Matrices of Native and Oxidized Pectin and Ferrous Bisglycinate and Their In Vitro Behavior through Gastrointestinal Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Ferrous BisGlycinate Sample Chelation
2.2. Pectin Characterization
2.3. Pectin Oxidation
2.4. Preparation of Pectin, Glycine, Iron, and Ferrous Bisglycinate Matrices
2.5. In Vitro Digestion
2.6. Scanning Electron Microscopy (SEM)
2.7. Fourier Transform Infrared Spectroscopy, FTIR
2.8. Thermal Analyses
2.9. Swelling Degree
2.10. Iron Release Quantification
2.11. Statistical Analysis
3. Results and Discussion
3.1. Pectin Characterization
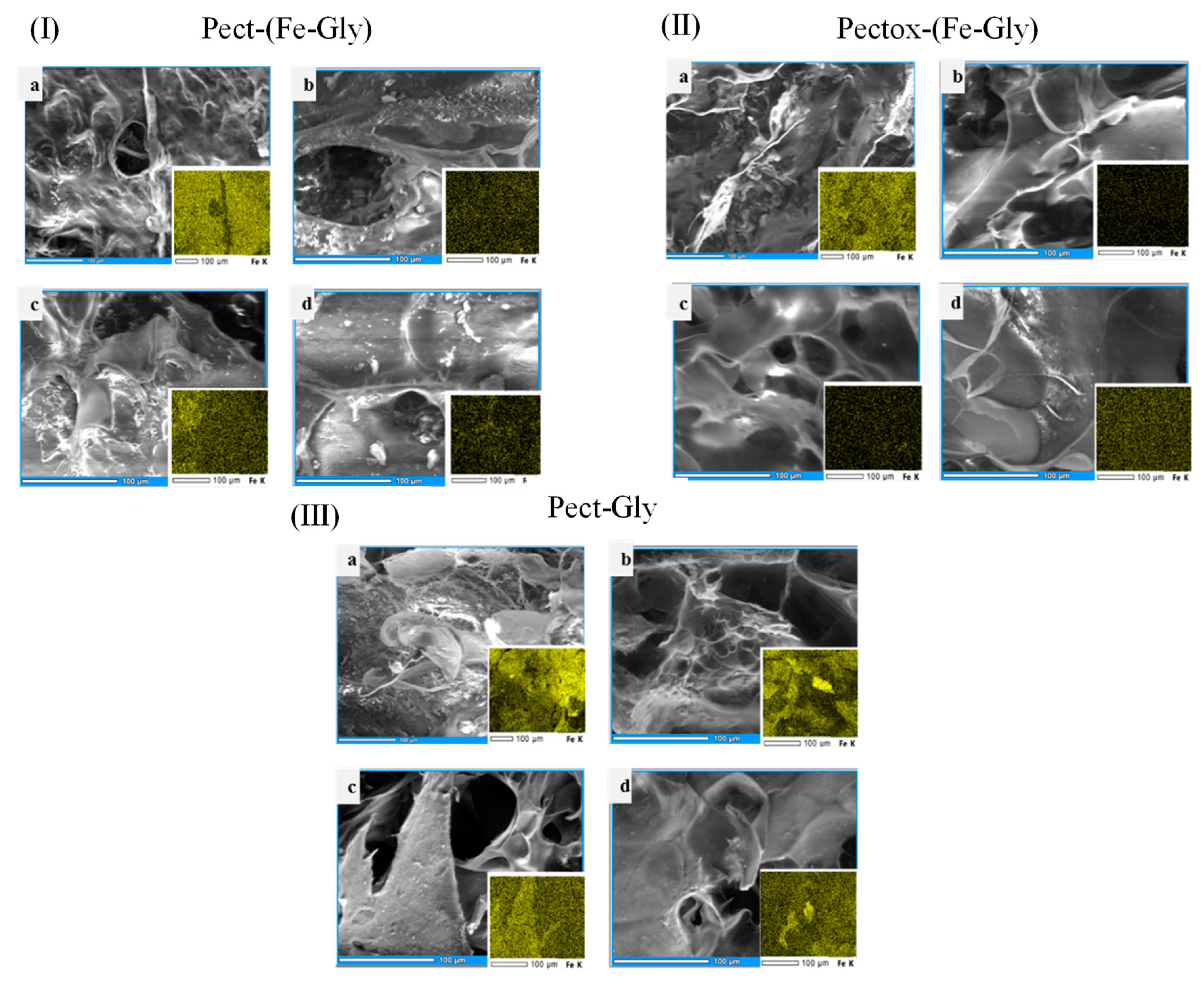
3.1.1. Matrix Morphology
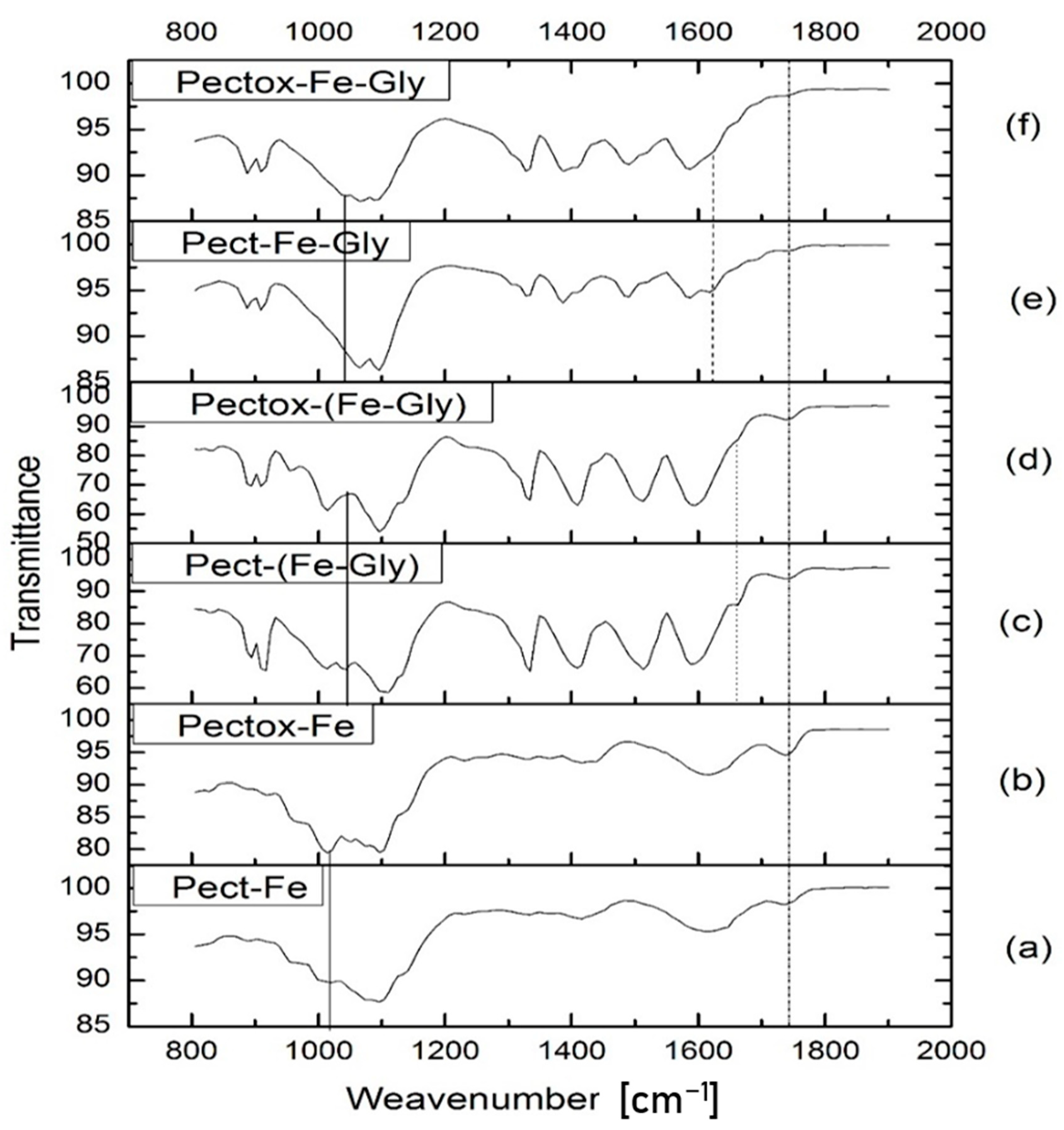
3.1.2. Analysis of Matrix Interactions through FTIR Spectra
- 1000–1150 cm−1: C-O of pectin carboxyl and methoxyl groups.
