Abstract
The effectiveness of copper-based composites, specifically cupric ion (Cu2+)-modified phyllosilicate minerals, was evaluated in reducing the concentration of infectious agents in the environment while minimizing metal ion release. The phyllosilicate minerals, vermiculite, exfoliated and unexfoliated, and sepiolite, all modified with Cu2+, were compared with copper oxide for their antiviral activity against non-enveloped porcine parvovirus (PPV) and enveloped human coronavirus 229E (HCoV). Sepiolite effectively removed PPV and HCoV from the solution, regardless of Cu2+ presence, while vermiculite showed substantial viral clearance only when Cu2+ was present. The kinetics of viral clearance was fast, with complete clearance within one hour in many cases. To better understand the mechanism of virus clearance, EDTA was added at different times during the clearance study for PPV. EDTA prevented virus clearance in all vermiculite samples, whereas sepiolite containing copper still demonstrated substantial virus clearance. The addition of BSA before the virus binding was able to block binding in all cases. It was determined that binding is the key mechanism, and PPV can be eluted from the minerals with EDTA and still be infectious. This study provides the potent antiviral mechanisms of Cu2+-modified phyllosilicate minerals, offering insights for designing paints and plastics for high-touch surfaces to reduce viral transmission and enhance public health significantly.
1. Introduction
Innovative approaches for virus inactivation or entrapment that prioritize efficacy and long functioning lifetime are needed. Metal-based surfaces designed with virucidal properties employ a mechanism of virus entrapment or inactivation to mitigate virus transmission via fomites [1,2,3,4,5,6]. Such surfaces can be tailored utilizing various forms of metals, including nanoparticles [5], ions [7,8,9], alloys [10], or metallic compounds [1,5]. These metallic materials have been comprehensively studied against abundant non-enveloped (adenovirus, norovirus, and bacteriophage) and enveloped (influenza, HIV, human coronavirus 229E, and herpes simplex virus) viruses [10,11,12,13,14,15]. Generally, metal-based materials have a toxic effect against viruses by releasing metal ions in the solution. For example, Cu2O immobilized on glass decreased the titer of bacteriophage Qβ by ~4 log reduction value within 30 min of incubation, and the dominant mechanism of antiviral action was due to the release of copper ions [16].
Metals can inhibit viral replication by contact with metal ions or generating reactive oxygen species (ROS) in the presence of oxidizing agents [17]. The antiviral efficacy of iron oxide increased against bacteriophage MS2 due to increased ROS generated when coupled with the photo-Fenton reaction [18]. However, releasing and accumulating metal ions and ROS in the environment may harm aquatic organisms and plants [6]. To overcome this toxicity, metal-based composites, like metal-infused minerals, polymers, and glasses, present promising strategies for mitigating environmental toxicity by reducing the concentration of metal released [4]. Despite their potential, the underlying mechanism of action for these composites remains to be determined.
Phyllosilicate minerals are suitable carriers for different metallic ions. These chemically inert minerals have a high ion exchange capacity, surface area, and thermal resistance [19]. Vermiculite and sepiolite are naturally occurring phyllosilicate minerals with charged interlayer regions filled with exchangeable magnesium ions. With abundant nano-scaled pores, phyllosilicate minerals are lightweight and have a large surface area and cation exchange capacity [20,21]. Due to these properties, vermiculite has improved the physical and mechanical properties of wood plastic composites when used as a gap filler [22,23]. It is also used as a filler in paints to impart fire resistance to wood coatings [24]. Vermiculite comes in two main forms. Unexfoliated vermiculite can be expanded after physicochemical treatment to become exfoliated vermiculite, which has a larger pore structure for adsorption. Sepiolite is often used as a carrier material for catalysis [25] and an absorbent of heavy metals [26]. Chemically, vermiculite [(Mg,Fe,Al)3(Al,Si)4O10(OH)2·4(H2O)] is a hydrous magnesium aluminosilicate while sepiolite (Mg4Si6O15(OH)2·6H2O) is a hydrous magnesium silicate [27]. Since copper and magnesium have comparable charges and atomic radii [28,29], vermiculite and sepiolite are ideal carriers for loading ionic copper [29].
Copper and copper ions have exhibited antiviral activity. Copper ions offer a significant environmental advantage over copper nanoparticles and alloys. Copper ions immobilized within gel and polymer carriers reduce the release of potentially hazardous nanoparticles or metal ions into the environment. While both the ionic forms of copper are effective against viruses, cupric ions (Cu2+) have a stronger virucidal effect because of their higher oxidative capabilities, hydration enthalpy, and stability compared to cuprous ions (Cu1+) [10,30]. Cu2+ has shown antiviral effects against subtypes H9, H5, and H1 of influenza virus, herpes simplex virus, and dengue virus by affecting viral structural integrity [31,32]. Cu2+-infused zeolite showed that the H5 influenza virus subtypes had different sensitivity to the Cu2+ within 30 s to 10 min of incubation. Furthermore, the human influenza virus and West Nile virus were inactivated in a time-dependent manner after being filtered through Cu2+ impregnated in cotton fibers, latex, or polyester [33]. While the ionic copper-impregnated composites have become a subject of considerable interest, the mechanism of their antiviral action remains to be determined, whether these composites inactivate the viruses or remove them from the system by adsorption.
Viruses are broadly categorized as non-enveloped and enveloped. Viral clearance is highly dependent on these categories. Non-enveloped viruses are very stable and difficult to inactivate, due to the protein capsid structure that surrounds the nucleic acids [34,35]. Enveloped viruses contain a labile lipid bilayer that can be disrupted to inactivate and clear viruses. To test the clearance of viruses by Cu2+-modified phyllosilicate materials, non-enveloped porcine parvovirus (PPV) and enveloped human coronavirus 229E (HCoV) viruses were tested. PPV contains single-stranded DNA encapsulated by a protein capsid composed of three capsid proteins: VP1, VP2, and VP3. All three are made from the same genomic transcript; thus, VP2 and VP3 are truncations of VP1 [36]. PPV is considered highly resistant to physiochemical disinfectants [37]. HCoV is a model for SARS-CoV-2 and other enveloped viruses that remain infectious on surfaces for a prolonged time [38,39,40]. The envelope of HCoV is composed of various structural proteins, including the spike protein (S), membrane protein (M), and enveloped protein (E). This envelope encases the viral RNA bound by nucleoproteins and forms a helical symmetry within the nucleocapsid [41]. Both non-enveloped and enveloped viruses need to be explored because they could have different viral clearance mechanisms due to differences in lability.
This study evaluated the virus clearance efficacy of Cu2+-modified phyllosilicate minerals, exfoliated and unexfoliated vermiculite and sepiolite, using non-enveloped PPV and enveloped HCoV. Furthermore, we explored the interaction mechanisms between Cu2+ and virus particles to determine whether virus clearance resulted from the adsorption of virus particles onto Cu2+ or the inactivation of the viruses with Cu2+. The results of this study provide a mechanistic understanding of how phyllosilicate minerals can be designed as antiviral fillers for plastics and paints for high touch-point surfaces in healthcare facilities to reduce viral disease transmission.
