Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing
Abstract
1. Introduction
2. Measuring Principle
2.1. Overview of In Situ Sizing Techniques
2.2. Theories of DLS
2.3. Advantages and Limitations
- ➢ DLS is a non-invasive method with a short experiment duration.
- ➢ Low numbers of samples and less sample preparation are required.
- ➢ Repeatability of the diameter obtained by DLS is very good.
- ➢ Analysis could be achieved with a wide range of temperature and modest development costs.
- ➢ Temperature and solvent viscosity have a significant impact on DLS results. Therefore, the temperature must be maintained at a constant level and the solvent viscosity should be determined.
- ➢ Resolution of DLS technique is limited by the cumulants procedure.
- ➢ Artifact peaks from bubbles, opalescent and reflective particles, optical mode.
- ➢ Inability to study concentrated solutions/systems with the classical device design.
- ➢ Strong distortion of results in the presence of even minimal numbers of larger particles in the system.
- ➢ Low concentration DLS measurements can be difficult to make due to the low signal-to-noise ratio. To improve the accuracy, it is important to use a high-sensitivity detector, to equip a sample cell with a large volume and to employ a sample that is well dispersed and has a high refractive index.
3. Instrument Design
3.1. Ex-Situ and In Situ Configuration
3.2. Sample Preparation and Measurement Conditions
3.3. Reaction Cell Design for In Situ Configuration
3.4. Latest Methods and DLS Setups
4. Nanoparticle Preparation Monitored by DLS
4.1. Ex-Situ Cases
4.2. In Situ Cases
4.2.1. TiO2
4.2.2. Doped-TiO2
4.2.3. ZrO2
4.2.4. ZrxTi1-xO2 and VxTi1-xO2
4.2.5. Zeolite
4.2.6. Metal and Metal-Polymer
4.2.7. Biological Materials
4.2.8. Others
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oskam, G. Metal Oxide Nanoparticles: Synthesis, Characterization and Application. J. Sol. Gel Sci. Technol. 2006, 37, 161–164. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and Metal Oxide Nanoparticles in Chemiresistors: Does the Nanoscale Matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Bayal, N.; Jeevanandam, P. Synthesis of TiO2−MgO Mixed Metal Oxide Nanoparticles via a Sol−Gel Method and Studies on Their Optical Properties. Ceram. Int. 2014, 40, 15463–15477. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Park, J.Y. Molecular Factors of Catalytic Selectivity. Angew. Chem. Int. Ed. 2008, 47, 9212–9228. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic Degradation for Environmental Applications–a Review. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Photolysis-Decomposition of Water at the Surface of an Irradiated Semiconductor. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly Enhanced Photocatalytic Performance of ZnO via Graphene Hybridization and the Mechanism Study. Appl. Catal. B Environ. 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Huang, S.-Y.; Hsiao, Y.-S.; Wei, P.-C.; Chou, C.-M.; Hsiao, V.K.S. Pulsed-Laser Induced Photolysis of Synthesizing Magnetic Fe3O4 Nanoparticles for Visible-Light Photocatalysis. Catalysts 2022, 12, 1459. [Google Scholar] [CrossRef]
- Fu, X.; Tang, W.; Ji, L.; Chen, S. V2O5/Al2O3 Composite Photocatalyst: Preparation, Characterization, and the Role of Al2O3. Chem. Eng. J. 2012, 180, 170–177. [Google Scholar] [CrossRef]
- Fauzi, A.A.; Jalil, A.A.; Hassan, N.S.; Aziz, F.F.A.; Azami, M.S.; Hussain, I.; Saravanan, R.; Vo, D.-V. A Critical Review on Relationship of CeO2-Based Photocatalyst towards Mechanistic Degradation of Organic Pollutant. Chemosphere 2022, 286, 131651. [Google Scholar] [CrossRef] [PubMed]
- Akpan, U.G.; Hameed, B.H. The Advancements in Sol–Gel Method of Doped-TiO2 Photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Wong, C.L.; Tan, Y.N.; Mohamed, A.R. A Review on the Formation of Titania Nanotube Photocatalysts by Hydrothermal Treatment. J. Environ. Manag. 2011, 92, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sheng, G.; Fu, J.; An, T.; Wang, X.; Hu, X. Novel Preparation of Nanosized ZnO–SnO2 with High Photocatalytic Activity by Homogeneous Co-Precipitation Method. Mater. Lett. 2005, 59, 3641–3644. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Hydrothermal/Solvothermal Synthesis and Treatment of TiO2 for Photocatalytic Degradation of Air Pollutants: Preparation, Characterization, Properties, and Performance. Chemosphere 2019, 219, 804–825. [Google Scholar] [CrossRef]
- Di, L.; Zhang, X.; Xu, Z.; Wang, K. Atmospheric-Pressure Cold Plasma for Preparation of High Performance Pt/TiO2 Photocatalyst and Its Mechanism. Plasma Chem. Plasma Process. 2014, 34, 301–311. [Google Scholar] [CrossRef]
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet Chemical Synthesis of Metal Oxide Nanoparticles: A Review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Ishak, N.M.; Kamarudin, S.K.; Timmiati, S.N. Green Synthesis of Metal and Metal Oxide Nanoparticles via Plant Extracts: An Overview. Mater. Res. Express 2019, 6, 112004. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of Metal and Metal Oxide Nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–Gel Based Materials for Biomedical Applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Li, Y.-F.; Liu, Z.-P. Particle Size, Shape and Activity for Photocatalysis on Titania Anatase Nanoparticles in Aqueous Surroundings. J. Am. Chem. Soc. 2011, 133, 15743–15752. [Google Scholar] [CrossRef]
- Stroyuk, A.L.; Kryukov, A.I.; Kuchmii, S.Y.; Pokhodenko, V.D. Quantum Size Effects in Semiconductor Photocatalysis. Theor. Exp. Chem. 2005, 41, 207–228. [Google Scholar] [CrossRef]
- Bałdyga, J.; Pohorecki, R. Turbulent Micromixing in Chemical Reactors—A Review. Chem. Eng. J. Biochem. Eng. J. 1995, 58, 183–195. [Google Scholar] [CrossRef]
- Barth, H.G. Modern Methods of Particle Size Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1984; Volume 97, ISBN 0-471-87571-6. [Google Scholar]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of Magnetic Nanoparticle by Dynamic Light Scattering. Nanoscale Res. Lett. 2013, 8, 1–14. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic Light Scattering: A Practical Guide and Applications in Biomedical Sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of Particles in Solution–a Perspective on Light Scattering and Comparative Technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Hassan, P.A.; Rana, S.; Verma, G. Making Sense of Brownian Motion: Colloid Characterization by Dynamic Light Scattering. Langmuir 2015, 31, 3–12. [Google Scholar] [CrossRef]
- Xu, R. Light Scattering: A Review of Particle Characterization Applications. Particuology 2015, 18, 11–21. [Google Scholar] [CrossRef]
- ISO 22412:2017(En); Particle Size Analysis—Dynamic Light Scattering (DLS). ISO: Geneva, Switzerland.
