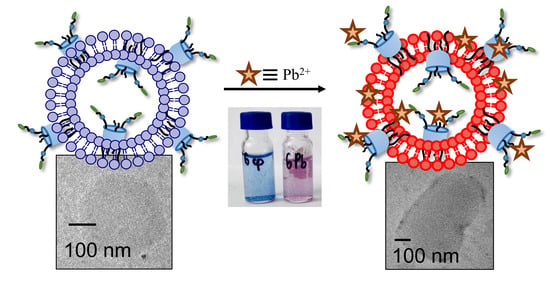
Sulfonate Thiacalixarene-Modified Polydiacetylene Vesicles as Colorimetric Sensors for Lead Ion Detection
Abstract
1. Introduction
2. Materials and Methods
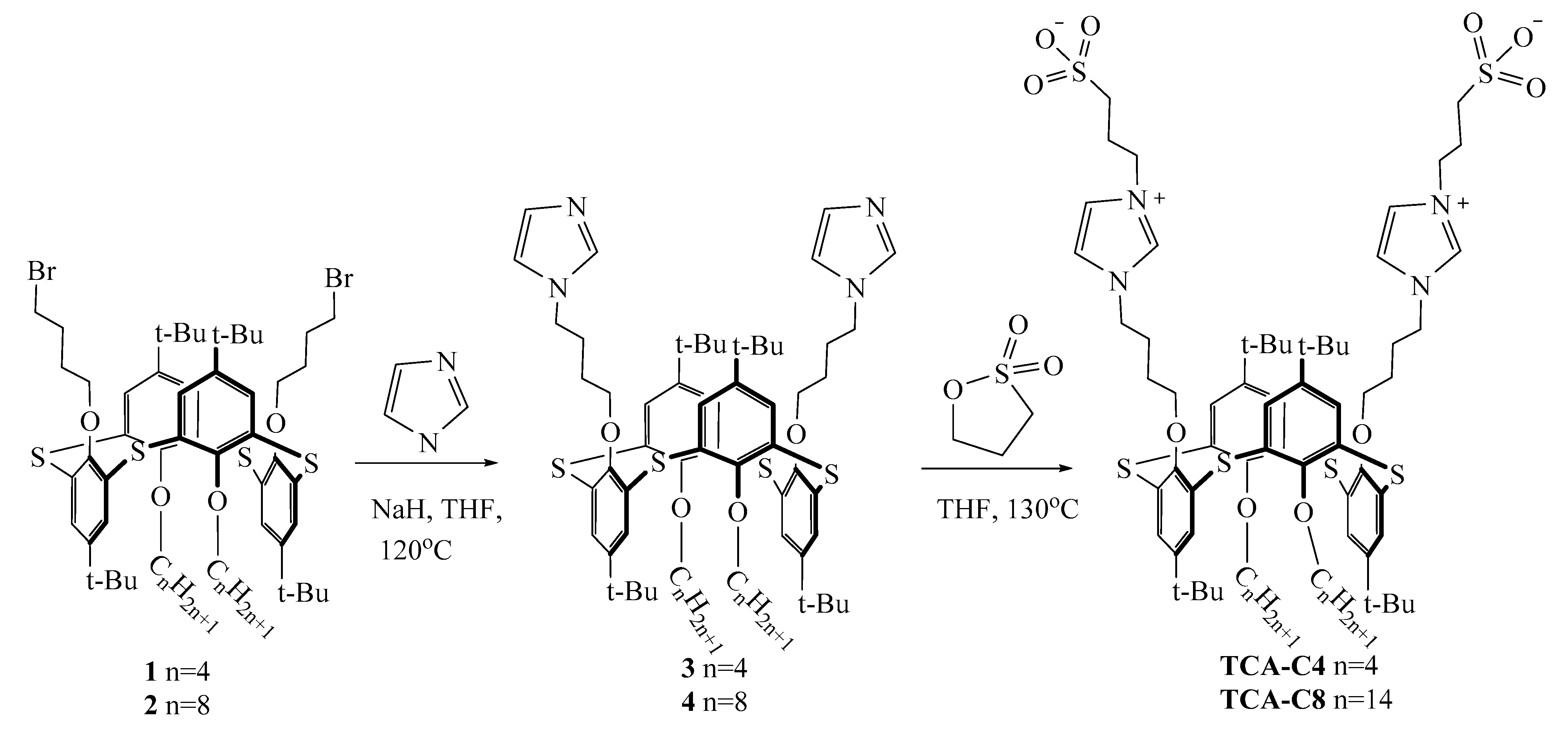
2.1. General Procedure for the Synthesis of Imidazole-Thiacalixarene Derivatives
- 5,11,17,23-tetra-tret-butyl-25,27-dibutyloxy-26,28-bis[1-(imidazole) butyloxy]-2,8,14,20-tetra-thiacalix[4]arene (3)
- 5,11,17,23-tetra-tret-butyl-25,27-dioctyloxy-26,28-bis[1-(imidazole) butyloxy]-2,8,14,20-tetra-thiacalix[4]arene (4)
2.2. General Procedure for the Synthesis of N-Sulfopropylimidazole Derivatives
- 5,11,17,23-tetra-tret-butyl-25,27-dibutyloxy-26,28-bis[4-(3-N-sulfopropylimidazolium) butyloxy-]-2,8,14,20-tetra-thiacalix[4]arene
- 5,11,17,23-tetra-tret-butyl-25,27-dioctyloxy-26,28-bis[4-(3-N-sulfopropylimidazolium) butyloxy-]-2,8,14,20-tetra-thiacalix[4]arene
2.3. General Procedure of PDA and Modified PDA Preparation
3. Results and Discussion
3.1. Synthesis of Zwitterionic Sulfonate Derivatives of Thiacalix[4]arenes
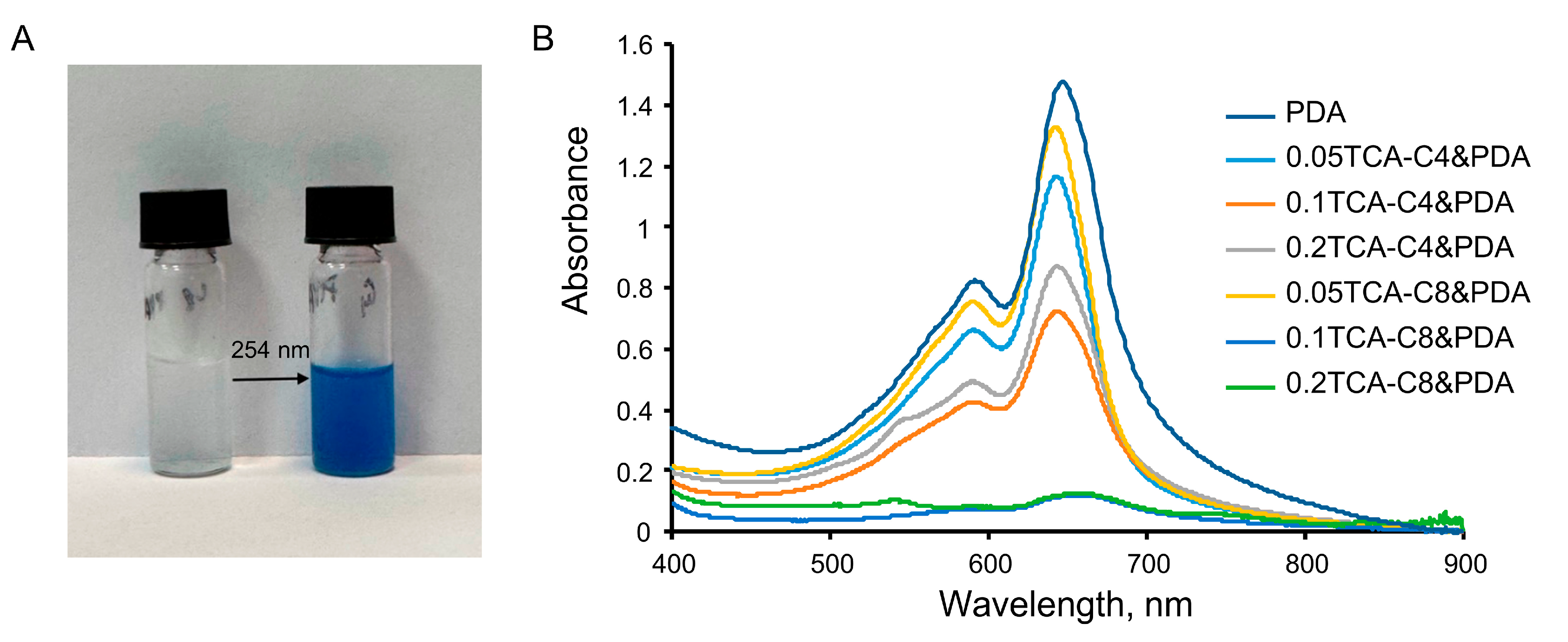
3.2. Preparation of Modified Thiacalixarene-PDA Systems
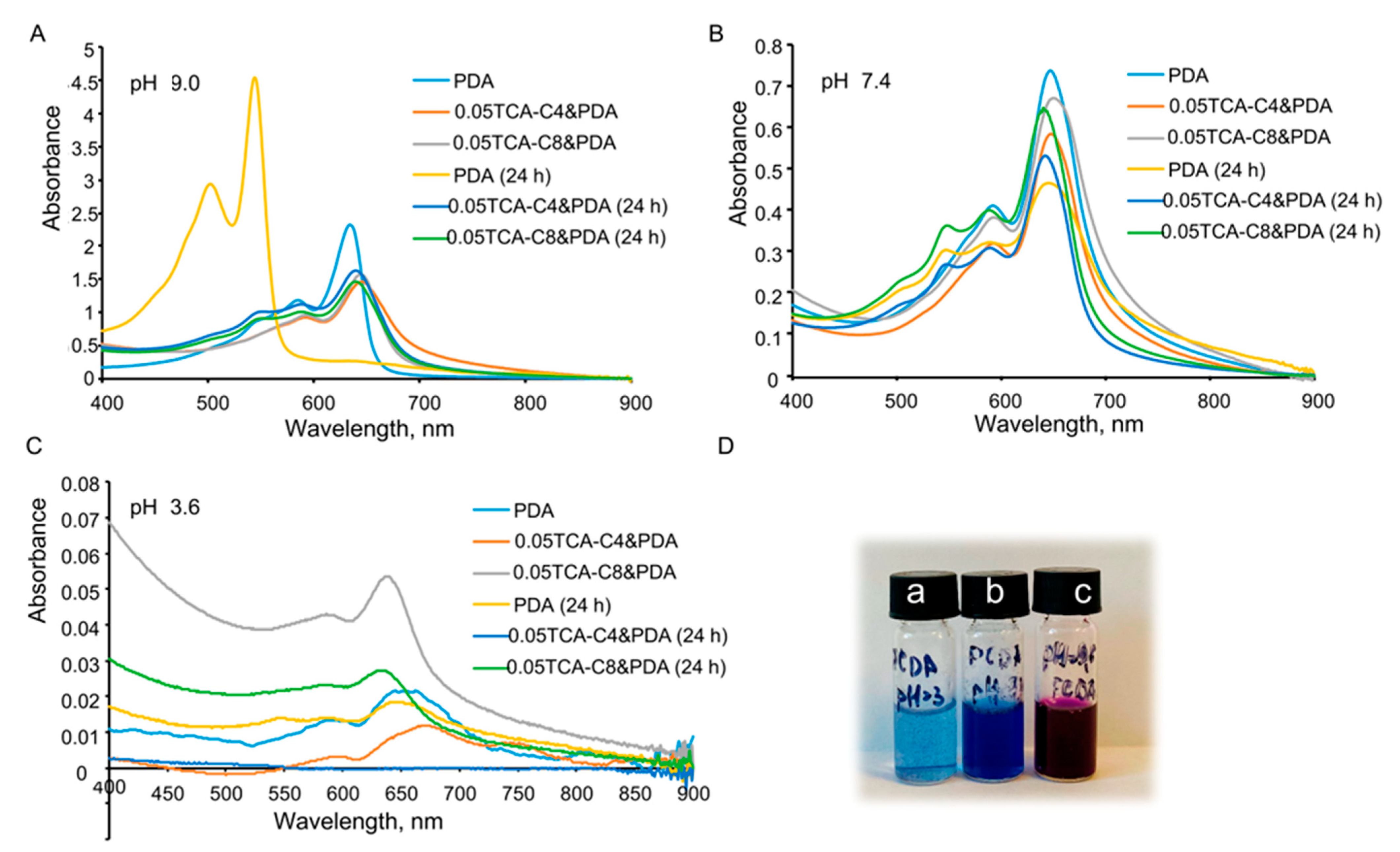
3.3. The Effect of pH on Polymerization Thiacalixarene-Modified Polydiacetylene Vesicles
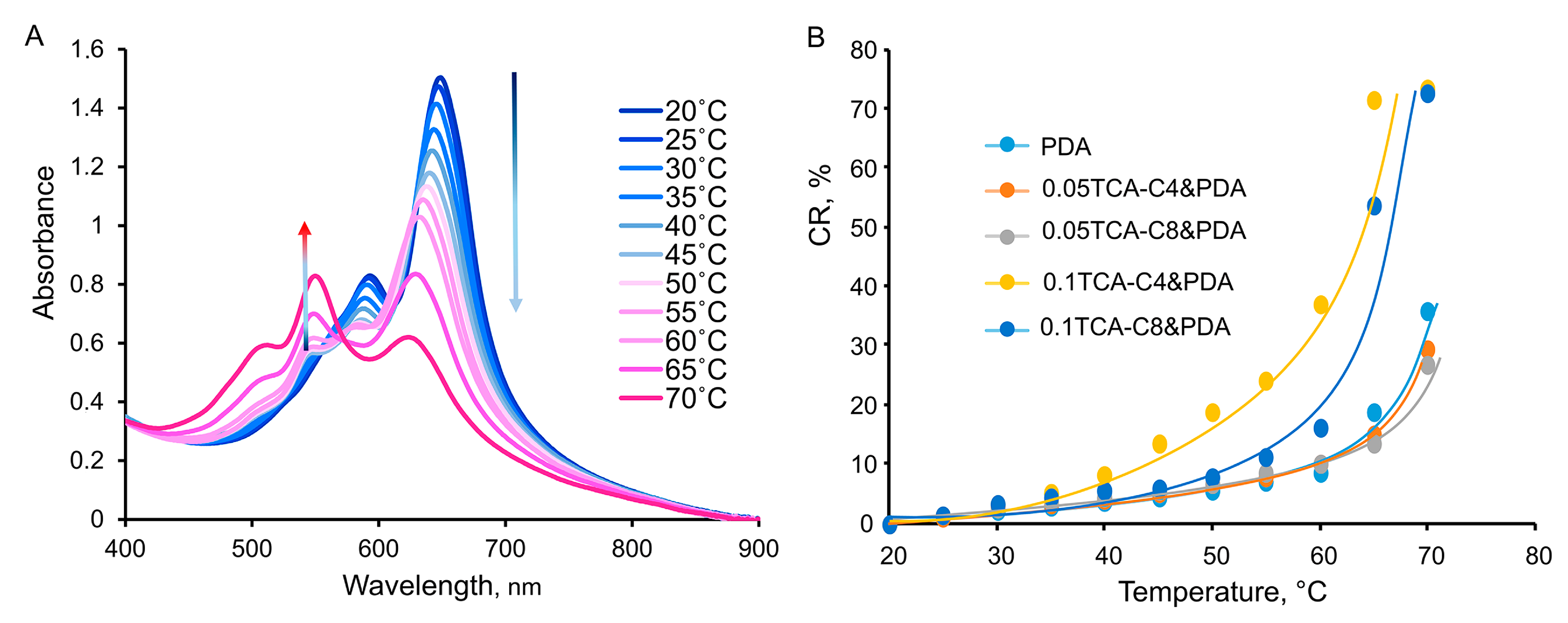
3.4. Thermochromism of Thiacalixarene-Modified Polydiacetylene Vesicles
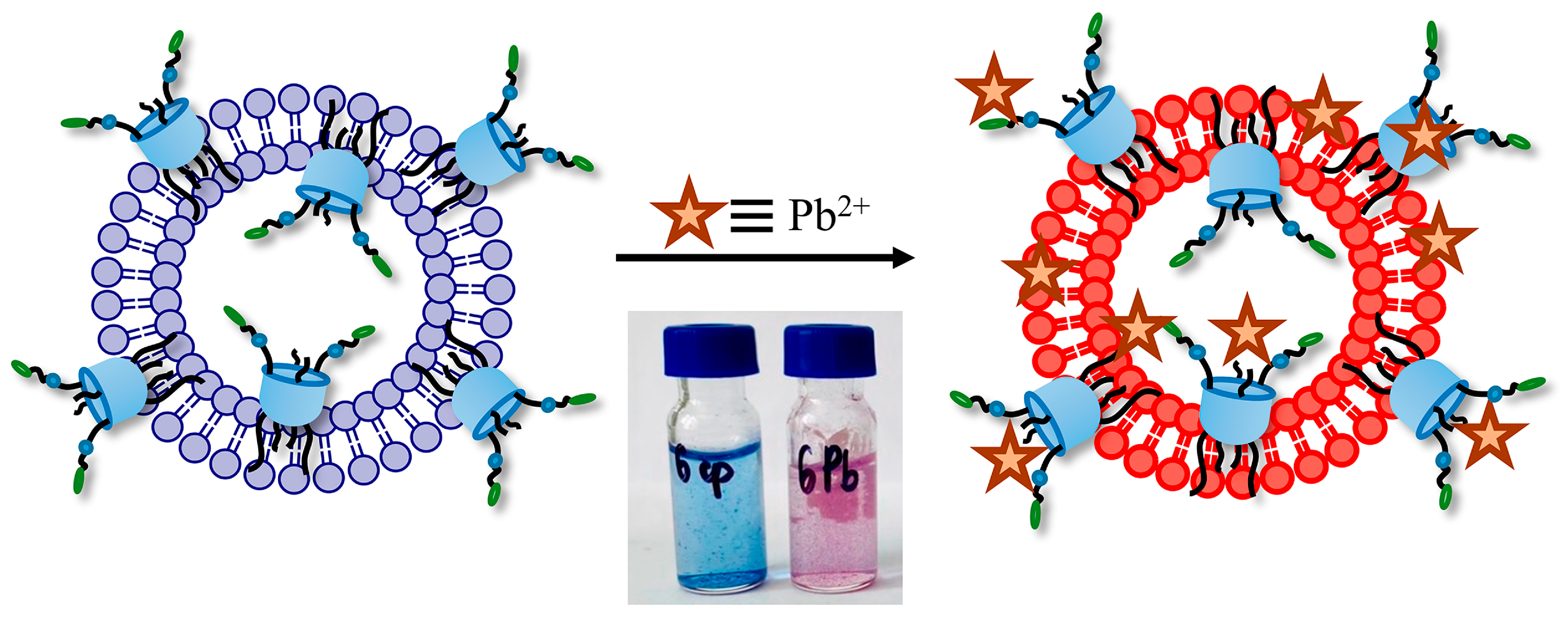
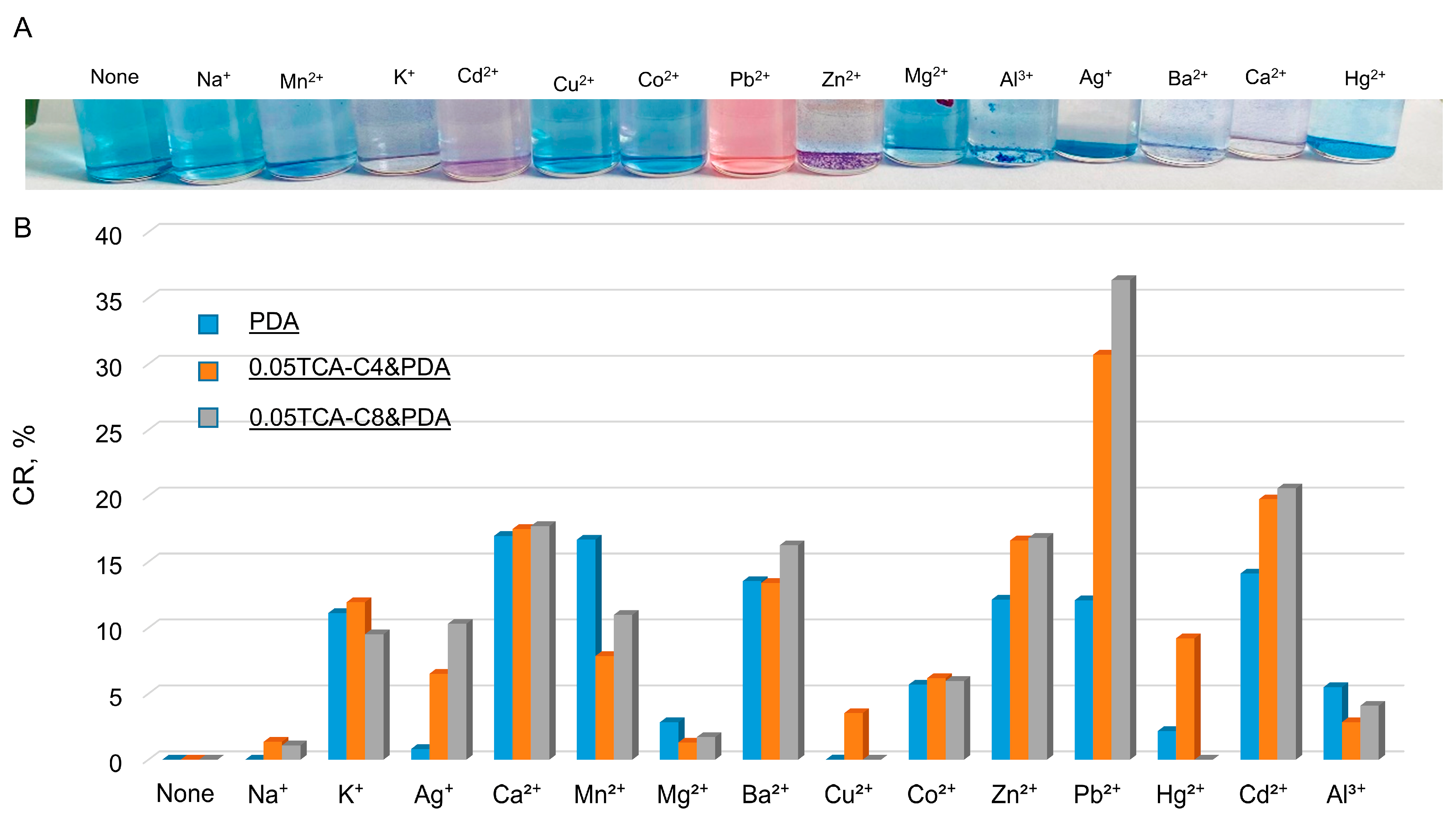
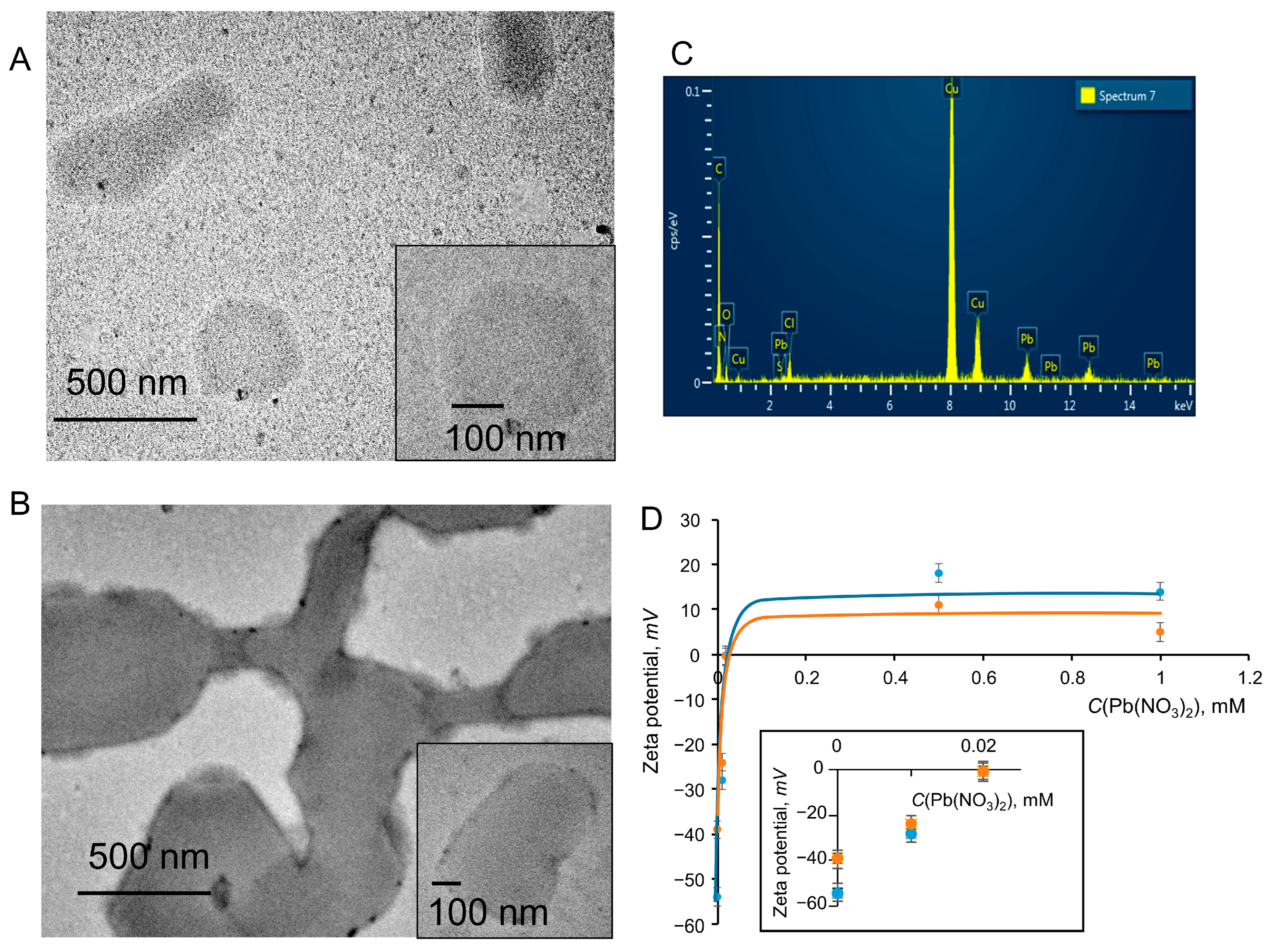
3.5. The Application of PDA Systems as Sensors for Metal Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, F.; Anslyn, E.V. Introduction: Supramolecular Chemistry. Chem. Rev. 2015, 115, 6999–7000. [Google Scholar] [CrossRef] [PubMed]
- Lackinger, M.; Heckl, W.M. Carboxylic Acids: Versatile Building Blocks and Mediators for Two-Dimensional Supramolecular Self-Assembly. Langmuir 2009, 25, 11307–11321. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Lee, S.-L. Supramolecular Chemistry: Host–Guest Molecular Complexes. Molecules 2021, 26, 3995. [Google Scholar] [CrossRef]
- Truong, P.L.; Yin, Y.; Lee, D.; Ko, S.H. Advancement in COVID-19 detection using nanomaterial-based biosensors. Exploration 2023, 3, 20210232. [Google Scholar] [CrossRef] [PubMed]
- Cebula, J.; Fink, K.; Boratyński, J.; Goszczyński, T.M. Supramolecular chemistry of anionic boron clusters and its applications in biology. Coord. Chem. Rev. 2023, 477, 214940. [Google Scholar] [CrossRef]
- Friedman, S.; Kolusheva, S.; Volinsky, R.; Zeiri, L.; Schrader, T.; Jelinek, R. Lipid/Polydiacetylene Films for Colorimetric Protein Surface-Charge Analysis. Anal. Chem. 2008, 80, 7804–7811. [Google Scholar] [CrossRef]