2. Materials and Methods
2.1. Preparation of Mineral Samples Modified with Cu2+
Both vermiculite and sepiolite powders were used as carriers of ionic copper and controls for antiviral assays in this study. There were three types of vermiculite powders and two types of sepiolite powders employed: vermiculite control (named V), exfoliated vermiculite loaded with ionic copper (named V-Cu), unexfoliated vermiculite loaded with ionic copper (named UnV-Cu), sepiolite control (S), and sepiolite loaded with ionic copper (S-Cu). In addition, cuprous oxide (Cu2O) was used as a positive control for comparing investigations. All the powders used in the study are discussed in Table 1.

Table 1.
Modified and unmodified phyllosilicate mineral samples with their copper content measured with ICP. Released Cu2+ was leached with 0.025 M HEPES buffer.
V was taken from the exfoliated vermiculite commercial product of Strong Company Inc., Pine Bluff, AR, USA. The sample was screened to pass 150 µm for use. V-Cu is a modified powder from V. First, 100 g of V was blended with 1000 mL of 0.2 M CuSO4 (copper sulfate, Sigma-Aldrich, St. Louis, MO, USA) solution at pH 3, heated to 80 °C for 3 h, cooled to room temperature, filtered, and washed with distilled water until the color of the solution on the slurry changed from blue to colorless. Then, the wet cake of V-Cu was dried at 105 °C and re-ground into a powder. UnV-Cu was prepared from unexfoliated vermiculite (Virginia Vermiculite LLC, Louisa, VA, USA). The sample was jet-milled into a fine powder and then treated with ionic copper (process same as V-Cu). S was a commercial product of Lhiost Group, Fort Worth, TX, USA. S-Cu was a modified powder from S with copper solution under the same conditions as V-Cu. Cu2O was a cuprous oxide purchased from Sigma-Aldrich, St. Louis, MO, USA (CAS No. 1317-39-1), with the purity of Cu2O > 99% and particle size < 20 µm.
2.2. Cell Maintenance
Porcine kidney cells (PK-13, ATCC, cat# CRL-6489) were cultured in Eagle’s minimum essential media (EMEM) (Invitrogen, Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) (Thermofisher Scientific, Waltham, MA, USA) and 1% (v/v) pen/strep (Invitrogen). The cells were passaged at 70% confluency at a split ratio of 1:5 and incubated at 37 °C with 5% CO2 and 100% humidity [42]. Human lung fibroblast cells (MRC-5, ATCC, cat# CCL-171) were cultured in EMEM supplemented with 10% (v/v) FBS, 1% (v/v) pen/strep, 1% (v/v) 100 mM sodium pyruvate (Thermofisher Scientific), and 1% (v/v) non-essential amino acids (MEM NEAA) (Thermofisher scientific). The cells were passaged the same as PK-13 [43].
2.3. Virus Propagation and Titration
PPV generously provided by Dr. Carbonell’s lab (North Carolina State University) and HCoV 229E (BEI Resources, Manassas, VA, USA) were propagated on PK-13 and MRC-5, respectively. PPV was propagated as described previously [42], and the viral stock was clarified after three freeze–thaw cycles by centrifuging in a TX-400 swing bucket rotor in an ST16R centrifuge (Thermofisher Scientific) at 4752× g at 4 °C for 15 min and stored at −80 °C [34]. HCoV was propagated as previously described [35]. Briefly, HCoV 229E was cultured on a monolayer of MRC-5 cells. Infected cells were incubated at 35 °C, 5% CO2, and 100% humidity until 100% CPE was observed. After two freeze–thaw cycles, HCoV supernatant was collected after centrifugation and stored at −80 °C with 10% (v/v) glycerol [43].
Virus titration was performed with the colorimetric MTT cell viability assay to determine the concentration of infectious virus [44]. Briefly, PK-13 cells for PPV and MRC-5 for HCoV were seeded in 96-well plates. PPV or HCoV was added to the wells in quadruplicate, with a serial dilution of 1:5 across the plate. After the plates were incubated for six days, 5 mg/mL MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Thermofisher scientific) was added. After four hours, a solubilization solution of 10% (w/v) SDS (sodium dodecyl sulfate 99%, VWR, Atlanta, GA, USA) and 0.01 M hydrochloric acid (HCl) was added to dissolve the formazan crystals. Once between 4 and 24 h, the absorbance was determined at 550 nm with a SynergyTX Mx microplate reader from BioTek (Winoski, VT, USA). The 50% infectious dose of the virus was determined by finding the concentration where 50% of the cells were viable, measured in MTT50/mL units [43,44].
2.4. Morphological and Chemical Characterization of Vermiculite and Sepiolite Samples
The morphological characteristics of the phyllosilicate mineral samples before and after 0.025 M HEPES, pH 7.2 (99% HEPES, Thermofisher Scientific) buffer treatment were analyzed using a Hitachi S-4700 Field Emission Scanning Electron Microscope (FE-SEM) (Thermofisher Scientific, Waltham, MA, USA). Before imaging, 0.02 g of loose phyllosilicate mineral was incubated with 0.025 M HEPES at 4 °C for 2 h with 360° rotating motion. Following incubation, samples were pelleted using a TX-400 swing bucket rotor in an ST16R centrifuge at 12,298× g for 15 min, and the supernatant was removed. Pelleted samples were re-suspended in 1 mL of 0.025 M HEPES buffer containing 7.14 μL of 70% glutaraldehyde (Sigma Aldrich) and incubated for 1 h at room temperature to cross-link and inactivate the virus. Cross-linked samples were pelleted by centrifugation, and the supernatant was removed. The pellet was washed with 1 mL HEPES buffer to remove residual glutaraldehyde and pelleted again. Samples were stained with 2% (w/v) uranyl acetate solution (Electron Microscopy Sciences, Hatfield, PA, USA) and dried in a fume hood overnight. The dried powder was applied to a carbon-tape-coated pin (Electron Microscopy Sciences) mount the following day. Compressed air was briefly used to remove loose particles from the mounted sample. A 208HR Sputter Coater (Cressington Scientific Instruments, Liverpool, UK) was used to coat the sample with a 2.5 nm iridium film, improving the sample conductivity to prevent charging in the FE-SEM. The iridium target was purchased from Rave Scientific (Somerset, NJ, USA). An accelerating voltage of 3 kV, a condenser lens setting of 12, an emission current of 7 eV, and a working distance of 3 mm were used to acquire FE-SEM images of the samples.
An FEI Philips XL 40 Environmental Scanning Microscope (ESEM, Hatfield, PA, USA) associated with an Oxford AZtec energy dispersive spectrometer (EDS, Hatfield, PA, USA) was employed to determine the state of copper in the mineral samples (sterilized samples). The operational parameters of the ESEM were an accelerated voltage of 10–20 kV and a working distance of 10 mm.
X-ray diffraction (XRD) was used to determine the mineral compositions of the samples for virus clearance and whether the sample contained metal copper, including copper nanoparticles. The instrument used was a Scintag XDS2000 Powder Diffractometer (Bruker, Billerica, MA, USA). The operational parameters for data collection were 45 kV, 35 mA, continuous scan, scan angle from 5° to 60°, and scan speed 2°/min.
