Promising Nanotechnology-Based Strategies for Melanoma Treatment
Abstract
1. Introduction
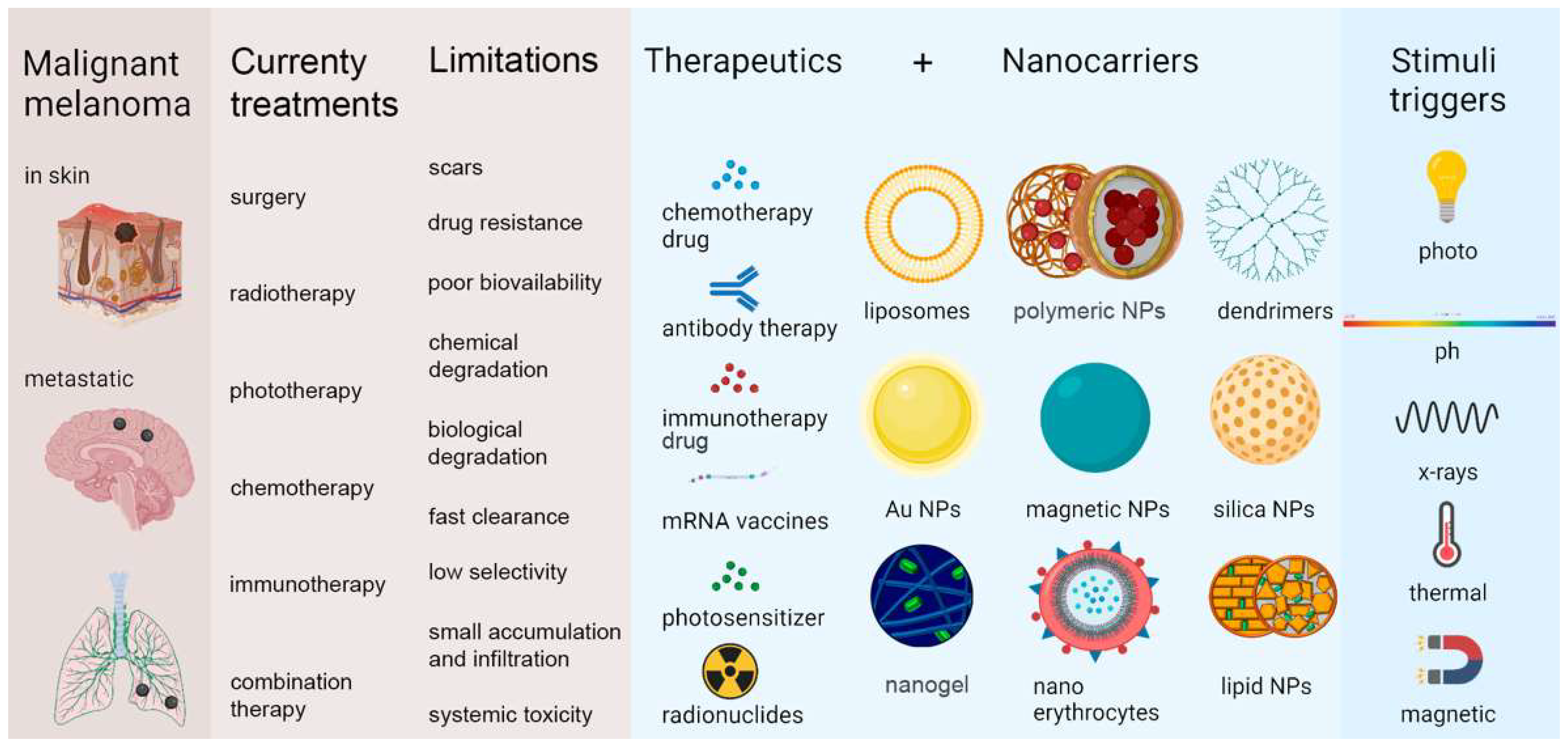
2. Melanoma: Definition, Causes, Occurrence, and Evaluation
3. Current Treatments for Melanoma, Their Limitations, and Promising Nanotechnology Contributions
| Current Treatments | General Aspects | Limitations | Promising Nanotechnology Contributions |
|---|---|---|---|
| Surgery | Consists of the removal of affected skin tissue with a safety margin of 5 cm around the lesion, including deep fascia, for tumors in the limbs and trunk and 2.5 cm for tumors in the face [1]. | It is considered a risky intervention, especially in elderly patients who generally have other health problems that can hamper healing, such as diabetes. Discomfort with the scar, considering that the highest incidence of melanoma is on the face and other regions generally exposed [1]. | Selective accumulation of nanoparticles (NPs) at disease sites enables early tumor visualization, improving diagnosis and reducing toxic side effects on healthy tissues [43,44,45]. |
| Radiotherapy (RT) | RT is an adjuvant treatment that supports cure or palliation and enhances immunotherapy effects [10,46,47]. | RT alone does not improve survival, limited by factors like poor tumor vascularization, hypoxia, and radiation absorption [48]. Cutaneous melanoma is considered relatively radioresistant [47,49,50]. | Metallic inorganic nanoparticles, such as gold (AuNP) and platinum (PtNP), act as radiosensitizers that enhance X-ray absorption, improving radiotherapy effectiveness against melanoma [48]. |
| Phototherapy (PT) | PT is a specific, localized outpatient treatment with low side effects. It includes photothermal therapy, which uses near-infrared light to heat and kill tumor cells, and photodynamic therapy, which activates photosensitizers to produce reactive oxygen species or heat, causing tumor death [51,52,53,54,55]. | PT alone has limited tumor ablation due to shallow light penetration, especially in melanin-rich melanoma cells that block radiation. Therefore, PDT requires photosensitizers like Indocyanine Green (ICG), which absorbs longer wavelengths for better effectiveness [56,57,58,59]. | Nanocarriers improve ICG stability and concentration in target tissues. Developing new nanomaterials for combined PTT and PDT allows for precise, controlled drug release using light-responsive nanoparticles, modulated by light intensity, wavelength, and exposure time [60,61,62]. |
| Chemotherapy (CT) | Chemotherapeutic drugs are toxic compounds that inhibit the rapid proliferation of cancer cells [63]. | CT causes side effects by affecting healthy fast-growing cells, and its drugs often have poor pharmacokinetics and wide distribution, reducing treatment efficiency [12,64]. Multidrug resistance is habitual, caused by internal factors, including mutations [65,66], gene amplification [67,68], deletions [69,70], and chromosomal rearrangements [71,72], or external factors, such as pH [73], hypoxia [74], and paracrine signaling interactions with stromal cells [11,75,76]. | LPs loaded with vemurafenib improve skin drug delivery and reduce toxicity [77]. SLNs loaded with temozolomide and SLNs loaded with paclitaxel show strong antimelanoma effects without toxicity [78,79]. PNPs loaded with flavone apigenin loaded with apigenin and functionalized with DMSA enhance drug release, bioavailability, lung targeting, and efficacy against melanoma metastases [80]. |
| Immunotherapy (IT) | It aims to modulate the systemic immune system so that it defends itself against cancer, focusing on the body’s defence system, not on the cancer cells themselves, as chemotherapy [81]. | It has great challenges, such as drug resistance from immune control point inhibitors, low immunogenicity of therapeutic vaccines [82], significant immunological adverse events (iRAE), off-target side effects and drug resistance [83]. The results of vaccines, cytokines, and cell therapies are only significant in a very small portion of patients with metastatic melanoma (MM), partially due to melanoma-induced immunosuppression [11,84,85,86]. | NLC loaded with LEM2 improved drug release and its solubility and bioavailability problems and does not interfere with molecular action, due to the adhesion and occlusion properties of NLC [87]. A polyethyleneimine-CpG PNP (CpG @ PEI) was tested as an in situ vaccine to enhance anti-cancer immunity, increasing cell stability and CpG internalization, improving innate and adaptive immunity, increasing NK cell numbers and infiltrating T cells in the tumor as well as the expression of CD80 on dendritic cells (DCs) and therefore effectively inhibiting the growth of murine B16F10 melanoma [88]. Dosta et al. developed dendrimers containing an interferon gene stimulator (STING) in the cytosol and concluded that NPs were advantageous in the delivery of gene-based immunomodulators [89]. Nanogels that selectively release IL-2 in response to activation of T cell receptors after T cell antigen recognition on tumor cells have been developed, showing improved efficiency and reduced severity of side effects [90]. |
| Chemoimmunotherapy | Chemotherapeutic drugs can induce immunomodulation, mainly by increasing the intrinsic immunogenicity of tumor cells [91], regulating the suppressive influence of T cells [92] and impacting the function of other cells, such as myeloid-derived suppressor cells (MDSC) [93] and dendritic cells (DC) [94], positively regulating the expression of tumor antigens [95] and the main class I histocompatibility complex (MHC-I) [96], inducing the expression of co-stimulatory molecules [97]. Chemotherapeutics can also act by negatively regulating the immunological checkpoint molecules expressed on the surface of the tumor cell [98], inducing the death of the tumor cell by secretion of adenosine triphosphate (ATP) or expression of calreticulin [91] and others [99,100]. | Chemoimmunotherapy has shown remarkable clinical results, however, there are also worrying effects, such as an inadequate T-cell response, a large discrepancy in the curative effect among patients, in addition to the fact that many patients do not present a satisfactory response [101]. Thus, developments in the use of chemoimmunotherapy are still needed to increase the effects of the immune response [102]. When there is a combination of drugs, as in chemoimmunotherapy, it is necessary to guarantee the ideal synergistic antitumor efficacy, considering the different pharmacokinetics and insufficient in vivo distribution of both agents, drug specificity for tumor cells and insufficient accumulation of drugs in the tumor and severe systemic side effects [103]. | The use of NP as drug carriers can improve pharmacokinetic behaviours in vivo, increase the stability and perform a controlled release of drugs and even targeted them to tumor cells, being, therefore, a promising tool for chemoimmunotherapy [104] |
4. Nanoparticles and Melanoma Treatment
4.1. Drug Targeting Through Nanoparticles
4.2. Passive Targeting
4.3. Active Targeting
5. Types of Nanoparticles, Their Advantages and Disadvantages, and Improvements in Tumor Treatments
6. Summary of Recent Studies Applying NPs to Melanoma Treatment
| Functionalization | Therapy | Anticancer Agent(s) | Results | Ref. | |
|---|---|---|---|---|---|
| Liposomes (LPs) | Stearyl chain (C18) fused pH-sensitive cell-penetrating peptide (TR) | Combination therapy | Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Paclitaxel (PTX) | TRAIL-[Lip-PTX] C18-TR exhibited a tumor inhibition rate of 93.8%, improving TRAIL-based therapy. Moreover, this system showed a better IC50 in vitro compared to free drugs and liposomal drugs separately; therefore, synergism of the two types of drugs in a single system was observed. | [144] |
| Tf-PEG-PE (Tf = transferrin) | Combination therapy | Mitoxantrone (MIT) Anacardic acid and Ammonium ascorbate | This peptide-modified co-delivery system was effective for use with chemotherapeutics and natural bioactive substances, showing an enhanced active tumor-targeting effect and therapeutic efficacy, due to the increased level of apoptosis and cell death in melanoma cell lines, which was not verified in normal cells. | [145] | |
| T-cell receptor (TCR)-like antibody (scFv G8 and Hyb3) | Combination therapy | Doxorrubicin (DXR) | These LPs effectively and selectively target antigen-positive melanoma and coupled to anti-M1/-A1 scFv inflict a significant antitumor response. This NPs can be used for drug delivery, immunotherapy, potentially imaging, and diagnosis of melanoma. | [45] | |
