1. Introduction
Currently, the development of polymer hydrogels is of great interest to many researchers. The significant practical interest in hydrogels is based on the fact that they can be used in various fields: as implants [
1,
2,
3] or drug carriers [
4,
5], for the regeneration of damaged tissues [
6,
7,
8], and in the development of composite matrices for biosensors [
9,
10,
11,
12]. Despite the large number of publications on the synthesis of hydrogels, research in this area continues, since new problems arise every year that determine the requirements for hydrogels.
One of the currently unresolved problems is the development of hydrogels that are promising for the treatment of corneal diseases [
13,
14,
15]. The cornea is often subject to injuries and diseases that can lead to significant and irreversible vision loss. The cornea consists of five layers: the epithelium, Bowman′s layer, the stroma, Descemet’s membrane, and the endothelium [
16]. At the same time, for the treatment and correction of disease or damage to any of the layers, it is necessary to develop appropriate polymer hydrogels. For more than 60 years, there has been a search for polymeric materials based on both natural and synthetic polymers that can effectively treat various corneal diseases [
17]. The main part of the cornea is made up of parallel collagen fibrils, which determines its regular structure and ensures the transparency of the cornea [
18,
19]. It is known that the cornea is characterized by biomechanical heterogeneity [
20]. In this regard, when developing polymer hydrogels that are promising for the treatment of various areas of the cornea, the possibility of forming hydrogels with specified mechanical properties should also be considered. In addition, a number of requirements are imposed on polymers considered as a potential basis for artificial corneas, such as biocompatibility, transparency, absence of an immune response, and permeability to physiological solutions and oxygen [
21,
22]. Unfortunately, although a wide range of polymer hydrogels have been developed to date for the treatment of corneal diseases, there is not a single one that meets all the necessary requirements. The first polymer corneas were made on the basis of poly(methyl methacrylate) [
23]. However, studies soon showed that the use of a homopolymer resulted in very low cell adhesion to the surface of the implanted hydrogel and also caused extrusion of fibrous tissue. The search for the synthesis of polymer hydrogels based on hydroxyethyl methacrylate [
24], biodegradable natural and synthetic polymers based on lactide [
25], caprolactone [
26], collagen and gelatin [
27,
28], and lactic and glycolic acids was continued [
29]. Although the synthesis of hydrogels based on natural and biodegradable polymers is certainly a promising and interesting direction, poor reproducibility and uncontrollable properties in the process of creating hydrogels based on such polymers remain the main unresolved problems.
A literature review shows that 2-acrylamido-2-methyl-1-propanesulfonic acid is a monomer used in the synthesis of nanoparticles that are promising for in vivo drug delivery [
30,
31]. The search for ways to synthesize hydrogels with specified mechanical and macroporous properties on its basis is currently the subject of research. Previous studies by our group have shown that a developed system of macropores can be realized under cryotropic gelation conditions [
32,
33]. Cryogels are highly elastic three-dimensional polymer materials consisting of a network of interconnected macropores. In addition, the introduction of comonomers during cryotropic gelation allows the formation of mesopores with a diameter of 2 to 50 nm in the structure of polymer walls. The presence of such small pores should ensure effective adsorption of various substances such as metal ions [
34,
35] and biologically active substances or drugs [
36,
37]. Oguz Okay [
38] investigated the process of cryogel formation based on 2-acrylamido-2-methyl-1-propanesulfonic acid. It was shown that the cryogels were produced in two stages, with the first stage being the preliminary cooling of the cryogel at −196 °C, and the second stage being cryotropic gelation at −18 °C. As a result, the obtained cryogels were characterized by a radial distribution of macropores no larger than 60 μm. However, it is known that one of the main factors determining the ability of cells to freely attach to the surface of hydrogels is the presence of a developed structure of diffusion pores with a diameter of 100 to 400 μm [
39]. Macropores of such a size ensure cell migration and effective diffusion of physiological fluids. Therefore, continuing research in the field of cryotropic gelation of the 2-acrylamido-2-methyl-1-propanesulfonic acid monomer in the presence of comonomers that differ in the degree of hydrophobicity is an urgent task. Thus, it should be noted that although studies on the preparation of cryogels based on 2-acrylamido-2-methyl-1-propanesulfonic acid have already been described in the scientific literature, there is no understanding of the effect of the nature of the functional comonomer on the physicochemical properties of the resulting cryogels. In addition, there is no information on the influence of the nature of the functional comonomer and the concentration of the crosslinking agent on the size and size distribution of pores in cryogels, their transparency in the swollen state, and their biocompatibility. The introduction of acrylate comonomers into the composition of cryogels with 2-acrylamido-2-methyl-1-propanesulfonic acid seems promising, since they are known to improve cell adhesion in implanted hydrogels.
As is known from the literature, biocompatible nanoparticles and films based on AMP and SBMA can be obtained, which justifies the choice of 2-acrylamido-2-methyl-1-propanesulfonic acid and
N-(3-sulfopropyl)-
N-(methacryloxyethyl)-
N,
N-dimethylammonium betaine as sulfo-containing comonomers in our study [
40,
41]. The presence of sulfonate and zwitterionic groups in the monomer unit of the polymer chain ensures good water absorption. Obtaining homopolymers based on the above monomers leads to the formation of a water-soluble material. The aim of this work was to demonstrate the fundamental possibility of synthesizing cryogels using the selected sulfo-containing monomers.