- Akhmadeev, B.S.; Podyachev, S.N.; Katsyuba, S.A.; Spicher, S.; Sudakova, S.N.; Gimazetdinova, G.S.; Syakaev, V.V.; Sinyashin, O.G.; Mustafina, A.R. The incorporation of upper vs. lower rim substituted thia- and calix[4]arene ligands into polydiacethylene polymeric bilayers for rational design of sensors to heavy metal ions. Polymer 2022, 245, 124728. [Google Scholar] [CrossRef]
- Chernikova, E.Y.; Berdnikova, D.V. Cucurbiturils in nucleic acids research. Chem. Commun. 2020, 56, 15360–15376. [Google Scholar] [CrossRef]
- Sharma, V.S.; Vishwakarma, V.K.; Shrivastav, P.S.; Ammathnadu Sudhakar, A.; Sharma, A.S.; Shah, P.A. Calixarene Functionalized Supramolecular Liquid Crystals and Their Diverse Applications. ACS Omega 2022, 7, 45752–45796. [Google Scholar] [CrossRef]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, W.; Ai, K.; Liu, J. Highly Sensitive Polydiacetylene Ensembles for Biosensing and Bioimaging. Front. Chem. 2020, 8, 565782. [Google Scholar] [CrossRef]
- Pankaew, A.; Traiphol, N.; Traiphol, R. Tuning the sensitivity of polydiacetylene-based colorimetric sensors to UV light and cationic surfactant by co-assembling with various polymers. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125626. [Google Scholar] [CrossRef]
- Jannah, F.; Kim, J.-H.; Lee, J.-W.; Kim, J.-M.; Kim, J.-M.; Lee, H. Immobilized Polydiacetylene Lipid Vesicles on Polydimethylsiloxane Micropillars as a Surfactin-Based Label-Free Bacterial Sensor Platform. Front. Mater. 2018, 5, 57. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-Y.; Chen, X.; Yoon, J. Recent progress in stimuli-induced polydiacetylenes for sensing temperature, chemical and biological targets. Chem. Commun. 2016, 52, 9178–9196. [Google Scholar] [CrossRef]
- Okada, S.; Peng, S.; Spevak, W.; Charych, D. Color and Chromism of Polydiacetylene Vesicles. Acc. Chem. Res. 1998, 31, 229–239. [Google Scholar] [CrossRef]
- Chakarborty, S.; Suklabaidya, S.; Bhattacharjee, D.; Arshad Hussain, S. Polydiacetylene (PDA) Film: A unique sensing element. Mater. Today Proc. 2018, 5, 2367–2372. [Google Scholar] [CrossRef]
- Jannah, F.; Kim, J.-M. pH-sensitive colorimetric polydiacetylene vesicles for urease sensing. Dye. Pigment. 2019, 169, 15–21. [Google Scholar] [CrossRef]
- He, L.; Lu, Y.; Wang, F.; Gao, X.; Chen, Y.; Liu, Y. Bare eye detection of Hg(II) ions based on enzyme inhibition and using mercaptoethanol as a reagent to improve selectivity. Microchim. Acta 2018, 185, 174. [Google Scholar] [CrossRef]