| Immunotherapy | Interleukin (IL)−2 Agonistic anti-CD137 | This immunoliposome delivery system achieved antitumor activity in multiple tumor models like both free drugs, but overcame the important challenge of IL-2/anti-CD137 toxicity. | [123] | ||
| Biological-inspired peptide TD (ACSSSPSKHCG) | Chemotherapy | Vemurafenib | The effective antitumor ability of the drug carrier delivered via the skin was better than that of oral and intravenous administrations; in terms of toxicity in the liver, kidney, and lungs, it was negligible and antitumor efficacy was increased. Moreover, surface modification with the peptide TD significantly enhanced the transdermal delivery of the drug. | [77] | |
| Solid lipid nanoparticles (SLN) | Chemotherapy | Temozolomide | The drug-loaded LP showed better inhibitory effects on cell proliferation, neoangiogenesis, growth, and vascularization of melanoma when compared to the free drug, with no apparent toxic effect. | [78] | |
| Immunotherapy | Programmed cell death siRNA-1 (PD-1) | PD-1 siRNA-SLN significantly decreased PD-1 expression in cultured macrophages and tumor tissues, as well as tumor growth in vivo, compared to negative control. | [143] | ||
| Tyr-3-octreotide | Chemotherapy | Paclitaxel (PTX) | Compared to dacarbazine (DTIC), the carrier system exerted more apoptotic and anti-invasive effects in vitro; in addition, an interesting immune modulation was observed, resulting in effective reduction in tumor volume in vivo. Furthermore, a reduction in the number of nodule formations of pulmonary metastases was observed. | [79] | |
| Nanostructured lipid carriers (NLC) | Immunotherapy | LEM2 | NLC improved LEM2 delivery and bioavailability issues and did not interfere with the drug’s molecular mechanism of action, as it showed greater cytotoxicity against the A375 melanoma cell line than uncharged NLCs, probably due to the adhesion and occlusive properties of NLC in the stratum corneum. | [87] | |
| Chemotherapy | Bupivacaine Lavender or Melaleuca essential oils | According to cytotoxicity tests, IC50 values of encapsulated bupivacaine decreased, relative to the free drug, in mice and human melanoma cells by ~80% and 62% (containing lavender oil) and 80% and 25% (containing melaleuca oil), respectively. In addition, anesthesia time doubled. | [188] | ||
| Polymeric nanoparticles (PNP) | Acid folic | Combination therapy | Metformin Doxorubicin | This drug carrier was developed with a new pH-sensitive homopolymer, based on the conjugation of sodium alginate, cholesterol, and folic acid, where metformin and doxorubicin were encapsulated. This system proved to be promising for effective combined drug delivery, increasing the antimelanoma effects. | [187] |
| Immunotherapy | siRNA | A nano-carrier developed for siRNA inhibited proliferation of B16F10 cells and significantly inhibited the growth and metastasis of melanoma in vivo. | [159] | ||
| Immunotherapy | CpG oligodeoxynucleotides | The nanocomplex effectively inhibited the growth of melanoma, the effect of which was attributed to the activation of both the innate and adaptive immune response. | [88] | ||
| meso-2,3-dimercaptosuccinic acid (DMSA) | Chemotherapy | Apigenin | The formulation of NP loaded with flavone apigenin and conjugated with DMSA improved bioavailability, increased antitumor and antimetastatic efficacy. | [80] | |
| Inorganic nanoparticles (INP) | Radiotherapy | Iode-131 (131I) | Combining grafted hybrid polymer (poly(methacrylic acid) NPs (PMAA-AuNPs) for systemic radioiodine therapy showed the effectiveness of a clinically relevant approach; therefore, this system is promising for use as a radiosensitizer in the field of RT internal radioisotopes. | [170] | |
| Photothermal | Carbon xerogel NPs converted NIR light to heat efficiently and within a duration of 10 min, successfully induced cell death, reduced necrosis by 70%, and resulted in significant reductions in tumors. | [214] | |||
| MSH | Radiotherapy | Lutetium-177 (177Lu) | Surface modification of the developed targeted ultra-small radiotherapeutic silica NPs, modified to have favorable pharmacokinetic properties, increased efficacy and survival. | [215] | |
| Dendrimers (DM) | Arginine or mixture of arginine/lysine cationic polypeptides | Immunotherapy | Stimulator of interferon genes agonist | Arginine-modified dendrimer formulations were efficiently internalized into THP-1 cells, activating IRF3 and NF-κB, and showed less toxicity than unmodified dendrimers. The combination of the polypeptide-modified dendrimer-based system with anti-PD-1 induced large tumor regression. | [89] |
| Methoxy polyethylene glycol (mPEG) | Immunotherapy | siRNA | Functionalized Au DENPs were able to deliver siPD-L1, resulting in a knock down of PD-L1 in tumors with an efficiency of 59%, thus mediating tumor immunotherapy (based on immune checkpoint blockade) and immune responses. | [216] | |
| Nanogels (NGs) | Combination therapy | Doxorrubicin (DXR) cytosine–phosphate–guanine (CpG) | The use of gel prolonged the action time of DOX-CpG NPs and allowed for sustained release of DOX and CpG NP, increasing the bioavailability of drugs and adjuvants. DOX-induced tumor cell damage provided tumor-associated antigens that promoted the immune system response, followed by the CpG NP effect, further improving the immune response and tumor inhibition. | [206] | |
| Immunotherapy | Interleukin-2 (IL-2) | The designed system expanded the transferred tumor-reactive T cells 80 times more than free IL-2/Fc and showed no effects on tumor infiltration regulatory T cell expansion, with no overt toxicity. | [90] | ||
| Biomimetic nanoparticles (BNP) | Chemotherapy | Doxorubicin (DXR) | Leukosomes, biomimetic NPs generated by combining lipids with membrane proteins derived from lipopolysaccharide-stimulated macrophages, showed more potent anticancer activity than LPs, reducing tumor volume and increasing survival, due to significantly greater accumulation in B16 tumors in vivo. This can be explained by the interaction of leukosome membrane proteins and tumor-associated vasculature. | [212] | |
| Folate modified red blood cell membrane | Combination therapy | Paclitaxel (PTX) indoleamine 2,3-dioxygenase (IDO) | The nanoplatform prolonged blood circulation and improved accumulation in tumor tissues, efficiently increasing uptake by specific cells (B16F10 and type M2 tumor-associated macrophages (TAM2)) and drug release. | [213] | |
| Antigenic peptide, a toll-like receptor 9 agonist and galactose-inserted erythrocyte membrane | Immunotherapy | Baicaina | PLGA biomimetic NPs reverted TAM phenotype M2 to M1 transition, resulting in effective T cell activation and induction of cytotoxic T cell responses and, consequently, significant suppression of melanoma tumor growth in vivo. | [217] |
7. Clinical Trials
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Orzan, O.A.; Șandru, A.; Jecan, C.R. Controversies in the diagnosis and treatment of early cutaneous melanoma. J. Med. Life 2015, 8, 132–141. [Google Scholar]
- Cullen, J.K.; Simmons, J.L.; Parsons, P.G.; Boyle, G.M. Topical treatments for skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 54–64. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA A Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Sacchetto, L.; Zanetti, R.; Comber, H.; Bouchardy, C.; Brewster, D.H.; Broganelli, P.; Chirlaque, M.D.; Coza, D.; Galceran, J.; Gavin, A.; et al. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur. J. Cancer 2018, 92, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.-J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.P. Cutaneous Malignant Melanoma: A Review of Early Diagnosis and Management. World J. Oncol. 2021, 12, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Lopes, J.; Rodrigues, C.M.; Gaspar, M.M.; Reis, C.P. Melanoma management: From epidemiology to treatment and latest advances. Cancers 2022, 14, 4652. [Google Scholar] [CrossRef]
- Beiu, C.; Giurcaneanu, C.; Grumezescu, A.M.; Holban, A.M.; Popa, L.G.; Mihai, M.M. Nanosystems for improved targeted therapies in melanoma. J. Clin. Med. 2020, 9, 318. [Google Scholar] [CrossRef]