- 1620–1690 cm−1: Schiff Base C=N expected to form between oxidized pectin free carbonyl and glycine amino group.
- 1640–1715 cm−1: C=O of pectin carboxyls.
- 1690–1750 cm−1: C=O of oxidized pectin free carbonyls.
3.1.3. Matrix Thermal Stability Analysis with TGA and DSC
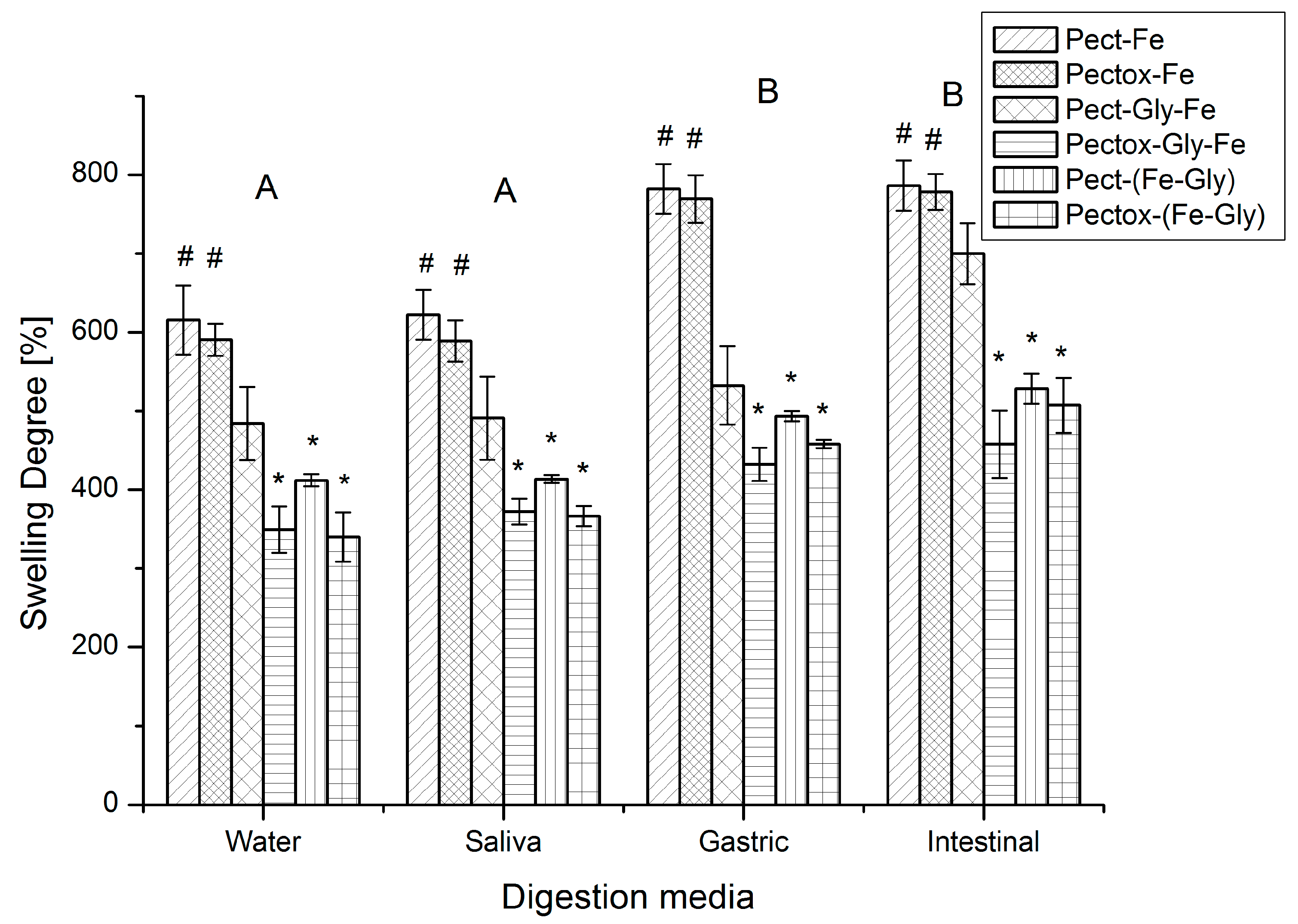
3.1.4. Swelling Degree
3.1.5. Iron Release
- Amino-chelate with native pectin increases matrix stability and iron retention within the matrix, compared with pectin–Fe. The greatest release was observed in saliva.
