Gel Polymer Electrolytes with High Thermal Stability for Safe Lithium Metal Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
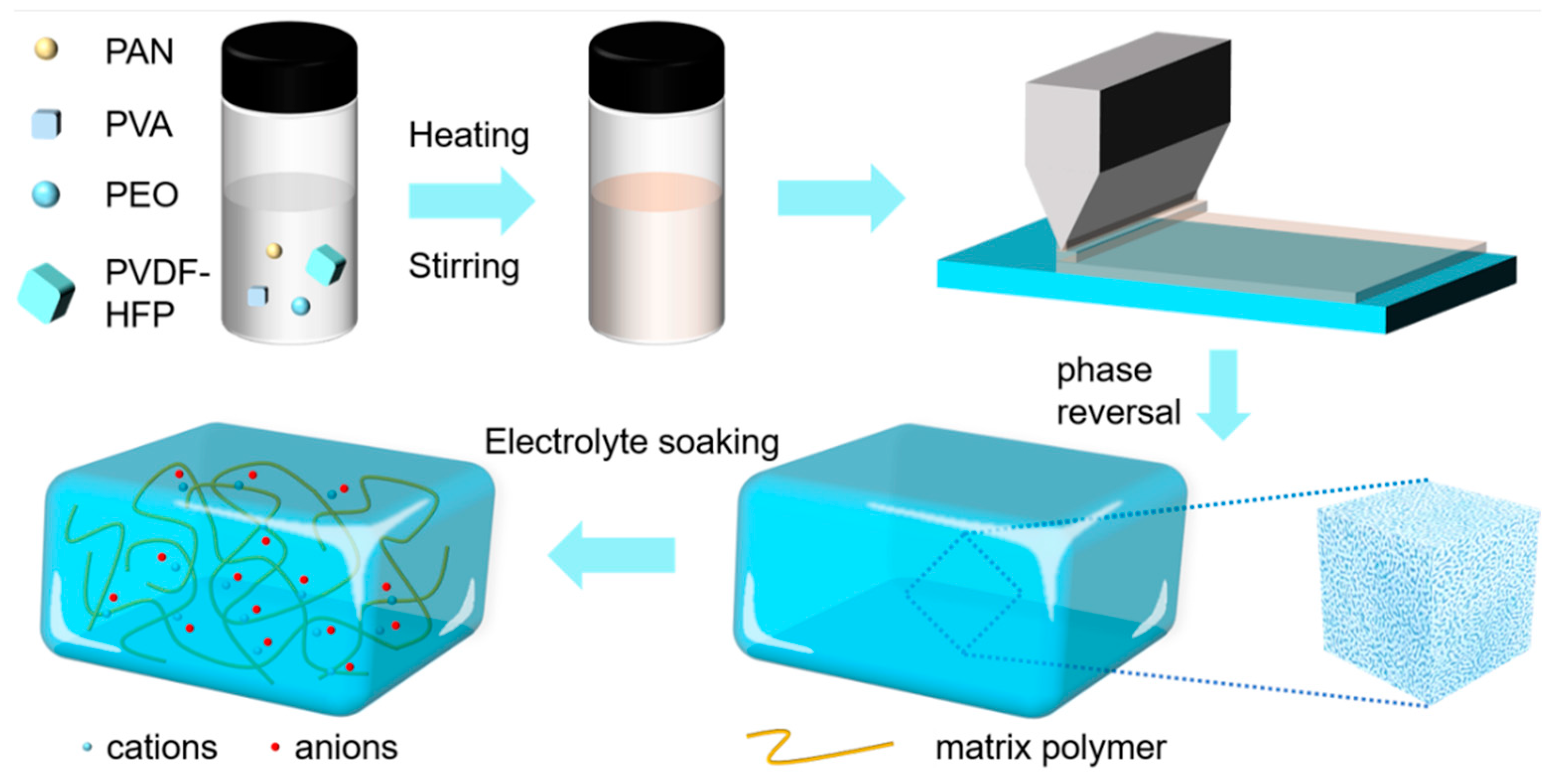
2.2. Preparation of b-PVDF and b-GPE
2.3. Preparation of LiCoO2 Cathode
2.4. Material Characterizations
2.5. Assembly and Electrochemical Measurements of LMBs
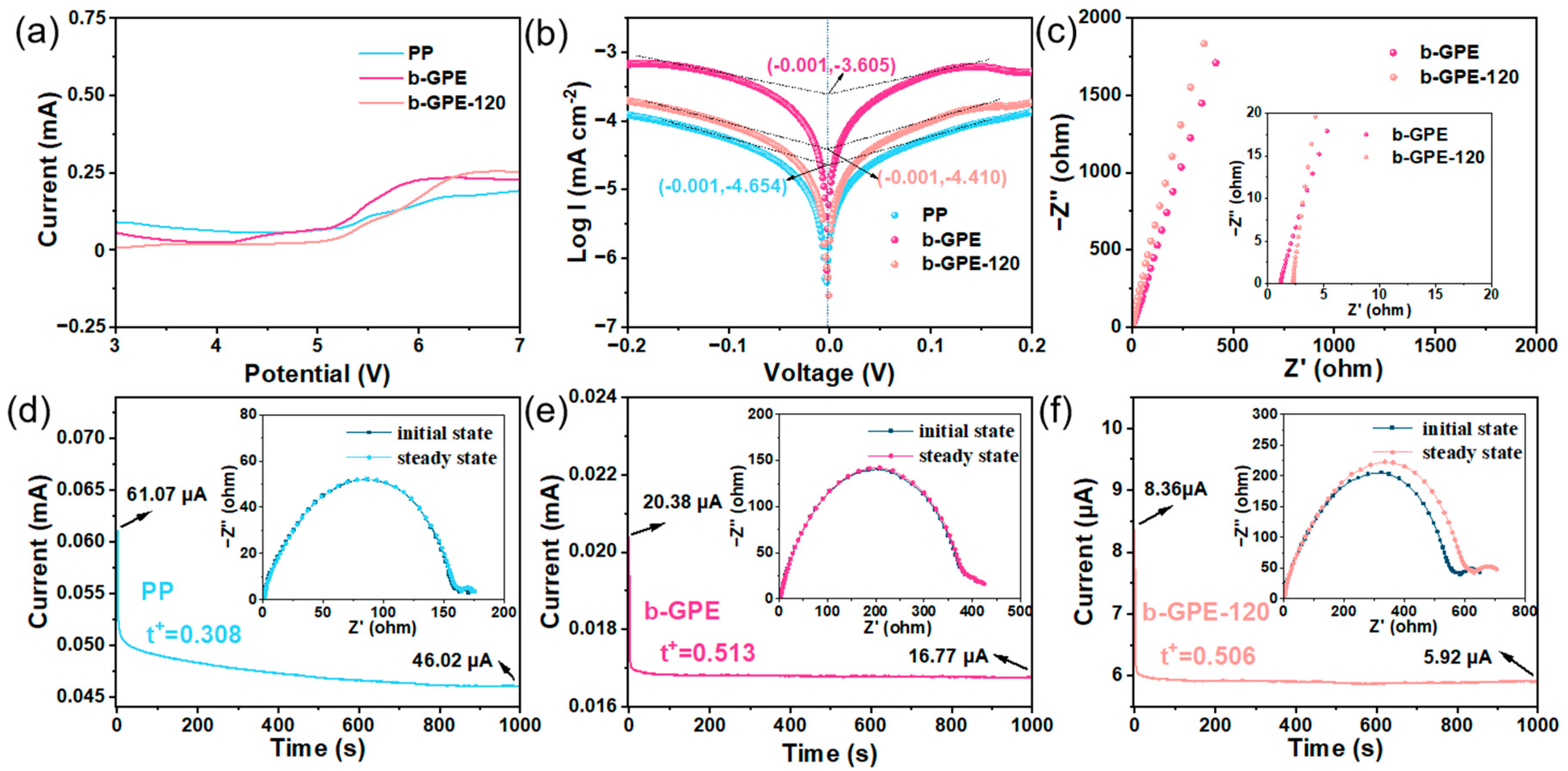
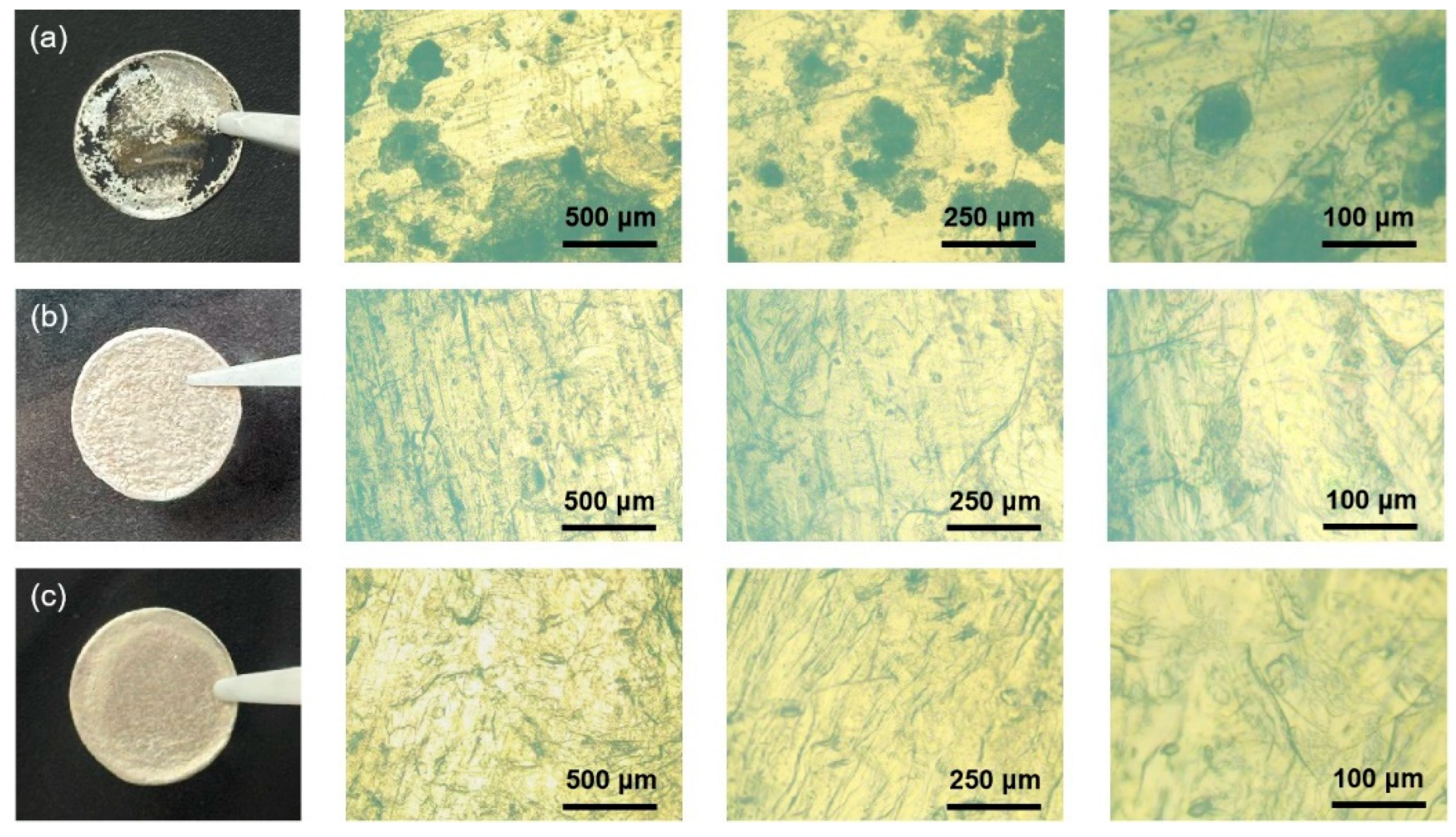
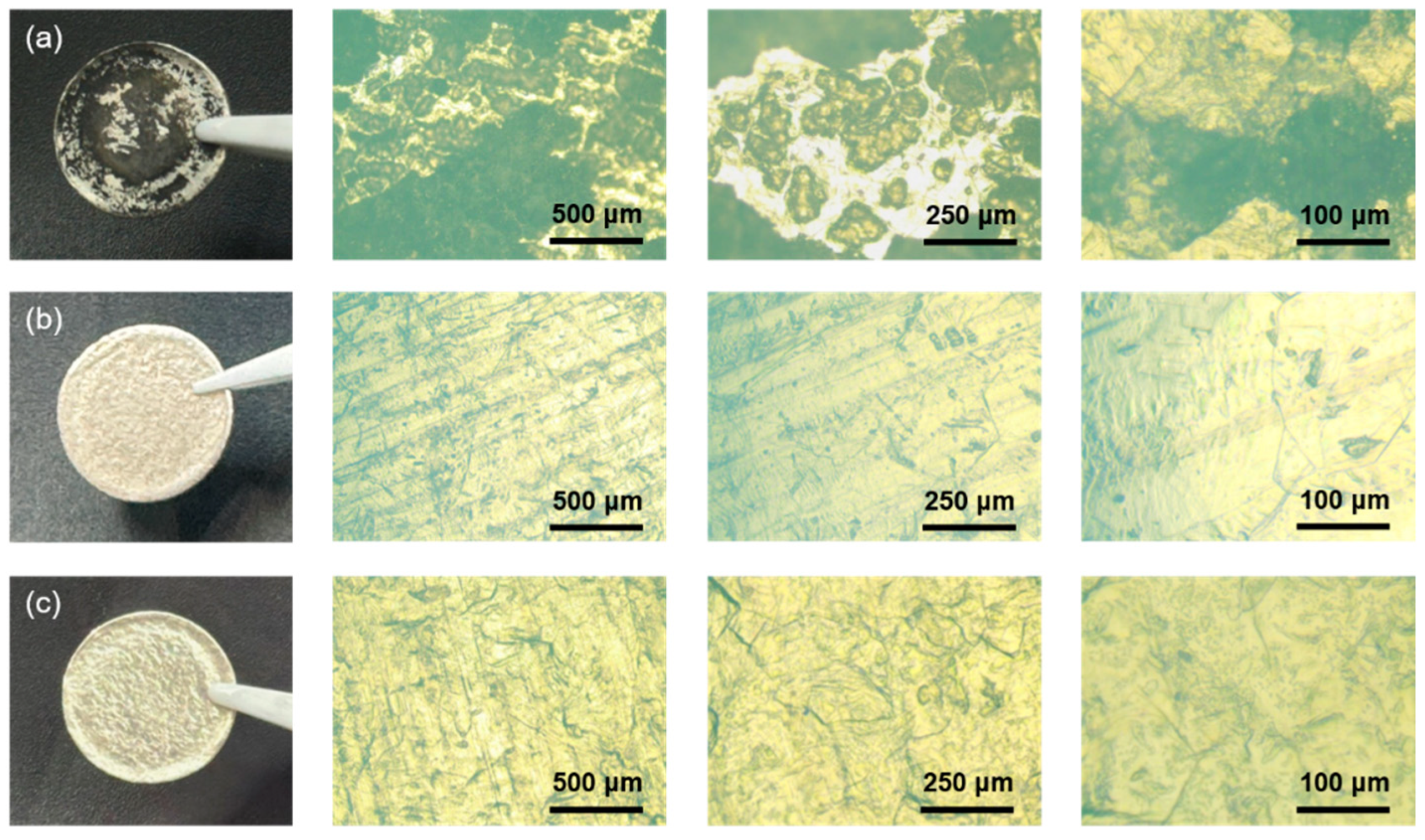
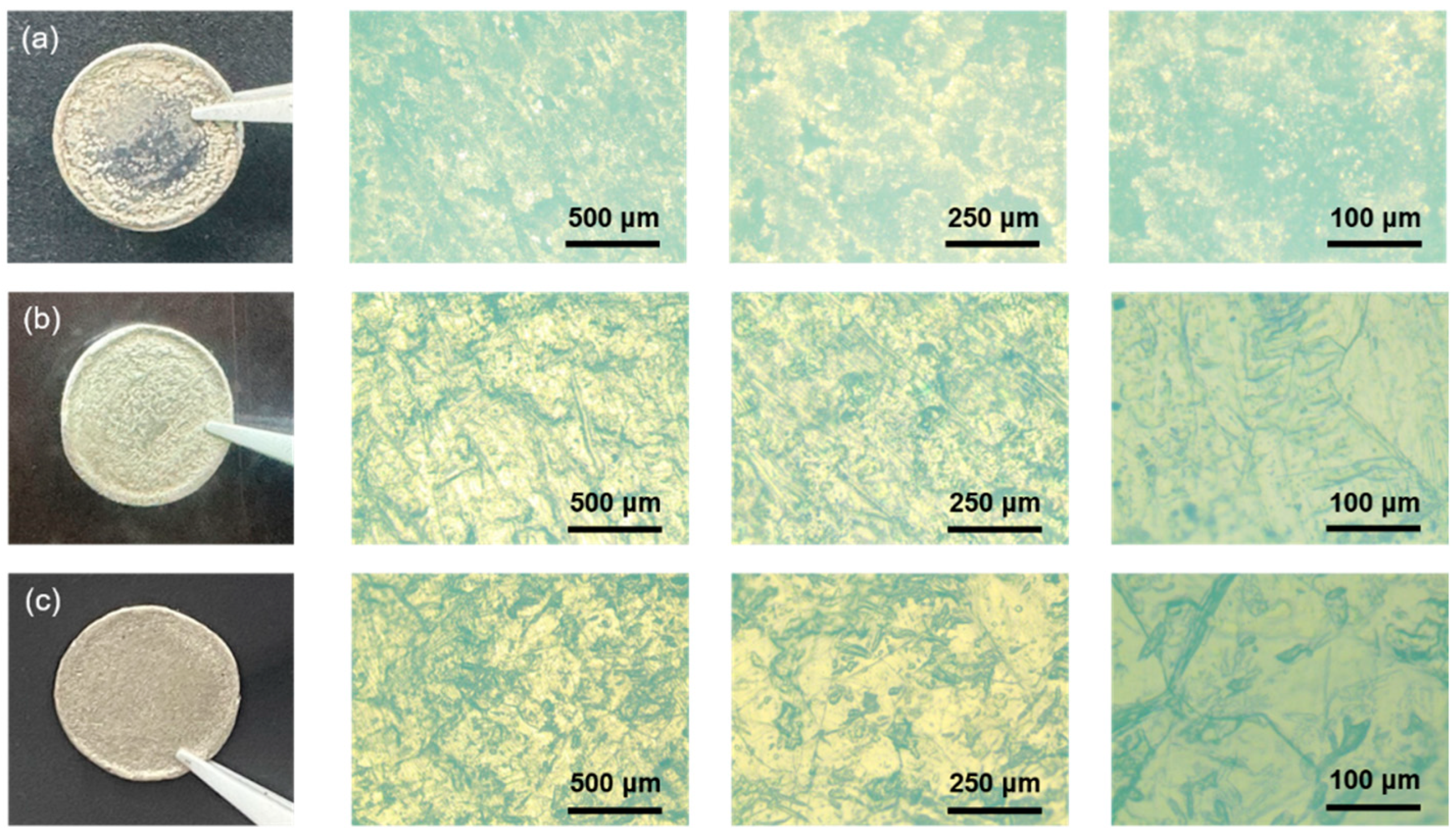
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Zhu, M.; Kang, P.; Zhu, T.; Yuan, H.; Lan, J.; Yang, X.; Sui, G. Self-Enhancing Gel Polymer Electrolyte by In Situ Construction for Enabling Safe Lithium Metal Battery. Adv. Sci. 2022, 9, 2103663. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Liu, Z.K.; Wang, Y.; Li, N.W.; Yu, L. Multiscale Structural Gel Polymer Electrolytes with Fast Li+ Transport for Long-Life Li Metal Batteries. Adv. Funct. Mater. 2023, 33, 2209837. [Google Scholar] [CrossRef]
- Zhou, Q.; Fu, C.; Li, R.; Zhang, X.; Xie, B.; Gao, Y.; Yin, G.; Zuo, P. Poly (vinyl ethylene carbonate)-based dual-salt gel polymer electrolyte enabling high voltage lithium metal batteries. Chem. Eng. J. 2022, 437, 135419. [Google Scholar] [CrossRef]
- Luo, B.; Wang, W.; Wang, Q.; Ji, W.; Yu, G.; Liu, Z.; Zhao, Z.; Wang, X.; Wang, S.; Zhang, J. Facilitating ionic conductivity and interfacial stability via oxygen vacancies-enriched TiO2 microrods for composite polymer electrolytes. Chem. Eng. J. 2023, 460, 141329. [Google Scholar] [CrossRef]