- Song, M.; Liu, C.; Chen, S.; Zhang, W. Nanocarrier-Based Drug Delivery for Melanoma Therapeutics. Int. J. Mol. Sci. 2021, 22, 1873. [Google Scholar] [CrossRef]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nano-Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- López-Estévez, A.M.; Lapuhs, P.; Pineiro-Alonso, L.; Alonso, M.J. Personalized cancer nanomedicine: Overcoming biological barriers for intracellular delivery of biopharmaceuticals. Adv. Mater. 2024, 36, e2309355. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, F.B.; Webster, C.A.; Moncrieff, M.; Sherwood, V. The scope of nanoparticle therapies for future metastatic melanoma treatment. Lancet Oncol. 2014, 15, e22–e32. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Cuconato, M.; Calviello, G.; Serini, S.; Trombino, S. Recent advances in nanotechnology for the treatment of melanoma. Molecules 2021, 26, 785. [Google Scholar] [CrossRef]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.; Yoong, C.; Robertson, T.A.; Soyer, H.P. Nanopar-ticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Liang, R.; An, X.; Wang, K.; Shen, G.; Tu, Y.; Zhu, J.; Tao, J. Recent advances in targeted nanoparticles drug delivery to melanoma. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 769–794. [Google Scholar] [CrossRef]
- Manconi, M.; Sinico, C.; Caddeo, C.; Vila, A.O.; Valenti, D.; Fadda, A.M. Penetration enhancer containing vesicles as carriers for dermal delivery of tretinoin. Int. J. Pharm. 2011, 412, 37–46. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Sinico, C.; Fadda, A.M. Vesicular carriers for dermal drug delivery. Expert Opin. Drug Deliv. 2009, 6, 813–825. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, Z.; Almotairy, A.; Repka, M.A. Development and evaluation of polymeric mixed micelles prepared using hotmelt extrusion for extended delivery of poorly water-soluble drugs. J. Pharm. Sci. 2023, 112, 2869–2878. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I Through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Miller, A.J.; Mihm, C.M., Jr. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef]
- Burns, D.; George, J.; Aucoin, D.; Bower, J.; Burrell, S.; Gilbert, R.; Bower, N. The pathogenesis and clinical management of cutaneous melanoma: An evidence-based review. J. Med. Imaging Radiat. Sci. 2019, 50, 460–469.e1. [Google Scholar] [CrossRef]
- Smith, A.J.; Lambert, P.C.; Rutherford, M.J. Understanding the impact of sex and stage differences on melanoma cancer patient survival: A SEER-based study. Br. J. Cancer 2021, 124, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Lesage, C.; Barbe, C.; Le Clainche, A.; Lesage, F.-X.; Bernard, P.; Grange, F. Sex-related location of head and neck melanoma strongly argues for a major role of sun exposure in cars and photoprotection by hair. J. Investig. Dermatol. 2013, 133, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Molinero, L.; Bolen, C.R.; Sosman, J.A.; Muñoz-Couselo, E.; Kluger, H.M.; McDermott, D.F.; Powderly, J.D.; Sarkar, I.; Ballinger, M.; et al. Safety, clinical activity, and biological correlates of response in patients with metastatic melanoma: Results from a phase I trial of atezolizumab. Clin. Cancer Res. 2019, 25, 6061–6072. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Hodi, F.S.; Fisher, D.E. From genes to drugs: Targeted strategies for melanoma. Nat. Rev. Cancer 2012, 12, 349–361. [Google Scholar] [CrossRef]
- Hill, V.K.; Gartner, J.J.; Samuels, Y.; Goldstein, A.M. The genetics of melanoma: Recent advances. Annu. Rev. Genom. Hum. Genet. 2013, 14, 257–279. [Google Scholar] [CrossRef]
- Jacobs, J.F.; Nierkens, S.; Figdor, C.G.; de Vries, I.J.M.; Adema, G.J. Regulatory T cells in melanoma: The final hurdle towards effective immunotherapy? Lancet Oncol. 2012, 13, e32–e42. [Google Scholar] [CrossRef]
- Pautu, V.; Leonetti, D.; Lepeltier, E.; Clere, N.; Passirani, C. Nanomedicine as a potent strategy in melanoma tumor microenvironment. Pharmacol. Res. 2017, 126, 31–53. [Google Scholar] [CrossRef]
- Popescu, M.; Munteanu, A.; Isvoranu, G.; Suciu, L.; Pavel, B.; Marinescu, B.; Zagrean, L. Dynamics of endothelial progenitor cells following sevoflurane preconditioning. Roum. Arch. Microbiol. Immunol. 2011, 70, 109–113. [Google Scholar]
- Mihai, M.M.; Holban, A.M.; Călugăreanu, A.; Orzan, O.A. Recent advances in diagnosis and therapy of skin cancers through nanotechnological approaches. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–306. [Google Scholar]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Adnane, F.; El-Zayat, E.; Fahmy, H.M. The combinational application of photodynamic therapy and nanotechnology in skin cancer treatment: A review. Tissue Cell 2022, 77, 101856. [Google Scholar] [CrossRef] [PubMed]
- Bahreyni, A.; Mohamud, Y.; Luo, H. Recent advancements in immunotherapy of melanoma using nanotechnology-based strategies. Biomed. Pharmacother. 2023, 159, 114243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Y.; Wang, C.; Li, J.; Pang, Y. Advances in the Application of Nanomaterials to the Treatment of Melanoma. Pharmaceutics 2022, 14, 2090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Han, T.; Xia, H.; Dong, L.; Chen, L.; Lei, L. Advances in Photodynamic Therapy Based on Nanotechnology and Its Application in Skin Cancer. Front. Oncol. 2022, 12, 836397. [Google Scholar] [CrossRef]
- Pereira, T.A.; Ramos, D.N.; Sobral, L.M.; Martins, Y.A.; Petrilli, R.; Fantini, M.d.A.C.; Leopoldino, A.M.; Lopez, R.F.V. Liquid crystalline nanogel targets skin cancer via low-frequency ultrasound treatment. Int. J. Pharm. 2023, 646, 123431. [Google Scholar] [CrossRef]
- e Silva, S.A.M.; de Souza, J.G.; Melo, P.S.; Moreno, I.A.M.; Alencar, S.M.; Lopez, R.F.V.; Leonardi, G.R. Target action of antioxidants using iontophoresis. J. Cosmet. Dermatol. 2021, 20, 664–676. [Google Scholar] [CrossRef]
- Vale, D.L.; Martinez, R.M.; Medeiros, D.C.; da Rocha, C.; Sfeir, N.; Lopez, R.F.V.; Vicentini, F.T.M.C.; Verri, W.A.; Georgetti, S.R.; Baracat, M.M.; et al. A topical formulation containing quercetin-loaded microcapsules protects against oxidative and inflammatory skin alterations triggered by UVB irradiation: Enhancement of activity by microencapsulation. J. Drug Target. 2021, 29, 983–997. [Google Scholar] [CrossRef]
- Martin, B.A.; Dalmolin, L.F.; Lemos, C.N.; Vaidergorn, M.d.M.; Emery, F.d.S.; Vargas-Rechia, C.G.; Ramos, A.P.; Lopez, R.F.V. Electrostimulable polymeric films with hyaluronic acid and lipid nanoparticles for simultaneous topical delivery of macromolecules and lipophilic drugs. Drug Deliv. Transl. Res. 2024, 14, 2499–2519. [Google Scholar] [CrossRef]
- Ceci, P.; Vannucci, L.; Falvo, E.; Fornara, M.; Micco, D.; Benada, O.; Krizan, J.; Svoboda, J.; Capkova, H.; Morea, V.; et al. Selective targeting of melanoma by PEG-masked protein-based multifunctional nanoparticles. Int. J. Nanomed. 2012, 7, 1489–1509. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing nanoparticles with cancer-targeting antibodies: A comparison of strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Saeed, M.; Zalba, S.; Seynhaeve, A.L.; Debets, R.; Hagen, T.L.M.T. Liposomes targeted to MHC-restricted antigen improve drug delivery and antimelanoma response. Int. J. Nanomed. 2019, 14, 2069–2089. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Wang, X.; Dai, W.; Wang, J.; Zhang, X.; Li, Z.; Zhang, Q. Materializing sequential killing of tumor vasculature and tumor cells via targeted polymeric micelle system. J. Control. Release 2011, 149, 299–306. [Google Scholar] [CrossRef]
- Dossgg, L.L.; Memula, N. The radioresponsiveness of melanoma. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1131–1134. [Google Scholar] [CrossRef]
- Daneshvar, F.; Salehi, F.; Karimi, M.; Vais, R.D.; Mosleh-Shirazi, M.; Sattarahmady, N. Combined X-ray radiotherapy and laser photothermal therapy of melanoma cancer cells using dual-sensitization of platinum nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 203, 111737. [Google Scholar] [CrossRef] [PubMed]
- Testori, A.; Rutkowski, P.; Marsden, J.; Bastholt, L.; Chiarion-Sileni, V.; Hauschild, A.; Eggermont, A.M.M. Surgery and radiotherapy in the treatment of cutaneous melanoma. Ann. Oncol. 2009, 20, vi22–vi29. [Google Scholar] [CrossRef] [PubMed]