- Amino-chelate with oxidized pectin increases the probability of bonding with ferrous bisglycinate because the free carbonyl group presence. It displays greatest stability and iron retention, compared to the other formulations.
- Nonchelated iron and glycine with native pectin presented lower matrix stability and iron retention capacity than the matrices with chelated iron.
- Nonchelated iron and glycine with oxidized pectin presented the lowest stability and iron retention capacity, when compared to the chelated forms and the non-chelated with native pectin.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yunarti, R.T.; Zulys, A.; Harahap, L.Y.; Saroh, M.; Pramukti, A. Effectiveness of Iron Fortification on Soy-Based Foods Using Ferrous Bisglycinate in the Presence of Phytic Acid. Makara J. Sci. 2013, 17, 11–16. [Google Scholar]
- Rahman, S.; Shaheen, N. Phytate-iron molar ratio and bioavailability of iron in Bangladesh. Trop. Med. Int. Health 2022, 27, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Rimbach, G. Iron absorption and the iron binding and anti-oxidant properties of phytic acid. Int. J. Food Sci. Technol. 2002, 37, 741–748. [Google Scholar] [CrossRef]
- Hurrell, R.F. Ensuring the Efficacious Iron Fortification of Foods: A Tale of Two Barriers. Nutrients 2022, 14, 1609. [Google Scholar] [CrossRef]
- Abdel Moety, G.A.F.; Ali, A.M.; Fouad, R.; Ramadan, W.; Belal, D.S.; Haggag, H.M. Amino acid chelated iron versus an iron salt in the treatment of iron deficiency anemia with pregnancy: A randomized controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 242–246. [Google Scholar] [CrossRef]
- Tepavitcharova, S.; Rabadjieva, D.; Havlíček, D.; Němec, I.; Vojtíšek, P.; Plocek, J.; Koleva, Z. Crystallization and characterization of the compounds Gly·MSO4·mH2O (M = Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Zn2+; m = 0, 3, 5, 6. J. Mol. Struct. 2012, 1018, 113–121. [Google Scholar] [CrossRef]
- Bagna, R.; Spada, E.; Mazzone, R.; Saracco, P.; Boetti, T.; Cester, E.A.; Bertino, E.; Coscia, A. Efficacy of Supplementation with Iron Sulfate Compared to Iron Bisglycinate Chelate in Preterm Infants. Curr. Pediatr. Rev. 2018, 14, 123–129. [Google Scholar] [CrossRef]
- Pineda, O.; Ashmead, H.D.W. Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate. Nutrition 2001, 17, 381–384. [Google Scholar] [CrossRef]
- Bovell-Benjamin, A.C.; Viteri, F.E.; Allen, L.H. Iron absorption from ferrous bisglycinate and ferric trisglycinate in whole maize is regulated by iron status. Am. J. Clin. Nutr. 2000, 71, 1563–1569. [Google Scholar] [CrossRef]
- Sun, X.; Sarteshnizi, R.A.; Boachie, R.T.; Okagu, O.D.; Abioye, R.O.; Neves, R.P.; Ohanenye, I.C.; Udenigwe, C.C. Peptide-Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency. Foods 2020, 9, 1402. [Google Scholar] [CrossRef]