- Xie, Z.; Lai, Q.; Dou, Y.; Chen, X.; Yang, Y. Electrospun gel composite electrolyte of solvent-free SiO2 nanofluids coupled polyacrylonitrile nanofibers for high-rate lithium-metal batteries. Chem. Eng. J. 2024, 481, 148510. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Liu, Y.; Li, Y.; Zhang, H.; He, X. Safety perceptions of solid-state lithium metal batteries. Etransportation 2023, 16, 100239. [Google Scholar] [CrossRef]
- Chen, W.; Xing, Z.; Wei, Y.; Zhang, X.; Zhang, Q. High thermal safety and conductivity gel polymer electrolyte composed of ionic liquid [EMIM][BF4] and PVDF-HFP for EDLCs. Polymer 2023, 268, 125727. [Google Scholar] [CrossRef]
- Chen, S.; Wen, K.; Fan, J.; Bando, Y.; Golberg, D. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: From liquid to solid electrolytes. J. Mater. Chem. A 2018, 6, 11631–11663. [Google Scholar] [CrossRef]
- Li, L.-X.; Li, R.; Huang, Z.-H.; Liu, M.-Q.; Xiang, J.; Shen, X.-Q.; Jing, M.-X. High-performance gel electrolyte for enhanced interface compatibility and lithium metal stability in high-voltage lithium battery. Colloid Surf. A 2022, 651, 129665. [Google Scholar] [CrossRef]
- Li, R.; Chen, Q.; Jian, J.; Hou, Y.; Liu, Y.; Liu, J.; Xie, H.; Zhu, J. A 3D network-structured gel polymer electrolyte with soluble starch for enhanced quasi-solid-state lithium-sulfur batteries. J. Power Sources 2024, 624, 235521. [Google Scholar] [CrossRef]
- Liu, K.; Liu, M.; Cheng, J.; Dong, S.; Wang, C.; Wang, Q.; Zhou, X.; Sun, H.; Chen, X.; Cui, G. Novel cellulose/polyurethane composite gel polymer electrolyte for high performance lithium batteries. Electrochim. Acta 2016, 215, 261–266. [Google Scholar] [CrossRef]
