Water-Based Bi2S3 Nano-Inks Obtained with Surfactant-Assisted Liquid Phase Exfoliation and Their Direct Processing into Thin Films
Abstract
1. Introduction
2. Materials and Methods
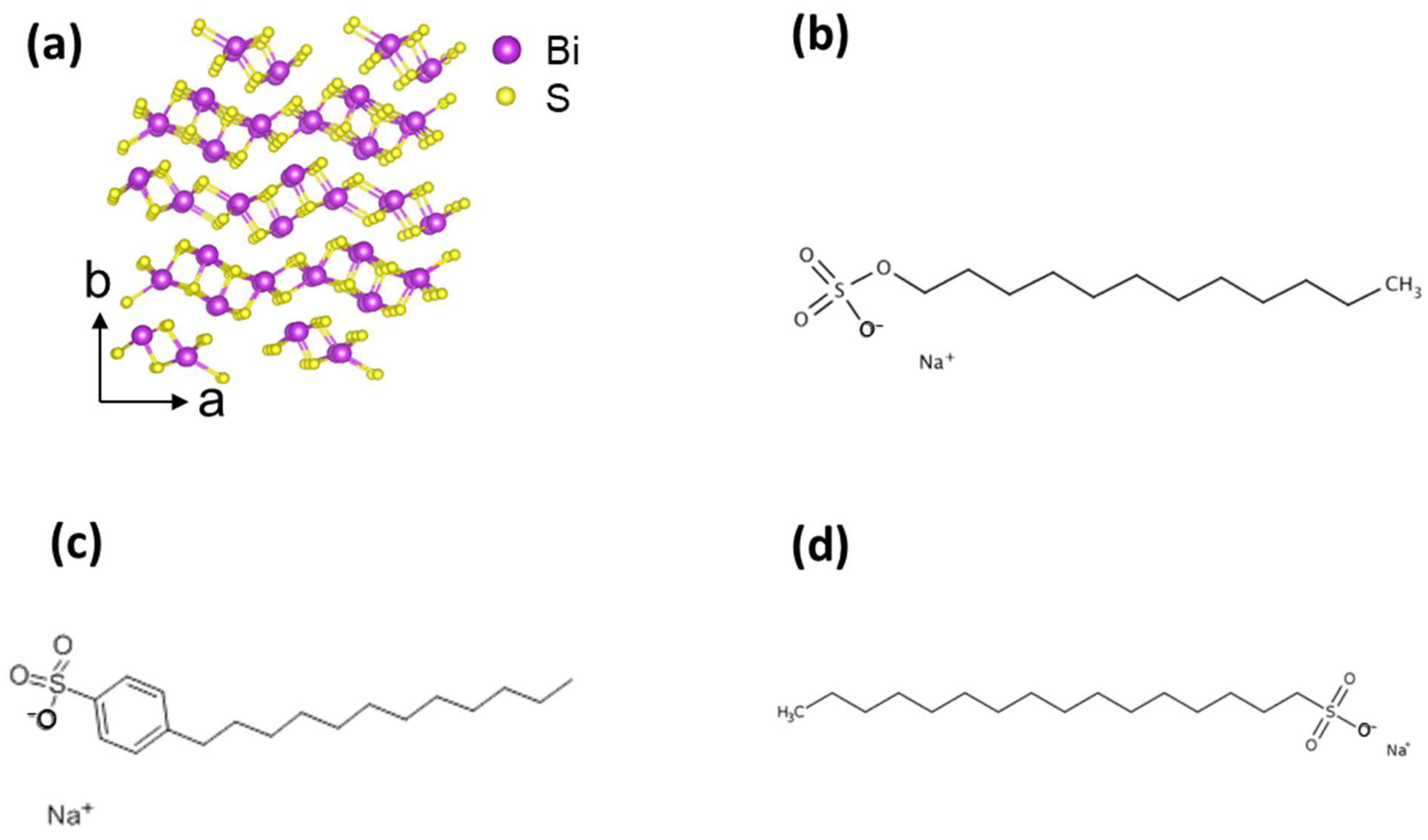
2.1. Materials and LPE Process
2.2. Characterizations
2.3. Calculation Methods of the Sample Concentration
3. Results and Discussion
3.1. Characterizations of the Resulting Nanomaterials
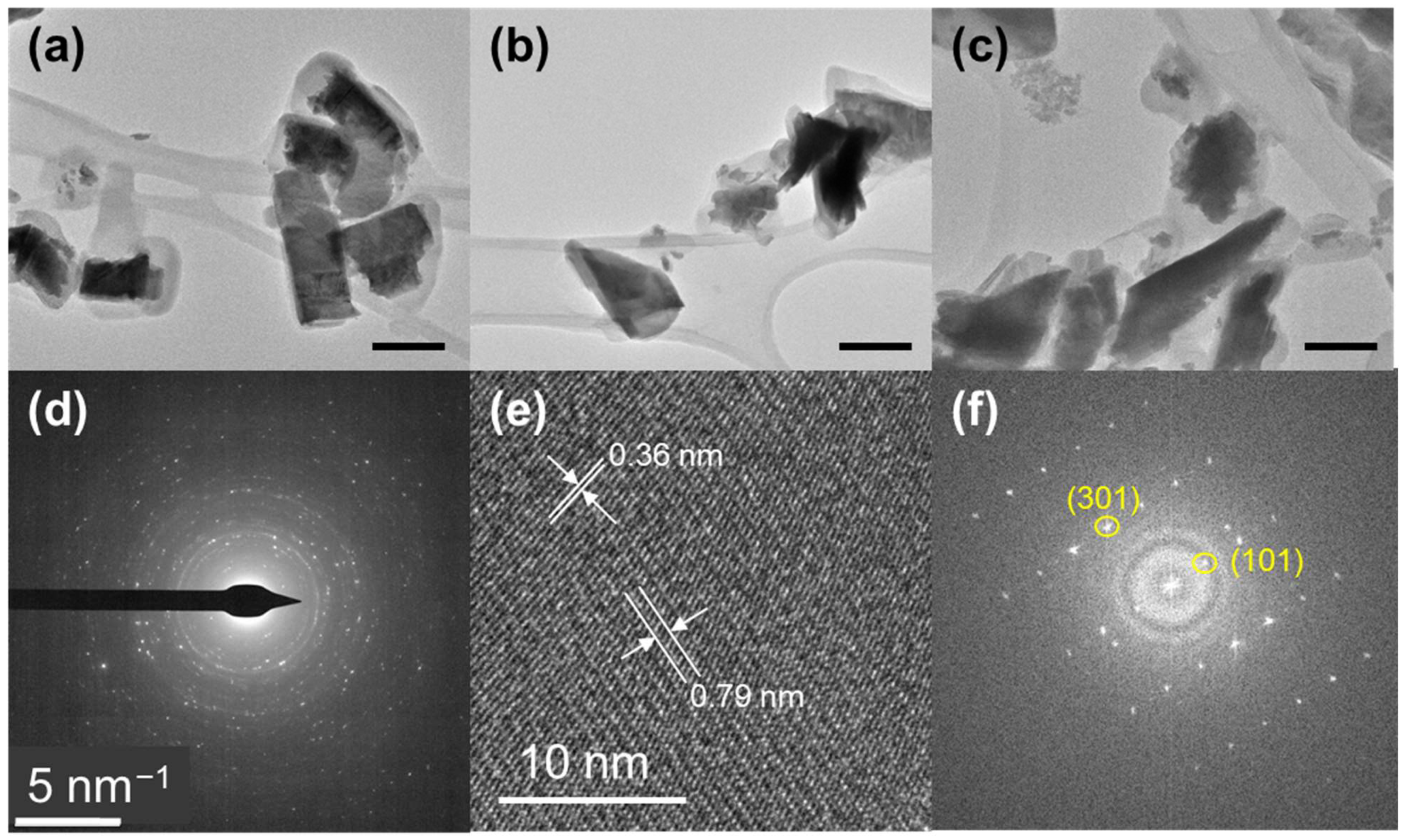
3.1.1. TEM Analysis
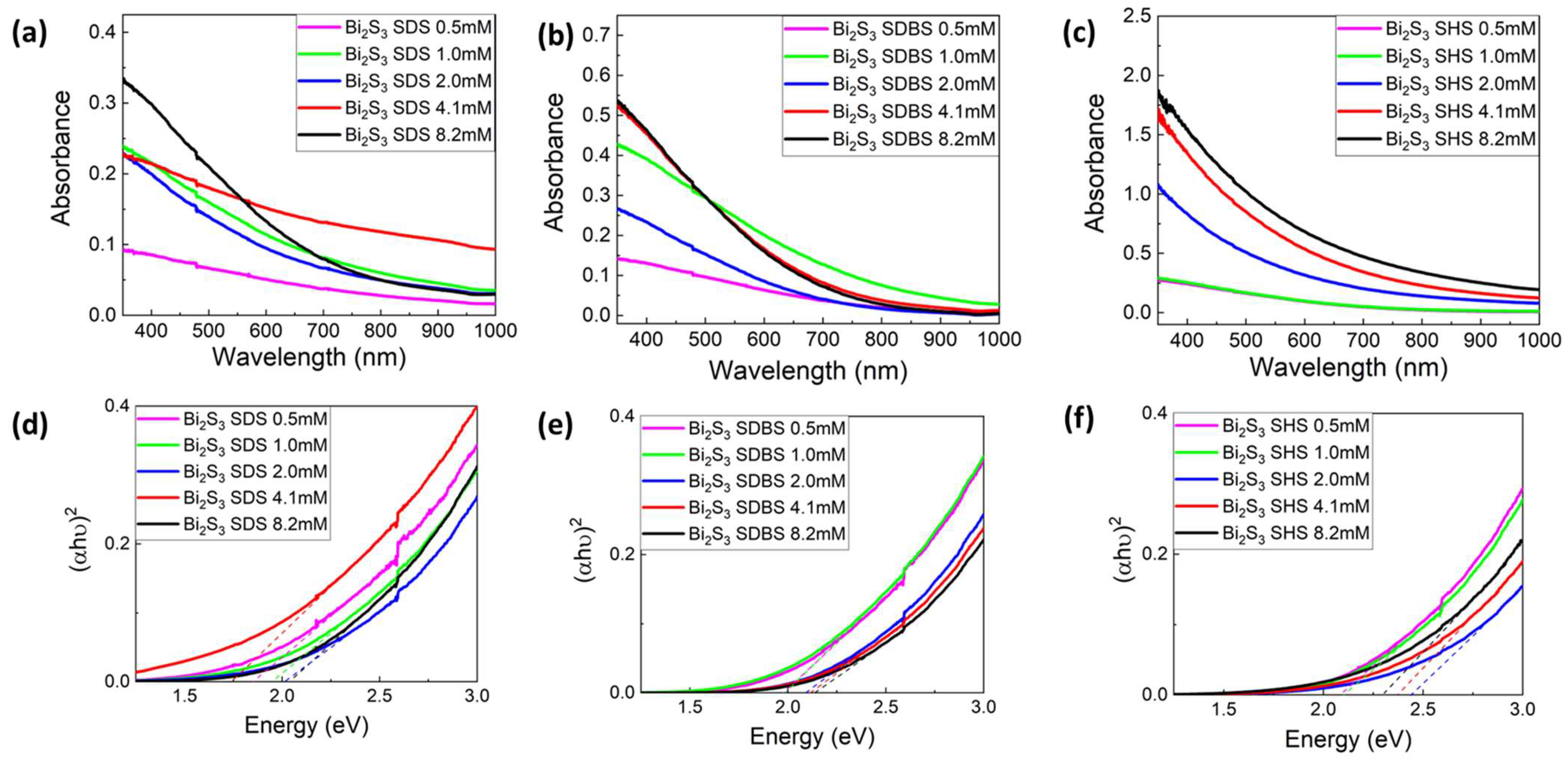
3.1.2. UV-Vis Analysis
3.1.3. Raman Analysis
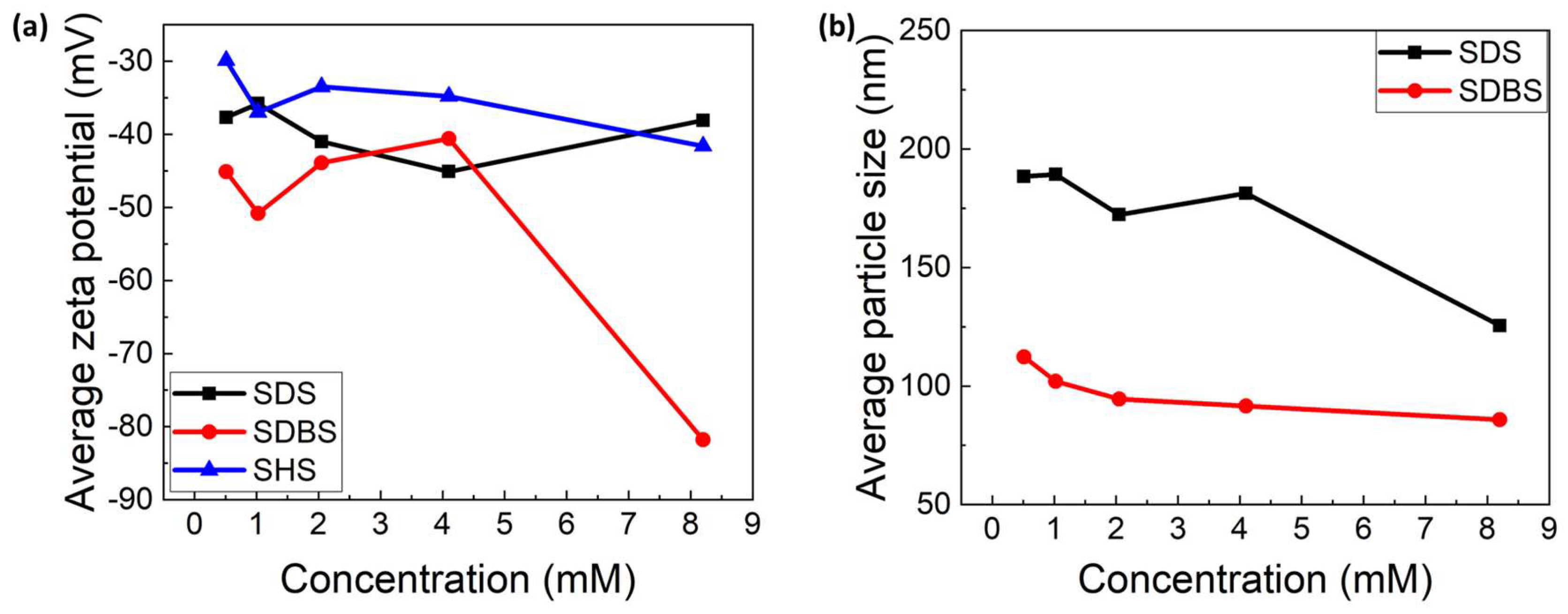
3.1.4. Zeta Potenzial and DLS
3.2. Yield and Final Concentration of the Nanomaterials
3.3. Production of Thin Films with Ultrasonic Spray-Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shawky, A.; Alahmadi, N.; Mohamed, R.M.; Zaki, Z.I. Bi2S3-Sensitized TiO2 Nanostructures Prepared by Solution Process for Highly Efficient Photoreduction of Hexavalent Chromium Ions in Water Under Visible Light. Opt. Mater. 2022, 124, 111964. [Google Scholar] [CrossRef]
- Ott, S.; Wolff, N.; Rashvand, F.; Rao, V.J.; Zaumseil, J.; Backes, C. Impact of the MoS2 Starting Material on the Dispersion Quality and Quantity after Liquid Phase Exfoliation. Chem. Mater. 2019, 31, 8424–8431. [Google Scholar] [CrossRef]
- Han, M.; Jia, J. The Interlace of Bi2S3 Nanowires with TiO2 Nanorods: An Effective Strategy for High Photoelectrochemical Performance. J. Colloid Interface Sci. 2016, 481, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, Y.; Shi, Z.; Xia, H.; Ma, M.; Wang, Y.; Huang, K.; Wu, Y.; Gong, Y.; Fei, H.; et al. High-Pressure Synthesis of Single-Crystalline SnS Nanoribbons. Nano Lett. 2023, 23, 7449–7455. [Google Scholar] [CrossRef] [PubMed]