- Mellican, R.I.; Li, J.; Mehansho, H.; Nielsen, S.S. The Role of Iron and the Factors Affecting Off-Color Development of Polyphenols. J. Agric. Food Chem. 2003, 51, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Pectin based multi-particulate carriers for colon-specific delivery of therapeutic agents. Int. J. Pharm. 2021, 605, 120814. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Tamburino, A.; Carnaroglio, D.; Cravotto, G.; Ilharco, L.M.; Pagliaro, M. Controlling the Degree of Esterification of Citrus Pectin for Demanding Applications by Selection of the Source. ACS Omega 2017, 2, 7991–7995. [Google Scholar] [CrossRef]
- Bochek, A.M.; Zabivalova, N.M.; Petropavlovskii, G.A. Determination of the esterification degree of polygalacturonic acid. Russ. J. Appl. Chem. 2001, 74, 796–799. [Google Scholar] [CrossRef]
- Ghibaudo, F.; Gerbino, E.; Hugo, A.A.; Simões, M.G.; Alves, P.; Costa, B.F.O.; Campo Dall’ Orto, V.; Gómez-Zavaglia, A.; Simões, P.N. Development and characterization of iron-pectin beads as a novel system for iron delivery to intestinal cells. Colloids Surf. B Biointerfaces 2018, 170, 538–543. [Google Scholar] [CrossRef]
- Ralet, M.C.; Bonnin, E.; Thibault, J.F. Chromatographic study of highly methoxylated lime pectins deesterified by different pectin methyl-esterases. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 157–166. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Garrido, C. Extracción y Modificación de Pectina para la Formación de Hidrogeles con Potencial para la Administración Controlada de Fármacos. 2017. Available online: http://dx.doi.org/10.1024/0300-9831.77.4.243 (accessed on 12 January 2023).
- Zhang, Z.; Feng, S.S. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)–tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials 2006, 27, 4025–4033. [Google Scholar] [CrossRef]
- Gupta, B.; Tummalapalli, M.; Deopura, B.L.; Alam, M.S. Functionalization of pectin by periodate oxidation. Carbohydr. Polym. 2013, 98, 1160–1165. [Google Scholar] [CrossRef]
- Siggia, S.; Maxcy, W. Analytical Chemistry, 12th ed.; ACS: Washington, DC, USA, 1947; Volume 19. [Google Scholar]
- Lobaina, C.; Ricardo, C.A.; Orue, T.; Castel, Z. Obtención de bases de schiff por condensación del o-hidroxibenzaldehido con anilina y p-derivados. Rev. Cuba. Química 2005, 17, 33–43. Available online: https://www.redalyc.org/articulo.oa?id=443543686007 (accessed on 12 January 2023).
- Begam, T.; Nagpal, A.K.; Singhal, R. A comparative study of swelling properties of hydrogels based on poly(acrylamide-co-methyl methacrylate) containing physical and chemical crosslinks. J. Appl. Polym. Sci. 2003, 89, 779–786. [Google Scholar] [CrossRef]
- Capparelli, M.V.; Moulatlet, G.M.; Abessa, D.M.d.S.; Lucas-Solis, O.; Rosero, B.; Galarza, E.; Tuba, D.; Carpintero, N.; Ochoa-Herrera, V.; Cipriani-Avila, I. An integrative approach to identify the impacts of multiple metal contamination sources on the Eastern Andean foothills of the Ecuadorian Amazonia. Sci. Total Environ. 2020, 709, 136088. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.; Sepulcre, L.; Artigas, D.; García, F. Estudio de Quelados de Glicina Mediante Espectroscopía FTIR, 2017–2018. Master’s Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2017. Available online: https://upcommons.upc.edu/handle/2117/108359 (accessed on 23 January 2023).