An inductively coupled plasma optical emission spectrometer (Perkin Elmer Optima 7000DV ICP-OES, Waltham, MA, USA) determined the copper concentrations in the mineral powder samples. The mineral samples were decomposed by fusion with lithium metaborate and dissolved in a 10% hydrochloric solution. The ICP determinations were conducted in triplicate, and the metal concentration was reported as the average.
2.5. Leaching of Cu2+ into the Solution
ICP-OES was performed to understand the leaching of Cu2+ into buffer solutions. First, 2% (w/v) of all the phyllosilicate minerals (V-Cu, UnV-Cu, V, S-Cu, S, Cu2O) was prepared in 1X, pH 7.4 PBS (phosphate buffered saline, Gibco™, Grand Island, NY, USA), 0.025 M HEPES, and deionized water. All buffers were made in deionized water. The solution was vortexed and incubated in 360° motion at 4 °C. Samples were extracted at 24 and 48 h and then centrifuged at 12,298× g for 15 min, and the supernatant was removed and stored at 4 °C until testing. Before ICP-OES analysis, the solutions were diluted 2.5-fold with 10% HNO3 solution to fit the instrumental requirement.
2.6. Virus Clearance and Adsorption Studies with Cu2+-Modified Phyllosilicate Minerals
All phyllosilicate minerals and buffer solutions were prepared 24 h before the experiment and stored at room temperature and 4 °C, respectively. The phyllosilicate minerals were weighed in low-binding microcentrifuge tubes. The virus solution in 0.025 M HEPES at a final concentration of 106 MTT50/mL was added to the minerals to obtain a concentration of 2% (w/v). The virus–mineral suspension was incubated at 4 °C with 360° rotation. After incubation, the phyllosilicate minerals were separated from the virus suspension by centrifugation (12,298× g for 15 min). The supernatant was collected at different times (1, 2, 4, and 24 h) and stored at 4 °C until tested.
Ethylenediaminetetraacetic acid (EDTA, 99.4–100.6% powder, cat# E9884 Sigma Aldrich) stock was prepared in Nanopure water to chelate the Cu2+ released from the phyllosilicate mineral in the HEPES buffer. For the pre-EDTA condition, the EDTA was added to the virus solution for a final concentration of 0.001 M. This solution was then added to the phyllosilicate mineral for a final mineral concentration of 2% (w/v) and then incubated at 4 °C for 2 h. For the post-EDTA condition, after incubation of the viral–mineral suspension at 4 °C for 2 h, stock EDTA was added to a final concentration of 0.001 M and then the solution was vortexed and centrifuged at 4 °C to collect the supernatant for virus titration. For no EDTA, 0.025 M HEPES was added to account for the concentration difference for the pre- and post-EDTA conditions. After 2 h, the phyllosilicate minerals were separated from the virus suspension by centrifugation at 12,298× g for 15 min, and the supernatants containing the treated PPV were collected and stored at 4 °C until titrated.
To understand the interaction mechanism between the virus particles and the model phyllosilicate minerals tested by blocking the active sites of the virus on the phyllosilicate mineral with bovine serum albumin (BSA, ≥98% Sigma Aldrich). A stock solution of BSA was prepared in Nanopure water. For the BSA conditions, stock BSA was added to the virus solution to a final concentration of 1% (w/v) BSA. For without-BSA conditions, 0.025 M HEPES buffer was added to compensate for the amount of BSA added in with-BSA conditions. However, for both the with- and without-BSA solutions, Nanopure water was added to the mineral–virus suspension for a final mineral concentration of 2% (w/v). After 2 h at 4 °C, the phyllosilicate mineral powders were separated from the virus suspension by centrifugation at 12,298× g for 15 min, and the supernatants containing the treated PPV and HCoV were collected and stored at 4 °C until titrated.
2.7. Virus Reduction Kinetics and Rate Constant
Clearance kinetics was calculated by fitting the inactivation data to a first-order kinetic model [45] using Equation (1).
where Cf is the final concentration of the virus in MTT50/mL and Ci is the initial concentration of the virus in MTT50/mL. k represents the rate constant of the inactivation kinetics in (s−1), and t is the time of incubation of the virus with the phyllosilicate minerals in (h).
2.8. Toxicity Analysis of the Phyllosilicate Minerals Against PK-13 and MRC-5 Cells
MTT cell viability assay was used to test the toxicity of Cu2+-modified phyllosilicate minerals against PK-13 and MRC-5 cell lines. First, 2% (w/v) phyllosilicate mineral was treated in 0.025 M HEPES buffer at 4 °C with a 360° rotation motion. After 24 h of incubation, the supernatant was separated from the minerals with centrifugation (12,298× g for 15 min) and stored at 4 °C until titrated. PK-13 and MRC-5 cells were seeded in 96-well plates at a seeding density of 8 × 104 cells/mL and 1 × 105 cells/mL, respectively. Then, 25 μL of supernatant was added to the first well in quadruplet and serially diluted 1:5 across the plate for PK-13- and MRC-5-containing 96-well plates. After six days of incubation at 37 °C, 5 mg/mL MTT was added to the plates. After four hours, a solubilization solution of 10% (w/v) SDS and HCl was added to dissolve the formazan crystals. Between 4 and 24 h, the absorbance was determined at 550 nm with a SynergyTX Mx microplate reader. Cell viability was calculated with Equation (2).
2.9. Statistical Analysis
Statistical analysis was performed with one-way ANOVA analysis to compare the means of the independent variables. With the assumption of equal variance between the two samples, the p-value < 0.05 was considered significant. All the data sets were performed in triplicates. The standard error and R2 for the inactivation kinetics were calculated using the linear regression model in Microsoft Excel.
3. Results
3.1. Morphological and Chemical Characterization of Vermiculite and Sepiolite Modified with Cu2+
Cu2+-modified phyllosilicate minerals were prepared by incubation of different minerals with CuSO4 at a low pH. The minerals utilized in this study are detailed in Table 1. Despite being treated with the same concentrations of CuSO4, the three minerals exhibited varying levels of Cu2+ uptake in the mineral and release into the buffer, as presented in Table 1. The cation exchange capacity of the phyllosilicate minerals is in reverse order of the amount of copper ion found in the mineral. UnV has a range of 130–165 meq/100 g of cation exchange capacity [46]. V is less, as the structure collapses during exfoliation, and S is the lowest at 40–90 meq/100 g [47]. High amounts of CuSO4 were used to saturate the minerals as much as possible with Cu2+. The Cu2+ incorporation was between 26% for the UnV-Cu and 43% for the S-Cu. After copper modification, the Cu2+ content was not greatly different for the different minerals. The UnV-Cu released the most Cu2+ into solution; see Table 1. This likely demonstrates that the Cu2+ may not have exchanged with the magnesium ion in UnV-Cu and there was only surface adsorption. Upon contact with buffer, some Cu2+ were released. For UnV-Cu, <1% of the Cu2+ incorporated was leached.