Here, for the first time, we show the effect of the nature of the comonomer (2-hydroxyethyl methacrylate, ethyl acrylate, vinyl acetate) on the structure of the resulting cryogel based on sodium 2-acrylamido-2-methyl-1-propanesulfonate. The pore structure of the resulting cryogels was studied using SEM and optical microscopy; the chemical structure of the cryogels was confirmed by FTIR spectroscopy. In addition, the swelling degree, gel fraction, and optical transparency of the cryogels were assessed; the mechanical properties and cytotoxicity of the resulting cryogels were also studied.
2. Materials and Methods
Materials. The synthesis of polymer cryogels was carried out using monomers from Sigma Aldrich (Taufkirchen, Germany). 2-Acrylamido-2-methyl-1-propanesulfonic acid (AMP) and N-(3-sulfopropyl)-N-(methacryloxyethyl)-N,N-dimethylammonium betaine (SBMA) were used without preliminary purification. Vinyl acetate (VA), ethyl acrylate (EA), and 2-hydroxyethyl methacrylate (HEMA) were purified by vacuum distillation according to standard methods. N,N′-methylenebisacrylamide (MBA) and potassium peroxodisulfate (K2S2O8) were purified by recrystallization from water, and N,N,N′,N′-tetramethylethylenediamine (TEMED) and NaCl were used without additional purification.
Bidistilled water was used to prepare solutions. Water was distilled twice from an all-glass apparatus. The specific electrical conductivity of water (3 μS/cm) and the surface tension (72 mN/m) indicated the absence of surface-active impurities. Deionized water (DI) with conductivity of no more than 1 μS/cm (VODOLEY-M deionization system, Saint-Petersburg, Russia) was used to carry out polymerization.
Preparation of cryogels. Before synthesis, AMP was converted into sodium 2-acrylamido-2-methyl-1-propanesulfonate by adding the prepared NaOH solution under pH control (pH value was 10) (pH-meter pH-150MI, Izmeritelnaya Tekhnika LLC, Moscow, Russia).
Cryogels were synthesized according to the following procedure: The initial reaction solutions consisting of water (1 mL), 0.6 mmol sulfo-containing monomers (AMP or SBMA), 0.7 mmol comonomer (HEMA or EA, or VA), initiator (K2S2O8, 1.3 mol.% to Mn.), and N,N′-methylenebisacrylamide were prepared within 2 min at 20 °C; then, the accelerator was quickly added to the system, and all reagents were immediately placed in plastic syringes and kept in a deep freezer at −24 °C for 1 day. The syringes were then removed from the freezer and brought to room temperature, and the resulting cryogels were washed 3 times with excess deionized water in a 600 mL beaker to remove unbounded reaction products and unreacted monomers, crosslinker excess, and oligomeric and polymer non-crosslinked chains. Finally, the cryogels were freeze-dried before further use (FreeZone Freeze Dry System, Labconco Corporation, Kansas City, MO, USA). Freeze-drying allows for preserving the structure of the swollen system in a dry state.
The compositions of the reaction mixtures and properties of the obtained cryogels discussed below (swelling degree, pore size, gel fraction) are given in
Table 1.
Characterization
Swelling tests. Dried cryogels of an appropriate size were weighed and then immersed in distilled water at 25 °C to ensure complete equilibration. The samples were left to swell for up to one day. After removing the swollen samples from the liquid, they were weighed after wiping off the surface water with a paper towel. The swelling ratios of each sample were measured using the gravimetric method. Swelling tests were carried out under identical conditions for all cryogels. The solvent volume was 600 mL, and the gel size was 8 mm in diameter and 15 mm in length. The swelling degree (SW) was defined as
where m
swollen cryogel is the weight of the swollen cryogel and m
dry cryogel is the weight of the freeze-dried cryogel. Dried and swollen cryogels were weighed on an Ohaus balance (OHAUS Europe GmbH, Nänikon, Switzerland).
Determination of gel fraction. The gel fractions of the cryogels were determined by continuous extraction in a Soxhlet apparatus (C. Gerhardt GmbH & Co. KG, Königswinter, Germany). Extraction was carried out in deionized water at 100 °C for 5 h. After extraction, the remaining polymer was dried.
Gel fraction was calculated using the formula below:
Determination of specific surface area of dried cryogel. The specific surface area of the dried cryogels was estimated using a nitrogen gas sorption analyzer (“NOVA 1200e” Quantachrome, Boynton Beach, FL, USA), and the Brunauer–Emmett–Teller (BET) multipoint method was used to calculate the pore parameters. Water was preliminarily removed from the cryogels by freeze-drying. Before measurements, the sample was degassed in a nitrogen flow at reduced pressure.
SEM, optical microscopy, and FTIR spectroscopy.
The structure of the cryogels was assessed by optical (MIKMED-5 microscope, LOMO, Saint-Petersburg, Russia) and scanning electron microscopy (SEM) (SUPRA 55 VP microscope, Carl Zeiss, Oberkochen, Germany). Optical images were taken at 300× magnification; to obtain a clear optical image, thin sections of the cryogels were contrasted with a methylene blue solution. For SEM evaluation, the cryogel samples were kept in water until equilibrium was reached, quickly frozen, and freeze-dried. The gels were then carefully cut with a sharp scalpel without applying pressure to monitor the state of the walls in cross-section. SEM images were captured at 100× and 10,000–20,000× magnification at 5.00 kV. The SEM aperture size was 30.00 μm.