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Hutin, A. Size Analysis of Dispersions in Liquid Phase; Zenodo: Geneva, Switzerland, 2022. [Google Scholar]
- Alexander, M.; Dalgleish, D.G. Dynamic Light Scattering Techniques and Their Applications in Food Science. Food Biophys. 2006, 1, 2–13. [Google Scholar] [CrossRef]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Demeler, B.; Nguyen, T.-L.; Gorbet, G.E.; Schirf, V.; Brookes, E.H.; Mulvaney, P.; El-Ballouli, A.O.; Pan, J.; Bakr, O.M.; Demeler, A.K. Characterization of Size, Anisotropy, and Density Heterogeneity of Nanoparticles by Sedimentation Velocity. Anal. Chem. 2014, 86, 7688–7695. [Google Scholar] [CrossRef] [PubMed]
- Wynn, E.J.W.; Hounslow, M.J. Coincidence Correction for Electrical-Zone (Coulter-Counter) Particle Size Analysers. Powder Technol. 1997, 93, 163–175. [Google Scholar] [CrossRef]
- Kim, J.S.; LaGrange, T.; Reed, B.W.; Taheri, M.L.; Armstrong, M.R.; King, W.E.; Browning, N.D.; Campbell, G.H. Imaging of Transient Structures Using Nanosecond in Situ TEM. Science 2008, 321, 1472–1475. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Raabe, D. In-Situ SEM Observation of Phase Transformation and Twinning Mechanisms in an Interstitial High-Entropy Alloy. Acta Mater. 2018, 147, 236–246. [Google Scholar] [CrossRef]
- Hall, B.D.; Zanchet, D.; Ugarte, D. Estimating Nanoparticle Size from Diffraction Measurements. J. Appl. Crystallogr. 2000, 33, 1335–1341. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khlebtsov, N.G. On the Measurement of Gold Nanoparticle Sizes by the Dynamic Light Scattering Method. Colloid J. 2011, 73, 118–127. [Google Scholar] [CrossRef]
- Tyndall, J. On the Blue Color of the Sky, the Polarization of Skylight, and Polarization of Light by Cloudy Matter Generally. J. Frankl. Inst. 1869, 88, 34–40. [Google Scholar] [CrossRef]
- Strutt, J.W.X.V. On the Light from the Sky, Its Polarization and Colour. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1871, 41, 107–120. [Google Scholar] [CrossRef]
- Mie, G. Beiträge Zur Optik Trüber Medien, Speziell Kolloidaler Metallösungen. Ann. Der Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Soloviev, A. Sol-Gel Process: DLS Studies of Particles Growth Kinetics during Hydrolysis-Condensation of Titanium Isopropoxide (IV). Ph.D. Thesis, Sorbonne Paris Nord University, Paris, France, 2000. [Google Scholar]
- Frisken, B.J. Revisiting the Method of Cumulants for the Analysis of Dynamic Light-Scattering Data. Appl. Opt. 2001, 40, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Dyott, R.B. The Fibre-Optic Doppler Anemometer. IEE J. Microw. Opt. Acoust. UK 1978, 2, 13. [Google Scholar] [CrossRef]
- Auweter, H.; Horn, D. Fiber-Optical Quasi-Elastic Light Scattering of Concentrated Dispersions. J. Colloid Interface Sci. 1985, 105, 399–409. [Google Scholar] [CrossRef]
- Egelhaaf, S.U.; Schurtenberger, P. A Fiber-optics-based Light Scattering Instrument for Time-resolved Simultaneous Static and Dynamic Measurements. Rev. Sci. Instrum. 1996, 67, 540–545. [Google Scholar] [CrossRef]
- Alargova, R.G.; Deguchi, S.; Tsujii, K. Dynamic Light Scattering Study of Polystyrene Latex Suspended in Water at High Temperatures and High Pressures. Colloids Surf. A Physicochem. Eng. Asp. 2001, 183–185, 303–312. [Google Scholar] [CrossRef]
- Rivallin, M.; Benmami, M.; Kanaev, A.; Gaunand, A. Sol–Gel Reactor with Rapid Micromixing: Modelling and Measurements of Titanium Oxide Nano-Particle Growth. Chem. Eng. Res. Des. 2005, 83, 67–74. [Google Scholar] [CrossRef]
- Pristinski, D.; Chastek, T.Q. A Versatile, Low-Cost Approach to Dynamic Light Scattering. Meas. Sci. Technol. 2009, 20, 045705. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Li, W.; Xia, M.; Meng, Z.; Xia, L. A Dual-Angle Fiber Dynamic Light Scattering System Integrated with Microfluidic Chip for Particle Size Measurement. Opt. Laser Technol. 2022, 150, 107891. [Google Scholar] [CrossRef]
- Soloviev, A.; Jensen, H.; Søgaard, E.G.; Kanaev, A.V. Aggregation Kinetics of Sol-Gel Process Based on Titanium Tetraisopropoxide. J. Mater. Sci. 2003, 38, 3315–3318. [Google Scholar] [CrossRef]
- Chastek, T.Q.; Beers, K.L.; Amis, E.J. Miniaturized Dynamic Light Scattering Instrumentation for Use in Microfluidic Applications. Rev. Sci. Instrum. 2007, 78, 072201. [Google Scholar] [CrossRef]
- Falke, S.; Dierks, K.; Blanchet, C.; Graewert, M.; Cipriani, F.; Meijers, R.; Svergun, D.; Betzel, C. Multi-Channel in Situ Dynamic Light Scattering Instrumentation Enhancing Biological Small-Angle X-Ray Scattering Experiments at the PETRA III Beamline P12. J. Synchrotron Radiat. 2018, 25, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Badaire, S.; Poulin, P.; Maugey, M.; Zakri, C. In Situ Measurements of Nanotube Dimensions in Suspensions by Depolarized Dynamic Light Scattering. Langmuir 2004, 20, 10367–10370. [Google Scholar] [CrossRef] [PubMed]
- Lehner, D.; Lindner, H.; Glatter, O. Determination of the Translational and Rotational Diffusion Coefficients of Rodlike Particles Using Depolarized Dynamic Light Scattering. Langmuir 2000, 16, 1689–1695. [Google Scholar] [CrossRef]
- Zarghani, M.; Akhlaghinia, B. Fe 3 O 4 Magnetic Nanoparticles (MNPs) as an Efficient Catalyst for Selective Oxidation of Benzylic and Allylic C–H Bonds to Carbonyl Compounds with Tert-Butyl Hydroperoxide. RSC Adv. 2016, 6, 38592–38601. [Google Scholar] [CrossRef]
- Dutta, S.; Jaiswal, K.K.; Verma, R.; Basavaraju, D.M.; Ramaswamy, A.P. Green Synthesis of Zinc Oxide Catalyst under Microwave Irradiation Using Banana (Musa Spp.) Corm (Rhizome) Extract for Biodiesel Synthesis from Fish Waste Lipid. Biocatal. Agric. Biotechnol. 2019, 22, 101390. [Google Scholar] [CrossRef]
- He, X.; Gan, J.; Fakhri, A.; Dizaji, B.F.; Azarbaijan, M.H.; Hosseini, M. Preparation of Ceric Oxide and Cobalt Sulfide-Ceric Oxide/Cellulose-Chitosan Nanocomposites as a Novel Catalyst for Efficient Photocatalysis and Antimicrobial Study. Int. J. Biol. Macromol. 2020, 143, 952–957. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Chen, Y.; Cheng, Z.; Luo, X.; Mo, S.; Jia, L.; Lin, P.; Yang, Z. Improved Hydrogen Production from Glycerol Photoreforming over Sol-Gel Derived TiO2 Coupled with Metal Oxides. Chem. Eng. J. 2017, 317, 522–532. [Google Scholar] [CrossRef]
- Larichev, Y.V. Application of DLS for Metal Nanoparticle Size Determination in Supported Catalysts. Chem. Pap. 2021, 75, 2059–2066. [Google Scholar] [CrossRef]
- Bayat, A.; Baghdadi, M.; Bidhendi, G.N. Tailored Magnetic Nano-Alumina as an Efficient Catalyst for Transesterification of Waste Cooking Oil: Optimization of Biodiesel Production Using Response Surface Methodology. Energy Convers. Manag. 2018, 177, 395–405. [Google Scholar] [CrossRef]
- Tran, U.P.N.; Le, K.K.A.; Phan, N.T.S. Expanding Applications of Metal−Organic Frameworks: Zeolite Imidazolate Framework ZIF-8 as an Efficient Heterogeneous Catalyst for the Knoevenagel Reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Catalytic and Physicochemical Properties of Fe-Polymer Nanocatalysts of Fischer–Tropsch Synthesis: Dynamic Light Scattering and FTIR Spectroscopy Study|SpringerLink. Available online: https://link.springer.com/article/10.1134/S0965544116120033 (accessed on 31 August 2022).
