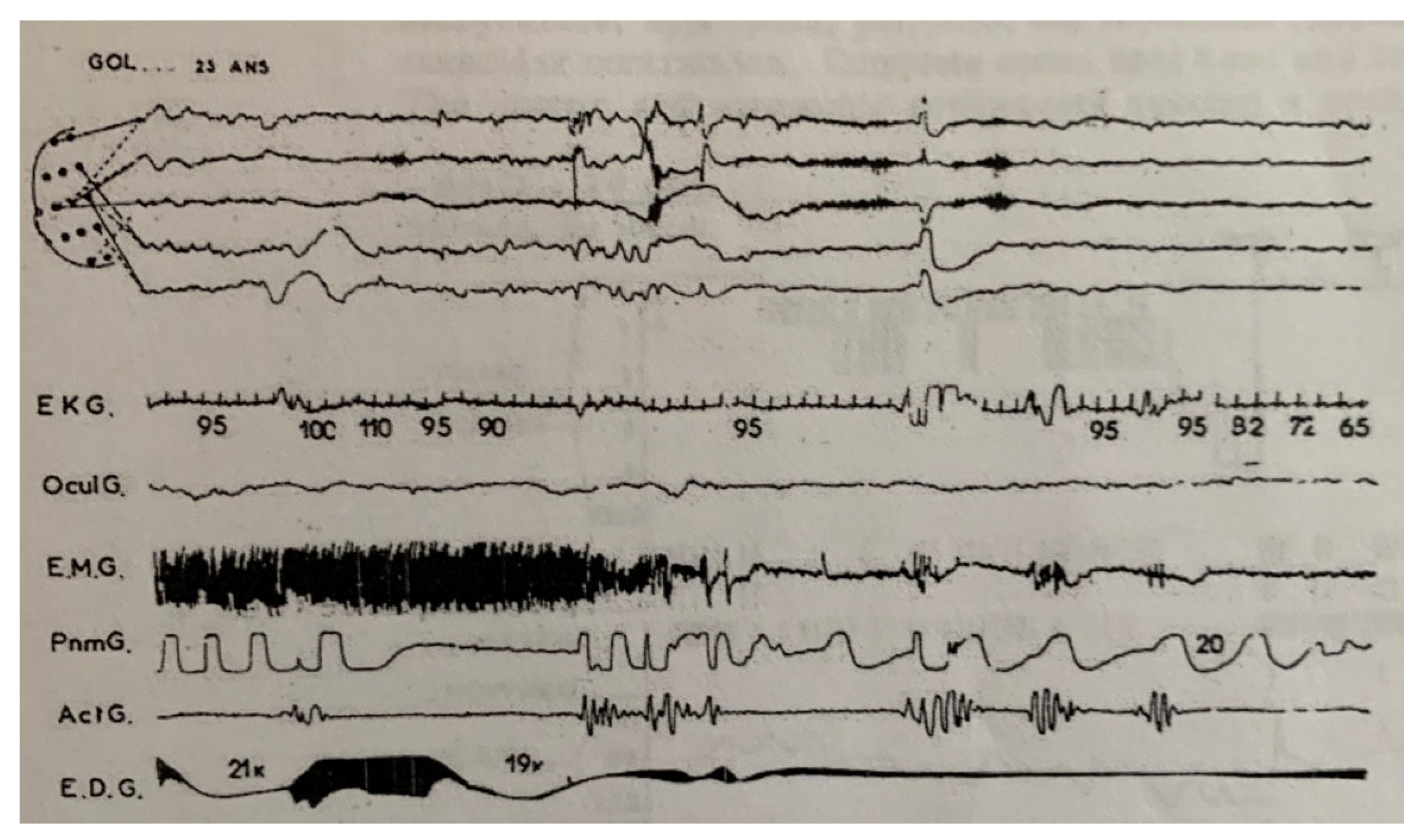

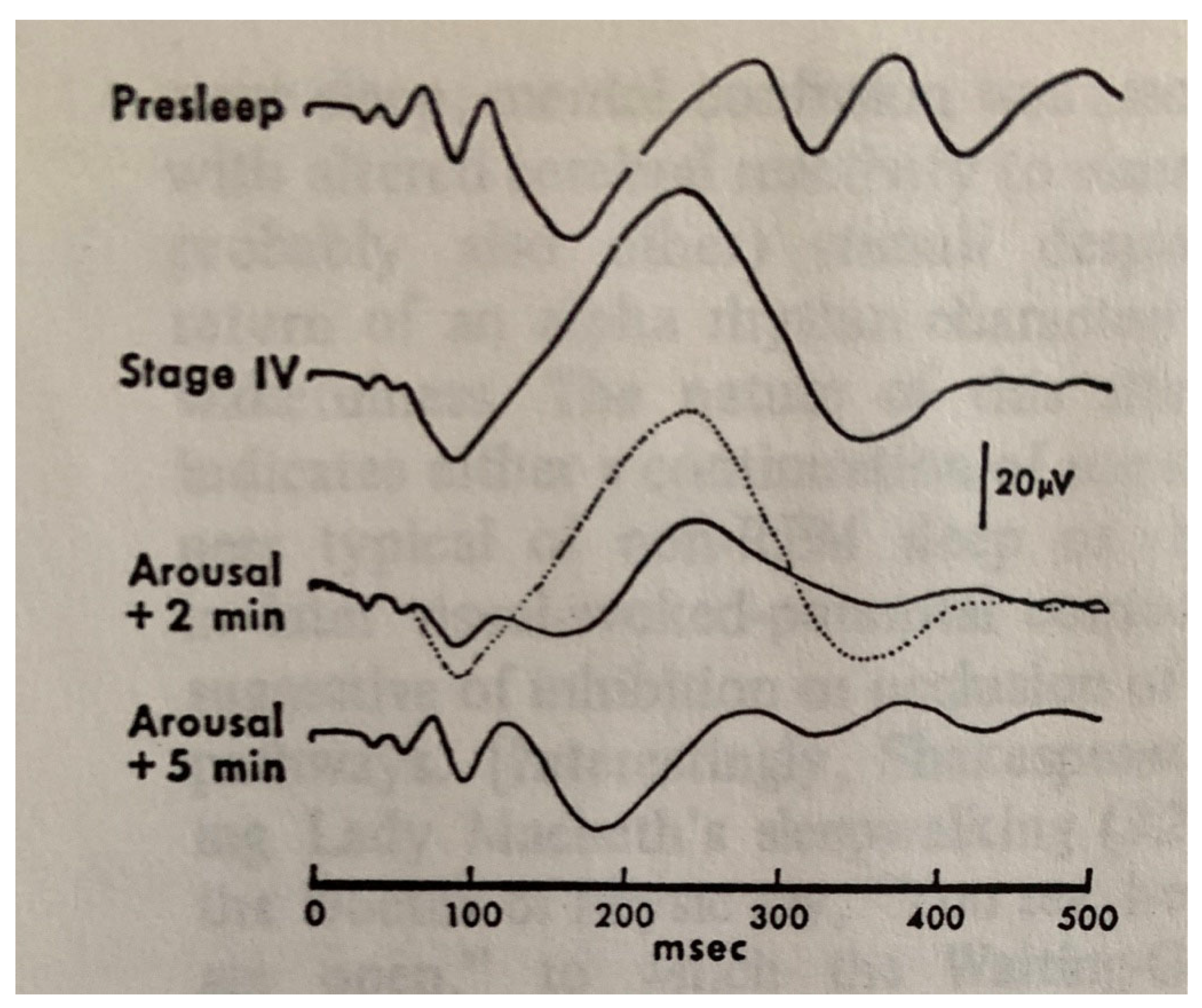
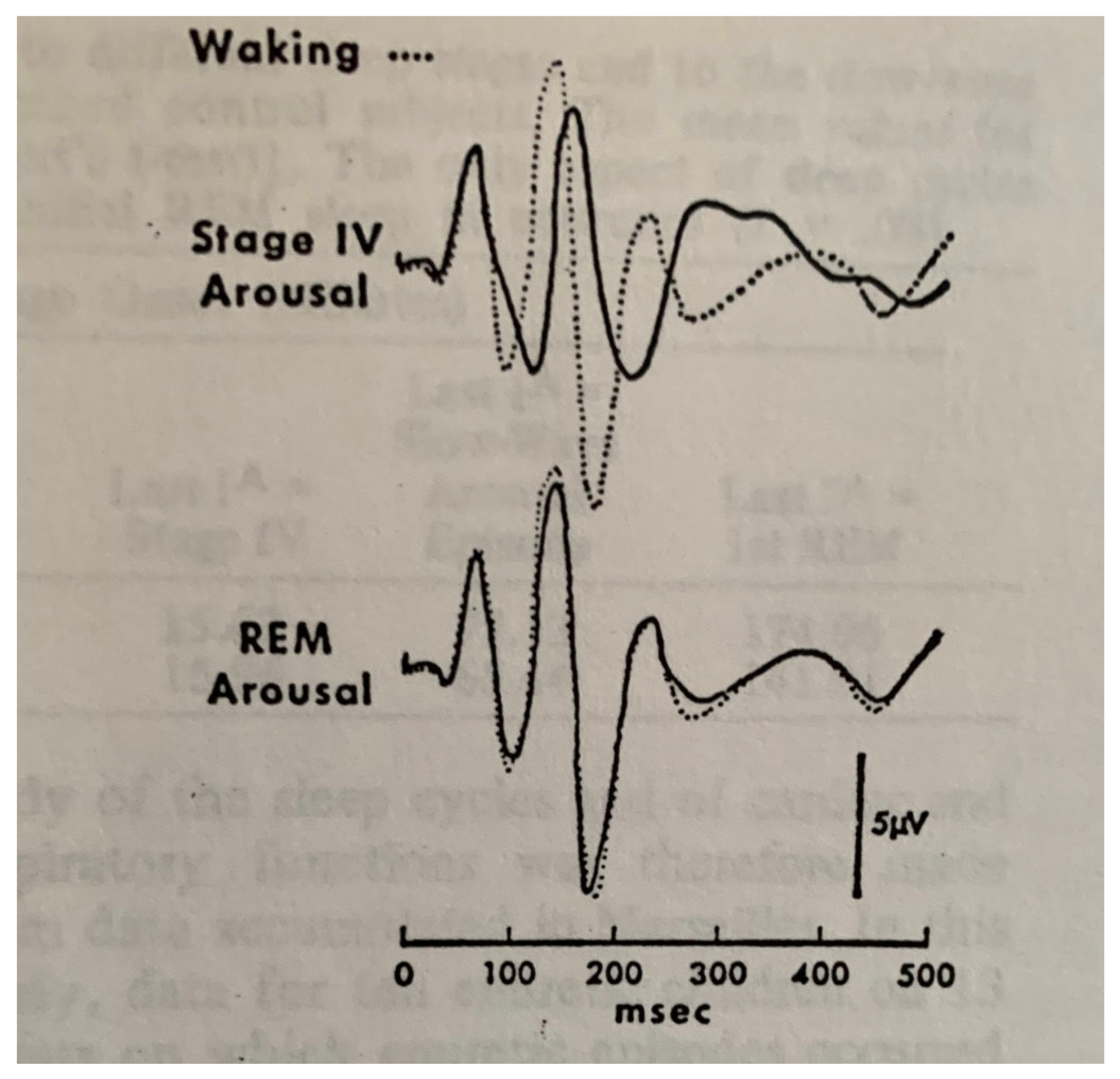
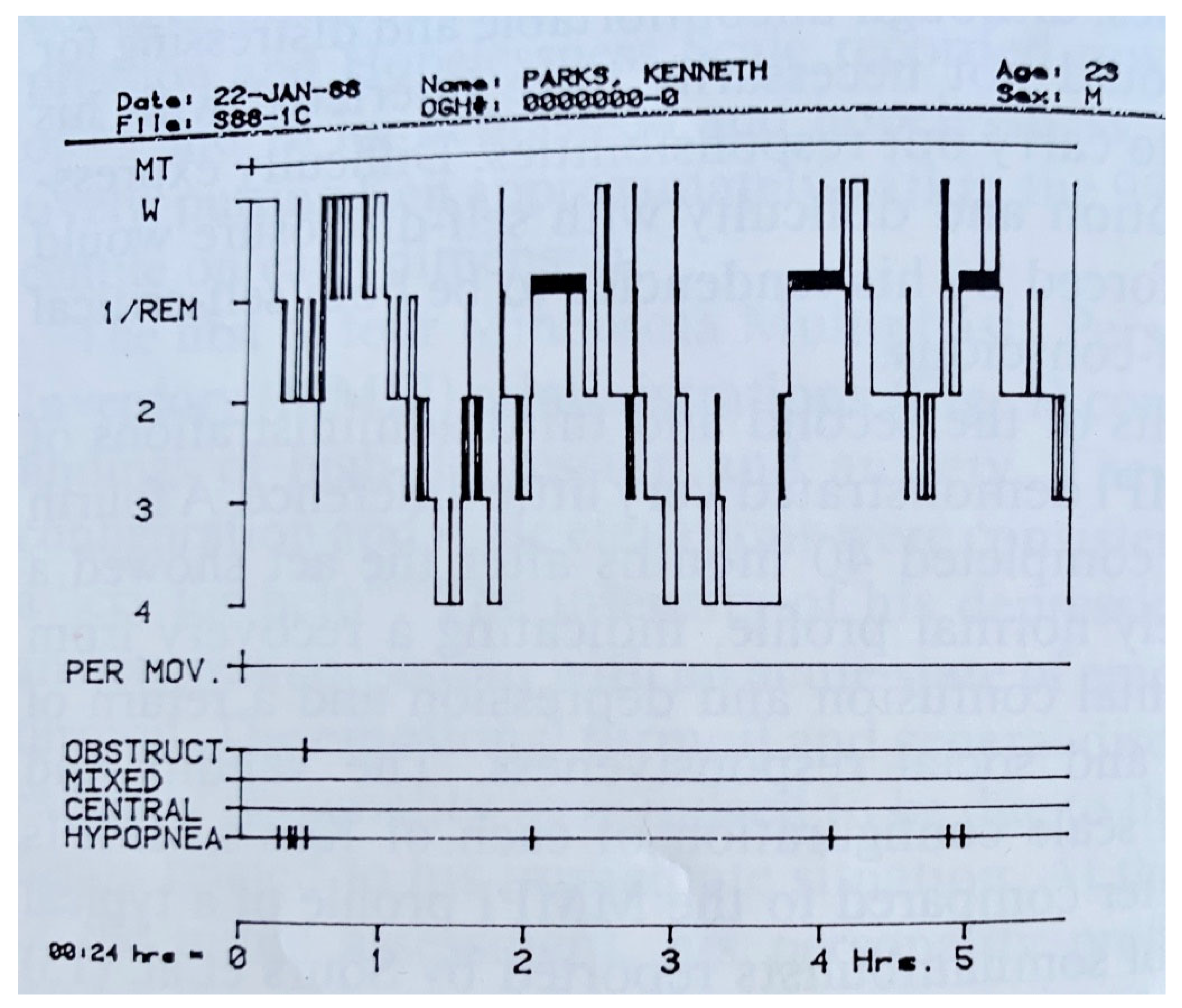

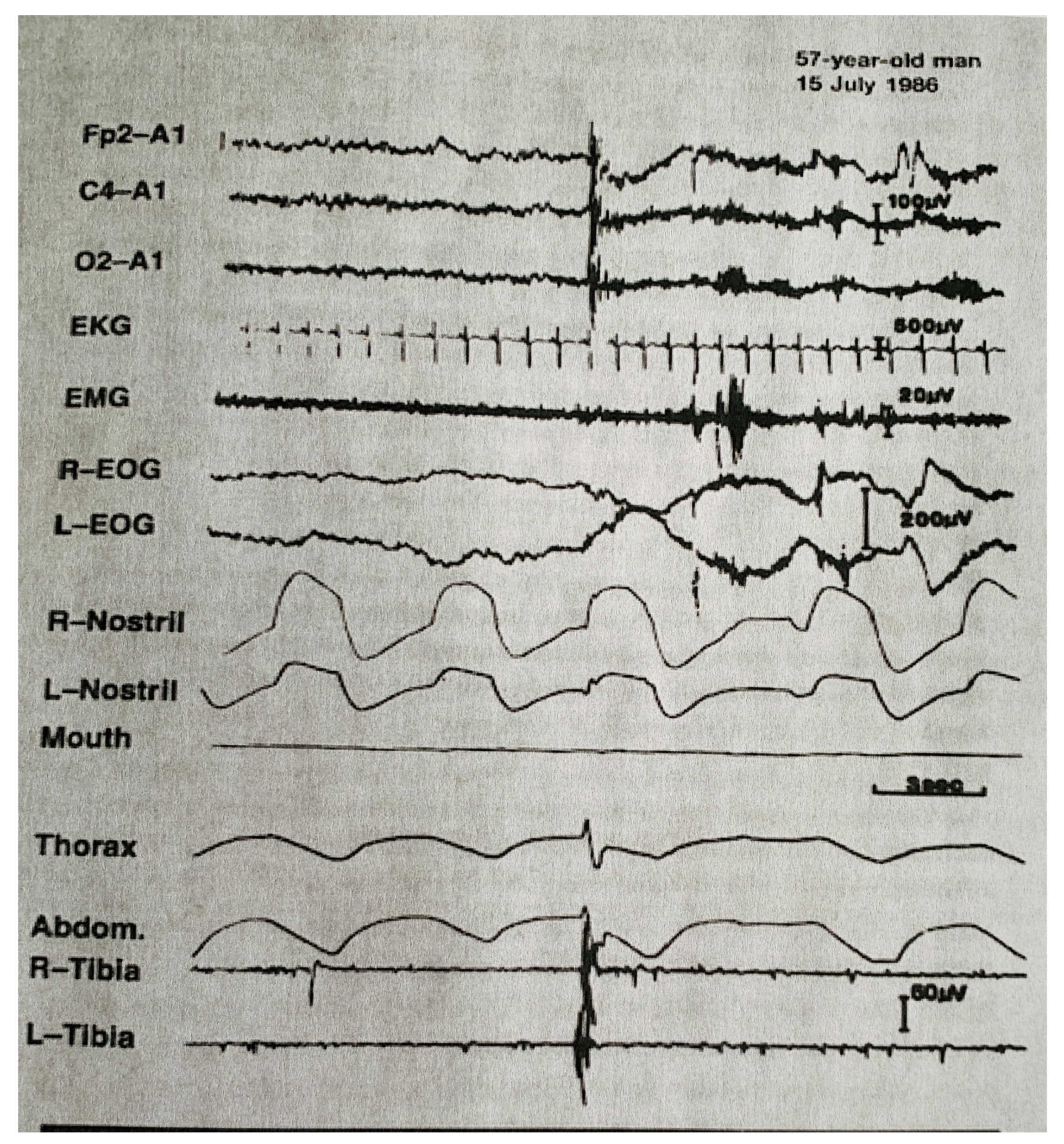
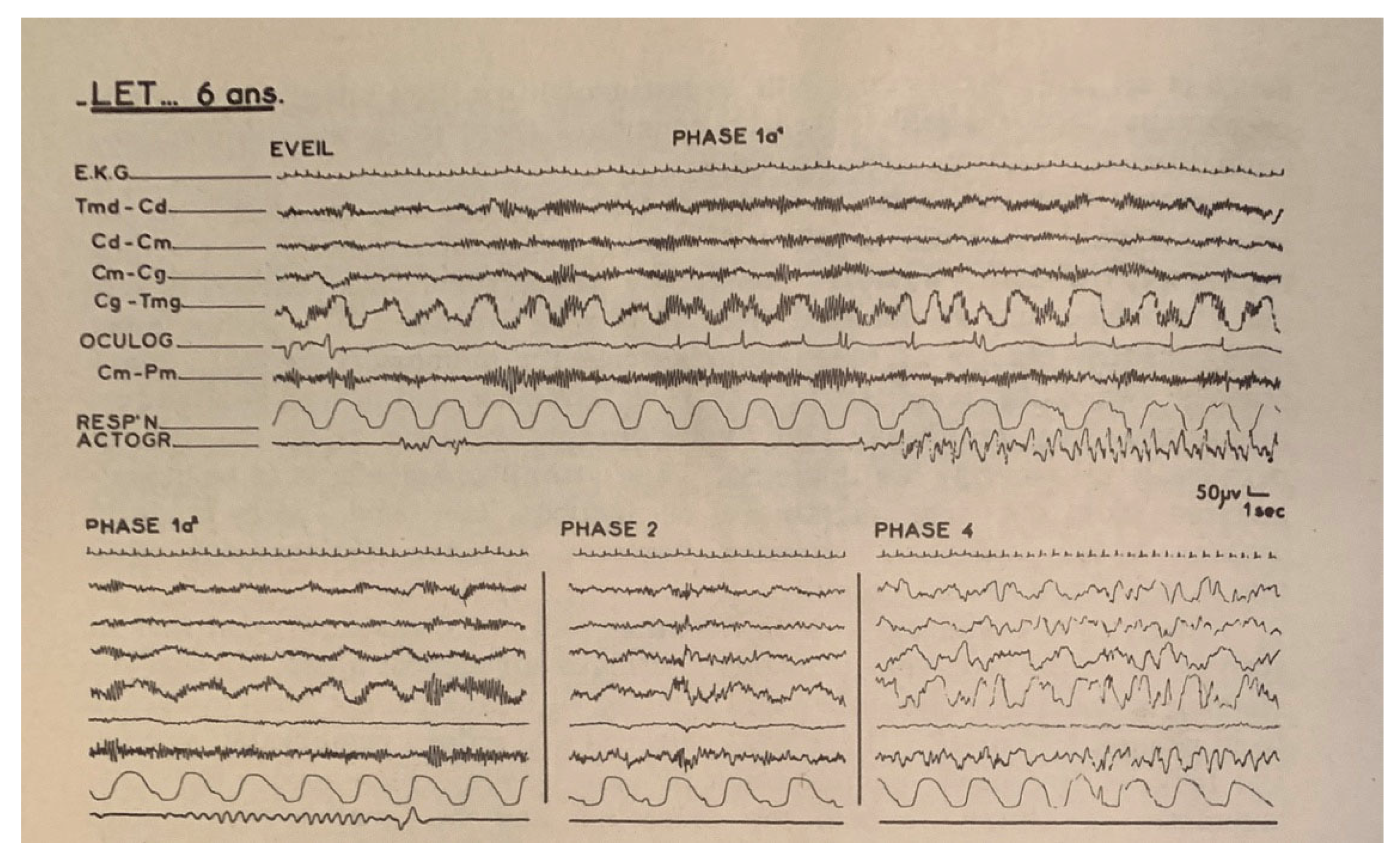
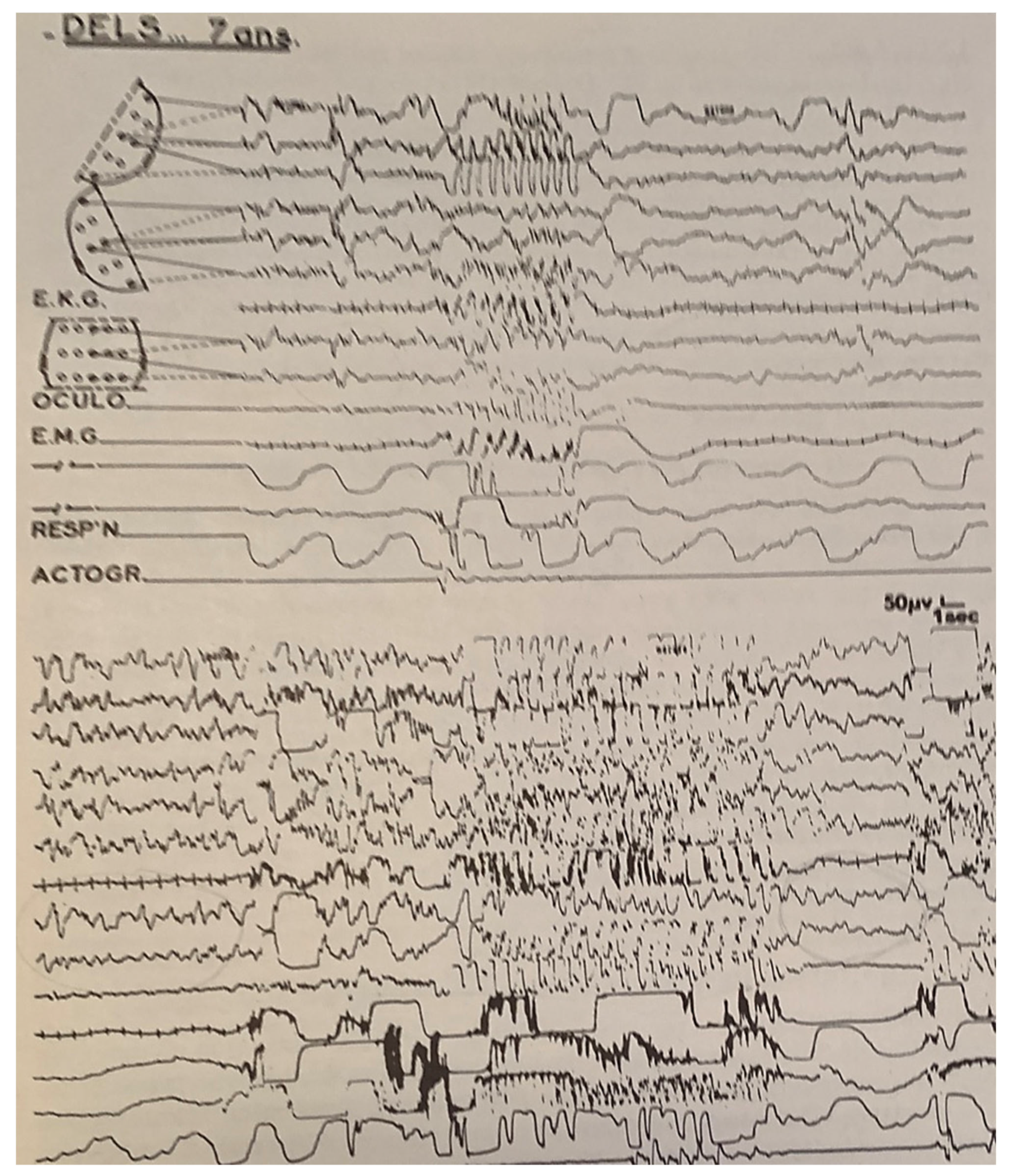