- Nie, L.; Gao, R.; Zhang, M.; Zhu, Y.; Wu, X.; Lao, Z.; Zhou, G. Integration of Porous High-Loading Electrode and Gel Polymer Electrolyte for High-Performance Quasi-Solid-State Battery. Adv. Energy Mater. 2024, 14, 2302476. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Song, K.; Chen, W. An effective solid-electrolyte interphase for stable solid-state batteries. Chem 2021, 7, 3195–3197. [Google Scholar] [CrossRef]
- Li, Z.-W.; Liang, Y.-L.; Wang, J.; Yan, J.-M.; Liu, J.-W.; Huang, G.; Liu, T.; Zhang, X.-B. An In Situ Gelled Polymer Electrolyte to Stabilize Lithium–Air Batteries. Adv. Energy Mater. 2024, 14, 2304463. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Shuai, H.; Luo, Z.; Wang, B.; Fang, S.; Zou, G.; Hou, H.; Peng, H.; Ji, X. Recent advances of composite electrolytes for solid-state Li batteries. J. Energy Chem. 2022, 67, 524–548. [Google Scholar] [CrossRef]
- Geng, Z.; Huang, Y.; Sun, G.; Chen, R.; Cao, W.; Zheng, J.; Li, H. In-situ polymerized solid-state electrolytes with stable cycling for Li/LiCoO2 batteries. Nano Energy 2022, 91, 106679. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Chen, X.; Zhou, J.; Xue, J.; Zhao, H.; Wang, C.; Liu, F.; Li, L. In Situ Forming Asymmetric Gel Polymer Electrolyte Enhances the Performance of High-Voltage Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2024, 16, 55395–55406. [Google Scholar] [CrossRef]