- Zhu, G.; Glass, E.N.; Zhao, C.; Lv, H.; Vickers, J.W.; Geletii, Y.V.; Musaev, D.G.; Song, J.; Hill, C.L. A Nickel Containing Polyoxometalate Water Oxidation Catalyst. Dalton Trans. 2012, 41, 13043. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, R.; Loganathan, B.; Raghu, K. Green Synthesis of Au–Ag Bimetallic Nanocomposites Using Silybum Marianum Seed Extract and Their Application as a Catalyst. RSC Adv. 2015, 5, 31691–31699. [Google Scholar] [CrossRef]
- Baruah, B.; Gabriel, G.J.; Akbashev, M.J.; Booher, M.E. Facile Synthesis of Silver Nanoparticles Stabilized by Cationic Polynorbornenes and Their Catalytic Activity in 4-Nitrophenol Reduction. Langmuir 2013, 29, 4225–4234. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.G.; Ciminelli, V.S.; Mohallem, N.D.S. A Comparison of TEM and DLS Methods to Characterize Size Distribution of Ceramic Nanoparticles. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2016; Volume 733, p. 012039. [Google Scholar]
- Chen, Z.H.; Kim, C.; Zeng, X.; Hwang, S.H.; Jang, J.; Ungar, G. Characterizing Size and Porosity of Hollow Nanoparticles: SAXS, SANS, TEM, DLS, and Adsorption Isotherms Compared. Langmuir 2012, 28, 15350–15361. [Google Scholar] [CrossRef]
- Pabisch, S.; Feichtenschlager, B.; Kickelbick, G.; Peterlik, H. Effect of Interparticle Interactions on Size Determination of Zirconia and Silica Based Systems—A Comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 2012, 521, 91–97. [Google Scholar] [CrossRef]
- Flower, G.L.; Latha, S.V.; Rao, K.V. Novel Characterization of Nanosilver Fluid through Ultrasonic Studies Supported by UV–Vis Spectroscopy, DLS and TEM Studies. J. Mol. Liq. 2016, 221, 333–338. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.-C. Oleic Acid Coating on the Monodisperse Magnetite Nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Panchal, J.; Kotarek, J.; Marszal, E.; Topp, E.M. Analyzing Subvisible Particles in Protein Drug Products: A Comparison of Dynamic Light Scattering (DLS) and Resonant Mass Measurement (RMM). AAPS J. 2014, 16, 440–451. [Google Scholar] [CrossRef]
- Phenrat, T.; Kim, H.-J.; Fagerlund, F.; Illangasekare, T.; Tilton, R.D.; Lowry, G.V. Particle Size Distribution, Concentration, and Magnetic Attraction Affect Transport of Polymer-Modified Fe0 Nanoparticles in Sand Columns. Environ. Sci. Technol. 2009, 43, 5079–5085. [Google Scholar] [CrossRef]
- Tieng, S.; Azouani, R.; Chhor, K.; Kanaev, A. Nucleation-Growth of TiO2 Nanoparticles Doped with Iron Acetylacetonate. J. Phys. Chem. C 2011, 115, 5244–5250. [Google Scholar] [CrossRef]
