Composition-Dependent Optical Behavior of SnS1−xSex Nanosheet Arrays Films
Abstract
1. Introduction
2. Experimental Methods
2.1. Sample Preparation
2.2. Structural Characterization and Performance Testing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamal, F.; Rafique, A.; Moeen, S.; Haider, J.; Nabgan, W.; Haider, A.; Imran, M.; Nazir, G.; Alhassan, M.; Ikram, M.; et al. Review of Metal Sulfide Nanostructures and their Applications. ACS Appl. Nano Mater. 2023, 6, 7077–7106. [Google Scholar] [CrossRef]
- He, H.Y.; Lu, J.; Cao, L.Y.; Li, M. Photodegradation of methyl orange from wastewater on TiO2/SnS combined powders. Res. Chem. Intermed. 2012, 38, 537–547. [Google Scholar] [CrossRef]
- Sarkar, A.S.; Mushtaq, A.; Kushavah, D.; Pal, S.K. Liquid exfoliation of electronic grade ultrathin tin(II) sulfide (SnS) with intriguing optical response. npj 2D Mater. Appl. 2020, 4, 1. [Google Scholar] [CrossRef]
- Abrikosov, N.K.; Bankina, V.F.; Poretskaya, L.V.; Shelimova, L.E.; Skudnova, E.V. (Eds.) AIVBVI Compounds. In Semiconducting II–VI, IV–VI, and V–VI Compounds; Springer: New York, NY, USA, 1969; pp. 65–157. [Google Scholar]
- Kumar, G.M.; Fu, X.; Ilanchezhiyan, P.; Yuldashev, S.U.; Lee, D.J.; Cho, H.D.; Kang, T.W. Highly Sensitive Flexible Photodetectors Based on Self-Assembled Tin Monosulfide Nanoflakes with Graphene Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 32142–32150. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Zhao, C.J.; Du, B.S.; Wu, R.; Lai, X.F.; He, Y.; Jian, J.K. SnSe nanosheet arrays film for trace NO2 detection at room temperature. Sens. Actuators B Chem. 2022, 370, 132407. [Google Scholar] [CrossRef]
- Yang, W.L.; Chen, Y.C.; Yin, X.X.; Lai, X.F.; Wang, J.; Jian, J.K. SnSe Nanosheet Array on Carbon Cloth as a High-Capacity Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 42811–42822. [Google Scholar] [CrossRef]