- Akhmadeev, B.S.; Retyunskaya, O.O.; Podyachev, S.N.; Katsyuba, S.A.; Gubaidullin, A.T.; Sudakova, S.N.; Syakaev, V.V.; Babaev, V.M.; Sinyashin, O.G.; Mustafina, A.R. Supramolecular Optimization of Sensory Function of a Hemicurcuminoid through Its Incorporation into Phospholipid and Polymeric Polydiacetylenic Vesicles: Experimental and Computational Insight. Polymers 2023, 15, 714. [Google Scholar] [CrossRef]
- Chen, X.; Kang, S.; Kim, M.J.; Kim, J.; Kim, Y.S.; Kim, H.; Chi, B.; Kim, S.; Lee, J.Y.; Yoon, J. Thin-Film Formation of Imidazolium-Based Conjugated Polydiacetylenes and Their Application for Sensing Anionic Surfactants. Angew. Chem. Int. Ed. 2010, 49, 1422–1425. [Google Scholar] [CrossRef]
- Meng, Y.; Jiang, J.; Liu, M. Self-assembled nanohelix from a bolaamphiphilic diacetylene via hydrogelation and selective responsiveness towards amino acids and nucleobases. Nanoscale 2017, 9, 7199–7206. [Google Scholar] [CrossRef]
- dos Santos Pires, A.C.; de Fátima Ferreira Soares, N.; da Silva, L.H.M.; de Andrade, N.J.; Silveira, M.F.A.; de Carvalho, A.F. Polydiacetylene as a Biosensor: Fundamentals and Applications in the Food Industry. Food Bioprocess Technol. 2010, 3, 172–181. [Google Scholar] [CrossRef]
- Demikhovsky, Y.; Kolusheva, S.; Geyzer, M.; Jelinek, R. Polydiacetylene-supported silica films formed at the air/water interface. J. Colloid Interface Sci. 2011, 364, 428–434. [Google Scholar] [CrossRef]
- Chanakul, A.; Traiphol, N.; Traiphol, R. Controlling the reversible thermochromism of polydiacetylene/zinc oxide nanocomposites by varying alkyl chain length. J. Colloid Interface Sci. 2013, 389, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, K. Polydiacetylene (PDA) Liposome-Based Immunosensor for the Detection of Exosomes. Biomacromolecules 2019, 20, 3392–3398. [Google Scholar] [CrossRef]
- Thakuri, A.; Acharya, R.; Banerjee, M.; Chatterjee, A. A polydiacetylene (PDA) based dual-mode optical sensor for the ppb level selective detection of biogenic polyamines. Sens. Actuators B Chem. 2024, 409, 135573. [Google Scholar] [CrossRef]
- Jang, H.; Jeon, J.; Shin, M.; Kang, G.; Ryu, H.; Kim, S.M.; Jeon, T.-J. Polydiacetylene (PDA) Embedded Polymer-Based Network Structure for Biosensor Applications. Gels 2025, 11, 66. [Google Scholar] [CrossRef]
- Yang, G.; Nie, Z.; Zhang, S.; Ge, Z.; Zhao, J.; Zhang, J.; Li, B. Sensitive Colorimetric Sensor for Lead Ions and VOCs Based on Histidine-Functionalized Polydiacetylene. Macromol. Res. 2020, 28, 1192–1197. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yin, X.; Ding, B.; Sun, G.; Ke, T.; Chen, J.; Yu, J. Colorimetric strips for visual lead ion recognition utilizing polydiacetylene embedded nanofibers. J. Mater. Chem. A 2014, 2, 18304–18312. [Google Scholar] [CrossRef]