- Azouani, R.; Tieng, S.; Chhor, K.; Bocquet, J.-F.; Eloy, P.; Gaigneaux, E.M.; Klementiev, K.; Kanaev, A.V. TiO 2 Doping by Hydroxyurea at the Nucleation Stage: Towards a New Photocatalyst in the Visible Spectral Range. Phys. Chem. Chem. Phys. 2010, 12, 11325–11334. [Google Scholar] [CrossRef] [PubMed]
- Azouani, R.; Michau, A.; Hassouni, K.; Chhor, K.; Bocquet, J.-F.; Vignes, J.-L.; Kanaev, A. Elaboration of Pure and Doped TiO2 Nanoparticles in Sol–Gel Reactor with Turbulent Micromixing: Application to Nanocoatings and Photocatalysis. Chem. Eng. Res. Des. 2010, 88, 1123–1130. [Google Scholar] [CrossRef]
- Labidi, S.; Jia, Z.; Amar, M.B.; Chhor, K.; Kanaev, A. Nucleation and Growth Kinetics of Zirconium-Oxo-Alkoxy Nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 2651–2659. [Google Scholar] [CrossRef]
- Cheng, K.; Chhor, K.; Brinza, O.; Vrel, D.; Kanaev, A. From Nanoparticles to Bulk Crystalline Solid: Nucleation, Growth Kinetics and Crystallisation of Mixed Oxide ZrxTi1−xO2 Nanoparticles. CrystEngComm 2017, 19, 3955–3965. [Google Scholar] [CrossRef]
- Sanchez Mendez, M.; Jia, Z.; Traore, M.; Ben Amar, M.; Nikravech, M.; Kanaev, A. Nucleation and Growth of Mixed Vanadium-Titanium Oxo-Alkoxy Nanoparticles in Sol-Gel Synthesis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125636. [Google Scholar] [CrossRef]
- Mintova, S.; Valtchev, V. Effect of the Silica Source on the Formation of Nanosized Silicalite-1: An in Situ Dynamic Light Scattering Study. Microporous Mesoporous Mater. 2002, 55, 171–179. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.; Chang, A.; Peng, Y.; Xu, W.; Wu, W. Responsive Au@polymer Hybrid Microgels for the Simultaneous Modulation and Monitoring of Au-Catalyzed Chemical Reaction. J. Mater. Chem. A 2014, 2, 9514. [Google Scholar] [CrossRef]
- Cheng, K.; Chhor, K.; Kanaev, A. Solvent Effect on Nucleation-Growth of Titanium-Oxo-Alkoxy Nanoparticles. Chem. Phys. Lett. 2017, 672, 119–123. [Google Scholar] [CrossRef]
- Benmami, M.; Chhor, K.; Kanaev, A.V. High Photocatalytic Activity of Monolayer Nanocoatings Prepared from Non-Crystalline Titanium Oxide Sol Nanoparticles. Chem. Phys. Lett. 2006, 422, 552–557. [Google Scholar] [CrossRef]
- Bouslama, M.; Amamra, M.C.; Jia, Z.; Ben Amar, M.; Chhor, K.; Brinza, O.; Abderrabba, M.; Vignes, J.-L.; Kanaev, A. Nanoparticulate TiO2–Al2O3 Photocatalytic Media: Effect of Particle Size and Polymorphism on Photocatalytic Activity. Acs Catal. 2012, 2, 1884–1892. [Google Scholar] [CrossRef]