- Liu, S.; Bai, S.; Wen, Y.; Lou, J.; Jiang, Y.; Zhu, Y.; Liu, D.; Li, Y.; Shi, H.; Liu, S.; et al. Quadruple-band synglisis enables high thermoelectric efficiency in earth-abundant tin sulfide crystals. Science 2025, 387, 202–208. [Google Scholar] [CrossRef]
- Chang, C.; Wu, M.H.; He, D.S.; Pei, Y.L.; Wu, C.F.; Wu, X.F.; Yu, H.L.; Zhu, F.Y.; Wang, K.D.; Chen, Y.; et al. 3D charge and 2D phonon transports leading to high out-of-plane ZT in n-type SnSe crystals. Science 2018, 360, 778–782. [Google Scholar] [CrossRef]
- Guo, J.; Jian, J.K.; Liu, J.; Cao, B.L.; Lei, R.B.; Zhang, Z.H.; Song, B.; Zhao, H.Z. Synthesis of SnSe nanobelts and the enhanced thermoelectric performance in its hot-pressed bulk composite. Nano Energy 2017, 38, 569–575. [Google Scholar] [CrossRef]
- Wang, W.H.; Zhang, T.; Seliverstov, A.; Zhang, H.H.; Wang, Y.C.; Wang, F.Z.; Peng, X.L.; Lu, Q.Q.; Qin, C.; Pan, X.H.; et al. Layer-Dependent Optoelectronic Properties of 2D van der Waals SnS Grown by Pulsed Laser Deposition. Adv. Electron. Mater. 2020, 6, 1901020. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.L.; Shao, Y.C.; Deng, B.C.; Guo, Q.S.; Ma, C.; Xia, F.N. Valley-Selective Linear Dichroism in Layered Tin Sulfide. ACS Photonics 2018, 5, 3814–3819. [Google Scholar] [CrossRef]
- Xie, Z.J.; Zhang, F.; Liang, Z.M.; Fan, T.J.; Li, Z.J.; Jiang, X.T.; Chen, H.; Li, J.Q.; Zhang, H. Revealing of the ultrafast third-order nonlinear optical response and enabled photonic application in two-dimensional tin sulfide. Photonics Res. 2019, 7, 494–502. [Google Scholar] [CrossRef]
- Zhang, H.D.; Balaji, Y.; Mehta, A.N.; Heyns, M.; Caymax, M.; Radu, I.; Vandervorst, W.; Delabie, A. Formation mechanism of 2D SnS2 and SnS by chemical vapor deposition using SnCl4 and H2S. J. Mater. Chem. C 2018, 6, 6172–6178. [Google Scholar] [CrossRef]
- Xia, J.; Li, X.Z.; Huang, X.; Mao, N.N.; Zhu, D.D.; Wang, L.; Xu, H.; Meng, X.M. Physical vapor deposition synthesis of two-dimensional orthorhombic SnS flakes with strong angle/temperature-dependent Raman responses. Nanoscale 2016, 8, 2063–2070. [Google Scholar] [CrossRef]