- Barraza-Lopez, S.; Fregoso, B.M.; Villanova, J.W.; Parkin, S.S.P.; Chang, K. Colloquium: Physical Properties of Group-IV Monochalcogenide Monolayers. Rev. Mod. Phys. 2021, 93, 011001. [Google Scholar] [CrossRef]
- Villanova, J.W.; Kumar, P.; Barraza-Lopez, S. Theory of Finite-Temperature Two-Dimensional Structural Transformations in Group-IV Monochalcogenide Monolayers. Phys. Rev. B 2020, 101, 184101. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, Y.; Gao, C.; Liu, L.; Zhang, Y.; Chen, Y.; Shao, Y. Construction of AgBr/BiOBr S-Scheme Heterojunction Using Ion Exchange Strategy for High-Efficiency Reduction of CO2 to CO under Visible Light. Sep. Purif. Technol. 2022, 303, 122288. [Google Scholar] [CrossRef]
- Bai, Y.; Ouyang, T.; Li, X.; Yan, Y.; Kong, Z.; Ma, X.; Li, Z.; Li, Z.; Cai, X.; Cai, J.; et al. Boosting the Thermoelectric Performance of n-Type Bi2S3 by Compositing rGO. J. Alloys Compd. 2023, 933, 167814. [Google Scholar] [CrossRef]
- Zhao, F.; Sheng, H.; Sun, Q.; Wang, J.; Liu, Q.; Hu, Z.; He, B.; Wang, Y.; Li, Z.; Liu, X. Harvesting the Infrared Part of Solar Light to Promote Charge Transfer in Bi2S3/WO3 Photoanode for Enhanced Photoelectrochemical Water Splitting. J. Colloid Interface Sci. 2022, 621, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, B.; Xu, L.; Zhang, G.; Cao, L.; Liu, N.; Wang, W.; Qian, H.; Shao, M. Urchin-like Fe3O4@Bi2S3 Nanospheres Enable the Destruction of Biofilm and Efficiently Antibacterial Activities. ACS Appl. Mater. Interfaces 2023, 16, 3215–3231. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Jamali-Sheini, F.; Cheraghizade, M. Visible-Range and Self-Powered Bilayer p-Si/n-Bi2S3 Heterojunction Photodetector: The Effect of Au Buffer Layer on the Optoelectronics Performance. J. Alloys Compd. 