- Gorbovyi, P.; Uklein, A.; Tieng, S.; Brinza, O.; Traore, M.; Chhor, K.; Museur, L.; Kanaev, A. Novel Nanostructured PHEMA-TiO2 Hybrid Materials with Efficient Light-Induced Charge Separation. Nanoscale 2011, 3, 1807–1812. [Google Scholar] [CrossRef]
- Gorbovyi, P.; Uklein, A.; Traore, M.; Museur, L.; Kanaev, A. Formation of Gel of Preformed Size-Selected Titanium-Oxo-Alkoxy Nanoparticles: Towards Organic-Inorganic Hybrid Material with Efficient Interfacial Electron Transfer. Mater. Res. Express 2014, 1, 045039. [Google Scholar] [CrossRef]
- Luu, T.T.H.; Jia, Z.; Kanaev, A.; Museur, L. Photopolymerization of TiO2-Based Hybrid Materials: Effect of Nanoparticles Loading and Photosensitive 1D Microstructures Fabrication. J. Mater. Sci. 2023, 58, 1127–1138. [Google Scholar] [CrossRef]
- Jia, Z.; Ben Amar, M.; Brinza, O.; Astafiev, A.; Nadtochenko, V.; Evlyukhin, A.B.; Chichkov, B.N.; Duten, X.; Kanaev, A. Growth of Silver Nanoclusters on Monolayer Nanoparticulate Titanium-Oxo-Alkoxy Coatings. J. Phys. Chem. C 2012, 116, 17239–17247. [Google Scholar] [CrossRef]
- Jia, Z.; Amar, M.B.; Yang, D.; Brinza, O.; Kanaev, A.; Duten, X.; Vega-González, A. Plasma Catalysis Application of Gold Nanoparticles for Acetaldehyde Decomposition. Chem. Eng. J. 2018, 347, 913–922. [Google Scholar] [CrossRef]
- Sauce, S.; Vega-González, A.; Jia, Z.; Touchard, S.; Hassouni, K.; Kanaev, A.; Duten, X. New Insights in Understanding Plasma-Catalysis Reaction Pathways: Study of the Catalytic Ozonation of an Acetaldehyde Saturated Ag/TiO2/SiO2 Catalyst. Eur. Phys. J. Appl. Phys. 2015, 71, 20805. [Google Scholar] [CrossRef]
- Jia, Z.; Vega-Gonzalez, A.; Amar, M.B.; Hassouni, K.; Tieng, S.; Touchard, S.; Kanaev, A.; Duten, X. Acetaldehyde Removal Using a Diphasic Process Coupling a Silver-Based Nano-Structured Catalyst and a Plasma at Atmospheric Pressure. Catal. Today 2013, 208, 82–89. [Google Scholar] [CrossRef]
- Azouani, R.; Soloviev, A.; Benmami, M.; Chhor, K.; Bocquet, J.-F.; Kanaev, A. Stability and Growth of Titanium-Oxo-Alkoxy Ti x O y (OiPr) z Clusters. J. Phys. Chem. C 2007, 111, 16243–16248. [Google Scholar] [CrossRef]
- Guiot, C.; Spalla, O. Stabilization of TiO2 Nanoparticles in Complex Medium through a PH Adjustment Protocol. Environ. Sci. Technol. 2013, 47, 1057–1064. [Google Scholar] [CrossRef]
- Tieng, S.; Kanaev, A.; Chhor, K. New Homogeneously Doped Fe(III)–TiO2 Photocatalyst for Gaseous Pollutant Degradation. Appl. Catal. A: Gen. 2011, 399, 191–197. [Google Scholar] [CrossRef]
- dos Santos, V.; da Silveira, N.P.; Bergmann, C.P. In-Situ Evaluation of Particle Size Distribution of ZrO2-Nanoparticles Obtained by Sol–Gel. Powder Technol. 2014, 267, 392–397. [Google Scholar] [CrossRef]