- Wang, M.; Wang, F.; Wang, Y.; Zhang, W.; Chen, X. Polydiacetylene-based sensor for highly sensitive and selective Pb2+ detection. Dye. Pigment. 2015, 120, 307–313. [Google Scholar] [CrossRef]
- Lee, C.G.; Kang, S.; Oh, J.; Eom, M.S.; Oh, J.; Kim, M.-G.; Lee, W.S.; Hong, S.; Han, M.S. A colorimetric and fluorescent chemosensor for detection of Hg2+ using counterion exchange of cationic polydiacetylene. Tetrahedron Lett. 2017, 58, 4340–4343. [Google Scholar] [CrossRef]
- Chen, S.-W.; Chen, X.; Li, Y.; Yang, Y.; Dong, Y.; Guo, J.; Wang, J. Polydiacetylene-based colorimetric and fluorometric sensors for lead ion recognition. RSC Adv. 2022, 12, 22210–22218. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Kim, Y.K.; Park, J.B.; Jeon, S.; Ahn, J.; Yim, Y.; Yoon, J.; Lee, S. A Selective Colorimetric and Fluorometric Chemosensor Based on Conjugated Polydiacetylenes for Cadmium Ion Detection. ChemPhotoChem 2019, 3, 1133–1137. [Google Scholar] [CrossRef]
- Valiyakhmetova, A.M.; Sultanova, E.D.; Burilov, V.A.; Solovieva, S.E.; Antipin, I.S. New DNA-sensor based on thiacalix [4]arene-modified polydiacetylene particles. Russ. Chem. Bull. 2019, 68, 1067–1074. [Google Scholar] [CrossRef]
- Burilov, V.; Valiyakhmetova, A.; Mironova, D.; Safiullin, R.; Kadirov, M.; Ivshin, K.; Kataeva, O.; Solovieva, S.; Antipin, I. “Clickable” thiacalix[4]arene derivatives bearing polymerizable 1,3-butadiyne fragments: Synthesis and incorporation into polydiacetylene vesicles. RSC Adv. 2016, 6, 44873–44877. [Google Scholar] [CrossRef][Green Version]
- Sultanova, E.; Gazalieva, A.; Makarov, E.; Belov, R.; Evtugyn, V.; Burilov, V.; Solovieva, S.; Antipin, I. Novel aminocalixarene-modified polydiacetylene vesicles: Synthesis and naked-eye detection of ATP. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127642. [Google Scholar] [CrossRef]
- Needleman, H. Lead Poisoning. Annu. Rev. Med. 2004, 55, 209–222. [Google Scholar] [CrossRef]
- Finkelstein, Y. Low-level lead-induced neurotoxicity in children: An update on central nervous system effects. Brain Res. Rev. 1998, 27, 168–176. [Google Scholar] [CrossRef]
- Xing, H.; Song, Y.; Ma, L.; Wang, F.; Zhang, M.; Li, Z.; Wan, Z.; Cui, X.; Yang, G. Preparation of Bis-Polydiacetylene with Reversible Thermochromism and High Sensitivity toward Pb2+ Detection. ACS Appl. Polym. Mater. 2024, 6, 15035–15042. [Google Scholar] [CrossRef]
- Sagong, H.Y.; Son, M.H.; Park, S.W.; Kim, J.S.; Li, T.; Jung, Y.K. Dual-signal optical detection of Lead(II) ions (Pb2+) using galloyl group-functionalized polydiacetylene. Analytica Chimica Acta 2022, 1230, 340403. [Google Scholar] [CrossRef]