- Chetouani, A.; Elkolli, M.; Bounekhel, M.; Benachour, D. Characterization and bioevaluation of new class of hydrogels based on oxidized pectin crosslinked to gelatin. J. Biomater. Tissue Eng. 2014, 4, 465–470. [Google Scholar] [CrossRef]
- Günter, E.A.; Popeyko O v Melekhin, A.K.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G. Preparation and properties of the pectic gel microparticles based on the Zn2+, Fe3+ and Al3+ cross-linking cations. Int. J. Biol. Macromol. 2019, 138, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef]
- Almeida, E.A.M.S.; Facchi, S.P.; Martins, A.F.; Nocchi, S.; Schuquel, I.T.A.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Synthesis and characterization of pectin derivative with antitumor property against Caco-2 colon cancer cells. Carbohydr. Polym. 2015, 115, 139–145. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, R.K.; Banthia, A.K. Development of pectin based hydrogel membranes for biomedical applications. Int. J. Plast. Technol. 2011, 14, 213–223. [Google Scholar] [CrossRef]
- Amin, M.R.; Chowdhury, M.A.; Kowser, M.A. Characterization and performance analysis of composite bioplastics synthesized using titanium dioxide nanoparticles with corn starch. Heliyon 2019, 5, e02009. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, S.; Shen, J.; Chen, Y.; Xiao, Y. A Composite Hydrogel Based on Pectin/Cellulose via Chemical Cross-Linking for Hemorrhage. Front. Bioeng. Biotechnol. 2021, 8, 1582. [Google Scholar] [CrossRef]
- Jiang, W.; Qiao, X.; Sun, K. Mechanical and thermal properties of thermoplastic acetylated starch/poly(ethylene-co-vinyl alcohol) blends. Carbohydr. Polym. 2006, 65, 139–143. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kunzek, H.; Dongowski, G. Thermal analysis of chemically and mechanically modified pectins. Food Hydrocoll. 2007, 21, 1101–1112. [Google Scholar] [CrossRef]
- Popov, S.; Paderin, N.; Chistiakova, E.; Ptashkin, D.; Markov, P.A. Effect of Cross-Linking Cations on In Vitro Biocompatibility of Apple Pectin Gel Beads. Int. J. Mol. Sci. 2022, 23, 14789. [Google Scholar] [CrossRef] [PubMed]
- Nowok, A.; Cieślik, W.; Grelska, J.; Jurkiewicz, K.; Makieieva, N.; Kupka, T.; Alemán, J.; Musioł, R.; Pawlus, S. Simple Rules for Complex Near-Glass-Transition Phenomena in Medium-Sized Schiff Bases. Int. J. Mol. Sci. 2022, 23, 5185. [Google Scholar] [CrossRef] [PubMed]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical description of hydrogel swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. Available online: https://sid.ir/paper/561994/en (accessed on 12 January 2023).


| Pect + Fe | Pectox + Fe | Pect + (Gly-Fe) | Pectox + (Gly-Fe) | Pect + Fe + Gly | Pectox + Fe + Gly | |
|---|---|---|---|---|---|---|
| Concentration (w/v %) | 10 + 10 | 10 + 10 | 10 + 10 | 10 + 10 | 10 + 5 + 5 | 10 + 5 + 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, M.; Viteri, D.; Oña, D.; Leon, M.; Ochoa-Herrera, V.; Carpintero, N.; Sepulcre, F.; Alvarez-Barreto, J.F. Matrices of Native and Oxidized Pectin and Ferrous Bisglycinate and Their In Vitro Behavior through Gastrointestinal Conditions. Colloids Interfaces 2023, 7, 35. https://doi.org/10.3390/colloids7020035
Jimenez M, Viteri D, Oña D, Leon M, Ochoa-Herrera V, Carpintero N, Sepulcre F, Alvarez-Barreto JF. Matrices of Native and Oxidized Pectin and Ferrous Bisglycinate and Their In Vitro Behavior through Gastrointestinal Conditions. Colloids and Interfaces. 2023; 7(2):35. https://doi.org/10.3390/colloids7020035
Chicago/Turabian StyleJimenez, Martin, Daniela Viteri, Daniela Oña, Marco Leon, Valeria Ochoa-Herrera, Natalia Carpintero, Francesc Sepulcre, and Jose F. Alvarez-Barreto. 2023. "Matrices of Native and Oxidized Pectin and Ferrous Bisglycinate and Their In Vitro Behavior through Gastrointestinal Conditions" Colloids and Interfaces 7, no. 2: 35. https://doi.org/10.3390/colloids7020035
APA StyleJimenez, M., Viteri, D., Oña, D., Leon, M., Ochoa-Herrera, V., Carpintero, N., Sepulcre, F., & Alvarez-Barreto, J. F. (2023). Matrices of Native and Oxidized Pectin and Ferrous Bisglycinate and Their In Vitro Behavior through Gastrointestinal Conditions. Colloids and Interfaces, 7(2), 35. https://doi.org/10.3390/colloids7020035