- Li, F.; Ramin Moayed, M.M.; Klein, E.; Lesyuk, R.; Klinke, C. In-Plane Anisotropic Faceting of Ultralarge and Thin Single-Crystalline Colloidal SnS Nanosheets. J. Phys. Chem. Lett. 2019, 10, 993–999. [Google Scholar] [CrossRef]
- Higashitarumizu, N.; Kawamoto, H.; Nakamura, M.; Shimamura, K.; Ohashi, N.; Ueno, K.; Nagashio, K. Self-passivated ultra-thin SnS layers via mechanical exfoliation and post-oxidation. Nanoscale 2018, 10, 22474–22483. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Xie, Z.J.; Fan, T.J.; Li, J.G.; Wang, Y.Z.; Wu, L.M.; Ma, D.T.; Li, Z.J.; Ge, Y.Q.; Huang, Z.N.; et al. Black-phosphorus-analogue tin monosulfide: An emerging optoelectronic two-dimensional material for high-performance photodetection with improved stability under ambient/harsh conditions. J. Mater. Chem. C 2018, 6, 9582–9593. [Google Scholar] [CrossRef]
- Li, Q.; Wei, A.X.; Lu, J.T.; Tao, L.L.; Yang, Y.B.; Luo, D.X.; Liu, J.; Xiao, Y.; Zhao, Y.; Li, J.B. Synthesis of Submillimeter-Scale Single Crystal Stannous Sulfide Nanoplates for Visible and Near-Infrared Photodetectors with Ultrahigh Responsivity. Adv. Electron. Mater. 2018, 4, 1800154. [Google Scholar] [CrossRef]
- Xin, C.; Zheng, J.X.; Su, Y.T.; Li, S.K.; Zhang, B.K.; Feng, Y.C.; Pan, F. Few-Layer Tin Sulfide: A New Black-Phosphorus-Analogue 2D Material with a Sizeable Band Gap, Odd-Even Quantum Confinement Effect, and High Carrier Mobility. J. Phys. Chem. C 2016, 120, 22663–22669. [Google Scholar] [CrossRef]
- Gomes, L.C.; Carvalho, A. Phosphorene analogues: Isoelectronic two-dimensional group-IV monochalcogenides with orthorhombic structure. Phys. Rev. B 2015, 92, 085406. [Google Scholar] [CrossRef]
- Khan, H.; Mahmood, N.; Zavabeti, A.; Elbourne, A.; Rahman, M.A.; Zhang, B.Y.; Krishnamurthi, V.; Atkin, P.; Ghasemian, M.B.; Yang, J.; et al. Liquid metal-based synthesis of high performance monolayer SnS piezoelectric nanogenerators. Nat. Commun. 2020, 11, 3449. [Google Scholar] [CrossRef]