- Labidi, S.; Amar, M.B.; Abderrabba, M.; Passarello, J.-P.; Kanaev, A. Nanoparticulate ZrO2/SO42- Catalyst for Biofuel Production. Int. J. Adv. Appl. Phys. Res. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Cheng, K.; Chhor, K.; Passarello, J.-P.; Colbeau-Justin, C.; Kanaev, A. Photocatalytic Nanoparticulate ZrxTi1-XO2 Coatings with Controlled Homogeneity of Elemental Composition. ChemistrySelect 2018, 3, 11118–11126. [Google Scholar] [CrossRef]
- Kessler, V.G. The Chemistry behind the Sol–Gel Synthesis of Complex Oxide Nanoparticles for Bio-Imaging Applications. J. Sol-Gel Sci. Technol. 2009, 51, 264. [Google Scholar] [CrossRef]
- Sanchez Mendez, M.; Lemarchand, A.; Traore, M.; Perruchot, C.; Sassoye, C.; Selmane, M.; Nikravech, M.; Ben Amar, M.; Kanaev, A. Photocatalytic Activity of Nanocoatings Based on Mixed Oxide V-TiO2 Nanoparticles with Controlled Composition and Size. Catalysts 2021, 11, 1457. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Wu, Q.; Wen, Y.; Xiao, F.-S. Zeolite Nanosheets for Catalysis. Chem. Soc. Rev. 2022, 51, 2431–2443. [Google Scholar] [CrossRef]
- Kirschhock, C.E.; Ravishankar, R.; Looveren, L.V.; Jacobs, P.A.; Martens, J.A. Mechanism of Transformation of Precursors into Nanoslabs in the Early Stages of MFI and MEL Zeolite Formation from TPAOH− TEOS− H2O and TBAOH− TEOS− H2O Mixtures. J. Phys. Chem. B 1999, 103, 4972–4978. [Google Scholar] [CrossRef]
- Artioli, G.; Grizzetti, R.; Carotenuto, L.; Piccolo, C.; Colella, C.; Liguori, B.; Aiello, R.; Frontera, P. In Situ Dynamic Light Scattering and Synchrotron X-Ray Powder Diffraction Study of the Early Stages of Zeolite Growth. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2002; Volume 142, pp. 45–52. ISBN 978-0-444-51174-4. [Google Scholar]
- Meyer, A.; Dierks, K.; Hussein, R.; Brillet, K.; Brognaro, H.; Betzel, C. Systematic Analysis of Protein–Detergent Complexes Applying Dynamic Light Scattering to Optimize Solutions for Crystallization Trials. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 75–81. [Google Scholar] [CrossRef]
- Schubert, R.; Meyer, A.; Dierks, K.; Kapis, S.; Reimer, R.; Einspahr, H.; Perbandt, M.; Betzel, C. Reliably Distinguishing Protein Nanocrystals from Amorphous Precipitate by Means of Depolarized Dynamic Light Scattering. J. Appl. Crystallogr. 2015, 48, 1476–1484. [Google Scholar] [CrossRef]
- Glidden, M.; Muschol, M. Characterizing Gold Nanorods in Solution Using Depolarized Dynamic Light Scattering. J. Phys. Chem. C 2012, 116, 8128–8137. [Google Scholar] [CrossRef]