The unmodified and Cu2+-modified phyllosilicate minerals were treated in 0.025 M HEPES buffer and then imaged for morphological features under FE-SEM. Unmodified vermiculite (V) has a flaky layer appearance with a smooth surface and lightly coiled edges, which could be caused by the treatment of the buffer solution (Figure 1A). The pore size is less than 1 nm [48]. The exfoliated vermiculite carrying copper (V-Cu) also showed a flaky structure, but the laminates are broken into multiple small and thin fractions with coiled edges. This could result from the initial softening by the exfoliation [49] process and the later cation exchange process with CuSO4 solution at a low pH value before the treatment with buffer solution (Figure 1B). The pore size of the exfoliated vermiculite was not measured, but experimental results presented later lead one to believe it is less than 0.5 µm. The unexfoliated vermiculite carrying Cu2+ (UnV-Cu) displayed a thicker, flaky crystal shape without coiled edges owing to stiffer layers and edges of vermiculite plates without an exfoliation process (Figure 1C). Sepiolite powder (S) exhibited the characteristics of a fiber cluster with randomly oriented fibers (Figure 1D), and it has a pore size of less than 1 nm [50]. The individual sepiolite crystals are in an acicular shape with a 5–20 nm diameter and 0.5–1 µm length. Loading Cu2+ did not change the morphological feature of the sepiolite particles (Figure 1E). However, the energy dispersive spectra (EDS) of V-Cu indicate that the copper is modified in ionic form in the mineral (Figure 1F).
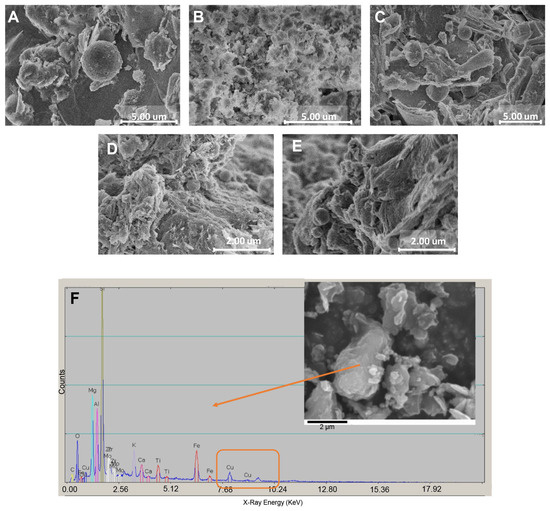
Figure 1.
Vermiculite and sepiolite clay minerals with and without copper treatment. The phyllosilicate minerals were contacted with 0.025 M HEPES buffer solution for 2 h and then dried. (A) V, (B) V-Cu, (C) UnV-Cu, (D) S, (E) S-Cu. (F) EDS of UnV-C for determining the Cu2+ concentration.
The phyllosilicate minerals were modified with Cu2+ and not elemental copper. The XRD spectra of the samples modified with copper exhibited no metal copper, copper oxide, or other copper compound grains (Figure S1). The XRD patterns of exfoliated vermiculite loaded with copper showed that V-Cu mainly consisted of vermiculite and phlogopite. The phlogopite is a phase occurring from vermiculite after exfoliation (Figure S1A). The UnV-Cu was missing the (001) peak of vermiculite, but a vermiculite–phlogopite mixing layer peak at 7.3° was found while maintaining the majority of vermiculite. This is caused by the pulverization of vermiculite into micron powder by jet-milling. The main component of S-Cu is sepiolite, while the sample contains a small amount of quartz and calcite from nature as an impurity (Figure S1B). However, all three samples did not display any peaks of metal copper, further indicating that the copper implanted in the mineral matrices existed in an ionic state.
We next wanted to determine if copper leached into a solution could be a driver for virus clearance. Deionized water, PBS, and HEPES were all tested as leaching solutions, and the concentration of leached Cu2+ was determined by ICP (Table S1). Since the highest amount of copper was leached into HEPES, we continued with that buffer for antiviral testing (Table 1). As expected, the unmodified vermiculate (V) and sepiolite (S) that were not exposed to CuSO4 exhibited undetectable Cu2+ release. The release of Cu2+ from the V-Cu sample was below the detection limit of 0.05 ppm. Copper release into the solution occurred for two copper-modified phyllosilicate minerals, UnV-Cu and S-Cu. As Cu2O was insoluble in the solution tested, there was a low release of Cu2+ from this control (Table 1). The minimum concentration for inactivating influenza virus has been shown to be 0.4 ppm of Cu1+ [7], so we were expecting only UnV-Cu to have antiviral activity.
3.2. Virus Clearance with Copper-Modified Phyllosilicate Mineral
We began by testing the effectiveness of Cu2+-modified phyllosilicate minerals in eliminating the infectious viruses, PPV and HCoV. The viruses were treated with 2% (w/v) phyllosilicate mineral at 4 °C with a 360° rotation motion for 4 h. All the virus clearance studies were performed at 4 °C as this is a harsher case for inactivation than room temperature or above. Additionally, HCoV is unstable at room temperature after 4 h. The toxicity of the minerals was tested against PK-13 and MRC-5 cells. As shown in Figure S3, MRC-5 was more sensitive to the minerals as compared to PK-13. However, both cell lines had >60% viability. The phyllosilicate minerals without copper had the lowest viability, demonstrating that the toxicity was the contact with the minerals, likely due to settling on the cells, and not leached copper.
The vermiculite samples containing Cu2+, either exfoliated or unexfoliated, achieved a similar clearance of the non-enveloped PPV. HEPES buffer exhibited no inactivation effect on PPV, maintaining a titer of 6.8 ± 0.2 logs MTT50/mL after incubation at 4 °C for 4 h. V-Cu reduced the PPV titer to 3.0 ± 0.4 logs MTT50/mL, while UnV-Cu achieved a similar reduction in PPV to 3.0 ± 0.8 logs MTT50/mL as shown in Figure 2A. PPV remained unchanged from the starting titer for the vermiculite without Cu2+. The difference between the antiviral activity of copper-modified vermiculite (V-Cu, UnV-Cu) and V suggests that Cu2+ plays some role in the antiviral activity of vermiculite.
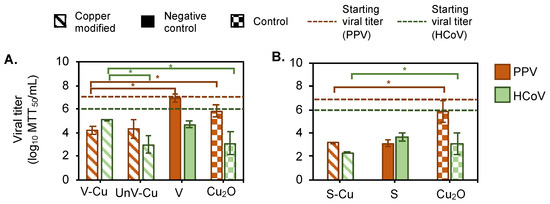
Figure 2.
Virus removal with cupric-modified phyllosilicate minerals. Viral titer after incubation of 2% (w/v) clay mineral at 4 °C for (A) vermiculite (left) and (B) sepiolite (right). Orange bars are titer for PPV; green bars are titer for HCoV after treatment with phyllosilicate mineral in 0.025 M HEPES buffer. Virus removal is performed with Cu2+-containing exfoliated vermiculite (V-Cu, diagonal strips), unexfoliated vermiculite (UnV-Cu, diagonal strips), and sepiolite (S-Cu, diagonal strips). Vermiculite and sepiolite without Cu2+ are the negative control (V and S, solid fill), and Cu2O is the control (Cu2O, small check) for the virus removal study. All the data sets were performed in triplicate. Error bars represent the standard deviation between the data.The line represents the starting viral titer for PPV (solid) and HCoV (dash), that is, the virus with 0.025 M HEPES was kept in the same condition as the test sample. * Represents significant difference between samples that had a p-value < 0.05.