- Gou, J.; Zhang, Z.; Wang, S.; Huang, J.; Cui, K.; Wang, H. An Ultrahigh Modulus Gel Electrolytes Reforming the Growing Pattern of Li Dendrites for Interfacially Stable Lithium-Metal Batteries. Adv. Mater. 2024, 36, 2309677. [Google Scholar] [CrossRef]
- Chen, M.; Fan, H.; Zhang, Y.; Liang, X.; Chen, Q.; Xia, X. Coupling PEDOT on Mesoporous Vanadium Nitride Arrays for Advanced Flexible All-Solid-State Supercapacitors. Small 2020, 16, 2003434. [Google Scholar] [CrossRef]
- Cui, S.; Wu, X.; Yang, Y.; Fei, M.; Liu, S.; Li, G.; Gao, X.-P. Heterostructured Gel Polymer Electrolyte Enabling Long-Cycle Quasi-Solid-State Lithium Metal Batteries. ACS Energy Lett. 2022, 7, 42–52. [Google Scholar] [CrossRef]
- Manuel Stephan, A. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, N.; Chen, J.; Cheng, B.; Kang, W. The recent research progress and prospect of gel polymer electrolytes in lithium-sulfur batteries. Chem. Eng. J. 2021, 413, 127427. [Google Scholar] [CrossRef]
- Guo, M.; Xiong, J.; Jin, X.; Lu, S.; Zhang, Y.; Xu, J.; Fan, H. Mussel stimulated modification of flexible Janus PAN/PVDF-HFP nanofiber hybrid membrane for advanced lithium-ion batteries separator. J. Membr. Sci. 2023, 675, 121533. [Google Scholar] [CrossRef]
- Jagadesan, P.; Cui, J.; Kalami, S.; Abrha, L.H.; Lee, H.; Khani, H. Perfluorinated Single-Ion Li+ Conducting Polymer Electrolyte for Lithium-Metal Batteries. J. Electrochem. Soc. 2024, 171, 1945. [Google Scholar] [CrossRef]
- Li, R.; Hua, H.; Yang, X.; Tian, J.; Chen, Q.; Huang, R.; Li, X.; Zhang, P.; Zhao, J. The deconstruction of a polymeric solvation cage: A critical promotion strategy for PEO-based all-solid polymer electrolytes. Energy Environ. Sci. 2024, 17, 5601–5612. [Google Scholar] [CrossRef]