The chemical characterization of the freeze-dried (FreeZone Freeze Dry System, Labconco Corporation, Kansas City, MO, USA) cryogels was performed by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) using an IR-Affinity-1S spectrometer (Shimadzu, Kyoto, Japan). All spectra were recorded in the range of 400 ÷ 2000 cm−1 in transmittance mode and represent the average of 30 scans taken. The resolution was set to 2 cm−1, and Happ–Genzel apodization was used.
Mechanical stress analysis. The mechanical properties were studied for the gels in the swollen state. For this purpose, the gels were placed in bidistilled water for at least 30 min. Compression tests were carried out using a tensile–compression test machine (DMA INSTRON 5943, INSTRON, Norwood, MA, USA). Cylindrical samples (~6 mm in diameter and ~6 mm in height) were compressed by two parallel plates at a maximum load of 0.01 N with a compression rate of 1 mm/min. The values of stress, strain (%), and impact strength were calculated using Trapezium X material testing software (Trapezium X Shimadzu, Kyoto, Japan). The compressive modulus was calculated from the linear portion of the stress–strain curve (0–25% strain). The results presented are the average of five independent runs.
Cytotoxicity test. Before the study, the cryogel samples were weighed and sterilized with ozone for 90 min. The appearance of the samples after sterilization did not change (
Figure S1).
For the cytotoxicity study, the human cell line FetMSCs (mesenchymal stromal cells) (Cell culture collection of Institute of Cytology, St. Petersburg) was used. The cells were cultured in a CO2 incubator at 37 °C in a humidified atmosphere containing air and 5% CO2 in DMEM/F12 growth medium containing 10% (v/v) heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT, USA), 1% L-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin.
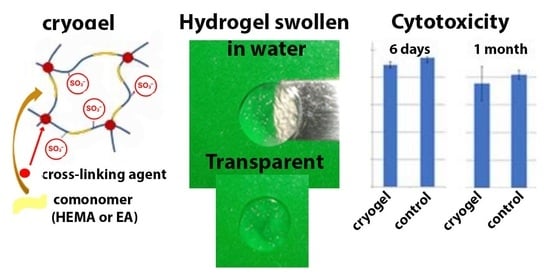
Cryogel samples of defined mass were filled with complete culture medium: 2 mL of complete culture medium was poured onto 0.01 g of matrices for cell culture and incubated for 6 days and 1 month (
Figure S2).
For the experiment, 5.0 × 103 cells/100 μL/well were seeded in 96-well plates; after 1 day, the medium was discarded and 100 μL of the medium from incubation with experimental samples was poured over and cultured for 72 h. At the end of the incubation period, the medium was removed and DMEM/F12 medium with MTT (0.1 mg/mL) was added at 50 μL/well. The cells were incubated in a CO2 incubator for 2 h at 37 °C. After the removal of the supernatant, formazan crystals formed by metabolically viable cells were dissolved in dimethyl sulfoxide (50 μL/well) and transferred to clean wells, and then the optical density was measured at 570 nm on a plate spectrophotometer (AIFR-01 UNIPLAN, “PICON”, Saint-Petersburg, Russia). Polynomial regression analysis in Microsoft Excel was used for calculations.
The % cytotoxicity was calculated as follows [
42]:
where A is the absorbance of the control and B is the absorbance of the samples.
Statistical analysis. One-way and two-way ANOVAs were performed using GraphPad Prism 10 statistical software (Prism 10). Student’s t-tests were performed using Microsoft Excel 2016. Values of p smaller than 0.05 were considered significant and denoted as ** p < 0.01 and **** p < 0.0001.
3. Results
3.1. Cryogel Formation
Polyelectrolyte cryogels based on the sulfonate-containing monomer sodium 2-acrylamido-2-methyl-1-propanesulfonate (reference samples based on
N-(3-sulfopropyl)-
N-(methacryloxyethyl)-
N,
N-dimethylammonium betaine (SBMA)) were synthesized. The influence of the nature of the comonomer (2-hydroxyethyl methacrylate, ethyl acrylate, vinyl acetate), as well as the concentration of the crosslinking agent
N,
N′-methylenebisacrylamide, on the structure and properties of the cryogels obtained was studied. The choice of
N,
N′-methylenebisacrylamide as a crosslinker is rationalized by its good solubility in water, which is important when crosslinking water-soluble monomers. The properties (swelling degree, pore size, gel fraction) of the cryogels obtained are given in
Table 2.
SBMA-based cryogels (CS series) were used as reference samples; their degree of crosslinking is comparable to that of the studied cryogels (CG series) (see
Table 1 and
Table 2). The synthesis of SBMA-based cryogels with a higher content of crosslinking agent leads to the formation of fragile and non-swelling cryogels.
The choice of medium for assessing the degree of swelling of a cryogel is very important. In fact, the degree of swelling of cryogels depends on the composition of the medium, which was demonstrated by us, among others, when analyzing the degree of swelling of sulfonic cryogels in water, 0.9% aqueous NaCl solution, and Knopp’s solution [
43].
A possible ophthalmic application of cryogel synthesis involves contact with biological fluids found in the eye, i.e., tears. Since tears comprise 98.2% water [
44], we assessed cryogel swelling in water correspondingly.