- Espenel, S.; Vallard, A.; Rancoule, C.; Garcia, M.-A.; Guy, J.-B.; Chargari, C.; Deutsch, E.; Magné, N. Melanoma: Last call for radiotherapy. Crit. Rev. Oncol. Hematol. 2017, 110, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Luo, M.; Zhang, F.; Zhang, L.; Wang, B.; Liu, P.; Zhang, Y.; Zhang, H.; Yang, D.; Zhang, G.; et al. Photothermal therapy enhanced the effectiveness of imiquimod against refractory cutaneous warts through boosting immune responses. J. Biophotonics 2019, 12, e201800149. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Nie, X.; Wen, T.; Ji, Y.; Wu, X.; Zhao, Y.; Chen, C. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J. Am. Chem. Soc. 2014, 136, 7317–7326. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Wang, X.; Kraatz, H.-B.; Ahmadi, S.; Gao, J.; Lv, Y.; Sun, X.; Huang, Y. A Trojan horse biomimetic delivery strategy using mesenchymal stem cells for PDT/PTT therapy against lung melanoma metastasis. Biomater. Sci. 2020, 8, 1160–1170. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, X.; Chen, J.; Hu, Y.; Sun, X.; Yu, Z. Nanoparticulate photosensitizer decorated with hyaluronic acid for photodynamic/photothermal cancer targeting therapy. Nanomedicine 2019, 14, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fang, Y.; Miao, Q.; Qi, X.; Ding, D.; Chen, P.; Pu, K. Regulating near-infrared photodynamic properties of semiconducting polymer nanotheranostics for optimized cancer therapy. ACS Nano 2017, 11, 8998–9009. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Leveraging engineering of indocyanine green-encapsulated polymeric nanocomposites for biomedical applications. Nanomaterials 2018, 8, 360. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Chen, H.; Lim, W.Q.; Phua, F.S.Z.; An, G.; Yang, P.; Zhao, Y. Reduction-sensitive fluorescence enhanced polymeric prodrug nanoparticles for combinational photothermal-chemotherapy. Biomaterials 2018, 163, 14–24. [Google Scholar] [CrossRef]
- You, Q.; Sun, Q.; Wang, J.; Tan, X.; Pang, X.; Liu, L.; Yu, M.; Tan, F.; Li, N. A single-light triggered and dual-imaging guided multifunctional platform for combined photothermal and photodynamic therapy based on TD-controlled and ICG-loaded CuS@ mSiO2. Nanoscale 2017, 9, 3784–3796. [Google Scholar] [CrossRef]
- Yang, R.; Hou, M.; Gao, Y.; Lu, S.; Zhang, L.; Xu, Z.; Li, C.M.; Kang, Y.; Xue, P. Biomineralization-inspired crystallization of manganese oxide on silk fibroin nanoparticles for in vivo Mr/fluorescence imaging-assisted tri-modal therapy of cancer. Theranostics 2019, 9, 6314–6333. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Liu, X.; Yan, H.; Xie, Z.; Sheng, Z.; Gong, X.; Wang, L.; Liu, X.; Zhang, P.; et al. Hybrid MoSe2–indocyanine green nanosheets as a highly efficient phototheranostic agent for photoacoustic imaging guided photothermal cancer therapy. Biomater. Sci. 2018, 6, 1503–1516. [Google Scholar] [CrossRef]
- Wen, L.; Hyoju, R.; Wang, P.; Shi, L.; Li, C.; Li, M.; Wang, X. Hydrogen-Peroxide-Responsive Protein Biomimetic Nanoparticles for Photothermal-Photodynamic Combination Therapy of Melanoma. Lasers Surg. Med. 2021, 53, 390–399. [Google Scholar] [CrossRef]
- Kil Song, C.; Lee, J.-H.; Jahn, A.; Choi, M.J.; Namgoong, S.K.; Hong, S.-S.; Chong, S.; Shim, C.-K.; Chung, S.-J.; Kim, D.-D. In vitro and in vivo evaluation of N,N,N-trimethylphytosphingosine-iodide (TMP) in liposomes for the treatment of angiogenesis and metastasis. Int. J. Pharm. 2012, 434, 191–198. [Google Scholar] [CrossRef]
- Qin, S.-Y.; Cheng, Y.-J.; Lei, Q.; Zhang, A.-Q.; Zhang, X.-Z. Combinational strategy for high-performance cancer chemotherapy. Biomaterials 2018, 171, 178–197. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, E.; Cui, Y.; Huang, Y. Nanotechnology-based combination therapy for overcoming multidrug-resistant cancer. Cancer Biol. Med. 2017, 14, 212–227. [Google Scholar] [CrossRef]
- Raaijmakers, M.I.G.; Widmer, D.S.; Narechania, A.; Eichhoff, O.; Freiberger, S.N.; Wenzina, J.; Cheng, P.F.; Mihic-Probst, D.; Desalle, R.; Dummer, R.; et al. Co-existence of BRAF and NRAS driver mutations in the same melanoma cells results in heterogeneity of targeted therapy resistance. Oncotarget 2016, 7, 77163–77174. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Vergani, E.; Vallacchi, V.; Frigerio, S.; Deho, P.; Mondellini, P.; Perego, P.; Cassinelli, G.; Lanzi, C.; Testi, M.A.; Rivoltini, L.; et al. Identification of MET and SRC activation in melanoma cell lines showing primary resistance to PLX4032. Neoplasia 2011, 13, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.; Infante, J.R.; Krepler, C.; Reyes-Uribe, P.; Samanta, M.; Chen, H.-Y.; Li, B.; Swoboda, R.K.; Wilson, M.; Vultur, A.; et al. Concurrent MEK2 mutation and BRAF amplification confer resistance to BRAF and MEK inhibitors in melanoma. Cell Rep. 2013, 4, 1090–1099. [Google Scholar] [CrossRef]
- Ablain, J.; Xu, M.; Rothschild, H.; Jordan, R.C.; Mito, J.K.; Daniels, B.H.; Bell, C.F.; Joseph, N.M.; Wu, H.; Bastian, B.C.; et al. Human tumor genomics and zebrafish modeling identify SPRED1 loss as a driver of mucosal melanoma. Science 2018, 362, 1055–1060. [Google Scholar] [CrossRef]
- Horn, S.; Leonardelli, S.; Sucker, A.; Schadendorf, D.; Griewank, K.G.; Paschen, A. Tumor CDKN2A-associated JAK2 loss and susceptibility to immunotherapy resistance. JNCI J. Natl. Cancer Inst. 2018, 110, 677–681. [Google Scholar] [CrossRef]
- Davis, E.; Teng, H.; Bilican, B.; Parker, I.M.; Liu, B.; Carriera, S.; Goding, C.R.; Prince, S. Ectopic Tbx2 expression results in polyploidy and cisplatin resistance. Oncogene 2008, 27, 976–984. [Google Scholar] [CrossRef]
- Pakneshan, S.; Salajegheh, A.; Smith, R.A.; Lam, A.K.-Y. Clinicopathological relevance of BRAF mutations in human cancer. Pathology 2013, 45, 346–356. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Margheri, F.; Laurenzana, A.; Fibbi, G.; Pimpinelli, N.; Calorini, L. Everolimus selectively targets vemurafenib resistant BRAFV600E melanoma cells adapted to low pH. Cancer Lett. 2017, 408, 43–54. [Google Scholar] [CrossRef]
- Walbrecq, G.; Lecha, O.; Gaigneaux, A.; Fougeras, M.R.; Philippidou, D.; Margue, C.; Nomigni, M.T.; Bernardin, F.; Dittmar, G.; Behrmann, I.; et al. Hypoxia-induced adaptations of miRNomes and proteomes in melanoma cells and their secreted extracellular vesicles. Cancers 2020, 12, 692. [Google Scholar] [CrossRef]
- Patel, D.; Gao, Y.; Son, K.; Siltanen, C.; Neve, R.M.; Ferrara, K.; Revzin, A. Microfluidic co-cultures with hydrogel-based ligand trap to study paracrine signals giving rise to cancer drug resistance. Lab A Chip 2015, 15, 4614–4624. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.J.; Saab, K.R.; Ma, J.; Schatton, T.; Pütz, P.; Zhan, Q.; Murphy, G.F.; Gasser, M.; Waaga-Gasser, A.M.; Frank, N.Y.; et al. ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit. Cancer Res. 2014, 74, 4196–4207. [Google Scholar] [CrossRef]
- Zou, L.; Ding, W.; Zhang, Y.; Cheng, S.; Li, F.; Ruan, R.; Wei, P.; Qiu, B. Peptide-modified vemurafenib-loaded liposomes for targeted inhibition of melanoma via the skin. Biomaterials 2018, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Clemente, N.; Ferrara, B.; Gigliotti, C.L.; Boggio, E.; Capucchio, M.T.; Biasibetti, E.; Schiffer, D.; Mellai, M.; Annovazzi, L.; Cangemi, L.; et al. Solid lipid nanoparticles carrying temozolomide for melanoma treatment. Preliminary in vitro and in vivo studies. Int. J. Mol. Sci. 2018, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; De, M.; Dey, G.; Bharti, R.; Chattopadhyay, S.; Ali, N.; Chakrabarti, P.; Reis, R.L.; Kundu, S.C.; Mandal, M. A peptide-modified solid lipid nanoparticle formulation of paclitaxel modulates immunity and outperforms dacarbazine in a murine melanoma model. Biomater. Sci. 2019, 7, 1161–1178. [Google Scholar] [CrossRef]
- Sen, R.; Ganguly, S.; Ganguly, S.; Debnath, M.C.; Chakraborty, S.; Mukherjee, B.; Chattopadhyay, D. Apigenin-Loaded PLGADMSA Nanoparticles: A Novel Strategy to Treat Melanoma Lung Metastasis. Mol. Pharm. 2021, 18, 1920–1938. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef]
- Fan, Y.; Moon, J.J. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines 2015, 3, 662–685. [Google Scholar] [CrossRef]
- Francis, D.M.; Thomas, S.N. Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy. Adv. Drug Deliv. Rev. 2017, 114, 33–42. [Google Scholar] [CrossRef]
- Lu, L.; Sun, Y.; Wan, C.; Hu, Y.; Lo, P.-C.; Lovell, J.F.; Yang, K.; Jin, H. Role of intravital imaging in nanomedicine-assisted anti-cancer therapy. Curr. Opin. Biotechnol. 2021, 69, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S. Cancer Nanomedicine: Lessons for Immuno-Oncology. Trends Cancer 2017, 3, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Malta, R.; Loureiro, J.B.; Costa, P.; Sousa, E.; Pinto, M.; Saraiva, L.; Amaral, M.H. Development of lipid nanoparticles containing the xanthone LEM2 for topical treatment of melanoma. J. Drug Deliv. Sci. Technol. 2021, 61, 102226. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Si, X.; Zhao, J.; Yu, H.; Ma, L.; Song, W.; Tang, Z. Polyethyleneimine-CpG Nanocomplex as an In Situ Vaccine for Boosting Anticancer Immunity in Melanoma. Macromol. Biosci. 2021, 21, e2000207. [Google Scholar] [CrossRef]