- Tian, W.R.; Li, N.J.; Chen, D.Y.; Xu, Q.F.; Li, H.; Yan, C.L.; Lu, J.M. Vibration-driven Reduction of CO2 to Acetate with 100% Selectivity by SnS Nanobelt Piezocatalysts. Angew. Chem. 2023, 62, e202306964. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, C.; Lee, H.; Ma, S.; Tan, J.; Jang, G.; Shim, S.G.; Park, Y.S.; Yun, J.; Kim, D.W.; et al. Elucidating the Synergistic Behavior of Orientation-Controlled SnS Nanoplates and Carbon Layers for High-Performance Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2103138. [Google Scholar] [CrossRef]
- Wei, H.; Su, Y.J.; Chen, S.Z.; Lin, Y.; Yang, Z.; Chen, X.S.; Zhang, Y.F. Novel SnSxSe1−x nanocrystals with tunable band gap: Experimental and first-principles calculations. J. Mater. Chem. 2011, 21, 12605–12608. [Google Scholar] [CrossRef]
- Gao, W.; Li, Y.T.; Guo, J.H.; Ni, M.X.; Liao, M.; Mo, H.J.; Li, J.B. Narrow-gap physical vapour deposition synthesis of ultrathin SnS1−xSex (0 ≤ x ≤ 1) two-dimensional alloys with unique polarized Raman spectra and high (opto)electronic properties. Nanoscale 2018, 10, 8787–8795. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.P.; Cao, M.; Jiang, Y.C.; Sun, L.Y.; Shen, Y.; Wang, L.J. SnSxSe1−x/Ag Nanosheet Thin Films for Solar Energy Water Splitting. ACS Appl. Nano Mater. 2021, 4, 13246–13256. [Google Scholar] [CrossRef]
- Ju, H.; Kim, K.; Park, D.; Kim, J. Fabrication of porous SnSeS nanosheets with controlled porosity and their enhanced thermoelectric performance. Chem. Eng. J. 2018, 335, 560–566. [Google Scholar] [CrossRef]
- Wu, R.Z.; Mao, J.P.; Li, H.; Yang, Y.C.; Hao, W.X.; Wang, Y.; Hao, J.Y. Revealing the relationship of NO2 sensing with energy level in 2D van der Waals SnS1-xSex alloys. Chem. Eng. J. 2023, 469, 144018. [Google Scholar] [CrossRef]