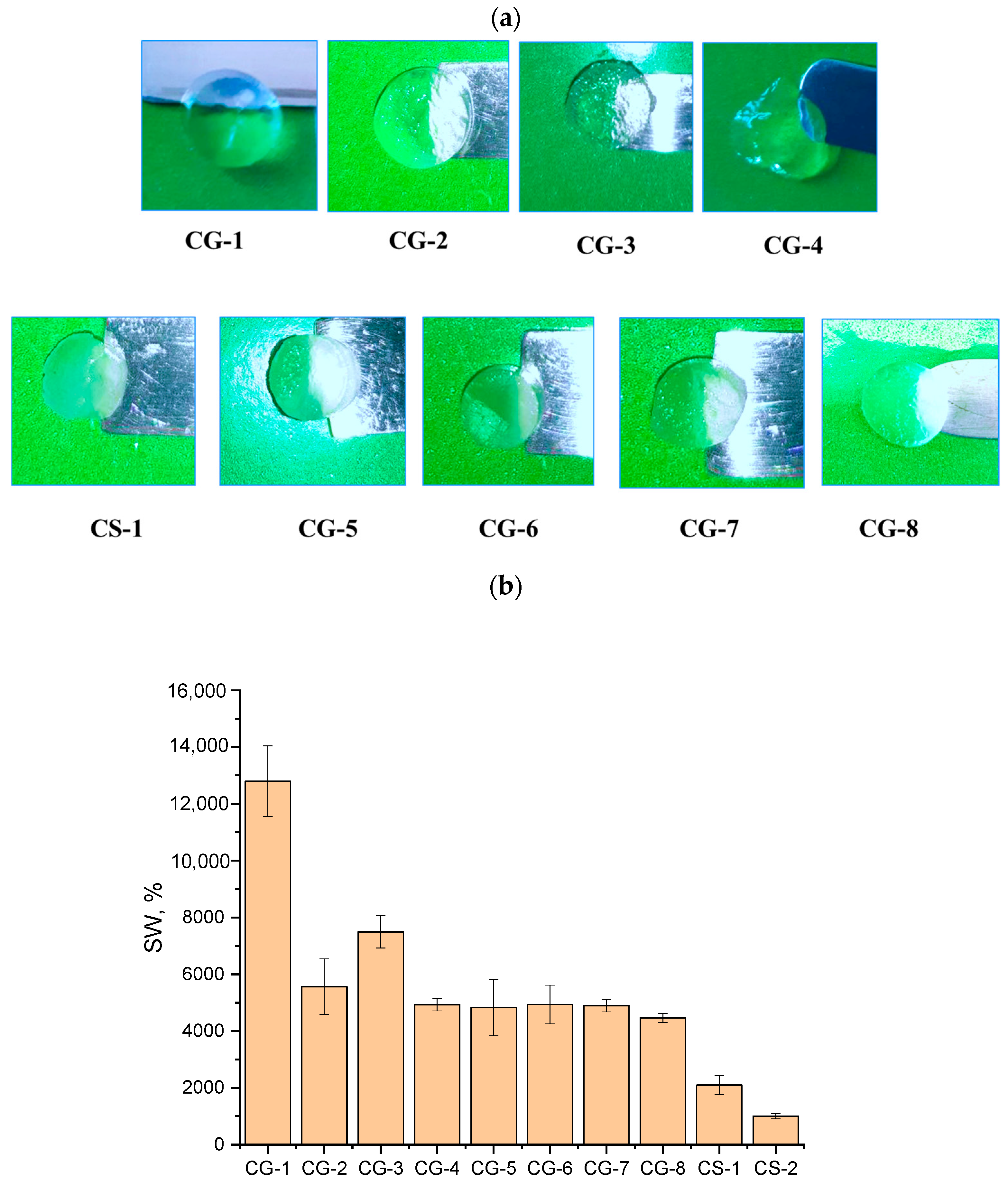
Figure 1a shows photographs of the cryogels after their swelling in water at room temperature (22–25 °C) (see more images in
Figure S3). In this work, two series of cryogels were obtained: in the first series, the nature of the comonomer was varied at a constant crosslinking agent concentration of 2.2 mol.%; in the second series, the content of the crosslinking agent was increased to 6.7 mol.%. It is known that, on the one hand, with an increase in the degree of crosslinking, the mechanical strength of the synthesized gels increases, but, on the other hand, a decrease in the transparency of the resulting gels is possible due to a decrease in the mobility of the polymer chains.
Successful crosslinking of the cryogels is also evidenced by the values determined for the gel fraction, which amounted to ~60–85% for all samples of the first series. The CG-3 sample typically had the highest value, which is consistent with the data on determining mechanical properties shown below. Increasing the amount of the crosslinking agent led to a higher gel fraction. Moreover, both when using 2.2% and 6.7% of crosslinker, the introduction of HEMA as a comonomer resulted in a reduction in the degree of crosslinking (decrease in the content of the gel fraction), and the introduction of VA, on the contrary, increased the degree of crosslinking (increased the content of the gel fraction) compared to the sample without a comonomer (CG-1).
Meanwhile, for samples based on SBMA, the gel fraction values were about ~80%, which indicates a sufficient amount of crosslinker in this system. In addition, the obtained gel fraction values were at the level of the values for the first series of samples with AMP (CG1-CG-4), justifying the choice of samples CS-1 and CS-2 as reference samples.
It turned out that cryogels were transparent when the concentration of the crosslinking agent was 2.2 mol.%, and the degree of transparency did not depend on the nature of the comonomer used (
Figure 1a). It should be noted that for cryogels obtained in the presence of different comonomers, the amount of sorbed water differed by more than 2 times (
Table 2,
Figure 1b).