- Dosta, P.; Cryer, A.M.; Prado, M.; Dion, M.Z.; Ferber, S.; Kalash, S.; Artzi, N. Delivery of Stimulator of Interferon Genes (STING) Agonist Using Polypeptide-Modified Dendrimer Nanoparticles in the Treatment of Melanoma. Adv. NanoBiomed Res. 2021, 1, 2100006. [Google Scholar] [CrossRef]
- Xie, Y.-Q.; Arik, H.; Wei, L.; Zheng, Y.; Suh, H.; Irvine, D.J.; Tang, L. Redox-responsive interleukin-2 nanogel specifically and safely promotes the proliferation and memory precursor differentiation of tumor-reactive T-cells. Biomater. Sci. 2019, 7, 1345–1357. [Google Scholar] [CrossRef]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Roessner, M.; de Wit, R.; Eisenberger, M.; Tannock, I.F. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: Relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin. Cancer Res. 2008, 14, 2763–2767. [Google Scholar] [CrossRef]
- Kodumudi, K.N.; Woan, K.; Gilvary, D.L.; Sahakian, E.; Wei, S.; Djeu, J.Y. A novel chemoimmunomodulating property of docetaxel: Suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. 2010, 16, 4583–4594. [Google Scholar] [CrossRef]
- Eralp, Y.; Wang, X.; Wang, J.-P.; Maughan, M.F.; Polo, J.M.; Lachman, L.B. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res. 2004, 6, R275-83. [Google Scholar] [CrossRef]
- Haggerty, T.J.; Dunn, I.S.; Rose, L.B.; Newton, E.E.; Martin, S.; Riley, J.L.; Kurnick, J.T. Topoisomerase inhibitors modulate expression of melanocytic antigens and enhance T cell recognition of tumor cells. Cancer Immunol. Immunother. 2011, 60, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.W.; Garnett, C.T.; Farsaci, B.; Palena, C.; Tsang, K.; Ferrone, S.; Gameiro, S.R. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int. J. Cancer 2013, 133, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Lehe, C.; Barhoush, E.; Al-Romaih, K.; Tulbah, A.; Al-Alwan, M.; Hendrayani, S.-F.; Manogaran, P.; Alaiya, A.; Al-Tweigeri, T.; et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010, 12, R48. [Google Scholar] [CrossRef] [PubMed]
- Lesterhuis, W.J.; Punt, C.J.; Hato, S.V.; Eleveld-Trancikova, D.; Jansen, B.J.; Nierkens, S.; Schreibelt, G.; de Boer, A.; Van Herpen, C.M.; Kaanders, J.H.; et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J. Clin. Investig. 2011, 121, 3100–3108. [Google Scholar] [CrossRef]
- Lynch, T.J.; Bondarenko, I.; Luft, A.; Serwatowski, P.; Barlesi, F.; Chacko, R.; Sebastian, M.; Neal, J.; Lu, H.; Cuillerot, J.-M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non–small-cell lung cancer: Re-sults from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 2012, 30, 2046–2054. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Assudani, D.; Nagaraj, S.; Hunter, T.; Cho, H.-I.; Antonia, S.; Altiok, S.; Celis, E.; Gabrilovich, D.I. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer mmunotherapy in mice. J. Clin. Investig. 2010, 120, 1111–1124. [Google Scholar] [CrossRef]
- Smyth, M.J.; Ngiow, S.F.; Ribas, A.; Teng, M.W.L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016, 13, 143–158. [Google Scholar] [CrossRef]
- Middleton, G.; Silcocks, P.; Cox, T.; Valle, J.; Wadsley, J.; Propper, D.; Coxon, F.; Ross, P.; Madhusudan, S.; Roques, T.; et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014, 15, 829–840. [Google Scholar] [CrossRef]
- He, C.; Tang, Z.; Tian, H.; Chen, X. Co-delivery of chemotherapeutics and proteins for synergistic therapy. Adv. Drug Deliv. Rev. 2016, 98, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.; Rueda, F.; Löwik, C.; Ossendorp, F.; Cruz, L.J. Combinatorial prospects of nano-targeted chemoimmunotherapy. Biomaterials 2016, 83, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Meewan, J.; Somani, S.; Almowalad, J.; Laskar, P.; Mullin, M.; MacKenzie, G.; Khadke, S.; Perrie, Y.; Dufès, C. Preparation of zein-based nanoparticles: Nanoprecipitation versus microfluidic-assisted manufacture, effects of PEGylation on nanoparticle characteristics and cellular uptake by melanoma cells. Int. J. Nanomed. 2022, 17, 2809–2822. [Google Scholar] [CrossRef] [PubMed]
- Wahlich, J.; Desai, A.; Greco, F.; Hill, K.; Jones, A.T.; Mrsny, R.J.; Pasut, G.; Perrie, Y.; Seib, F.P.; Seymour, L.W. Nanomedicines for the Delivery of Biologics. Pharmaceutics 2019, 11, 210. [Google Scholar] [CrossRef]
- Neubert, R.H. Mechanisms of penetration and diffusion of drugs and cosmetic actives across the human Stratum corneum. Eur. J. Pharm. Biopharm. 2024, 202, 114394. [Google Scholar] [CrossRef]
- Adem, A.A.; Belete, A.; Lai, K.K.; Hage, C.; Neubert, R.H.; Gebre-Mariam, T. Nanoemulgel formulation for topical delivery of plant glucosylceramide: Characterization and optimization. J. Drug Deliv. Sci. Technol. 2023, 79, 104056. [Google Scholar] [CrossRef]
- Neubert, R.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Marwah, M.; Perrie, Y.; Badhan, R.K.S.; Lowry, D. Intracellular uptake of EGCG-loaded deformable controlled release liposomes for skin cancer. J. Liposome Res. 2020, 30, 136–149. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P. Temperature and pH stimuli-responsive polymers and their applications in controlled and self-regulated drug delivery. J. Appl. Pharm. Sci. 2012, 2, 01–10. [Google Scholar]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- Sundarraj, K.; Raghunath, A.; Perumal, E. A review on the chemotherapeutic potential of fisetin: In vitro evidences. Biomed. Pharmacother. 2018, 97, 928–940. [Google Scholar] [CrossRef]
- Jaudoin, C.; Gehrke, M.M.; Grillo, I.; Cousin, F.; Ouldali, M.; Arteni, A.-A.; Ferrary, E.; Siepmann, F.; Siepmann, J.; Simelière, F.; et al. Release of liposomes from hyaluronic acid-based hybrid systems: Effects of liposome surface and size. Int. J. Pharm. 2023, 648, 123560. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, characterization and applica-tions of liposomes: State of the art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Bawarski, W.E.; Chidlowsky, E.; Bharali, D.J.; Mousa, S.A. Emerging nanopharmaceuticals. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 273–282. [Google Scholar] [CrossRef]
- Mei, K.-C.; Liao, Y.-P.; Jiang, J.; Chiang, M.; Khazaieli, M.; Liu, X.; Wang, X.; Liu, Q.; Chang, C.H.; Zhang, X.; et al. Liposomal delivery of mitoxantrone and a cholesteryl indoximod prodrug provides effective chemo-immunotherapy in multiple solid tumors. ACS Nano 2020, 14, 13343–13366. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zhang, W.; Huang, H.; Xie, Y.; Zhang, Y.; Guo, X.; Jin, C.; Liao, X.; Yao, S.; Chen, G.; et al. Cancer targeted gene therapy for inhibition of melanoma lung metastasis with eiF3i shRNA loaded liposomes. Mol. Pharm. 2019, 17, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, Q.; Shi, Y.; Huang, Y.; Zhang, J.; Zhang, X.; Lin, G. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J. Control. Release 2018, 286, 369–380. [Google Scholar] [CrossRef]
- Reddy, T.L.; Garikapati, K.R.; Reddy, S.G.; Reddy, B.V.S.; Yadav, J.S.; Bhadra, U.; Bhadra, M.P. Simultaneous delivery of Paclitaxel and Bcl-2 siRNA via pH-Sensitive liposomal nanocarrier for the synergistic treatment of melanoma. Sci. Rep. 2016, 6, 35223. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Suh, H.; Irvine, D.J. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun. 2018, 9, 6. [Google Scholar] [CrossRef]
- Sinico, C.; Manconi, M.; Peppi, M.; Lai, F.; Valenti, D.; Fadda, A.M. Liposomes as carriers for dermal delivery of tretinoin: In vitro evaluation of drug permeation and vesicle–skin interaction. J. Control. Release 2005, 103, 123–136. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Tzanova, M.M.; Nguyen, L.; Moretti, F.; Grassi, M.; Magnano, G.C.; Voinovich, D.; Stein, P.C.; Hiorth, M.; di Cagno, M.P. Interpreting permeability as a function of free drug fraction: The case studies of cyclodextrins and liposomes. Eur. J. Pharm. Sci. 2023, 189, 106559. [Google Scholar] [CrossRef]
- Muller, R.H.; Runge, S.A. Solid lipid nanoparticles (SLN) for controlled drug delivery. In Submicron Emulsions N Drug Targeting and Delivery; CRC Press: Boca Raton, FL, USA, 1998; Volume 22, pp. 219–234. [Google Scholar]
- Estanqueiro, M.; Amaral, M.H.; Conceição, J.; Lobo, J.M.S. Nanotechnological carriers for cancer chemotherapy: The state of the art. Colloids Surfaces B Biointerfaces 2015, 126, 631–648. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Kasongo, W.; Pardeike, J.; Müller, R.H.; Walker, R.B. Selection and characterization of suitable lipid excipients for use in the manufacture of didanosine-loaded solid lipid nanoparticles and nanostructured lipid carriers. J. Pharm. Sci. 2011, 100, 5185–5196. [Google Scholar] [CrossRef]