Vermiculite showed a different profile when inactivating HCoV. Following a similar trend as observed with PPV, HCoV remained active in the HEPES buffer, retaining a titer of 5.7 ± 0.2 logs MTT50/mL in the experimental conditions. Only UnV-Cu showed any virus titer reduction to 2.4 ± 0.7 logs MTT50/mL (Figure 2A). We hypothesize this may be due to the size difference between PPV and HCoV. The exfoliation process expands pores in the mineral [51]; therefore, PPV, because of its smaller size, likely had access to the pores of the V-Cu, but HCoV could not access the pores because the virus was too large. However, the surface of the UnV-Cu is smoother than the V-Cu and allows for the binding of the larger virus.
For the sepiolite, both the copper-modified (S-Cu) and control (S) minerals showed comparable levels of virus clearance for both PPV and HCoV. The unmodified sepiolite (S) showed a significant reduction in viral titer, around 3.6 ± 0.1 logs MTT50/mL for PPV and 1.8 ± 0.3 logs MTT50/mL for HCoV, as shown in Figure 2A. However, it is difficult to conclude if this reduction in viral titer results from viral inactivation, adsorption, or physical entrapment of virus particles between the powder particles.
Lastly, the Cu2O control showed variable clearance between PPV and HCoV. For PPV, a total of 0.9 ± 0.5 logs MTT50/mL was removed after 4 h of incubation (Figure 2A). However, the HCoV titer was reduced after 4 h of incubation by 2.4 ± 1.0 logs MTT50/mL. This result could be attributed to the difference in resistance to disinfectants. PPV is known to have high resistance against disinfectants [40] and thus was not affected by the cupric oxide. HCoV did have some susceptibility.
Inactivation kinetics studies were conducted to find the minimum time required for the copper-modified and unmodified minerals to remove PPV and HCoV from the solution. As shown in Figure 2B, V-Cu, Un-V, S-Cu, and S reduced the PPV titer within 2 h of incubation and maintained this reduction for up to 24 h. PPV clearance kinetics after 2 h of incubation with Cu2+ modified minerals were fit to a first-order kinetic inactivation model. The rate constant was calculated with Equation (1), and the equation fits are shown in Figure S3, which only used data from the first 2 h, which is the linear region before complete inactivation occurred. The rate constant values were found in the order of S-Cu > V-Cu > UnV-Cu, indicating S-Cu had the most rapid effect on PPV titer reduction. Among the vermiculite samples, V-Cu showed a faster clearance rate compared to UnV-Cu, as detailed in Table 2. In contrast, Cu2O cleared PPV after 24 h of incubation and showed a lower rate constant (0.3 s−1). The lack of virus clearance for Cu2O is likely due to its insolubility in the buffer used.

Table 2.
Rate constants for PPV and HCoV inactivation kinetics after contact with phyllosilicate minerals and cuprous oxide.
A reduction in the titer of HCoV with UnV-Cu, S-Cu, S, and Cu2O was observed within 2 h of incubation. However, after 24 h of incubation, V-Cu decreased the titer of HCoV by only 1 log MTT50/mL (Figure 3A) with a rate constant of 0.14 s−1 (Table 2). In contrast, UnV-Cu reduced HCoV within 1 h of incubation by 1.9 ± 0.7 logs MTT50/mL with a rate constant of 4.8 s−1. The reason behind the strong interaction behavior of UnV-Cu with HCoV is unclear, but the exfoliation process may cause structural disorder in vermiculite. Unexfoliated vermiculite, with its more crystalline structure, offers more sites for HCoV to interact with embedded Cu2+ [49]. S-Cu inactivated HCoV within 1 h of incubation, exhibiting the highest rate constant of 6.4 s−1. Cu2O inactivated HCoV 2.9 ± 0.5 logs MTT50/mL after 2 h of incubation, which was an hour longer than the time required for V-Cu to achieve the same effect. This difference may be due to the insolubility of Cu2O in the solution, as solid-state cuprous compounds have been observed to exhibit higher antiviral activity compared to leached copper ions [16].
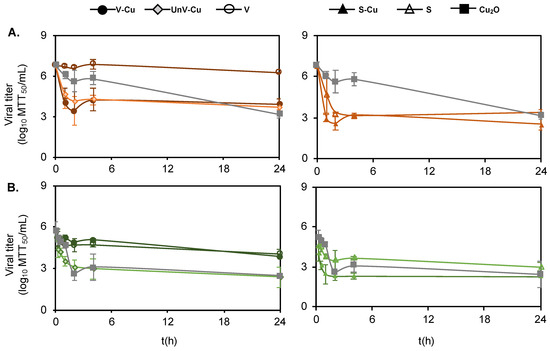
Figure 3.
Viral removal kinetics for (A) PPV (orange) and (B) HCoV (green). Viruses treated with V-Cu (solid circle), UnV-Cu (diamond), V (open circle), S-Cu (solid triangle), S (open triangle), and Cu2O (square) for different incubation time points. All the data sets were performed in triplicates, and the error bars represent the standard deviation.
Our findings demonstrated that Cu2+ within the phyllosilicate minerals contributed significantly to the antiviral activity of the minerals. For vermiculite, the clearance of viruses is primarily by facilitating via adsorption to Cu2+ in the mineral. The difference between the antiviral activity of copper-modified vermiculite (V-Cu, UnV-Cu) and V suggests that Cu2+ played some role in the antiviral activity of vermiculite. This role of Cu2+ is consistent with previously observed antibacterial activity, where copper-modified vermiculite showed enhanced effectiveness in removing Staphylococcus aureus [29] and Enterococcus faecalis [52] compared to unmodified vermiculite, indicating that copper ions impart antibacterial properties to the vermiculite. The difference in clearance patterns for PPV and HCoV, especially with UnV-Cu, may be due to the size difference between PPV and HCoV. The exfoliation process expands the layers of the vermiculite [51], creating pores that provide binding sites for the Cu2+. PPV, because of its smaller size, likely had access to the ions impregnated in the inner layer of the V-Cu, but HCoV could not access the pores because the virus was too large. The surface of the UnV-Cu is smoother than the V-Cu and allows for the binding of the Cu2+ to the external surface [49], which was accessible to both viruses.
In contrast, sepiolite exhibited robust viral clearance irrespective of the presence of Cu2+. The high adsorption capabilities of all sepiolite materials effectively removed viruses from the solution. The sepiolite powder particles used were finer in size and had more of an absorbent clay consistency [26,53,54]. The inherent ability of sepiolite to absorb cations and neutral molecules [53] has facilitated its use as a carrier for various applications, such as a carrier of phosphate-accumulating bacteria for wastewater treatment plants [26] or as an alternative carrier for peat-based microbial inoculants [55]. These findings suggest that adsorption could be the mechanism governing virus clearance by sepiolite. Nevertheless, we acknowledge the need for further rigorous experimentation to conclusively validate this hypothesis and explore any potential secondary mechanisms underlying their viral clearance mechanism.