Thus, the maximum swelling degree of 12,800% was recorded for the cryogel obtained by AMP polymerization without comonomers at 2.2 mol.% MBA (experiment CG-1), and the minimum degree of 4930% was recorded for the cryogel obtained in the presence of VA, the most hydrophobic, as a comonomer and 2.2 mol.% of MBA (experiment CG-4). For the cryogel based on the AMP-HEMA copolymer, the swelling values were more than 2 times lower than for the gel based on AMP only. Such experimental data contradict the results obtained earlier by us and other researchers [
45,
46]. It is generally accepted that a hydrogel containing HEMA units should have a higher swelling degree compared to a gel obtained without this comonomer [
32]. Apparently, in the P(AMP-HEMA-MBA) cryogel (experiment CG-2), strong intra- and interchain interactions are realized between the AMP and HEMA units, as shown in
Scheme 1. We assume that the mechanism of this interaction is based on ion–ion interactions, which are realized as a result of proton transfer from the hydroxyl group of HEMA with the formation of a hydroxyl anion to the amino group of AMP with the formation of an ammonium cation.
Scheme 1 also shows the monomer formulas with calculations of atomic charges using the Chem & Bio 3D 12.0 software with the extended Hückel method. After proton transfer, the charge of the oxygen atom of the hydroxyl group increases from −0.34 to −0.45, which leads to strong ion–ion interactions with neighboring units containing ammonium cations.
The introduction of EA and VA into the reaction system decreases the swelling degree of the synthesized cryogels. Both EA and VA are hydrophobic comonomers (the solubility of EA and VA in water is 1.5 wt.% and 2 wt.%, respectively), and the charge of the oxygen atom is comparable for both substances (
Scheme 1); however, as follows from
Table 1, the size of the macropores for the cryogel obtained in the presence of VA decreases to values of 50–170 μm. As a result, the swelling degree of this cryogel is lower.
Scheme 1 shows the formulas of EA and VA monomers with the calculated charges of the oxygen atoms of the carbonyl group. As can be seen, the charges of the carbonyl oxygen of EA and VA are close and equal to −0.66 and −0.64, respectively. However, unlike EA, in VA, the oxygen atom of the carbonyl group molecule is located further from the main polymer chain. As a result, the presence of VA units in the structure of the polymer chains leads to repulsion between the carbonyl group of VA and the carbonyl and sulfonate groups of AMP, as shown in
Scheme 1b. In addition, as seen from
Figure 1a, the most fragile cryogel after swelling was P(AMP-VA-MBA) (CG-4 sample), which indicates insufficient crosslinking of the polymer chains by MBA units. Since the gel fraction values of the CG4 sample were not minimal in the series of comonomers used (
Table 1), VA when copolymerized with AMP is not likely to provide sufficient homogeneity of crosslinking, which is indirectly confirmed by the SEM image (
Figure 2g). Thus, this indirectly confirms the insufficiently close arrangement of polymer chains in the cryogel due to the repulsion between the carbonyl group of VA and the carbonyl and sulfonate groups of AMP assumed above. On the contrary, in the structure of the P(AMP-EA-MBA) cryogel (CG-3 sample) containing ethyl acrylate units, in which the carbonyl group is localized close to the main chain, the interaction between the carbonyl and sulfonate groups is limited (
Scheme 1).
In the second series of experiments, while maintaining the weight ratio of AMP to HEMA (VA or EA) and their concentration relative to the aqueous phase, the concentration of the crosslinking agent was varied. It was shown that an increase in the MBA content by 3 times reduced the swelling degree of the resulting cryogels in water (
Table 2) to 4470–4940 (swelling percentage). These data are consistent with the data of ref. [
47], where the swelling degree of porous systems was decreased by increasing crosslinker content due to lower hydrophilicity.
However, a decrease in the transparency of cryogels with an increase in the degree of crosslinking turned out to be more important—the gels became cloudy, as can be seen from
Figure 1a. Thus, cryogels with a crosslinking agent content of 6.7 mol.% are not of interest in the further study of hydrogels for the possible treatment of corneal diseases.
Cryogels based on the zwitterionic sulfonate-containing monomer
N-(3-sulfopropyl)-
N-(methacryloxyethyl)-
N,
N-dimethylammonium betaine (SBMA) were used as reference samples. It is known that polymers obtained on the basis of zwitterionic monomers are widely studied as coating materials for various implants [
48]. It was shown that such polymers prevent fouling, since they have strong hydration properties and can resist non-specific adsorption of proteins. The SBMA-based cryogels obtained here are characterized by a minimum swelling degree of 1000 (swelling percentage), although the content of the crosslinking agent during their synthesis was 1 mol.%. Apparently, strong ion–ion interactions between charged nitrogen atoms and sulfonate groups are also realized in this case. It should be noted that the obtained P(SBMA-HEMA-MBA) and P(SBMA-VA-MBA) cryogels in the swollen state were not transparent (
Figure 1a). Here, we additionally measured the time to reach equilibrium swelling; the data are given in the
Supporting Information (please see
Figure S6). It turned out that samples CG-1, CG-2, and CG-3 achieved equilibrium swelling within a short period from 30 s to 4 min. Moreover, the introduction of comonomers (HEMA, EA) significantly reduced the swelling time. Then, the swollen cryogels retained their volume for at least a day. Thus, this work shows that the obtained cryogels quickly swell in water, which is one of the requirements for polymeric materials for ophthalmology.