2022, 905, 164119. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zhang, D.; Chen, C. A Sensitizing Photoelectrochemical Sensing Platform Strategy Based on Bio-Etching Preparation of Bi2S3/BiOCl p–n Heterojunction. Talanta 2018, 190, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; O’Neill, A.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G.; et al. Large-Scale Exfoliation of Inorganic Layered Compounds in Aqueous Surfactant Solutions. Adv. Mater. 2011, 23, 3944–3948. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Young, R.J.; Backes, C.; Zhao, W.; Zhang, X.; Zhukov, A.A.; Tillotson, E.; Conlan, A.P.; Ding, F.; Haigh, S.J.; et al. Mechanisms of Liquid-Phase Exfoliation for the Production of Graphene. ACS Nano 2020, 14, 10976–10985. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.S.; Konidakis, I.; Gagaoudakis, E.; Maragkakis, G.M.; Psilodimitrakopoulos, S.; Katerinopoulou, D.; Sygellou, L.; Deligeorgis, G.; Binas, V.; Oikonomou, I.M.; et al. Liquid Phase Isolation of SnS Monolayers with Enhanced Optoelectronic Properties. Adv. Sci. 2023, 10, 2201842. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, A.; Nepal, D.; Park, K.; Jespersen, M.; Qualley, A.; Mirau, P.; Drummy, L.F.; Vaia, R.A. Mechanism for Liquid Phase Exfoliation of MoS2. Chem. Mater. 2016, 28, 337–348. [Google Scholar] [CrossRef]
- Hu, C.X.; Shin, Y.; Read, O.; Casiraghi, C. Dispersant-Assisted Liquid-Phase Exfoliation of 2D Materials beyond Graphene. Nanoscale 2021, 13, 460–484. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, Q.; Yao, Z.; Si, K.; Zhou, Y.; Xu, X. Efficient Mixed-Solvent Exfoliation of Few-Quintuple Layer Bi2S3 and Its Photoelectric Response. Nanotechnology 2017, 28, 335602. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.S.; Stratakis, E. Dispersion Behaviour of Two Dimensional Monochalcogenides. J. Colloid Interface Sci. 2021, 594, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.; Nisi, K.; Pepper, J.; Harvey, A.; Szydłowska, B.M.; Coleman, J.N.; Backes, C. Effect of Surfactant Choice and Concentration on the Dimensions and Yield of Liquid-Phase-Exfoliated Nanosheets. Chem. Mater. 2020, 32, 2852–2862. [Google Scholar] [CrossRef]
- Ying, G.G. Fate, Behavior and Effects of Surfactants and their Degradation Products in the Environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Arunachalam, V.; Vasudevan, S. Water Dispersible, Positively and Negatively Charged MoS2 Nanosheets: Surface Chemistry and the Role of Surfactant Binding. J. Phys. Chem. Lett. 2015, 6, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, H. Self-Aggregation of the SDS Surfactant at a Solid-Liquid Interface. J. Phys. Chem. B 2007, 111, 4054–4059. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, H.; Dargusch, M.; Huang, Y. A Reliable and Highly Efficient Exfoliation Method for Water-Dispersible MoS2 Nanosheet. J. Colloid Interface Sci. 2018, 514, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Crisci, M.; Pavan, M.; Liu, Z.; Gallego, J.; Gatti, T. New Insights into the Surfactant-Assisted Liquid-Phase Exfoliation of Bi2S3 for Electrocatalytic Applications. Catalysts 2023, 13, 551. [Google Scholar] [CrossRef]