- Ektarawong, A.; Alling, B. Stability of SnSe1-xSx solid solutions revealed by first-principles cluster expansion. J. Phys.-Condens. Matter 2018, 30, 29LT01. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Gong, Y.R.; Dou, W.; Lu, B.C.; Zhang, X.M.; Zhu, H.; Ying, P.; Zhang, Q.T.; Liu, Y.Q.; Li, Y.A.; Huang, X.Q.; et al. Divacancy and resonance level enables high thermoelectric performance in n-type SnSe polycrystals. Nat. Commun. 2024, 15, 4231. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.F.; Ul Ain, Q.; Yaqoob, M.M.; Zhu, P.; Wang, D.L. Temperature dependence of exciton-phonon coupling and phonon anharmonicity in ZnTe thin films. J. Raman Spectrosc. 2022, 53, 1265–1274. [Google Scholar] [CrossRef]
- Zha, G.Z.; Zhao, Y.; Luo, H.; Jiang, W.L.; Xu, B.Q.; Yang, B. Evaporation Regularities of Elemental Selenium in the Vacuum Distillation Process. Metall. Mater. Trans. B 2022, 53, 3856–3864. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Kumar, R.R.; Selvan, V.T.; Sathyanarayanan, M.; Rao, K.N. Growth of ZnSe nano and microstructures at high vacuum by thermal evaporation. Appl. Nanosci. 2014, 4, 469–475. [Google Scholar] [CrossRef][Green Version]
- Wang, T.S.; Legut, D.; Fan, Y.C.; Qin, J.; Li, X.F.; Zhang, Q.F. Building Fast Diffusion Channel by Constructing Metal Sulfide/Metal Selenide Heterostructures for High-Performance Sodium Ion Batteries Anode. Nano Lett. 2020, 20, 6199–6205. [Google Scholar] [CrossRef]
- Xiong, X.S.; Zhang, J.; Cheng, Y.S.; Chen, C.; Zeng, J.H.; Xi, J.H.; Kong, Z.; Yuan, Y.J.; Ji, Z.G. Excellent photocatalytic and photoelectrochemical activities from 1D/2D FeSe2/SnSe heterojunction photocatalysts constructed by FeSe2 nanorods and SnSe nanosheets. Int. J. Hydrogen Energy 2022, 47, 7189–7201. [Google Scholar] [CrossRef]