The experimental data on the swelling degree of cryogels are consistent with the results from studying their structure using the SEM method. As can be seen from
Figure 2, cryogels based on AMP (CG-1) are characterized by the widest pore size distribution, with pore sizes being up to 350 μm. When AMP is copolymerized with EA, VA, or HEMA, the pore size decreases, and macropores with a diameter of 50–250 μm are detected. In contrast, SBMA-based cryogels consist of smaller macropores with diameters from 10 to 60 μm.
The introduction of HEMA into the polymer chains of the cryogel based on AMP does not change the structure of the polymer walls of the cryogel, which are characterized by a developed rough surface (
Figure 2b,d). The introduction of hydrophobic comonomers VA and EA leads to the walls of the polymer cryogels becoming more homogeneous (
Figure 2f,h).
The smoothest walls are characteristic of the cryogel obtained from the SBMA copolymer with VA (
Figure 2i,j). The insets in
Figure 2 show photographs of the cryogels obtained using optical microscopy.
As can be seen from the photographs, the structure of the swollen cryogels depends on the nature of the comonomers. Thus, the most uniform swelling—the absence of unevenly distributed dark (characteristic of cryogel polymer walls) and light areas (water-filled macropores in the cryogel structure)—is observed for the P(AMP-EA-MBA) cryogel (sample CG-3). Thus, morphological analysis shows that sample CG-3 is a cryogel that combines a homogeneous wall structure with uniform swelling.
3.2. FTIR
The obtained cryogels were characterized by FTIR (
Figure 3). The inclusion of comonomers in the gel composition was confirmed by the presence of vibration bands of the bonds of characteristic groups [
39]. All spectra contain intense vibration peaks of sulfonate groups, namely, a doublet asymmetric stretching (S=O)
2 band in the range of 1150–1186 cm
−1 and a symmetric stretching (S=O)
2 band at around 1040 cm
−1 (for cryogels containing AMP) or 1035 cm
−1 (for cryogels containing SBMA).
AMP also exhibits characteristic amide group vibration bands, namely, amide I (C=O stretching vibrations, 1651–1653 cm−1) and amide II (coupled N-H bending and C-N stretching of C-N-H group, 1539–1542 cm−1), as well as a band that also relates to the interactions between N-H bending and C-N stretching at 1386 cm−1 and out-of-plane N-H wagging at 765–770 cm−1. The quaternary ammonium group of SBMA has a vibration peak at 1473–1477 cm−1. The above confirms the successful incorporation of the functional sulfonate comonomer AMP or SBMA into the structure of the gels.
The incorporation of acrylate comonomers (EA or HEMA) is confirmed by the presence of an intense absorption band of C=O stretching in acrylates (1720–1724 cm−1); in addition, a vibrational peak of the C-O stretching of the OH group (1076 cm−1) is recorded for HEMA.
Thus, FTIR analysis has confirmed that both sulfonate and acrylate comonomer units are present in the cryogels.
3.3. N2 Adsorption Analysis
The N2 adsorption–desorption study shows that the specific surface area of the cryogels varies significantly—115 and 5.0 m2/g for samples CG-3 and CS-2, respectively.
While the values obtained for CS-2 are lower than the specific surface area values determined, for example, for cryogels based on PHEMA (~30 m
2/g [
37]), the values obtained for CG-3 are significantly higher. Thus, the use of AMP is justified for the formation of cryogels with an enhanced adsorption capacity.
The pore size distribution analyses by SEM (
Figure 2) and the N
2 adsorption–desorption study (
Figure 4) show that the cryogels are characterized by two types of pores: pores in place of ice crystals with a diameter of 10–350 microns (
Figure 2), and mesopores in the polymer walls of gels with a diameter of 2–15 nm (
Figure 4). The small diameter of large macropores and the minimal volume of mesopores (20 times less compared to the CG-3 sample) explain a more than 20-fold decrease in the specific surface area of the P(SBMA-VA-MBA) cryogel relative to P(AMP-VA- MBA).
The decrease in macro- and mesopores by an order of magnitude is one of the reasons for the lowest swelling degree of the P(SBMA-VA-MBA) cryogel in water. Thus, it has been shown that the nature of the introduced comonomer containing sulfonate groups significantly affects the porosity, specific surface area, and mesopore volume of the resulting cryogels.
3.4. Mechanical Stress Analysis
The mechanical properties of the obtained cryogels were determined by testing the uniaxial compression of the cryogels in the swollen state at a temperature of 23 ± 2 °C.