- Guan, Z.; Wang, C.; Li, W.; Luo, S.; Yao, Y.; Yu, S.; Sun, R.; Wong, C.P. A Facile and Clean Process for Exfoliating MoS2 Nanosheets Assisted by a Surface Active Agent in Aqueous Solution. Nanotechnology 2018, 29, 425702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Ghini, M.; Melcherts, A.E.M.; Zito, J.; Goldoni, L.; Infante, I.; Guizzardi, M.; Scotognella, F.; Kriegel, I.; et al. Colloidal Bi-Doped Cs2Ag1- XNaxInCl6 Nanocrystals: Undercoordinated Surface Cl Ions Limit Their Light Emission Efficiency. ACS Mater. Lett. 2020, 2, 1442–1449. [Google Scholar] [CrossRef]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-Concentration, Surfactant-Stabilized Graphene Dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef]
- Abreu, B.; Almeida, B.; Ferreira, P.; Fernandes, R.M.F.; Fernandes, D.M.; Marques, E.F. A Critical Assessment of the Role of Ionic Surfactants in the Exfoliation and Stabilization of 2D Nanosheets: The Case of the Transition Metal Dichalcogenides MoS2, WS2 and MoSe2. J. Colloid Interface Sci. 2022, 626, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yi, M.; Shen, Z. The Effect of Surfactants and Their Concentration on the Liquid Exfoliation of Graphene. RSC Adv. 2016, 6, 56705–56710. [Google Scholar] [CrossRef]
- Markarian, S.A.; Harutyunyan, L.R.; Harutyunyan, R.S. The Properties of Mixtures of Sodium Dodecylsulfate and Diethylsulfoxide in Water. J. Solution Chem. 2005, 34, 361–368. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Xing, B. Enhanced Soil Washing of Phenanthrene by Mixed Solutions of TX100 and SDBS. Environ. Sci. Technol. 2006, 40, 4274–4280. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, R.; Gente, G.; La Mesa, C.; Caponetti, E.; Chillura-Martino, D.; Pedone, L.; Saladino, M.L. Micelles in Mixtures of Sodium Dodecyl Sulfate and a Bolaform Surfactant. Langmuir 2006, 22, 6001–6009. [Google Scholar] [CrossRef]