- Hu, X.J.; Zhang, P.; Li, Y.S. The Preparation of TCAS-Loaded Resin and its Adsorption Property to Pb2+ Ion. Adv. Mater. Res. 2011, 396–398, 421–424. [Google Scholar] [CrossRef]
- Armarego, W.L. Purification of Laboratory Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123821614. [Google Scholar]
- Sultanova, E.D.; Gafiatullin, B.K.; Ocherednyuk, E.A.; Garipova, R.I.; Volodina, A.A.; Daminova, A.G.; Evtugyn, V.G.; Burilov, V.A.; Solovieva, S.E.; Antipin, I.S. N-Oxyethylimidazolium Calix[4]arenes and Thiacalix[4]arenes: Difference in Solubilization Property and Detection of Adenine-Containing Nucleotides. Macroheterocycles 2023, 16, 168–176. [Google Scholar] [CrossRef]
- Kolusheva, S.; Zadmard, R.; Schrader, T.; Jelinek, R. Color Fingerprinting of Proteins by Calixarenes Embedded in Lipid/Polydiacetylene Vesicles. J. Am. Chem. Soc. 2006, 128, 13592–13598. [Google Scholar] [CrossRef]
- Weston, M.; Pham, A.-H.; Tubman, J.; Gao, Y.; Tjandra, A.D.; Chandrawati, R. Polydiacetylene-based sensors for food applications. Mater. Adv. 2022, 3, 4088–4102. [Google Scholar] [CrossRef]
- Wilk-Kozubek, M.; Potaniec, B.; Gazińska, P.; Cybińska, J. Exploring the Origins of Low-Temperature Thermochromism in Polydiacetylenes. Polymers 2024, 16, 2856. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Lee, S.; Kim, J.-M. Recent conceptual and technological advances in polydiacetylene-based supramolecular chemosensors. Chem. Soc. Rev. 2009, 38, 1958. [Google Scholar] [CrossRef]
- Lee, D.-C.; Sahoo, S.K.; Cholli, A.L.; Sandman, D.J. Structural Aspects of the Thermochromic Transition in Urethane-Substituted Polydiacetylenes. Macromolecules 2002, 35, 4347–4355. [Google Scholar] [CrossRef]
- Charoenthai, N.; Pattanatornchai, T.; Wacharasindhu, S.; Sukwattanasinitt, M.; Traiphol, R. Roles of head group architecture and side chain length on colorimetric response of polydiacetylene vesicles to temperature, ethanol and pH. J. Colloid Interface Sci. 2011, 360, 565–573. [Google Scholar] [CrossRef]
- Kamphan, A.; Charoenthai, N.; Traiphol, R. Fine tuning the colorimetric response to thermal and chemical stimuli of polydiacetylene vesicles by using various alcohols as additives. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 103–112. [Google Scholar] [CrossRef]
- Tjandra, A.D.; Weston, M.; Tang, J.; Kuchel, R.P.; Chandrawati, R. Solvent injection for polydiacetylene particle synthesis—Effects of varying solvent, injection rate, monomers and needle size on polydiacetylene properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126497. [Google Scholar] [CrossRef]