Once it was demonstrated that many of the phyllosilicate minerals tested had the ability to clear the virus, then we wanted to better understand how the clearance was occurring. It could either be through adsorption or inactivation. Understanding whether these Cu2+-modified minerals inactivate viruses or facilitate their removal from the environment is important in their application in paints or as fillers for high-touch surfaces [5].
3.3. Virus Adsorption: The Mechanism of Virus Clearance
We used EDTA to chelate the leached Cu2+ in the solution to explore if the ions in the solution were the main mechanism of viral clearance. We looked at three conditions, adding EDTA to the mineral before the virus (pre-EDTA), adding EDTA after virus incubation (post-EDTA), and a no-EDTA condition. We expected that the pre-EDTA condition would chelate all of the ions in the solution, thus allowing us to understand if the soluble ions were the main compound for inactivation [16]. The post-EDTA condition was used to determine if EDTA had any effect on virus inactivation as shown in Figure 3A. The EDTA chelation was only performed with PPV due to simplicity. PPV did not bind to V regardless of EDTA addition, as shown in Figure 4A. However, both vermiculites with copper had less viral clearance with EDTA present regardless of addition time. Based on ICP testing, V-Cu exhibited minimal release of Cu2+ in 0.025 M HEPES buffer (Table 1), indicating that once the EDTA was added to the copper-containing phyllosilicate mineral, it immediately chelated the Cu2+ ion absorbed on the mineral layer. So, the pre-EDTA was expected to retain the virus titer. However, the clearance of the virus in the post-EDTA condition was surprising. We hypothesize that in the post-EDTA condition, the EDTA has a stronger binding for the Cu2+ in the mineral than the virus did. So, the EDTA eluted the virus off of the mineral. If this is true, then the V-Cu and UnV-Cu do not inactivate the virus. They only adsorb the virus, but it is still active when eluted.
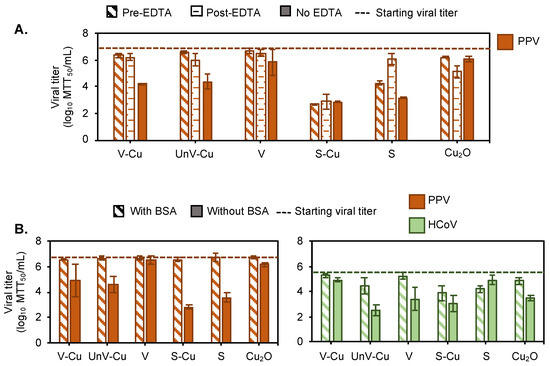
Figure 4.
Virus adsorption is a key factor in the antiviral efficacy of Cu2+-modified phyllosilicate minerals. (A) PPV inactivation was observed after contact with 2% (w/v) clay mineral powders for 2 h at 4 °C under three different conditions: (i) adding 1 mM EDTA to clay mineral powders prior to virus addition (diagonal stripes), (ii) adding 1 mM EDTA to clay mineral powders after virus incubation (horizontal grid), and (iii) in the absence of EDTA (solid). (B) Inactivation of PPV and HCoV after incubation of virus solution with 2% (w/v) clay mineral powders for 2 h at 4 °C, in blocking solution of 1% (w/v) BSA (diagonal strips) and control solution of 0.025 M HEPES (solid). The dashed line represents the starting viral titer that was kept in the same condition as the test samples.
There were different results for sepiolite. The EDTA did not change the virus clearance for S-Cu, even though copper was found in the solution. If the theory of binding described for vermiculite holds true for sepiolite, then the virus is not only bound to the Cu2+, but also sequestered in the more complex sepiolite surface and is not able to be eluted. It was also odd that adding EDTA reduced virus binding to S, even when copper was absent, as shown in Figure 3A. Hence, the chelator also acts as a blocking agent, as described for V-Cu and UnV-Cu. Overall, no change in PPV titer in the post-EDTA treatment indicates that EDTA eluted PPV from the copper-modified mineral and that the virus was adsorbed rather than inactivated.
Since adding EDTA showed that the mechanism behind the viral clearance could be the adsorption of the virus onto the Cu2+ ions, we tested if we could interfere directly with the binding mechanism by adding bovine serum albumin (BSA) as a blocking agent. As shown in Figure 3B, adding BSA to the virus–mineral solution inhibited the binding in the case of PPV and significantly reduced binding in the case of HCoV. These data demonstrated that a key mechanism for the virus clearance was binding to the phyllosilicate minerals. Thus, the amount of Cu2+ in the solution was insignificant.
The adsorbed Cu2+ did not directly inactivate the viruses, and infectious viruses could be eluted from the mineral. The viral clearance of copper could be due to three factors: reactive oxygen species (ROS), leached Cu2+, and/or Cu2+ adsorbed in the phyllosilicate minerals [6,10,16]. Since viruses are not metabolically active, they cannot produce superoxide (O2−•). This disproves the ROS-mediated antiviral action of copper [56]. Therefore, we explored leached vs. absorbed Cu2+ as a source of viral clearance. The EDTA study was able to help us discern whether the virus clearance was due to either direct viral interaction with Cu2+, adsorption, or physical entrapment of virus particles in the phyllosilicate minerals. Since EDTA chelates metal ions present in the solution, it was expected that if the leached Cu2+ were responsible for the viral clearance of vermiculite and sepiolite, the addition of EDTA would inhibit this action. However, our tests showed that even after the addition of EDTA following virus incubation with the minerals (post-EDTA), minimal antiviral activity was observed. This finding led us to conclude that EDTA was effective in displacing the virus from the Cu2+ absorbed by the minerals, indicating that the virus particles remained infectious after treatment. The Cu2+ was found to entrap the non-enveloped viruses primarily through adsorption rather than inactivation. To further confirm this mechanism, we used BSA to block the active binding sites on the phyllosilicate minerals. The results showed that adsorption was indeed the key mechanism responsible for viral clearance. This conclusion is supported by previous work showing that solid-state cuprous compounds have been observed to exhibit higher antiviral activity compared to leached copper ions [16].