- Long, M.-C.; Wang, T.; Duan, P.-H.; Gao, Y.; Wang, X.-L.; Wu, G.; Wang, Y.-Z. Thermotolerant and fireproof gel polymer electrolyte toward high-performance and safe lithium-ion battery. J. Energy Chem. 2022, 65, 9–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Bahi, A.; Ko, F.; Liu, J. Polyacrylonitrile-Reinforced Composite Gel Polymer Electrolytes for Stable Potassium Metal Anodes. Small 2022, 18, 2107186. [Google Scholar] [CrossRef]
- Chiu, L.-L.; Chung, S.-H. Composite gel-polymer electrolyte for high-loading polysulfide cathodes. J. Mater. Chem. A 2022, 10, 13719–13726. [Google Scholar] [CrossRef]
- Costa, C.M.; Cardoso, V.F.; Martins, P.; Correia, D.M.; Gonçalves, R.; Costa, P.; Correia, V.; Ribeiro, C.; Fernandes, M.M.; Martins, P.M.; et al. Smart and Multifunctional Materials Based on Electroactive Poly(vinylidene fluoride): Recent Advances and Opportunities in Sensors, Actuators, Energy, Environmental, and Biomedical Applications. Chem. Rev. 2023, 123, 11392–11487. [Google Scholar] [CrossRef]
- Konishi, Y.; Kokubo, H.; Tsuzuki, S.; Tatara, R.; Dokko, K. A partially fluorinated polymer network enhances the Li-ion transference number of sulfolane-based highly concentrated electrolytes. Chem. Comm. 2024, 60, 12896–12899. [Google Scholar] [CrossRef]
- Xu, D.; Su, J.; Jin, J.; Sun, C.; Ruan, Y.; Chen, C.; Wen, Z. In Situ Generated Fireproof Gel Polymer Electrolyte with Li6.4Ga0.2La3Zr2O12 As Initiator and Ion-Conductive Filler. Adv. Energy Mater. 2019, 9, 1900611. [Google Scholar] [CrossRef]