- Chanakul, A.; Traiphol, N.; Faisadcha, K.; Traiphol, R. Dual colorimetric response of polydiacetylene/Zinc oxide nanocomposites to low and high pH. J. Colloid Interface Sci. 2014, 418, 43–51. [Google Scholar] [CrossRef]
- Kew, S.J.; Hall, E.A.H. pH Response of Carboxy-Terminated Colorimetric Polydiacetylene Vesicles. Anal. Chem. 2006, 78, 2231–2238. [Google Scholar] [CrossRef]
- de Oliveira, C.P.; de Fátima Ferreira Soares, N.; Fontes, E.A.F.; de Oliveira, T.V.; Filho, A.M.M. Behaviour of polydiacetylene vesicles under different conditions of temperature, pH and chemical components of milk. Food Chem. 2012, 135, 1052–1056. [Google Scholar] [CrossRef]
- Kamphan, A.; Traiphol, N.; Traiphol, R. Versatile route to prepare reversible thermochromic polydiacetylene nanocomposite using low molecular weight poly(vinylpyrrolidone). Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 370–377. [Google Scholar] [CrossRef]
- Nakayama, S.; Suga, K.; Kamata, T.; Watanabe, K.; Namigata, H.; Welling, T.A.J.; Nagao, D. Bio-Inspired Polydiacetylene Vesicles for Controlling Stimulus Sensitivity. Macromol. React. Eng. 2025, 19, 2400016. [Google Scholar] [CrossRef]
- Filhol, J.-S.; Deschamps, J.; Dutremez, S.G.; Boury, B.; Barisien, T.; Legrand, L.; Schott, M. Polymorphs and Colors of Polydiacetylenes: A First Principles Study. J. Am. Chem. Soc. 2009, 131, 6976–6988. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.K.; Park, H.G. Colorimetric polydiacetylene (PDA) liposome-based assay for rapid and simple detection of GST-fusion protein. Sens. Actuators B Chem. 2019, 278, 190–195. [Google Scholar] [CrossRef]
- Traiphol, N.; Faisadcha, K.; Potai, R.; Traiphol, R. Fine tuning the color-transition temperature of thermoreversible polydiacetylene/zinc oxide nanocomposites: The effect of photopolymerization time. J. Colloid Interface Sci. 2015, 439, 105–111. [Google Scholar] [CrossRef]
- Huang, M.-R.; Ding, Y.-B.; Li, X.-G. Lead-ion potentiometric sensor based on electrically conducting microparticles of sulfonic phenylenediamine copolymer. Analyst 2013, 138, 3820. [Google Scholar] [CrossRef]
- Narkwiboonwong, P.; Tumcharern, G.; Potisatityuenyong, A.; Wacharasindhu, S.; Sukwattanasinitt, M. Aqueous sols of oligo(ethylene glycol) surface decorated polydiacetylene vesicles for colorimetric detection of Pb2+. Talanta 2011, 83, 872–878. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, B.; Yang, G. A Selective Colorimetric Sensor for Pb2+ Detection by Using Phenylboronic Acid Functionalized Polydiacetylene Liposomes. Macromol. Res. 2020, 28, 51–56. [Google Scholar] [CrossRef]