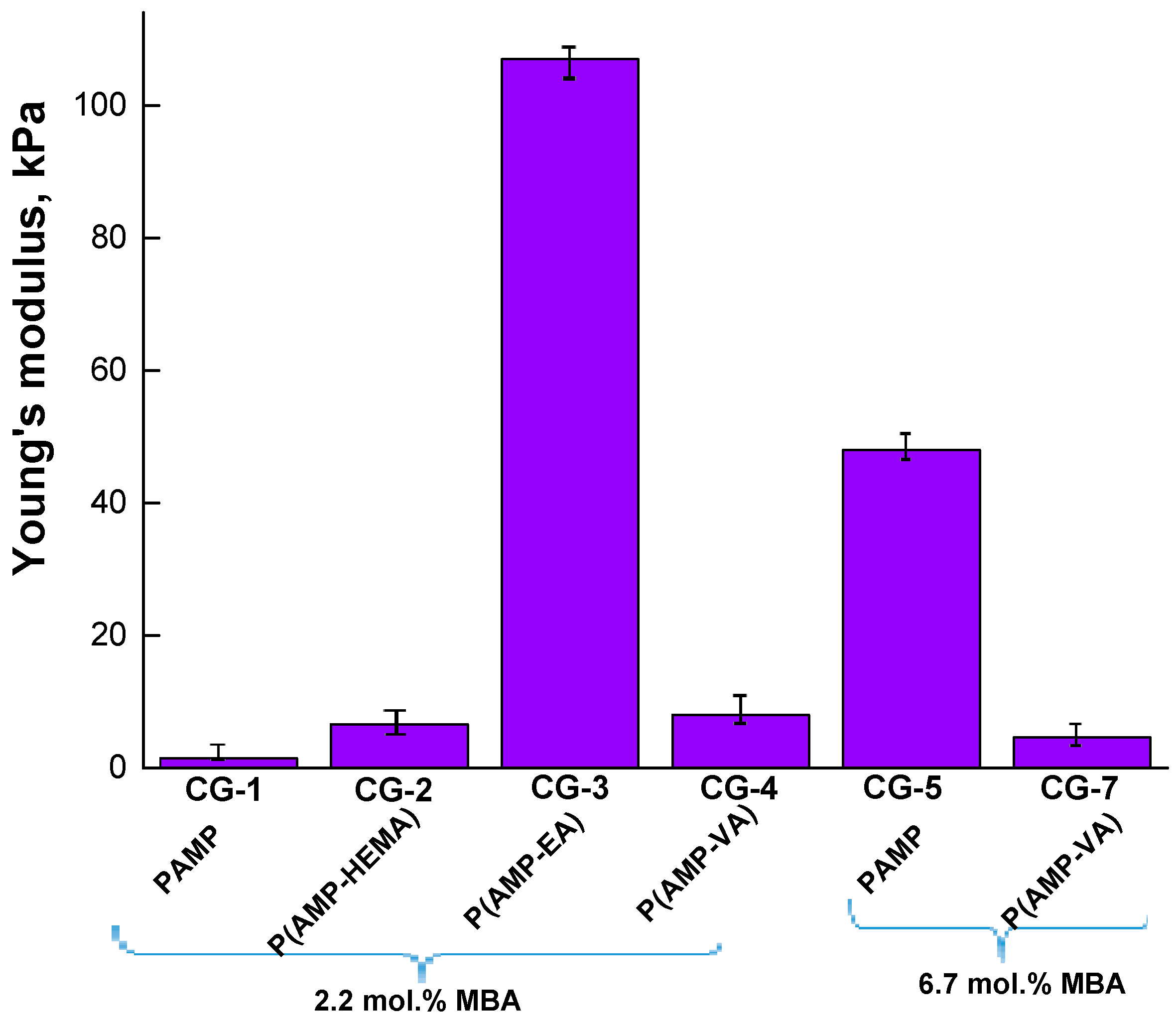
Figure 5 shows the data on the mechanical properties of the cryogels depending on the degree of crosslinking and the nature of the comonomer (typical stress–strain curves of the swollen cryogels corresponding to the dependence of the nominal stress on the strain are shown in
Figure S5). It is obvious that for a cryogel obtained only on the basis of AMP, with a 3-fold increase in the content of the crosslinking agent in the reaction system, Young’s modulus increases more than 30 times: from 1.5 to 48 kPa. The introduction of the VA comonomer into the reaction system with a simultaneously high content of MBA (6.7 mol.%) (CG-7 sample) does not allow the formation of cryogels, with Young’s modulus values of more than 5 kPa. A decrease in the MBA content in the reaction system leads to the formation of a P(AMP-VA-MBA) cryogel with Young’s modulus values equal to 8 kPa (CG-4 sample). The cryogel obtained by introducing EA into the reaction system as a comonomer (CG-3 sample) possesses the best mechanical properties. From the data presented in
Figure 5, it is evident that the nature of the comonomer, as well as the degree of crosslinking, has a significant effect on the strength of the polymer walls.
Thus, only for cryogels obtained in the presence of HEMA or VA (in the case of a highly crosslinked cryogel) is an elastic region initially observed in the stress–strain curves, followed by a quasi-plateau regime, during which the gel samples are easily deformed (see
Figure S5).
The appearance of a plateau regime in the stress–strain curves indicates the collapse of the porous structure of cryogels, as reported previously [
49]. However, for the cryogel obtained in the presence of EA, a rapidly increasing elastic regime is observed in the stress–strain curve of the hydrogel under compression, which indicates an increase in the mechanical strength of the pore walls that make up the network structure. Thus, it is shown that varying the composition of the monomer mixture makes it possible to form cryogels with variable mechanical properties, namely, Young’s modulus, lying in the range from 1.5 to 107 kPa. Analysis of the literature shows that each of the five corneal layers is characterized by its own Young’s modulus: epithelium—0.57 kPa; Bowman′s layer—109.8 kPa; stroma—33.1 kPa; Descemet’s membrane—50 kPa; endothelium—4.1 kPa [
16]. Thus, the synthesized cryogels are apparently not suitable for the treatment of the upper layer of the cornea—the epithelium. However, further studies of the obtained cryogels for the treatment of the other four corneal layers are possible.
3.5. Cytotoxicity Test
The cytotoxicity of the obtained cryogels was studied in vitro. In this work, the MTT test based on the formation of azure formazan in living cells was used to assess cytotoxicity, which allows us to evaluate the enzymatic activity of succinate dehydrogenase and thereby determine the viability of the cells. The study of the supernatant by the colorimetric method at a certain wavelength allows us to estimate the amount of formazan formed, which is proportional to the number of living cells.