- Hu, X.; Liu, K.; Zhang, S.; Shao, G.; Silva, S.R.P.; Zhang, P. A functional gel polymer electrolyte based on PVDF-HFP/gelatin toward dendrite-free lithium metal batteries. Nano Res. 2023, 17, 2790–2799. [Google Scholar] [CrossRef]
- Guo, Q.; Han, Y.; Wang, H.; Xiong, S.; Liu, S.; Zheng, C.; Xie, K. Preparation and characterization of nanocomposite ionic liquid-based gel polymer electrolyte for safe applications in solid-state lithium battery. Solid. State Ion. 2018, 321, 48–54. [Google Scholar] [CrossRef]
- Chen, Z.; Guan, M.; Cheng, Y.; Li, H.; Ji, G.; Chen, H.; Fu, X.; Awuye, D.E.; Zhu, Y.; Yin, X.; et al. Boehmite-enhanced poly(vinylidene fluoride-co-hexafluoropropylene)/polyacrylonitrile (PVDF-HFP/PAN) coaxial electrospun nanofiber hybrid membrane: A superior separator for lithium-ion batteries. Adv. Compos. Hybrid. Mater. 2023, 6, 219. [Google Scholar] [CrossRef]
- Tan, E.; Peng, W.; Li, Q.; Wang, D.; Li, X.; Duan, J.; Guo, H.; Yan, G.; Wang, J.; Wang, Z. Synergy of PVDF-HFP and ZIF-8 Promotes PEO Electrolyte Towards All-solid-state Lithium Battery. Electrochim. Acta 2023, 473, 143469. [Google Scholar] [CrossRef]
- Hu, X.; Silva, S.R.P.; Zhang, P.; Liu, K.; Zhang, S.; Shao, G. A multifunctional fire-retardant gel electrolyte to enable Li-S batteries with higher Li-ion conductivity and effectively inhibited shuttling of polysulfides. Chem. Eng. J. 2023, 467, 143378. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Liu, R.-J.; Hsu, C.-H.; Kuo, P.