- Antonioli Júnior, R.; Poloni, J.d.F.; Pinto, É.S.M.; Dorn, M. Interdisciplinary Overview of Lipopeptide and Protein-Containing Biosurfactants. Genes 2023, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Messalea, K.A.; Zavabeti, A.; Mohiuddin, M.; Syed, N.; Jannat, A.; Atkin, P.; Ahmed, T.; Walia, S.; McConville, C.F.; Kalantar-Zadeh, K.; et al. Two-Step Synthesis of Large-Area 2D Bi2S3 Nanosheets Featuring High In-Plane Anisotropy. Adv. Mater. Interfaces 2020, 7, 2001131. [Google Scholar] [CrossRef]
- Dhar, N.; Syed, N.; Mohiuddin, M.; Jannat, A.; Zavabeti, A.; Zhang, B.Y.; Datta, R.S.; Atkin, P.; Mahmood, N.; Esrafilzadeh, D.; et al. Exfoliation Behavior of van Der Waals Strings: Case Study of Bi2S3. ACS Appl. Mater. Interfaces 2018, 10, 42603–42611. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lu, C.; Ma, J.; Luo, M.; Zhao, Q.; Jin, Y.; Xu, X. Enhanced Nonlinear Saturable Absorption from Type III van Der Waals Heterostructure Bi2S3/MoS2 by Interlayer Electron Transition. Appl. Surf. Sci. 2021, 538, 147989. [Google Scholar] [CrossRef]
- Zumeta-Dubé, I.; Ortiz-Quiñonez, J.L.; Díaz, D.; Trallero-Giner, C.; Ruiz-Ruiz, V.F. First Order Raman Scattering in Bulk Bi2S3 and Quantum Dots: Reconsidering Controversial Interpretations. J. Phys. Chem. C 2014, 118, 30244–30252. [Google Scholar] [CrossRef]
- Clark, R.M.; Kotsakidis, J.C.; Weber, B.; Berean, K.J.; Carey, B.J.; Field, M.R.; Khan, H.; Ou, J.Z.; Ahmed, T.; Harrison, C.J.; et al. Exfoliation of Quasi-Stratified Bi2S3 Crystals into Micron-Scale Ultrathin Corrugated Nanosheets. Chem. Mater. 2016, 28, 8942–8950. [Google Scholar] [CrossRef]