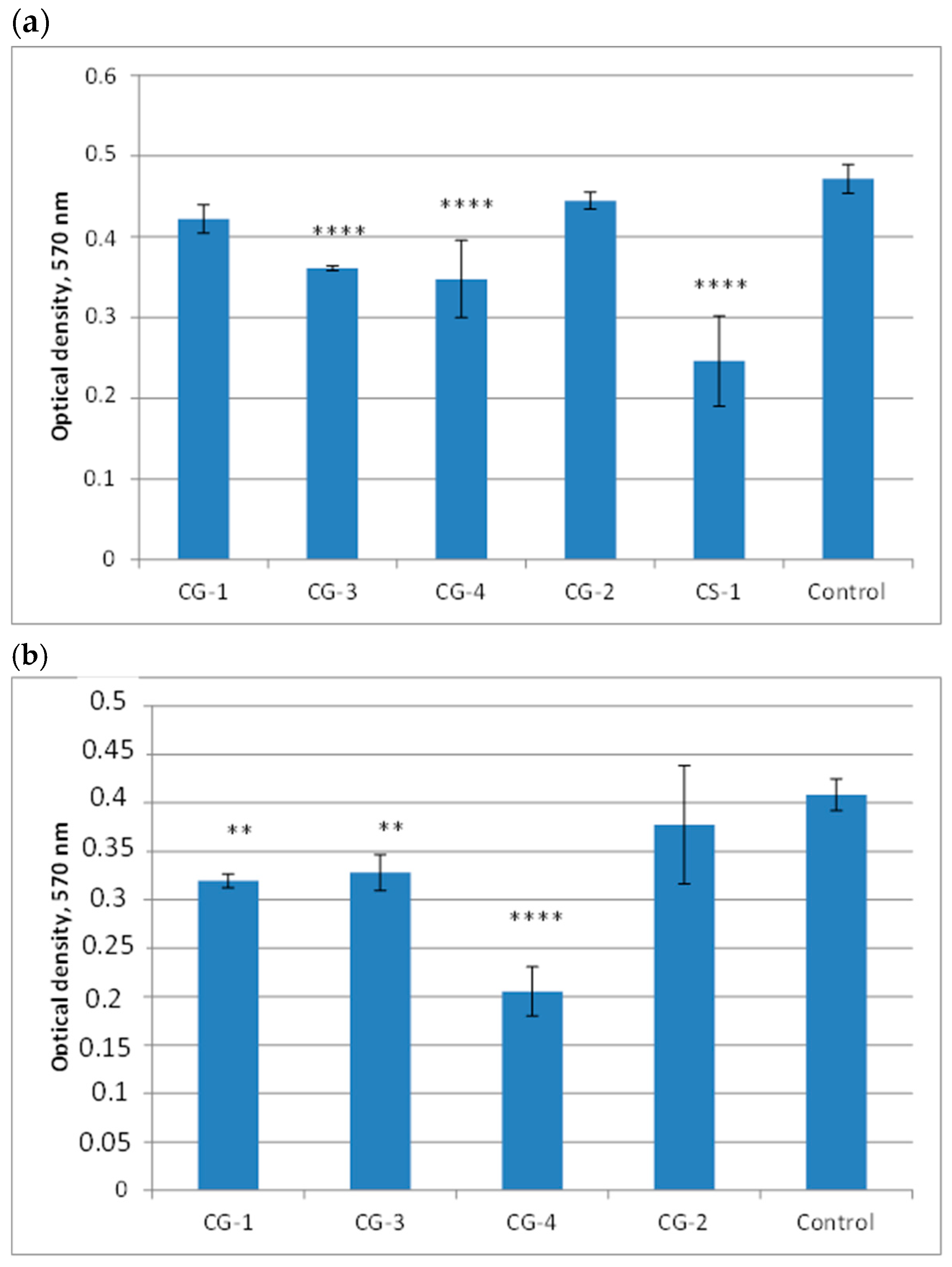
It turned out that the experimental samples did not change the pH of the medium, as evidenced by the color of the nutrient medium, which did not change its color even after 1 month of incubation with the experimental samples. According to the MTT test data, the smallest number of viable cells after 6 days of incubation with the samples was found for CG-4 and CS-1. The MTT test data (
Figure 6) are consistent with the optical microscopy data (
Figure 7).
The number of cells and their morphology in the presence of samples CG-4 and CS-1 were significantly inferior to the control. The viability percentages of the CG-1, CG-3, CG-4, CG-2, and CS-1 hydrogels after 6 days were 91%, 78%, 74%, 96%, and 52%, respectively. Thus, it is shown that the introduction of a comonomer such as VA into the cryogel structure significantly worsens biocompatibility. In addition, sodium 2-acrylamido-2-methyl-1-propanesulfonate showed promise as a sulfonate-containing comonomer, since the cryogel based on
N-(3-sulfopropyl)-
N-(methacryloxyethyl)-
N,
N-dimethylammonium betaine reduced cell viability after 6 days by almost 2 times compared to the control sample (
Figure 6).
The study of the cytotoxicity of the comparison sample CS-1 after one month was not carried out. The viability percentages of the CG-1, CG-3, CG-4, and CG-2 hydrogels after one month were 79%, 82%, 49%, and 94%, respectively. Thus, the most non-cytotoxic cryogels are those obtained by the copolymerization of AMP with HEMA or EA.