-L. High thermal and electrochemical stability of PVDF-graft-PAN copolymer hybrid PEO membrane for safety reinforced lithium-ion battery. RSC Adv. 2016, 6, 18082–18088. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; He, Y.; Li, F.; Ouyang, K.; Ma, D.; Feng, J.; Huang, J.; Zhao, J.; Yang, M.; et al. A Novel Ultrathin Multiple-Kinetics-Enhanced Polymer Electrolyte Editing Enabled Wide-Temperature Fast-Charging Solid-State Zinc Metal Batteries. Adv. Funct. Mater. 2023, 34, 2307736. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Gu, Q.; Li, P.; Xu, H.; Deng, Y.; Gao, P. 1 µm-Thick Robust Gel Polymer Electrolyte with Excellent Interfacial Stability for High-Performance Li Metal Batteries. Adv. Funct. Mater. 2024, 35, 2412287. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Zhang, W.; Jiang, X.; Han, L.; Wang, X.; Kan, Y.; Song, L.; Hu, Y. Flame-retardant in-situ formed gel polymer electrolyte with different valance states of phosphorus structures for high-performance and fire-safety lithium-ion batteries. Chem. Eng. J. 2024, 490, 151568. [Google Scholar] [CrossRef]
- Liu, S.; Tian, W.; Shen, J.; Wang, Z.; Pan, H.; Kuang, X.; Yang, C.; Chen, S.; Han, X.; Quan, H.; et al. Bioinspired gel polymer electrolyte for wide temperature lithium metal battery. Nat. Commun. 2025, 16, 2474. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qin, S.; Wang, Z.; Kui, M.; Cheng, D.; Xiao, Y.; Ren, Y.; Zhang, S.; Chen, J.; Xia, X.; et al. Synergistic enhancement effect of G4 and SN in gel polymer electrolyte reinforced by PET nonwoven for lithium metal batteries. Nano Energy 2025, 133, 110454. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Z.; Song, M.; Zhang, Y.; Jing, C.; Chen, L.; Ji, X.; Wei, W. Highly Oxidation-Resistant Ether Gel Electrolytes for 4.7 V High-Safety Lithium Metal Batteries. Adv. Energy Mater. 2023, 13, 2203870. [Google Scholar] [CrossRef]





| Matrix | Ionic Conductivity | Security | Mechanical Strength | Temperature Range | Disadvantages |
|---|---|---|---|---|---|
| PEO | ~10−4 S cm−1 | high | high | 40–100 °C | limited electrochemical stability |
| PAN | ~10−4 S cm−1 | high | high | - | passivation on contact with electrodes |
| PMMA | ~10−3 S cm−1 | high | low | −20–125 °C | poor mechanical strength |
| PVDF | ~10−3 S cm−1 | high | high | <150 °C | higher cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, X.; Li, X.; Xin, X. Gel Polymer Electrolytes with High Thermal Stability for Safe Lithium Metal Batteries. Colloids Interfaces 2025, 9, 30. https://doi.org/10.3390/colloids9030030
Chen X, Wang X, Li X, Xin X. Gel Polymer Electrolytes with High Thermal Stability for Safe Lithium Metal Batteries. Colloids and Interfaces. 2025; 9(3):30. https://doi.org/10.3390/colloids9030030
Chicago/Turabian StyleChen, Xianhui, Xue Wang, Xing Li, and Xing Xin. 2025. "Gel Polymer Electrolytes with High Thermal Stability for Safe Lithium Metal Batteries" Colloids and Interfaces 9, no. 3: 30. https://doi.org/10.3390/colloids9030030
APA StyleChen, X., Wang, X., Li, X., & Xin, X. (2025). Gel Polymer Electrolytes with High Thermal Stability for Safe Lithium Metal Batteries. Colloids and Interfaces, 9(3), 30. https://doi.org/10.3390/colloids9030030