- Souto, E.; Wissing, S.; Barbosa, C.; Müller, R. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef] [PubMed]
- Jores, K.; Mehnert, W.; Drechsler, M.; Bunjes, H.; Johann, C.; Mäder, K. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J. Control. Release 2004, 95, 217–227. [Google Scholar] [CrossRef]
- Jores, K.; Haberland, A.; Wartewig, S.; Mäder, K.; Mehnert, W. Solid lipid nanoparticles (SLN) and oil-loaded sln studied by spectrofluorometry and Raman spectroscopy. Pharm. Res. 2005, 22, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Mussi, S.V.; Torchilin, V.P. Recent trends in the use of lipidic nanoparticles as pharmaceutical carriers for cancer therapy and diagnostics. J. Mater. Chem. B 2013, 1, 5201–5209. [Google Scholar] [CrossRef]
- Lim, E.-K.; Jang, E.; Lee, K.; Haam, S.; Huh, Y.-M. Delivery of cancer therapeutics using nanotechnology. Pharmaceutics 2013, 5, 294–317. [Google Scholar] [CrossRef]
- Salehzadeh, R.; Abdullah, R. Solid lipid nanoparticles as new drug delivery system. Int. J. Biotechnol. Mol. Biol. Res. 2011, 2, 252–261. [Google Scholar]
- Hanafy, M.S.; Hufnagel, S.; Trementozzi, A.N.; Sakran, W.; Stachowiak, J.C.; Koleng, J.J.; Cui, Z. PD-1 siRNA-Encapsulated Solid Lipid Nanoparticles Downregulate PD-1 Expression by Macrophages and Inhibit Tumor Growth. AAPS PharmSciTech 2021, 22, 60. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Wang, L.; Liu, W.; Xiao, L.; Lin, Q.; Gong, T.; Sun, X.; He, Q.; Zhang, Z.; et al. Improved melanoma suppression with target-delivered TRAIL and Paclitaxel by a multifunctional nanocarrier. J. Control. Release 2020, 325, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Jaromin, A.; Piwoni, A.; Mahmud, M.; Sarisozen, C.; Torchilin, V.; Gubernator, J. A Triple Co-Delivery Liposomal Carrier That Enhances Apoptosis via an Intrinsic Pathway in Melanoma Cells. Cancers 2019, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Teeranachaideekul, V.; Souto, E.B.; Junyaprasert, V.B.; Müller, R.H. Cetyl palmitate-based NLC for topical delivery of Coen-zyme Q10–Development, physicochemical characterization and in vitro release studies. Eur. J. Pharm. Biopharm. 2007, 67, 141–148. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.; Lobo, J.S.; Silva, A. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Silva, A.C.; Loboa, J.M.S. Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: Present and future considerations. J. Pharm. Pharm. Sci. 2014, 17, 278–293. [Google Scholar] [CrossRef]
- Cunha, S.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Lipid nanoparticles for nasal/intranasal drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 257–282. [Google Scholar] [CrossRef]
- Tichota, D.M.; Silva, A.C.; Lobo, J.M.S.; Amaral, M.H. Design, characterization, and clinical evaluation of argan oil nanostruc-tured lipid carriers to improve skin hydration. Int. J. Nanomed. 2014, 9, 3855. [Google Scholar]
- Kurakula, M.; Chen, L.; Tiwari, A.K.; Srinivas, N.R.; Dash, R.P.; Panizzi, P.R.; Arnold, R.D.; Babu, R.J. Recent Advances in Li-pid-Based Nanovesicular Delivery Systems for Melanoma Therapy. Crit. Rev. Ther. Drug Carr. Syst. 2021, 38, 1–38. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, W.; Zhu, Q.; Burns, N.A.; Khan, S.A.; Mo, R.; Gu, Z. Furin-mediated sequential delivery of anticancer cytokine and small-molecule drug shuttled by graphene. Adv. Mater. 2015, 27, 1021–1028. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Peng, B. Chitosan-capped mesoporous silica nanoparticles as pH-responsive nanocarriers for controlled drug release. Chem.—Asian J. 2014, 9, 319–327. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Zheng, D.-W.; Chen, J.-L.; Zhu, J.-Y.; Rong, L.; Li, B.; Lei, Q.; Fan, J.-X.; Zou, M.-Z.; Li, C.; Cheng, S.-X.; et al. Highly integrated nano-platform for breaking the barrier between chemotherapy and immunotherapy. Nano Lett. 2016, 16, 4341–4347. [Google Scholar] [CrossRef]
- Ou, W.; Byeon, J.H.; Thapa, R.K.; Ku, S.K.; Yong, C.S.; Kim, J.O. Plug-and-Play nanorization of coarse black phosphorus for targeted chemo-photoimmunotherapy of colorectal cancer. ACS Nano 2018, 12, 10061–10074. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ye, X.; Wang, C.; Xing, C.; Miao, Q.; Xie, Z.; Chen, X.; Zhang, X.; Zhang, H.; Mei, L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control. Release 2019, 296, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, L.; Zhang, Y.; Xin, X.; Qi, L.; Jin, M.; Guan, Y.; Gao, Z.; Huang, W. Enhancing anti-melanoma outcomes in mice using novel chitooligosaccharide nanoparticles loaded with therapeutic survivin-targeted siRNA. Eur. J. Pharm. Sci. 2021, 158, 105641. [Google Scholar] [CrossRef]
- Li, B.; Lane, L.A. Probing the biological obstacles of nanomedicine with gold nanoparticles. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1542. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.B.R.; Choi, J.H.; Hong, J.W.; Song, K.W.; Lee, H.J.; Kim, U.K.; Kim, G.C. Selective killing of melanoma cells with non-thermal atmospheric pressure plasma and p-FAK antibody conjugated gold nanoparticles. Int. J. Med Sci. 2017, 14, 1101–1109. [Google Scholar] [CrossRef]
- Lomelí-Marroquín, D.; Cruz, D.M.; Nieto-Argüello, A.; Crua, A.V.; Chen, J.; Torres-Castro, A.; Webster, T.J.; Cholula-Díaz, J.L. Starch-mediated synthesis of mono- and bimetallic silver/gold nanoparticles as antimicrobial and anticancer agents. Int. J. Nanomed. 2019, 14, 2171–2190. [Google Scholar] [CrossRef]
- Zhang, X.; Teodoro, J.G.; Nadeau, J.L. Intratumoral gold-doxorubicin is effective in treating melanoma in mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1365–1375. [Google Scholar] [CrossRef]
- Tawagi, E.; Massmann, C.; Chibli, H.; Nadeau, J.L. Differential toxicity of gold-doxorubicin in cancer cells vs. cardiomyocytes as measured by real-time growth assays and fluorescence lifetime imaging microscopy (FLIM). Analyst 2015, 140, 5732–5741. [Google Scholar] [CrossRef]
- Labala, S.; Jose, A.; Chawla, S.R.; Khan, M.S.; Bhatnagar, S.; Kulkarni, O.P.; Venuganti, V.V.K. Effective melanoma cancer suppression by iontophoretic co-delivery of STAT3 siRNA and imatinib using gold nanoparticles. Int. J. Pharm. 2017, 525, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Labala, S.; Jose, A.; Venuganti, V.V.K. Transcutaneous iontophoretic delivery of STAT3 siRNA using layer-by-layer chitosan coated gold nanoparticles to treat melanoma. Colloids Surfaces B Biointerfaces 2016, 146, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Lin, C.-C.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef]
- Camerin, M.; Moreno, M.; Marín, M.J.; Schofield, C.L.; Chambrier, I.; Cook, M.J.; Coppellotti, O.; Jori, G.; Russell, D.A. Delivery of a hydrophobic phthalocyanine photosensitizer using PEGylated gold nanoparticle conjugates for the in vivo photodynamic therapy of amelanotic melanoma. Photochem. Photobiol. Sci. 2016, 15, 618–625. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Sazgarnia, A.; Rajabi, O.; Toosi, M.S. Comparative study of X-ray treatment and photodynamic therapy by using 5-aminolevulinic acid conjugated gold nanoparticles in a melanoma cell line. Artif. Cells Nanomed. Biotechnol. 2017, 45, 467–473. [Google Scholar] [CrossRef]
- Le Goas, M.; Paquet, M.; Paquirissamy, A.; Guglielmi, J.; Compin, C.; Thariat, J.; Vassaux, G.; Geertsen, V.; Humbert, O.; Renault, J.-P.; et al. Improving 131I radioiodine therapy by hybrid polymer-grafted gold nanoparticles. Int. J. Nanomed. 2019, 14, 7933–7946. [Google Scholar] [CrossRef]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer drug delivery in the nano era: An overview and perspectives. Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Vlasceanu, G.M.; Victor, L.; Maricica, H.; Raluca, T.; Vlad, O.; Gheorghe, I.; Bolocan, A.; Grumezescu, A.M.; Holban, A.M. Nanostructures for cancer therapy: From targeting to selective toxicology. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 831–847. [Google Scholar]
- O’REilly, R.K.; Hawker, C.J.; Wooley, K.L. Cross-linked block copolymer micelles: Functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006, 35, 1068–1083. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly (ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, H.; Kjøniksen, A.-L.; Hiorth, M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.R.; Cruz, L.; Mezzalira, G.; Soares, L.U.; Da Silveira, N.P.; Guterres, S.S. Structural model of polymeric nanospheres containing indomethacin ethyl ester and in vivo antiedematogenic activity. Int. J. Nanotechnol. 2007, 4, 454. [Google Scholar] [CrossRef]