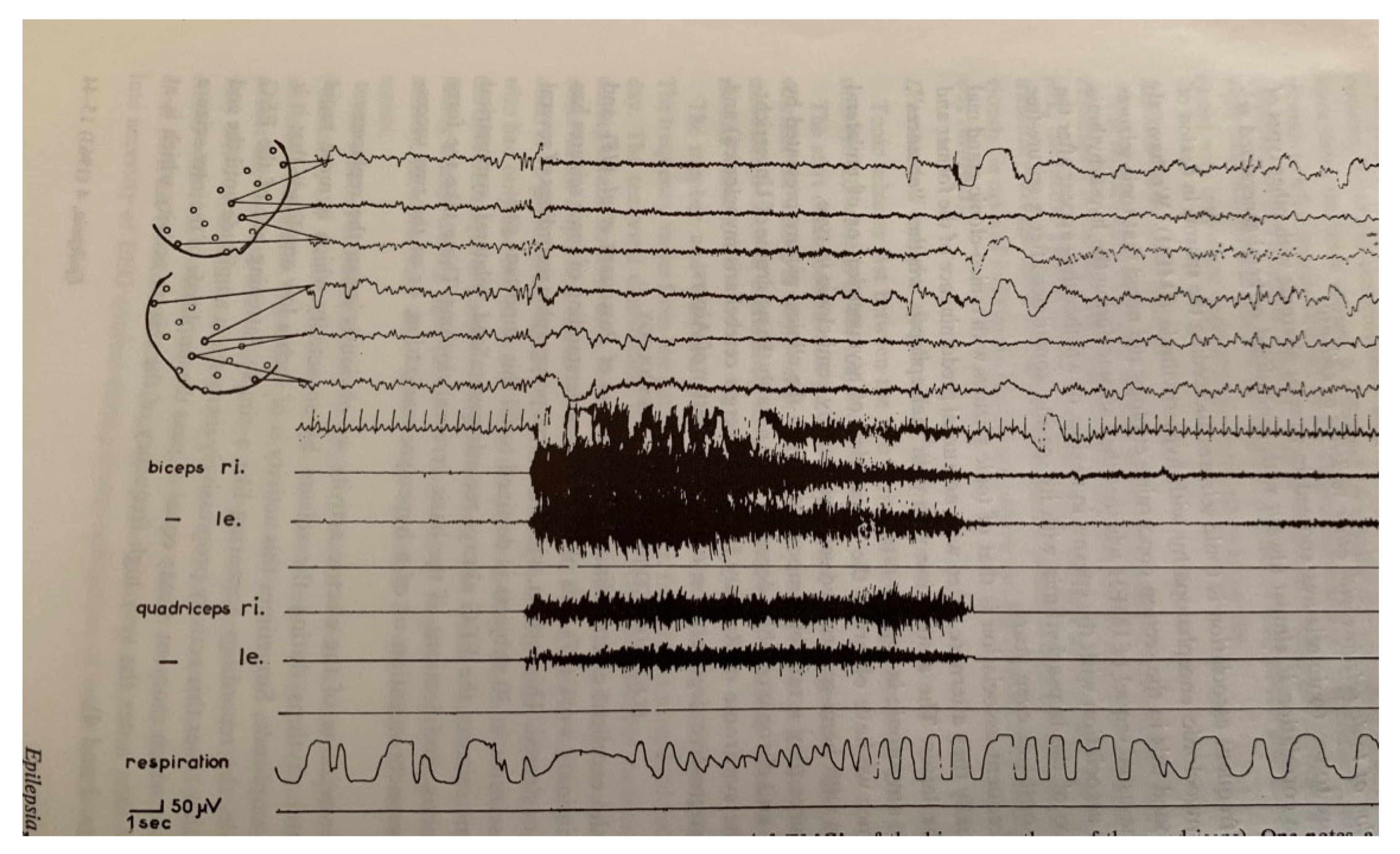

| From SWS | Can Induce | Automatism | Amnesia | Autonomic | Genetic | |
|---|---|---|---|---|---|---|
| Bedwetting | ++ | + | - | + | + | + |
| Sleepwalking | ++ | + | + | + | − | + |
| Sleep terrors | ++ | + | + | + | +++ | + |