- Ni, J.; Bi, X.; Jiang, Y.; Li, L.; Lu, J. Bismuth Chalcogenide Compounds Bi2×3 (X=O, S, Se): Applications in Electrochemical Energy Storage. Nano Energy 2017, 34, 356–366. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, S.; Cho, D.H.; Kang, B.; Kwon, H.; Kim, Y.; Park, S.O.; Jung, G.Y.; Shin, E.; Kim, W.G.; et al. Direct Exfoliation and Dispersion of Two-Dimensional Materials in Pure Water via Temperature Control. Nat. Commun. 2015, 6, 8294. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Dieng, M.; Vanel, J.C.; Florea, I.; Bouanis, F.Z.; Yassar, A. Liquid Shear Exfoliation of MoS2: Preparation, Characterization, and NO2-Sensing Properties. Nanomaterials 2023, 13, 2502. [Google Scholar] [CrossRef] [PubMed]
- Medles, M.; Benramdane, N.; Bouzidi, A.; Nakrela, A.; Tabet-Derraz, H.; Kebbab, Z.; Mathieu, C.; Khelifa, B.; Desfeux, R. Optical and Electrical Properties of Bi2S3 Films Deposited by Spray Pyrolysis. Thin Solid Film. 2006, 497, 58–64. [Google Scholar] [CrossRef]



| Surfactant | Surf. Concentration (mM) | Band Gap (eV) |
|---|---|---|
| SDS | 8.2 | 2.03 |
| 4.1 | 1.74 | |
| 2.0 | 2.01 | |
| 1.0 | 1.95 | |
| 0.5 | 1.85 | |
| SDBS | 8.2 | 2.13 |
| 4.1 | 2.11 | |
| 2.0 | 2.10 | |
| 1.0 | 1.99 | |
| 0.5 | 1.97 | |
| SHS | 8.2 | 2.30 |
| 4.1 | 2.37 | |
| 2.0 | 2.44 | |
| 1.0 | 2.10 | |
| 0.5 | 2.08 |
| 2D Material | Surfactant | Surf. Concentration (mM) | Ag/B1g Ratio |
|---|---|---|---|
| Bi2S3 | 8.2 | 1.20 | |
| 4.1 | 1.18 | ||
| SDS | 2.0 | 1.15 | |
| 1.0 | 1.07 | ||
| 0.5 | 1.32 | ||
| Bi2S3 | 8.2 | 1.32 | |
| 4.1 | 1.25 | ||
| SDBS | 2.0 | 1.19 | |
| 1.0 | 1.17 | ||
| 0.5 | 1.02 | ||
| Bi2S3 | 8.2 | 1.30 | |
| 4.1 | 1.12 | ||
| SHS | 2.0 | 1.30 | |
| 1.0 | 1.25 | ||
| 0.5 | 1.25 |
| Surfactant | Surfactant Concentration (mM) | Product Concentration with UV-Vis Absorption (mg/mL) | Product Concentration with Freeze-Drying (mg/mL) | Yield (%) |
|---|---|---|---|---|