- da Fonseca, L.S.; Silveira, R.P.; Deboni, A.M.; Benvenutti, E.V.; Costa, T.M.; Guterres, S.S.; Pohlmann, A.R. Nanocapsule@ xerogel microparticles containing sodium diclofenac: A new strategy to control the release of drugs. Int. J. Pharm. 2008, 358, 292–295. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int. J. Biol. Macromol. 2019, 125, 700–710. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Escrivani, O.D.; Lopes, M.V.; Poletto, F.; Ferrarini, S.R.; Sousa-Batista, A.J.; Steel, P.G.; Guterres, S.S.; Pohlmann, A.R.; Rossi-Bergmann, B. Encapsulation in lipid-core nanocapsules improves topical treatment with the potent antileishmanial compound CH8. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102121. [Google Scholar] [CrossRef]
- Jäger, A.; Stefani, V.; Guterres, S.S.; Pohlmann, A.R. Physico-chemical characterization of nanocapsule polymeric wall using fluorescent benzazole probes. Int. J. Pharm. 2007, 338, 297–305. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.C.; Tomoda, K.; Kwon, G.S. Polymeric micelles for multi-drug delivery in cancer. AAPS PharmSciTech 2015, 16, 10–20. [Google Scholar] [CrossRef]
- Kumar, R.; Kulkarni, A.; Nagesha, D.K.; Sridhar, S. In vitro evaluation of theranostic polymeric micelles for imaging and drug delivery in cancer. Theranostics 2012, 2, 714–722. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.; Rapoport, N. Doxorubicin as a molecular nanotheranostic agent: Effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S. Self-assembled polymeric nanocarrier-mediated co-delivery of metformin and doxorubicin for melanoma therapy. Drug Deliv. 2021, 28, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Geronimo, G.; da Silva, G.H.R.; de Moura, L.D.; Ribeiro, L.N.; Guilherme, V.A.; Mendonça, T.C.; Castro, S.R.; Breitkreitz, M.C.; de Paula, E. Development of S 75: R 25 bupivacaine-loaded lipid nanoparticles functionalized with essential oils for treating melanoma. J. Chem. Technol. Biotechnol. 2021, 96, 8. [Google Scholar]
- Herai, H.; Gratieri, T.; Thomazine, J.A.; Bentley, M.V.L.B.; Lopez, R.F.V. Doxorubicin skin penetration from monoolein-containing propylene glycol formulations. Int. J. Pharm. 2007, 329, 88–93. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef]
- Luu, Y.; Kim, K.; Hsiao, B.; Chu, B.; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef]
- Su, Z.; Xiao, Z.; Wang, Y.; Huang, J.; An, Y.; Wang, X.; Shuai, X. Codelivery of anti-PD-1 antibody and paclitaxel with matrix metalloproteinase and pH dual-sensitive micelles for enhanced tumor chemoimmunotherapy. Small 2020, 16, e1906832. [Google Scholar] [CrossRef]
- McCoubrey, L.E.; Ferraro, F.; Seegobin, N.; Verin, J.; Alfassam, H.A.; Awad, A.; Marzorati, M.; Verstrepen, L.; Ghyselinck, J.; De Munck, J.; et al. Poly(D,l-lactide-co-glycolide) particles are metabolised by the gut microbiome and elevate short chain fatty acids. J. Control. Release 2024, 369, 163–178. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Stability of aqueous polymeric controlled release film coatings. Int. J. Pharm. 2013, 457, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- STripathy; Das, M.K. Dendrimers and their applications as novel drug delivery carriers. J. Appl. Pharm. Sci. 2013, 3, 142–149. [Google Scholar]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Degim, I.T.; Burgess, D.J.; Papadimitrakopoulos, F. Carbon nanotubes for transdermal drug delivery. J. Microencapsul. 2010, 27, 669–681. [Google Scholar] [CrossRef]
- Siu, K.S.; Chen, D.; Zheng, X.; Zhang, X.; Johnston, N.; Liu, Y.; Yuan, K.; Koropatnick, J.; Gillies, E.R.; Min, W.-P. Non-covalently functionalized single-walled carbon nanotube for topical siRNA delivery into melanoma. Biomaterials 2014, 35, 3435–3442. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Khan, I.; Shukla, S.; Kang, S.-M.; Aziz, F.; Tripathi, K.M.; Saini, D.; Cho, H.-J.; Heo, N.S.; Sonkar, S.K. Multifunctional NP-doped carbon dots for regulation of apoptosis and autophagy in B16F10 melanoma cancer cells and in vitro imaging applications. Theranostics 2020, 10, 7841. [Google Scholar] [CrossRef]
- Manca, M.L.; Manconi, M.; Nacher, A.; Carbone, C.; Valenti, D.; Maccioni, A.M.; Sinico, C.; Fadda, A.M. Development of novel diolein–niosomes for cutaneous delivery of tretinoin: Influence of formulation and in vitro assessment. Int. J. Pharm. 2014, 477, 176–186. [Google Scholar] [CrossRef]
- Manconi, M.; Sinico, C.; Valenti, D.; Lai, F.; Fadda, A.M. Niosomes as carriers for tretinoin: III. A study nto the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int. J. Pharm. 2006, 311, 11–19. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Y.; Ye, H.; Yu, S.; He, C.; Chen, X. Interleukin-15 and cisplatin co-encapsulated thermosensitive polypeptide hydrogels for combined immuno-chemotherapy. J. Control. Release 2017, 255, 81–93. [Google Scholar] [CrossRef]
- Dong, X.; Yang, A.; Bai, Y.; Kong, D.; Lv, F. Dual fluorescence imaging-guided programmed delivery of doxorubicin and CpG nanoparticles to modulate tumor microenvironment for effective chemo-immunotherapy. Biomaterials 2020, 230, 119659. [Google Scholar] [CrossRef]
- Li, Y.; Fang, M.; Zhang, J.; Wang, J.; Song, Y.; Shi, J.; Li, W.; Wu, G.; Ren, J.; Wang, Z.; et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. OncoImmunology 2016, 5, e1074374. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Chen, X.; Chen, E.; Zhang, J.; Huang, D.; Yang, D.; Ding, Y.; Qian, H.; Feijen, J.; Chen, W. Folated pH-degradable nanogels for the simultaneous delivery of docetaxel and an IDO1-inhibitor in enhancing cancer chemo-immunotherapy. Biomater. Sci. 2019, 7, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Lefol, L.; Bawuah, P.; Zeitler, J.; Verin, J.; Danede, F.; Willart, J.; Siepmann, F.; Siepmann, J. Drug release from PLGA microparticles can be slowed down by a surrounding hydrogel. Int. J. Pharm. X 2023, 6, 100220. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; van der Lugt, S.A.; Annala, A.; Di Marco, G.; Sampon, T.; Siepmann, J.; Siepmann, F.; Hennink, W.E.; Vermonden, T. Thermo-responsive Diels-Alder stabilized hydrogels for ocular drug delivery of a corticosteroid and an anti-VEGF fab fragment. J. Control. Release 2023, 361, 334–349. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; He, R.; Xu, D.; Zang, J.; Weeranoppanant, N.; Dong, H.; Li, Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2020, 8, 552–568. [Google Scholar] [CrossRef]
- Molinaro, R.; Martinez, J.O.; Zinger, A.; De Vita, A.; Storci, G.; Arrighetti, N.; De Rosa, E.; Hartman, K.A.; Basu, N.; Taghipour, N.; et al. Leukocyte-mimicking nanovesicles for effective doxorubicin delivery to treat breast cancer and melanoma. Biomater. Sci. 2020, 8, 333–341. [Google Scholar] [CrossRef]
- Luo, K.; Lian, Y.; Zhang, M.; Yu, H.; Wang, G.; Li, J. Charge convertible biomimetic micellar nanoparticles for enhanced melanoma-targeted therapy through tumor cells and tumor-associated macrophages dual chemotherapy with IDO immunotherapy. Chem. Eng. J. 2021, 412, 128659. [Google Scholar] [CrossRef]
- Gorgizadeh, M.; Azarpira, N.; Veis, R.D.; Sattarahmady, N. Repression of melanoma tumor in vitro and in vivo by photothermal effect of carbon xerogel nanoparticles. Colloids Surfaces B Biointerfaces 2019, 176, 449–455. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Turker, M.Z.; Ma, K.; Zanzonico, P.; Gallazzi, F.; Shah, M.A.; Prater, A.R.; Wiesner, U.; Bradbury, M.S.; et al. Targeted melanoma radiotherapy using ultrasmall 177Lu-labeled α-melanocyte stimulating hormone-functionalized core-shell silica nanoparticles. Biomaterials 2020, 241, 119858. [Google Scholar] [CrossRef]
- Xue, X.; Li, J.; Fan, Y.; Shen, M.; Shi, X. Gene silencing-mediated immune checkpoint blockade for tumor therapy boosted by dendrimer-entrapped gold nanoparticles. Sci. China Mater. 2021, 64, 2045–2055. [Google Scholar] [CrossRef]
- Han, S.; Wang, W.; Wang, S.; Wang, S.; Ju, R.; Pan, Z.; Yang, T.; Zhang, G.; Wang, H.; Wang, L. Multifunctional biomimetic nanoparticles loading baicalin for polarizing tumor-associated macrophages. Nanoscale 2019, 11, 20206–20220. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/search?cond=Melanoma,%20Skin&term=nanoparticles (accessed on 8 August 2025).