- Zhou, L.; O’Brien, P. Mesocrystals Properties and Applications. J. Phys. Chem. Lett. 2012, 3, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Embrechts, H.E.; Zhang, S.; Hock, R.; Peukert, W.; Distaso, M. Observing Oriented Attachment during Mesocrystal Growth with in Situ Dynamic Light Scattering (DLS). Cryst. Growth Des. 2020, 20, 1266–1275. [Google Scholar] [CrossRef]
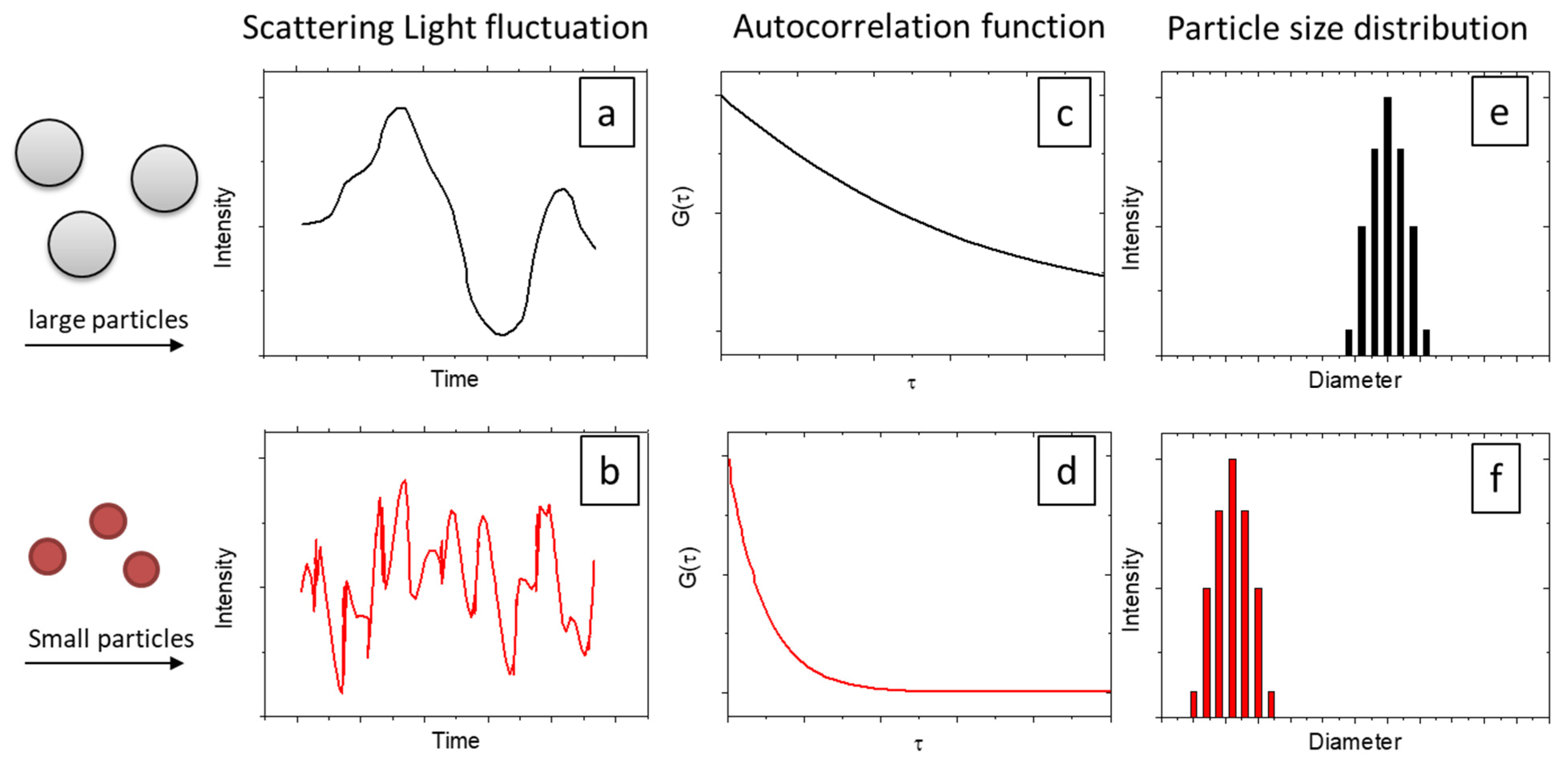
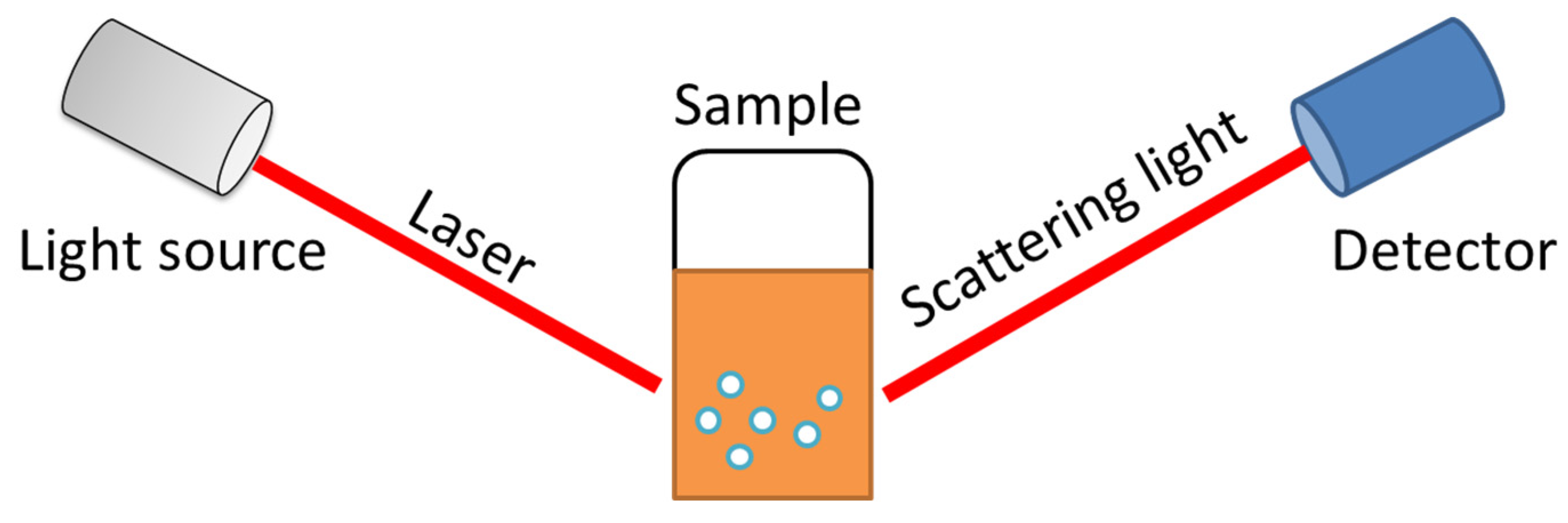
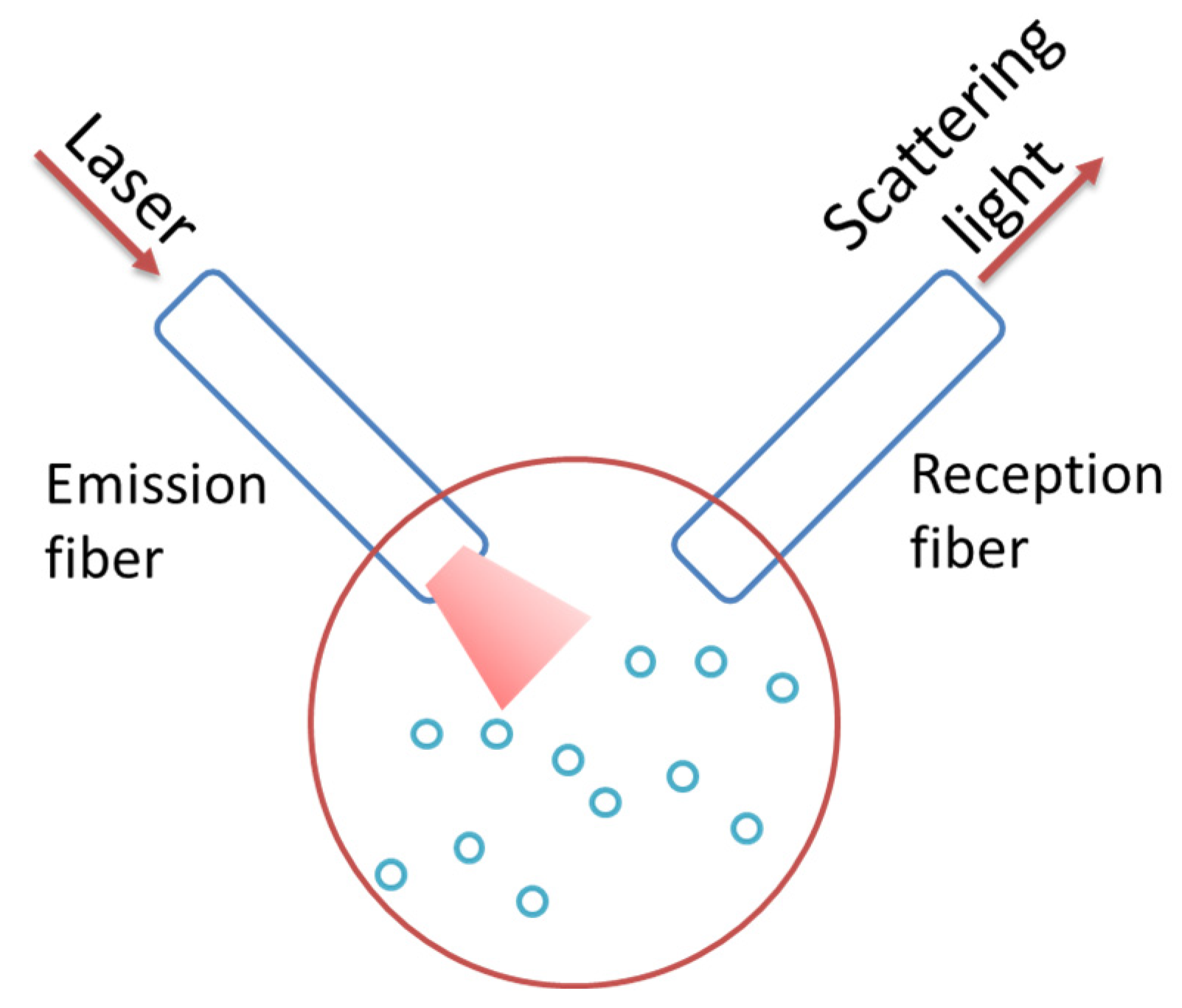
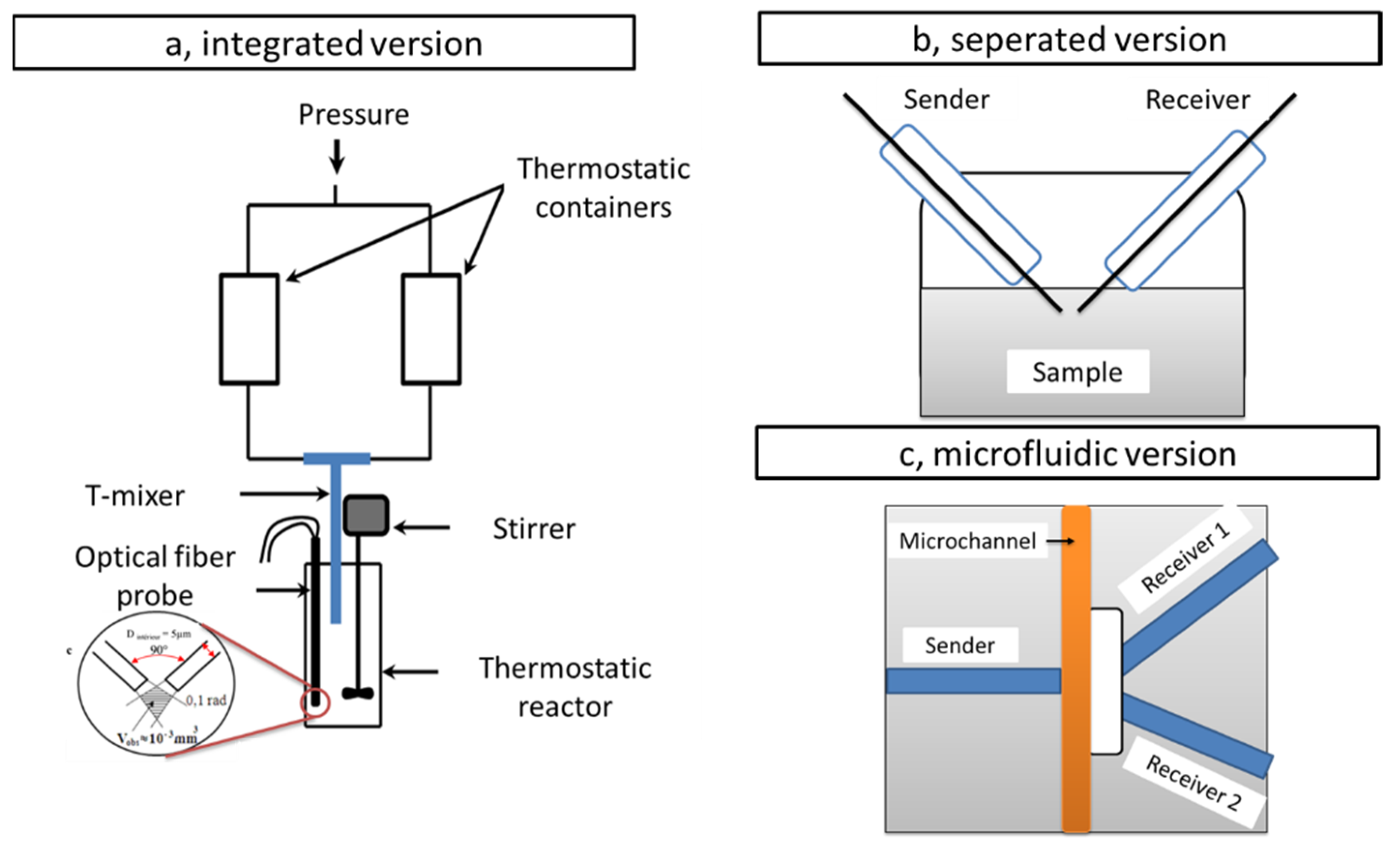
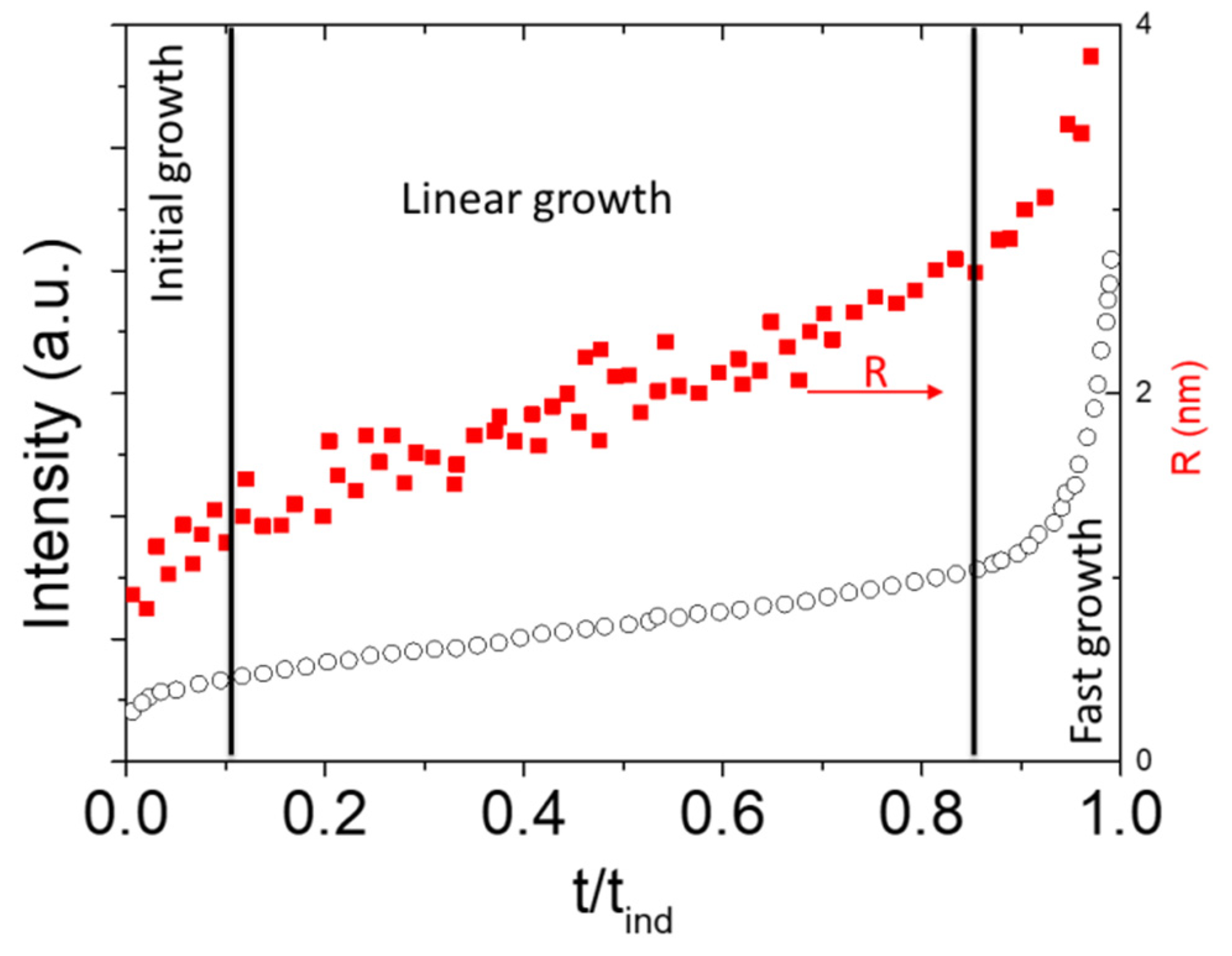
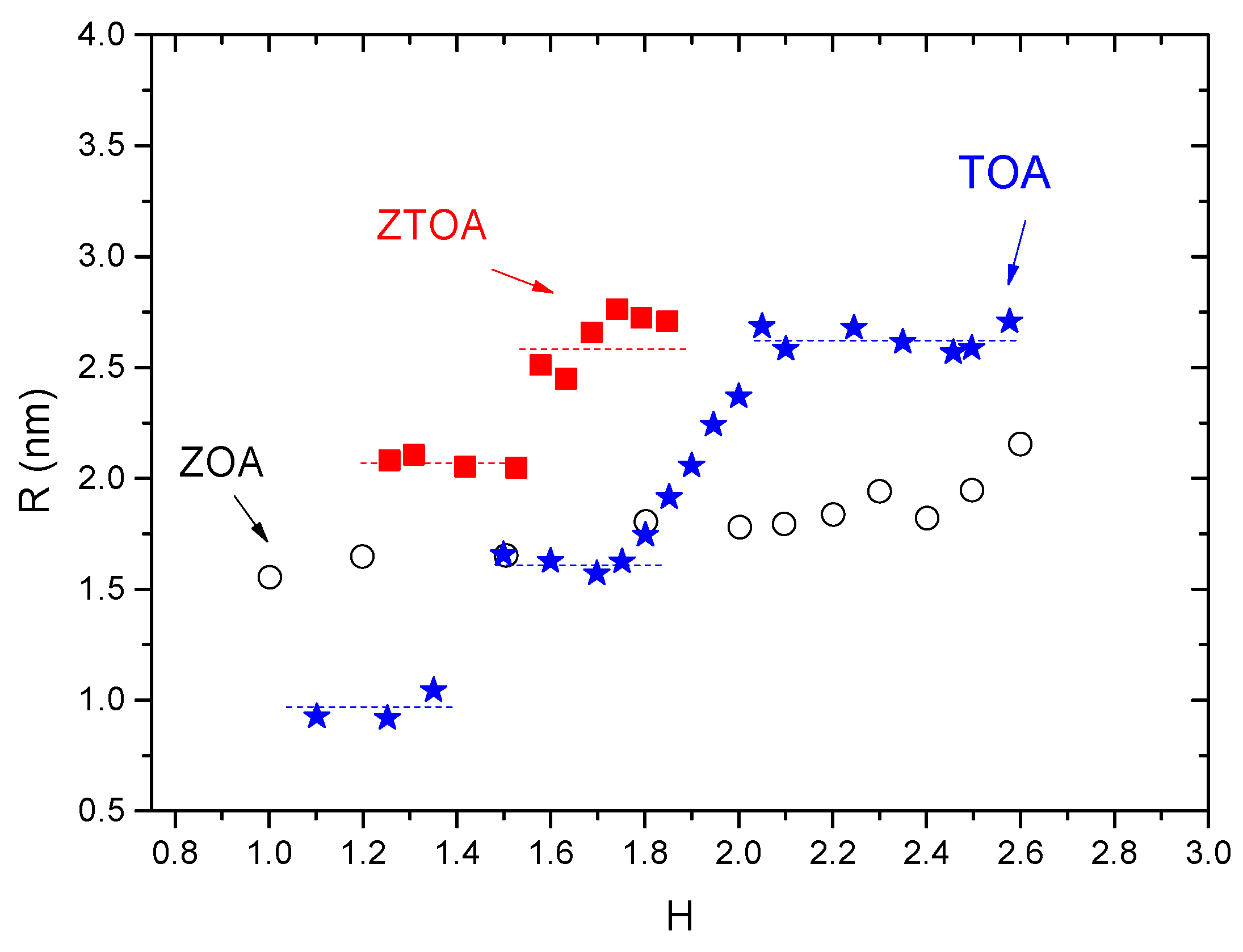
| Technique | Type of Size | Size (2R) Range |
|---|---|---|
| Dynamic light scattering | Hydrodynamic radius | 1 nm–1 µm |
| Electrical mobility | Electrical mobility | 2 nm–1 µm |
| Laser diffraction | Scatter diameter | 100 nm–104 µm |
| Optical microscopy | Shape/Structure | 800 nm–150 µm |
| Scanning electron microscopy | Shape/Structure | 10 nm–5 µm |
| Transmission electron microscopy | Shape/Structure | 0.5 nm–1 µm |
| X-ray diffraction | Crystallite size | 1 nm–1 µm |
| Ultrasound attenuation | Ultrasound attenuation | 10 nm–3000 µm |
| Designation | Criteria | |
|---|---|---|
| Device | Light source | Monochromatic light (Laser): semiconductor laser is preferable for the low cost and long lifetime. |
| Angle | 90° or another specified angle | |
| Detector | Sensitive photomultiplier | |
| Optical fibers | Single and multimode | |
| Calibration | Latex standard | |
| Sample | Concentration | Dilution or Enrichment (10−2–10−3% (v/v)) |
| Sample | Colloids | |
| Solvent | No influence of samples’ properties Good particles dispersion | |
| Temperature | Constant: Room or others (up to 275 °C [49]). | |
| Viscosity of solvent | Known |
| Integrated | Separated | Microfluidics | |
|---|---|---|---|
| Reference | [50] | [51] | [52] |
| Laser | He–Ne laser 632.8 nm | DPSS 532 nm | MGL-III 532 nm |
| Optical fiber | Mono et multi mode SEDI | LPC-07 series single mode | single-mode fiber probe, |
| Angle | 90° | 90° | 30° and 45° |
| Observed Volume(nL) | ~1 | 7 | ~1 |
| Calibration | 100 and 300 nm latex spheres 35 nm TiO2 sols | 107.6 and 64 nm latex spheres110 and 140 Carboxylated latex | 362–710 nm polystyrene particles |
| Catalysts | Synthesis Method | Hydrodynamic Diameter (nm) | Catalytic Application | Ref. |
|---|---|---|---|---|
| Fe3O4 | Coprecipitation | 16 nm | Benzylic and allylic C-H oxidation | [58] |
| ZnO | Microwave irradiation | 370 nm | Transesterification conversion | [59] |