| Confusional arousals | ++ | + | + | + | − | + |
| Terrifying Dreams | Sleep Terrors | |
|---|---|---|
| State of occurrence | REM sleep | SWS arousal |
| Stability of state | Stable (REM) | Changing |
| Circadian aspect | Late night | Early night |
| Preceding autonomic activation | Present | Absent |
| Intense behavioral arousal | Rare | Always |
| Heart rate increase | Mild | Marked |
| Respiratory rate increase | Mild | Marked |
| Muscle tone increase | Mild to moderate | Marked |
| Mental confusion | Minimal | Marked |
| Memory problems | Rare | Marked |
| Paralysis | Atonic | Hypertonic |
| Recalled mental activity | Dream-like | Single scene |
| Anxiety level | Mild to moderate | Intense |
| Genetic factors | Minimal | Important |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broughton, R.J. The Parasomnias and Sleep Related Movement Disorders—A Look Back at Six Decades of Scientific Studies. Clin. Transl. Neurosci. 2022, 6, 3. https://doi.org/10.3390/ctn6010003
Broughton RJ. The Parasomnias and Sleep Related Movement Disorders—A Look Back at Six Decades of Scientific Studies. Clinical and Translational Neuroscience. 2022; 6(1):3. https://doi.org/10.3390/ctn6010003
Chicago/Turabian StyleBroughton, Roger J. 2022. "The Parasomnias and Sleep Related Movement Disorders—A Look Back at Six Decades of Scientific Studies" Clinical and Translational Neuroscience 6, no. 1: 3. https://doi.org/10.3390/ctn6010003
APA StyleBroughton, R. J. (2022). The Parasomnias and Sleep Related Movement Disorders—A Look Back at Six Decades of Scientific Studies. Clinical and Translational Neuroscience, 6(1), 3. https://doi.org/10.3390/ctn6010003