- Moni, S.S.; Moshi, J.M.; Matou-Nasri, S.; Alotaibi, S.; Hawsawi, Y.M.; Elmobark, M.E.; Hakami, A.M.S.; Jeraiby, M.A.; Sulayli, A.A.; Moafa, H.N. Advances in Materials Science for Precision Melanoma Therapy: Nanotechnology-Enhanced Drug Delivery Systems. Pharmaceutics 2025, 17, 296. [Google Scholar] [CrossRef]


| Title | Status | Study Results | Conditions | Interventions | Outcome Measures | Phase |
|---|---|---|---|---|---|---|
| Sunscreen Based on Bioadhesive Nanoparticles | Completed | No Results Available | Melanoma and UV Ray Skin Damage | Drug: standard sunscreen (padimate O (7%) and oxybenzone (3%)) Device: bioadesive nanoparticles | Skin reaction | Phase 1 (13 enrolled) |
| Novel RNA-Nanoparticle Vaccine for the Treatment of Early Melanoma Recurrence Following Adjuvant Anti-PD-1 Antibody Therapy | Suspended | No Results Available | Melanoma | Biological: Autologous total tumor mRNA-loaded DOTAP liposome vaccine | Skin reaction | Phase 1 (18 enrolled) |
| Targeted Silica Nanoparticles for Real-Time Image-Guided Intraoperative Mapping of Nodal Metastases | Active, not recruiting | No Results Available | Head and Neck Melanoma | Drug: fluorescent dye-labeled particle (cRGDY-PEG-Cy5.5-C dots) | Feasibility of conducting pre-operative SLN mapping | Phase 1 Phase 2 (86 enrolled) |
| Bevacizumab and Temozolomide or Bevacizumab and Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin in Treating Patients With Stage IV Malignant Melanoma That Cannot Be Removed by Surgery | Completed | Has Results | Melanoma (Skin) | Biological: bevacizumab| Drug: carboplatin Drug: paclitaxel albumin-stabilized nanoparticle formulation Drug: temozolomide | Progression-free survival at 6 months; tumor response rate, calculated as a percentage along With its 95% confidence interval; overall survival | Phase 2 (95 enrolled) |
| Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Metastatic Melanoma of the Eye That Cannot Be Removed By Surgery | Completed | Has Results | Intraocular Melanoma | Drug: nab-paclitaxel (paclitaxel albumin-stabilized nanoparticle formulation) | Overall response rate; progression-free survival; overall survival | Phase 2 (4 enrolled) |
| ABI-007 in Treating Patients With Inoperable Locally Recurrent or Metastatic Melanoma | Completed | No Results Available | Melanoma (Skin) | Drug: paclitaxel albumin-stabilized nanoparticle formulation | Not included | Phase 2 |
| Carboplatin and ABI-007 in Treating Patients With Stage IV Melanoma That Cannot Be Removed By Surgery | Completed | Has Results | Melanoma (Skin) | Drug: carboplatin Drug: paclitaxel albumin-stabilized nanoparticle formulation | Tumor response sate, as measured by RECIST criteria; survival time; time to disease progression; duration of response; number of treatment cycles administered | Phase 2 (76 enrolled) |
| MR Lymphography and Magnetic Sentinel Lymph Node Biopsy in Melanoma Patients Measured With DiffMag | Unknown status | No Results Available | Melanoma (Skin) | Device: magnetic (super paramagnetic iron oxide (SPIO) nanoparticles) sentinel lymph node (SLN) biopsy by means of Magtrace®, in combination with SentiMag® and DiffMag | True positive/false negative rate for magnetic SLN detection measured via the DiffMag system and the Sentimag system compared to radioactive detection. True positive/false negative rate for metastatic SLN using ex vivo MRI. True positive/false negative rate for metastatic SLN using in vivo MRI. | Not Applicable (20 enrolled) |
| Stereotactic Brain-Directed Radiation With or Without Aguix Gadolinium-Based Nanoparticles in Brain Metastases | Recruiting | No Results Available | Brain Cancer, Brain Metastases, Melanoma, Lung Cancer, Breast Cancer, HER2-positive Breast Cancer, Colorectal Cancer, Gastrointestinal Cancer, SRS, SRT, Whole-Brain Radiation, Stereotactic Radiation, AGuIX Nanoparticles, Cystic Brain Tumor | Radiation: stereotactic radiation Drug: AGuIX gadolinium-based nanoparticles Other: placebo | Key clinical endpoints include local recurrence, overall survival (OS), progression-free survival (PFS), time to progression (TTP), neurologic-related death, performance status, daily living activities, and incidence/timing of new brain metastases, radiation necrosis, leptomeningeal disease, intracranial progression, salvage craniotomy, additional radiotherapy, seizures, and steroid use. Neurocognitive functions assessed cover verbal learning and memory, visual attention and task switching, verbal fluency, and overall cognitive impairment. | Phase 2 (112 enrolled) |
| Nab-Paclitaxel and Bevacizumab or Ipilimumab as First-Line Therapy in Treating Patients With Stage IV Melanoma That Cannot Be Removed by Surgery | Completed | Has Results | Metastatic Melanoma, Mucosal Melanoma, Stage IV Cutaneous Melanoma AJCC v6 and v7, Stage IV Uveal Melanoma AJCC v7, Unresectable Melanoma | Biological: bevacizumab Biological: ipilimumab Other: laboratory biomarker analysis Drug: nab-paclitaxel (paclitaxel albumin-stabilized nanoparticle formulation) | Progression-free survival (PFS); overall survival (OS); number of patients with tumor response; number of patients who experienced toxicity | Phase 2 (24 enrolled) |
| A Study of BIND-014 Given to Patients With Advanced or Metastatic Cancer | Completed | No Results Available | Metastatic Cancer, Cancer, Solid Tumors | Drug: BIND-014 (docetaxel nanoparticles for injectable suspension) | To assess the dose-limiting toxicities (DLTs) of BIND-014 when given on day 1 of a 21-day cycle or when given on day 1, 8, and 15 of a 28-day cycle. To characterize the pharmacokinetics of BIND-014 following an IV infusion. To assess any preliminary evidence of antitumor activity observed with BIND-014. To assess changes in serum tumor markers when appropriate. | Phase 1 (58 enrolled) |
| Dose Escalation Study of mRNA-2752 for Intratumoral Injection to Participants With Advanced Malignancies | Active, not recruiting | No Results Available | Dose Escalation: Relapsed, Refractory Solid Tumor Malignancies, or Lymphoma Dose Expansion: Triple-Negative Breast Cancer, HNSCC, Non-Hodgkins, Urothelial Cancer, Immune Checkpoint Refractory Melanoma, and NSCLC Lymphoma | Biological: mRNA-2752 Biological: durvalumab Device: lipid nanoparticle | Number of participants with dose-limiting toxicities (DLTs). Number of participants with adverse events (AEs). Arm B: overall response rate (ORR): percentage of participants with tumor response (partial or complete) based on response evaluation criteria in solid tumors version 1.1 in cutaneous melanoma. ORR: percentage of participants with tumor response (partial or complete) based on RECIST v1.1 and modified RECIST (iRECIST), and Cheson and lymphoma response to immunomodulatory therapy criteria (LYRIC) for participants with lymphoma. Pharmacokinetics: maximum observed concentration (Cmax) | Phase I (264 enrolled) |
| Nab-Paclitaxel and Bevacizumab in Treating Patients With Unresectable Stage IV Melanoma or Gynecological Cancers | Suspended | No Results Available | Stage IV Unresectable Melanoma, Cancer of the Cervix, Endometrium, Ovary, Fallopian Tube, or Peritoneal Cavity | Biological: bevacizumab laboratory biomarker analysis Drug: nab-paclitaxel (paclitaxel albumin-stabilized nanoparticle formulation) Pharmacological study | Maximum tolerated dose; tumor response; progression-free survival (PFS); overall survival (OS); incidence of adverse events (soft tissue expansion cohort) | Phase 1 (73 enrolled) |
| Safety Study of CALAA-01 to Treat Solid Tumor Cancers | Terminated | No Results Available | Cancer, Solid Tumor | Drug: CALAA-01 (a small interfering RNA (siRNA)) protected from nuclease degradation within a stabilized nanoparticle targeted to tumor cells | Objectives include assessing the tolerability, safety, and maximum tolerated dose (MTD) of intravenous CALAA-01; characterizing its pharmacokinetics (PK); evaluating preliminary tumor response for efficacy; recommending a dose for future studies; and measuring immune response by antibody, cytokine levels, and complement effects. | Phase 1 (24 enrolled) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sias-Fonseca, L.; Costa, P.C.; Saraiva, L.; Alves, A.; Amaral, M.H. Promising Nanotechnology-Based Strategies for Melanoma Treatment. Colloids Interfaces 2025, 9, 53. https://doi.org/10.3390/colloids9040053
Sias-Fonseca L, Costa PC, Saraiva L, Alves A, Amaral MH. Promising Nanotechnology-Based Strategies for Melanoma Treatment. Colloids and Interfaces. 2025; 9(4):53. https://doi.org/10.3390/colloids9040053
Chicago/Turabian StyleSias-Fonseca, Letícia, Paulo C. Costa, Lucília Saraiva, Ana Alves, and Maria Helena Amaral. 2025. "Promising Nanotechnology-Based Strategies for Melanoma Treatment" Colloids and Interfaces 9, no. 4: 53. https://doi.org/10.3390/colloids9040053
APA StyleSias-Fonseca, L., Costa, P. C., Saraiva, L., Alves, A., & Amaral, M. H. (2025). Promising Nanotechnology-Based Strategies for Melanoma Treatment. Colloids and Interfaces, 9(4), 53. https://doi.org/10.3390/colloids9040053