- Kuriki, R.; Ishii, Y.; Kuwabara, T. Exo- and endo -Isomers of Plumbylenes Supported by 1,3-Diethers of Calix[4]arene. Eur. J. Inorg. Chem. 2023, 26, e202200655. [Google Scholar] [CrossRef]
- Eddaif, L.; Shaban, A.; Telegdi, J. Sensitive detection of heavy metals ions based on the calixarene derivatives-modified piezoelectric resonators: A review. Int. J. Environ. Anal. Chem. 2019, 99, 824–853. [Google Scholar] [CrossRef]
- Ohto, K. Review of adsorbents incorporating calixarene derivatives used for metals recovery and hazardous ions removal: The concept of adsorbent design and classification of adsorbents. J. Incl. Phenom. Macrocycl. Chem. 2021, 101, 175–194. [Google Scholar] [CrossRef]
- Rathod, N.V.; Rao, A.; Kumar, P.; Ramakumar, K.L.; Malkhede, D.D. Complexation with calixarenes and efficient supercritical CO2 extraction of Pb(ii) from acidic medium. New J. Chem. 2014, 38, 5331–5340. [Google Scholar] [CrossRef]
- Ferreira, A.S.D.; Ascenso, J.R.; Marcos, P.M.; Schurhammer, R.; Hickey, N.; Geremia, S. Calixarene-Based lead receptors: An NMR, DFT and X-Ray synergetic approach. Supramol. Chem. 2021, 33, 231–244. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. Ligand design for selective complexation of metal ions in aqueous solution. Chem. Rev. 1989, 89, 1875–1914. [Google Scholar] [CrossRef]
- Burilov, V.; Valiyakhmetova, A.; Mironova, D.; Sultanova, E.; Evtugyn, V.; Osin, Y.; Katsyuba, S.; Burganov, T.; Solovieva, S.; Antipin, I. Novel amphiphilic conjugates of p-tert -butylthiacalix[4]arene with 10,12-pentacosadiynoic acid in 1,3-alternate stereoisomeric form. Synthesis and chromatic properties in the presence of metal ions. New J. Chem. 2018, 42, 2942–2951. [Google Scholar] [CrossRef]

| Macrocycle | n | Yield (%) | Time (h) |
|---|---|---|---|
| 3 | 4 | 65 | 40 |
| 4 | 8 | 70 | 30 |
| TCA-C4 | 4 | 70 | 20 |
| TCA-C8 | 8 | 75 | 27 |
| Concentration, mM | PDI | Hydrodynamic Diameter, nm | |
|---|---|---|---|
| TCA-C4 | 0.001 | - | |
| 0.01 | 0.06 | 32 | |
| 0.05 | 0.08 | 73 | |
| 0.1 | sedimentation | ||
| 0.5 | |||
| TCA-C8 | 0.001 | ||
| 0.01 | 0.05 | 41 | |
| 0.05 | 0.07 | 36 | |
| 0.1 | 0.01 | 43 | |
| 0.5 | 0.03 | 713 | |
| Before Polymerization | After Polymerization | ||||
|---|---|---|---|---|---|
| System | PDI | Hydrodynamic Diameter, nm | System | PDI | Hydrodynamic Diameter, nm |
| PCDA | 0.164 ± 0.018 | 228 ± 31 (86%), 53 ± 63 (14%) | PDA | 0.226 ± 0.013 | 270 ± 45 (77%), 95 ± 15 (23%) |
| 0.05TCA-C4+PCDA | 0.380 ± 0.019 | 483 ± 178 (68%), 545 ± 705 (34%) | 0.05TCA-C4&PDA | 0.413 ± 0.041 | 391 ± 68 (78%), 92 ± 82 (22%) |