| CeO2 CoS2-CeO2 | ultrasonic method | 56 nm 62 nm | Photocatalysis | [60] |
| TiO2 | Sol-gel | 215 nm | Photocatalysis | [61] |
| Au/Al2O3 | Deposition–precipitation | 5–2000 nm | CO oxidation | [62] |
| Fe3O4/Al2O3 | Coprecipitation | 196 nm | Transesterification reaction | [63] |
| ZIF-8 | Solvothermal | 143 µm | Knoevenagel reaction | [64] |
| Fe-polymer | Thermal decomposition | 2–932 nm | Fischer–Tropsch Synthesis | [65] |
| Ni-POM | Deposition–precipitation | 700–1300 nm | Water oxidation | [66] |
| Au-Ag | Successive reduction | 10–5000 nm | Methanol oxidation | [67] |
| Ag | Reduction | 0.2–30 nm | 4-Nitrophenol Reduction | [68] |
| Catalysts | DLS (nm) | TEM (nm) | Difference % | Ref. |
|---|---|---|---|---|
| TiO2 | 77.2 224.8 | 39.3 66.2 | 49.1 70.63 | [69] |
| Fe3O4 | 16.9 21.1 43.1 | 7.2 14.5 20.1 | 57.4 31.3 53.4 | [73] |
| CoFe2O4 | 27.9 84.5 | 10.8 45.8 | 61.3 45.8 | [69] |
| SiO2 | 64 | 53.5 | 16.4 | [71] |
| ZrO2 small ZrO2 Large | 17.8 54 | 3.8 15.2 | 78.7 71.9 | |
| SiO2/TiO2 | 28 59 108 | 26 57 105 | 7.1 3.4 2.8 | [70] |
| Catalysts | Precursor of Sol-Gel | Hydrodynamic Diameter (nm) | Catalytic Application | Ref. |
|---|---|---|---|---|
| TiO2 | Tianium tetraisopropoxide | 0.9–2.6 nm | Photocatalysis | [50] |
| Fe–TiO2 | Iron(acetylacetonate)3 Tianium tetraisopropoxide | 6 nm | Photocatalysis | [76] |
| N–TiO2 | Hydroxyurea Tianium tetraisopropoxide | 3.0–6.7 nm | Photocatalysis | [77,78] |
| ZrO2 | Zirconium n-butoxide | 1.5–2.1 nm | Esterification | [79] |
| ZrxTi1-xO2 | Zirconium n-butoxide Tianium tetraisopropoxide | 2.0–2.7 nm | Photocatalysis | [80] |
| VxTi1-xO2 | Vanadium(V) oxytripropoxide Tianium tetraisopropoxide | 2–7 nm | Photocatalysis | [81] |
| Silicalite | TEOS, Cab-O-Sil, Ludox LS | 2–50 nm | Petrochemistry | [82] |
| Au hybrid gel | HAuCl4 | 32 nm 260 nm | Au-catalyzed reaction | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids Interfaces 2023, 7, 15. https://doi.org/10.3390/colloids7010015
Jia Z, Li J, Gao L, Yang D, Kanaev A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids and Interfaces. 2023; 7(1):15. https://doi.org/10.3390/colloids7010015
Chicago/Turabian StyleJia, Zixian, Jiantao Li, Lin Gao, Dezheng Yang, and Andrei Kanaev. 2023. "Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing" Colloids and Interfaces 7, no. 1: 15. https://doi.org/10.3390/colloids7010015
APA StyleJia, Z., Li, J., Gao, L., Yang, D., & Kanaev, A. (2023). Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids and Interfaces, 7(1), 15. https://doi.org/10.3390/colloids7010015