- Barton, D.G.; Shtein, M.; Wilson, R.D.; Soled, S.L.; Iglesia, E. Structure and electronic properties of solid acids based on tungsten oxide nanostructures. J. Phys. Chem. B 1999, 103, 630–640. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Graetzel, M. Light-Induced Redox Reactions in Nanocrystalline Systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Hu, Y.; Wang, X.W.; Zhang, T.; Chen, W.; Wang, Q.B. Single-Layer Single-Crystalline SnSe Nanosheets. J. Am. Chem. Soc. 2013, 135, 1213–1216. [Google Scholar] [CrossRef]
- Sohila, S.; Rajalakshmi, M.; Ghosh, C.; Arora, A.K.; Muthamizhchelvan, C. Optical and Raman scattering studies on SnS nanoparticles. J. Alloys Compd. 2011, 509, 5843–5847. [Google Scholar] [CrossRef]
- Alanazi, H.T.A.; Alzaidy, G.A. Recent advancements and progress in development in chalcogenide (S, Se)-based thin films for high-performance photodetectors: A review. Phys. Scr. 2024, 99, 082001. [Google Scholar] [CrossRef]


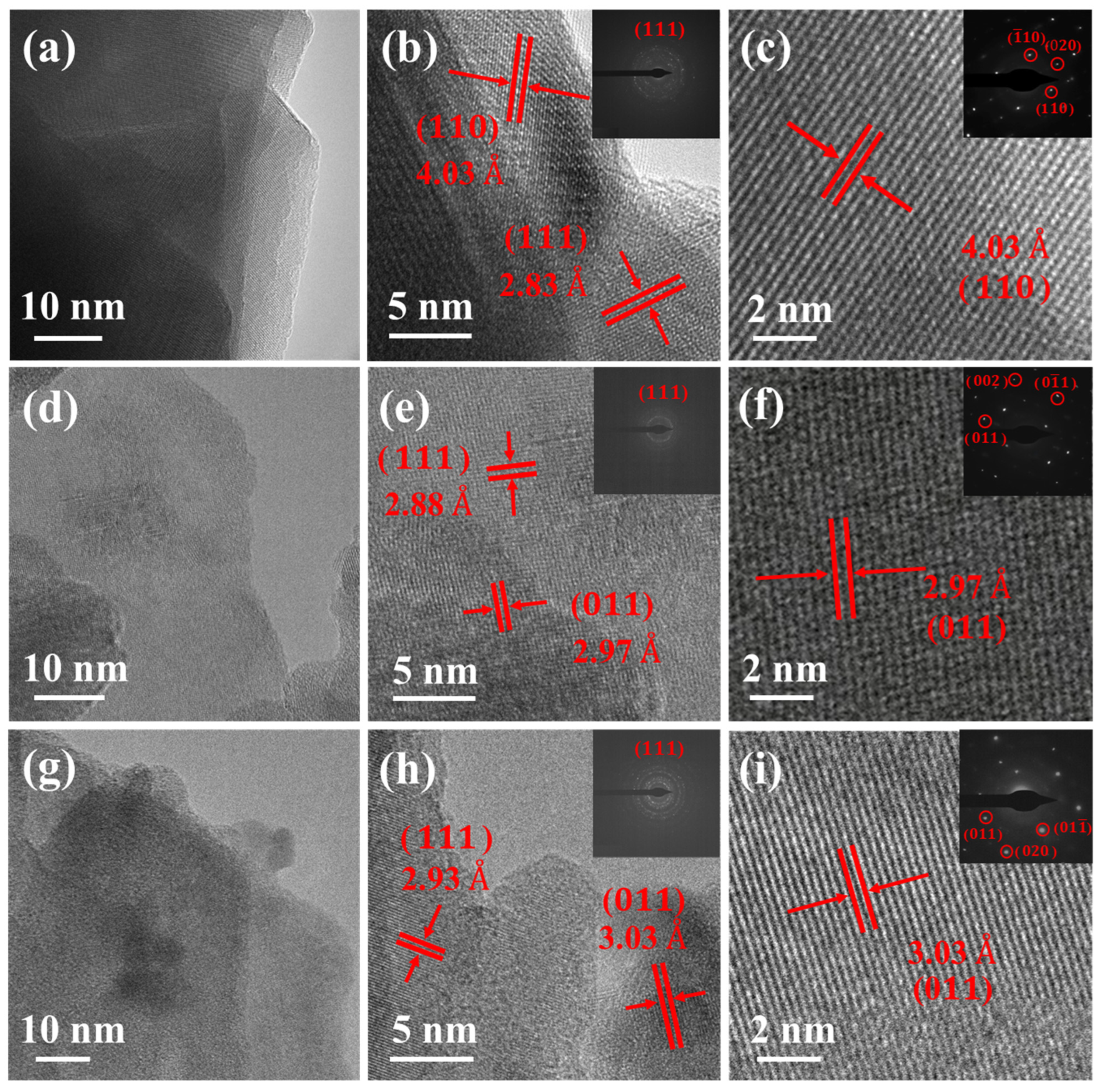
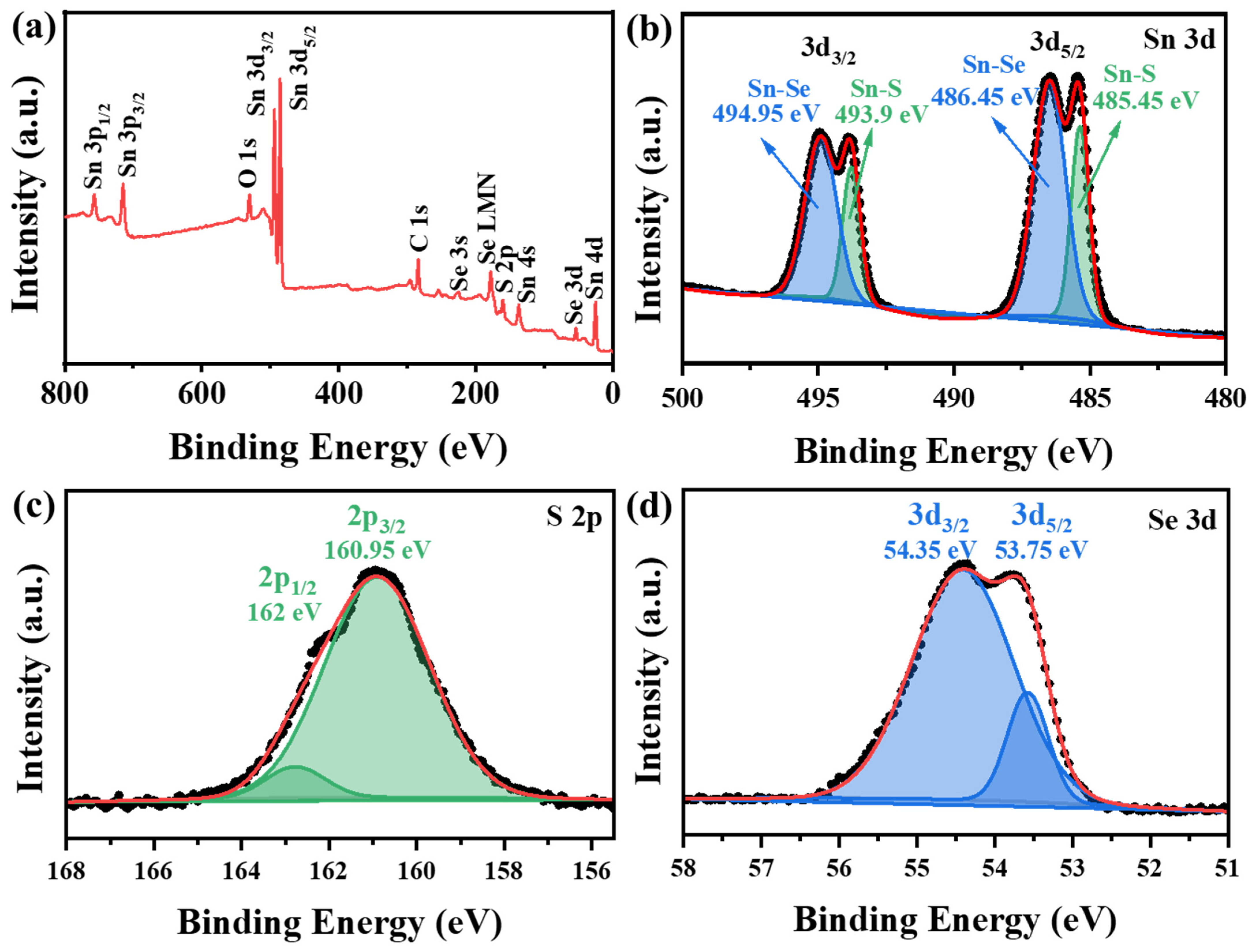

| Sample | Sn/% | S/% | Se/% |
|---|---|---|---|
| SnS | 49.38 | 50.62 | — |
| SnS0.8Se0.2 | 49.75 | 39.53 | 10.72 |
| SnS0.5Se0.5 | 49.46 | 24.93 | 25.61 |
| SnS0.2Se0.8 | 49.65 | 9.97 | 40.38 |
| SnSe | 49.33 | — | 50.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Lin, X.; Lai, X.; Jian, J. Composition-Dependent Optical Behavior of SnS1−xSex Nanosheet Arrays Films. Colloids Interfaces 2025, 9, 37. https://doi.org/10.3390/colloids9030037
Feng Y, Lin X, Lai X, Jian J. Composition-Dependent Optical Behavior of SnS1−xSex Nanosheet Arrays Films. Colloids and Interfaces. 2025; 9(3):37. https://doi.org/10.3390/colloids9030037
Chicago/Turabian StyleFeng, Yongzhao, Xinyi Lin, Xiaofang Lai, and Jikang Jian. 2025. "Composition-Dependent Optical Behavior of SnS1−xSex Nanosheet Arrays Films" Colloids and Interfaces 9, no. 3: 37. https://doi.org/10.3390/colloids9030037
APA StyleFeng, Y., Lin, X., Lai, X., & Jian, J. (2025). Composition-Dependent Optical Behavior of SnS1−xSex Nanosheet Arrays Films. Colloids and Interfaces, 9(3), 37. https://doi.org/10.3390/colloids9030037