4. Conclusions
Phyllosilicate minerals, vermiculite and sepiolite, were successfully modified with Cu2+, and this imparted antiviral properties to the phyllosilicate minerals. The degree of inactivation of the two model viruses was proportional to the contact time between the Cu2+-modified powders and viruses. However, the mechanism and extent of viral clearance varied between the powders and the viruses studied. This study underscores the crucial importance of understanding the antiviral properties provided by Cu2+-modified minerals. The potential application of these minerals as fillers in paints or plastics suggests a practical avenue for imparting antiviral characteristics to high-touch surfaces. Through this evaluation of copper-modified phyllosilicate minerals against two distinct viruses, PPV and HCoV, we have gained valuable insights into their mechanisms of viral clearance. These findings emphasize the critical role of direct viral interaction with Cu2+ embedded within the mineral matrix in facilitating viral clearance. This study advances our understanding of how Cu2+ contributes to viral clearance, particularly in the context of mineral-based applications. Therefore, this work lays a solid foundation for future research aimed at optimizing the design and application of such materials to combat viral spread via fomites.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/colloids9010013/s1: Figure S1: XRD patterns confirming the absence of metallic Cu. A, V-Cu; B, UnV-Cu; C, S-Cu. Figure S2: Graph for calculating rate constant for PPV and (E-H) HCoV inactivation kinetics for 2 h of incubation. A, V-Cu; B, UnV-Cu; C, S-Cu; D, Cu2O for PPV. (E-H) for HCoV. Figure S3: Toxicity of phyllosilicate minerals for PK-13 (orange) and MRC-5 (green). A: V-Cu (solid circle), UnV-Cu (diamond), V (open circle). B: S-Cu (solid triangle), S (open triangle), and Cu2O (square) for different mineral concentrations. The black dashed line represents the mark for 50% cell survival. All the data sets were performed in triplicate, and the error bars represent the standard deviation. Table S1: ICP analysis of control and copper-treated phyllosilicate mineral with test buffers (PBS, 0.025 M HEPES, and distilled water) after 2 h of incubation.
Author Contributions
Conceptualization, B.L. and C.L.H.; methodology, V.S. and S.S.; software, V.S. and S.S.; validation, C.L.H.; formal analysis, V.S. and S.S.; data collection, V.S., S.S., N.M.N., S.K. and B.L.; visualization, V.S., S.S., and C.L.H.; writing—original draft preparation, V.S. and S.S.; writing—review and editing, C.L.H.; writing—SEM characterization method, N.M.N.; writing—material characterization method, B.L.; supervision, C.L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the College of Engineering at Michigan Technological University and the NSF CBET [1818906].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Thanks to BEI for providing the original HCoV 229E virus stock and the ACMAL facility at Michigan Technological University for access to the SEM and XRD. Thanks to the Center for Applied Mathematics and Statistics (CAMS) for assistance with the statistical data analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Birkett, M.; Dover, L.; Cherian Lukose, C.; Wasy Zia, A.; Tambuwala, M.M.; Serrano-Aroca, A. Recent Advances in Metal-Based Antimicrobial Coatings for High-Touch Surfaces. Int. J. Mol. Sci. 2022, 23, 1162. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, U.; Khandelwal, M.; Deshpande, A.S. Antimicrobial surfaces: A review of synthetic approaches, applicability and outlook. J. Mater. Sci. 2021, 56, 17915–17941. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.T.; Neoh, K.G. Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 21159–21182. [Google Scholar] [CrossRef]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and metal oxide-based antiviral nanoparticles: Properties, mechanisms of action, and applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef]
- Tian, H.; He, B.; Yin, Y.; Liu, L.; Shi, J.; Hu, L.; Jiang, G. Chemical Nature of Metals and Metal-Based Materials in inactivation of Viruses. Nanomaterials 2022, 12, 2345. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Ogawa, H.; Yoshida, Y.; Yamada, K.; Hara, A.; Ozawa, K.; Matsuda, S.; Mizota, C.; Tani, M.; Yamamoto, Y.; et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch. Virol. 2008, 153, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Bright, K.R.; Sicairos-Ruelas, E.E.; Gundy, P.M.; Gerba, C.P. Assessment of the Antiviral Properties of Zeolites Containing Metal Ions. Food Environ. Virol. 2008, 1, 37–41. [Google Scholar] [CrossRef]
- Mertens, B.S.; Moore, M.D.; Jaykus, L.A.; Velev, O.D. Efficacy and Mechanisms of Copper Ion-Catalyzed Inactivation of Human Norovirus. ACS Infect. Dis. 2022, 8, 855–864. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.R.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral properties of copper and its alloys to inactivate COVID-19 virus: A review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Ogawa, H.; Bui, V.N.; Inoue, H.; Fukuda, J.; Ohba, M.; Yamamoto, Y.; Nakamura, K. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antivir. Res. 2012, 93, 225–233. [Google Scholar] [CrossRef]
- Sengupta, D.; Timilsina, U.; Mazumder, Z.H.; Mukherjee, A.; Ghimire, D.; Markandey, M.; Upadhyaya, K.; Sharma, D.; Mishra, N.; Jha, T. Dual activity of amphiphilic Zn (II) nitroporphyrin derivatives as HIV-1 entry inhibitors and in cancer photodynamic therapy. Eur. J. Med. Chem. 2019, 174, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.; Michels, H.; Keevil, C. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ko, Y.-S.; Jung, H.; Lee, C.; Woo, K.; Ko, G. Disinfection of waterborne viruses using silver nanoparticle-decorated silica hybrid composites in water environments. Sci. Total Environ. 2018, 625, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Vonnemann, J.; Sieben, C.; Wolff, C.; Ludwig, K.; Böttcher, C.; Herrmann, A.; Haag, R. Virus inhibition induced by polyvalent nanoparticles of different sizes. Nanoscale 2014, 6, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Giannakis, S.; Liu, S.; Carratalà, A.; Rtimi, S.; Amiri, M.T.; Bensimon, M.; Pulgarin, C. Iron oxide-mediated semiconductor photocatalysis vs. heterogeneous photo-Fenton treatment of viruses in wastewater. Impact of the oxide particle size. J. Hazard. Mater. 2017, 339, 223–231. [Google Scholar] [CrossRef]
- Barcelos, I.D.; de Oliveira, R.; Schleder, G.R.; Matos, M.J.S.; Longuinhos, R.; Ribeiro-Soares, J.; Barboza, A.P.M.; Prado, M.C.; Pinto, E.S.; Gobato, Y.G.; et al. Phyllosilicates as earth-abundant layered materials for electronics and optoelectronics: Prospects and challenges in their ultrathin limit. J. Appl. Phys. 2023, 134, 090902. [Google Scholar] [CrossRef]
- Brown, G. Crystal structures of clay minerals and related phyllosilicates. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1984, 311, 221–240. [Google Scholar]