| 8.2 | 0.03 | 0.3 | ||
| 4.1 | 0.02 | 0.2 | ||
| SDS | 2.0 | 0.02 | 0.2 | |
| 1.0 | 0.02 | 0.2 | ||
| 0.5 | 0.01 | 0.1 | ||
| 8.2 | 0.04 | 0.04 | 0.4 | |
| 4.1 | 0.04 | 0.4 | ||
| SDBS | 2.0 | 0.02 | 0.2 | |
| 1.0 | 0.04 | 0.4 | ||
| 0.5 | 0.01 | 0.1 | ||
| 8.2 | 0.14 | 0.08 | 1.4 | |
| 4.1 | 0.11 | 0.44 | 1.1 | |
| SHS | 2.0 | 0.07 | 0.7 | |
| 1.0 | 0.02 | 0.2 | ||
| 0.5 | 0.02 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzati, M.; Boll, F.; Crisci, M.; Domenici, S.; Scotognella, F.; Smarsly, B.; Gatti, T.; Wang, M. Water-Based Bi2S3 Nano-Inks Obtained with Surfactant-Assisted Liquid Phase Exfoliation and Their Direct Processing into Thin Films. Colloids Interfaces 2024, 8, 28. https://doi.org/10.3390/colloids8030028
Pozzati M, Boll F, Crisci M, Domenici S, Scotognella F, Smarsly B, Gatti T, Wang M. Water-Based Bi2S3 Nano-Inks Obtained with Surfactant-Assisted Liquid Phase Exfoliation and Their Direct Processing into Thin Films. Colloids and Interfaces. 2024; 8(3):28. https://doi.org/10.3390/colloids8030028
Chicago/Turabian StylePozzati, Micaela, Felix Boll, Matteo Crisci, Sara Domenici, Francesco Scotognella, Bernd Smarsly, Teresa Gatti, and Mengjiao Wang. 2024. "Water-Based Bi2S3 Nano-Inks Obtained with Surfactant-Assisted Liquid Phase Exfoliation and Their Direct Processing into Thin Films" Colloids and Interfaces 8, no. 3: 28. https://doi.org/10.3390/colloids8030028
APA StylePozzati, M., Boll, F., Crisci, M., Domenici, S., Scotognella, F., Smarsly, B., Gatti, T., & Wang, M. (2024). Water-Based Bi2S3 Nano-Inks Obtained with Surfactant-Assisted Liquid Phase Exfoliation and Their Direct Processing into Thin Films. Colloids and Interfaces, 8(3), 28. https://doi.org/10.3390/colloids8030028