| 0.1TCA-C4+PCDA | 0.448 ± 0.015 | 976 ± 120 (66%), 222 ± 57 (34%) | 0.1TCA-C4&PDA | 0.434 ± 0.012 | 888 ± 140 (64%), 188 ± 27 (36%) |
| 0.2TCA-C4+PCDA | 0.263 ± 0.02 | 243 ± 64 (79%), 1523 ± 2598 (21%) | 0.2TCA-C4&PDA | 0.266 ± 0.01 | 254 ± 57 (89%), 41 ± 72 (11%) |
| 0.05TCA-C8+PCDA | 0.229 ± 0.008 | 195 ± 21 (94%), 28 ± 48 (6%) | 0.05TCA-C8&PDA | 0.337 ± 0.003 | 251 ± 96 (65%), 177 ± 150 (35%) |
| 0.1TCA-C8+PCDA | 0.203 ± 0.024 | 252 ± 37 (82%), 101 ± 16 (18%) | 0.1TCA-C8&PDA | 0.257 ± 0.011 | 318 ± 46 (71%), 115 ± 19 (29%) |
| 0.2TCA-C8+PCDA | 0.373 ± 0.019 | 422 ± 42 (74%), 143 ± 19 (26%) | 0.2TCA-C8&PDA | 0.322 ± 0.024 | 356 ± 96 (74%), 273 ± 263 (26%) |
| MP AES After Diluted * | ASV After Diluted * | |||||||
|---|---|---|---|---|---|---|---|---|
| Entered, mg/L | Found, mg/L | Adsorbed Lead, % | RSD % | Entered, mg/L | Found, mg/L | Adsorbed Lead, % | RSD % | |
| PDA | 10.37 | 7.54 | 27 | 0.59 | 51.86 | 37.29 | 28 | 1.36 |
| 0.05TCA-C8&PDA | 10.37 | 5.96 | 42 | 0.57 | 51.86 | 29.01 | 44 | 2.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedoseeva, A.A.; Yespanova, I.; Sultanova, E.D.; Gafiatullin, B.K.; Ibragimova, R.R.; Darmagambet, K.K.; Il’ina, M.A.; Chibirev, E.O.; Evtugyn, V.G.; Appazov, N.O.; et al. Sulfonate Thiacalixarene-Modified Polydiacetylene Vesicles as Colorimetric Sensors for Lead Ion Detection. Colloids Interfaces 2025, 9, 20. https://doi.org/10.3390/colloids9020020
Fedoseeva AA, Yespanova I, Sultanova ED, Gafiatullin BK, Ibragimova RR, Darmagambet KK, Il’ina MA, Chibirev EO, Evtugyn VG, Appazov NO, et al. Sulfonate Thiacalixarene-Modified Polydiacetylene Vesicles as Colorimetric Sensors for Lead Ion Detection. Colloids and Interfaces. 2025; 9(2):20. https://doi.org/10.3390/colloids9020020
Chicago/Turabian StyleFedoseeva, Angelina A., Indira Yespanova, Elza D. Sultanova, Bulat Kh. Gafiatullin, Regina R. Ibragimova, Klara Kh. Darmagambet, Marina A. Il’ina, Egor O. Chibirev, Vladimir G. Evtugyn, Nurbol O. Appazov, and et al. 2025. "Sulfonate Thiacalixarene-Modified Polydiacetylene Vesicles as Colorimetric Sensors for Lead Ion Detection" Colloids and Interfaces 9, no. 2: 20. https://doi.org/10.3390/colloids9020020
APA StyleFedoseeva, A. A., Yespanova, I., Sultanova, E. D., Gafiatullin, B. K., Ibragimova, R. R., Darmagambet, K. K., Il’ina, M. A., Chibirev, E. O., Evtugyn, V. G., Appazov, N. O., Burilov, V. A., Solovieva, S. E., & Antipin, I. S. (2025). Sulfonate Thiacalixarene-Modified Polydiacetylene Vesicles as Colorimetric Sensors for Lead Ion Detection. Colloids and Interfaces, 9(2), 20. https://doi.org/10.3390/colloids9020020