- Cecilia, J.A.; Vilarrasa-García, E.; Cavalcante, C.L.; Azevedo, D.C.S.; Franco, F.; Rodríguez-Castellón, E. Evaluation of two fibrous clay minerals (sepiolite and palygorskite) for CO2 Capture. J. Environ. Chem. Eng. 2018, 6, 4573–4587. [Google Scholar] [CrossRef]
- Cura D’Ars de Figueiredo, J., Jr.; Diniz Pereira Marques, H.; Goulart Silva, G. Expanded vermiculite and polyvinyl acetate composite as gap filler for wooden objects conservation. J. Cult. Herit. 2022, 55, 88–94. [Google Scholar] [CrossRef]
- Diler, H.; Durmaz, S.; Acar, M.; Aras, U.; Erdil, Y.Z. The Effect of Vermiculite on Flame Retardancy, Physical and Mechanical Properties of Wood Plastic Composites. BioResources 2024, 19, 183. [Google Scholar] [CrossRef]
- Sethurajaperumal, A.; Manohar, A.; Banerjee, A.; Varrla, E.; Wang, H.; Ostrikov, K.K. A thermally insulating vermiculite nanosheet–epoxy nanocomposite paint as a fire-resistant wood coating. Nanoscale Adv. 2021, 3, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.; Gertisser, R.; Zholobenko, V. Structural features and stability of Spanish sepiolite as a potential catalyst. Appl. Clay Sci. 2018, 162, 297–304. [Google Scholar] [CrossRef]
- Hrenovic, J.; Tibljas, D.; Ivankovic, T.; Kovacevic, D.; Sekovanic, L. Sepiolite as carrier of the phosphate-accumulating bacteria Acinetobacter junii. Appl. Clay Sci. 2010, 50, 582–587. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.; Balek, V.; Poyato, J.; Perez-Rodriquez, J.; Šubrt, J.; Bountsewa, I.; Beckman, I.; Málek, Z. Study of natural and ion exchanged vermiculite by emanation thermal analysis, TG, DTA and XRD. J. Therm. Anal. Calorim. 2003, 71, 715–726. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Drelich, J.; Li, B.; Bowen, P.; Hwang, J.-Y.; Mills, O.; Hoffman, D. Vermiculite decorated with copper nanoparticles: Novel antibacterial hybrid material. Appl. Surf. Sci. 2011, 257, 9435–9443. [Google Scholar] [CrossRef]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Dim, S.; Edri, A.; Greenshpan, Y.; Ottolenghi, A.; Eisner, N.; Tzadka, S.; Pandey, A.; Ben Nun, H.; Le Saux, G.; et al. Nanocomposite coatings for the prevention of surface contamination by coronavirus. PLoS ONE 2022, 17, e0272307. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, W.A. A Surface Coating that Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Putting copper into action: Copper-impregnated products with potent biocidal activities. FASEB J. 2004, 18, 1728–1730. [Google Scholar] [CrossRef]
- Joshi, P.U.; Meingast, C.L.; Xu, X.; Holstein, M.; Feroz, H.; Ranjan, S.; Ghose, S.; Li, Z.J.; Heldt, C.L. Virus inactivation at moderately low pH varies with virus and buffer properties. Biotechnol. J. 2022, 17, e2100320. [Google Scholar] [CrossRef]
- Barone, P.W.; Wiebe, M.E.; Leung, J.C.; Hussein, I.T.M.; Keumurian, F.J.; Bouressa, J.; Brussel, A.; Chen, D.; Chong, M.; Dehghani, H.; et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nat. Biotechnol. 2020, 38, 563–572. [Google Scholar] [CrossRef]
- Molitor, T.; Joo, H.; Collett, M. Porcine parvovirus: Virus purification and structural and antigenic properties of virion polypeptides. J. Virol. 1983, 45, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Tarka, P.; Nitsch-Osuch, A. Evaluating the Virucidal Activity of Disinfectants According to European Union Standards. Viruses 2021, 13, 534. [Google Scholar] [CrossRef]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697-15. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Murallidharan, J.S.; Agrawal, A.; Bhardwaj, R. Why coronavirus survives longer on impermeable than porous surfaces. Phys. Fluids 2021, 33, 021701. [Google Scholar] [CrossRef] [PubMed]
- Firquet, S.; Beaujard, S.; Lobert, P.E.; Sane, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Env. 2015, 30, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.F.; Maile, J.; Heister, A.; Siddell, S.G. Characterization of functional domains in the human coronavirus HCV 229E receptor. J. Gen. Virol. 1996, 77, 2515–2521. [Google Scholar] [CrossRef]
- Turpeinen, D.G.; Joshi, P.U.; Kriz, S.A.; Kaur, S.; Nold, N.M.; O’Hagan, D.; Nikam, S.; Masoud, H.; Heldt, C.L. Continuous purification of an enveloped and non-enveloped viral particle using an aqueous two-phase system. Sep. Purif. Technol. 2021, 269, 118753. [Google Scholar] [CrossRef]
- Sorci, M.; Fink, T.D.; Sharma, V.; Singh, S.; Chen, R.; Arduini, B.L.; Dovidenko, K.; Heldt, C.L.; Palermo, E.F.; Zha, R.H. Virucidal N95 Respirator Face Masks via Ultrathin Surface-Grafted Quaternary Ammonium Polymer Coatings. ACS Appl. Mater. Interfaces 2022, 14, 25135–25146. [Google Scholar] [CrossRef] [PubMed]
- Heldt, C.L.; Hernandez, R.; Mudiganti, U.; Gurgel, P.V.; Brown, D.T.; Carbonell, R.G. A colorimetric assay for viral agents that produce cytopathic effects. J. Virol. Methods 2006, 135, 56–65. [Google Scholar] [CrossRef]
- Hiatt, C. Kinetics of the inactivation of viruses. Bacteriol. Rev. 1964, 28, 150–163. [Google Scholar] [CrossRef]
- Steudel, A.; Weidler, P.G.; Schuhmann, R.; Emmerich, K. Cation Exchange Reactions of Vermiculite With Cu-Triethylenetetramine as Affected by Mechanical and Chemical Pretreatment. Clays Clay Miner. 2024, 57, 486–493. [Google Scholar] [CrossRef]
- Erdoğan, B.; Esenli, F. Sepiolite as an efficient adsorbent for ethylene gas. Clay Miner. 2022, 56, 222–228. [Google Scholar] [CrossRef]
- Shirozu, H.; Bailey, S.W. Crystal structure of a two-layer Mg-vermiculite. Am. Mineral. 1966, 51, 1124–1143. [Google Scholar]
- Marcos, C. Structural changes in vermiculites induced by temperature, pressure, irradiation, and chemical treatments. In Clay Science and Technology; IntechOpen: London, UK, 2020. [Google Scholar]
- Nath, D.; Santhosh, R.; Pal, K.; Sarkar, P. Nanoclay-based active food packaging systems: A review. Food Packag. Shelf Life 2022, 31, 100803. [Google Scholar] [CrossRef]
- Hillier, S.; Marwa, E.M.M.; Rice, C.M. On the mechanism of exfoliation of ‘Vermiculite’. Clay Miner. 2018, 48, 563–582. [Google Scholar] [CrossRef]
- Hundáková, M.; Valášková, M.; Samlíková, M.; Pazdziora, E. Vermiculite With Ag and Cu Used as an Antibacterial Nanofiller in Polyethylene/ Vermikulit S Ag A Cu Použitý Jako Antibakteriální Nanoplnivo V Polyethylenu. GeoScience Eng. 2014, 60, 28–36. [Google Scholar] [CrossRef][Green Version]
- Alkan, M.; Demirbas, O.; Dogan, M. Electrokinetic properties of sepiolite suspensions in different electrolyte media. J. Colloid. Interface Sci. 2005, 281, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Can, M.F.; Çınar, M.; Benli, B.; Özdemir, O.; Çelik, M.S. Determining the fiber size of nano structured sepiolite using Atomic Force Microscopy (AFM). Appl. Clay Sci. 2010, 47, 217–222. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil. Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Borkow, G. Use of Biocidal Surfaces for Reduction of Healthcare Acquired Infections; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).