Boosting Water Oxidation Activity via Carbon–Nitrogen Vacancies in NiFe Prussian Blue Analogue Electrocatalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Reagents and Synthesis Conditions
2.2. Physical Characterization
2.3. Electrochemical Characterization
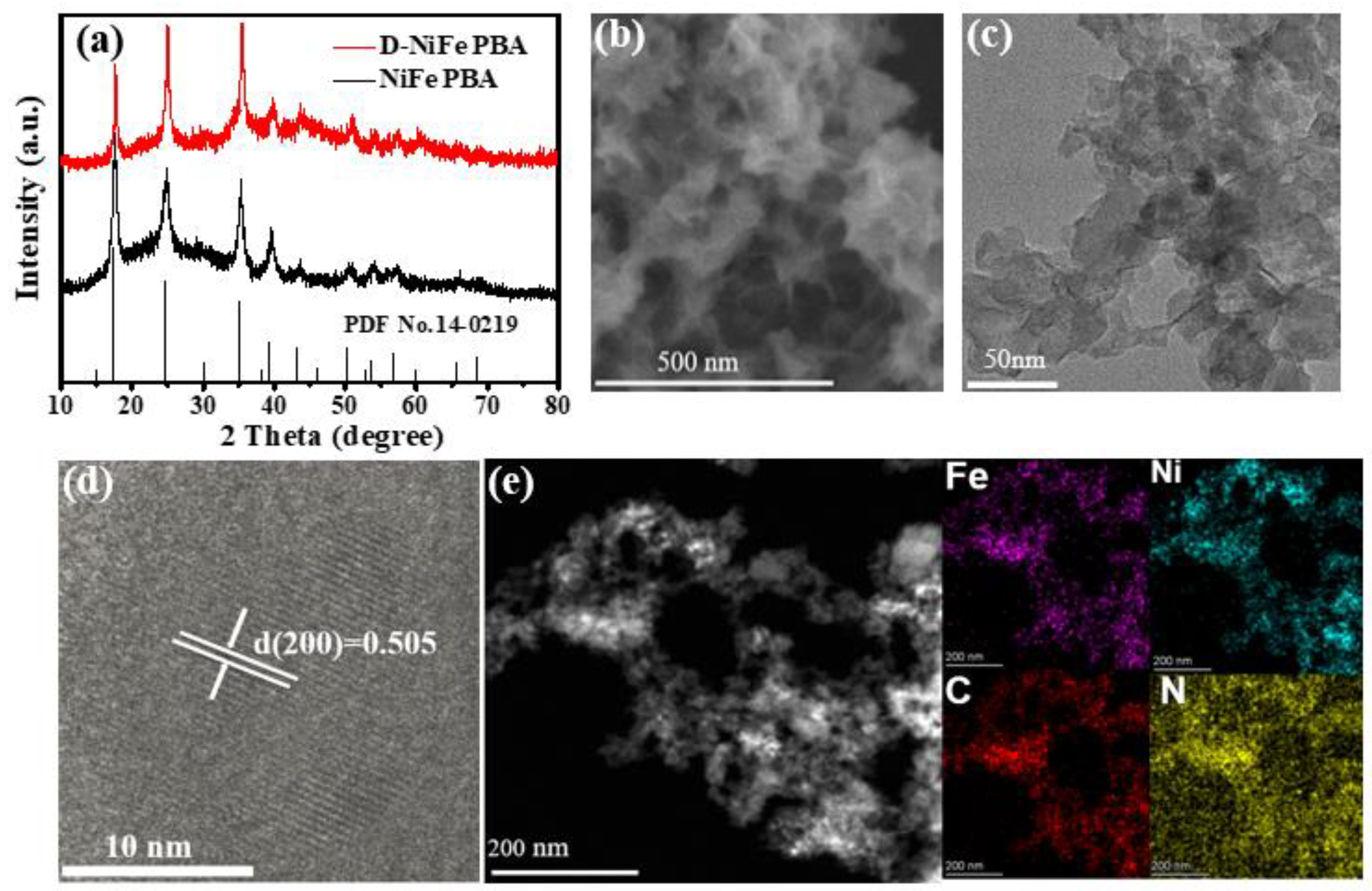
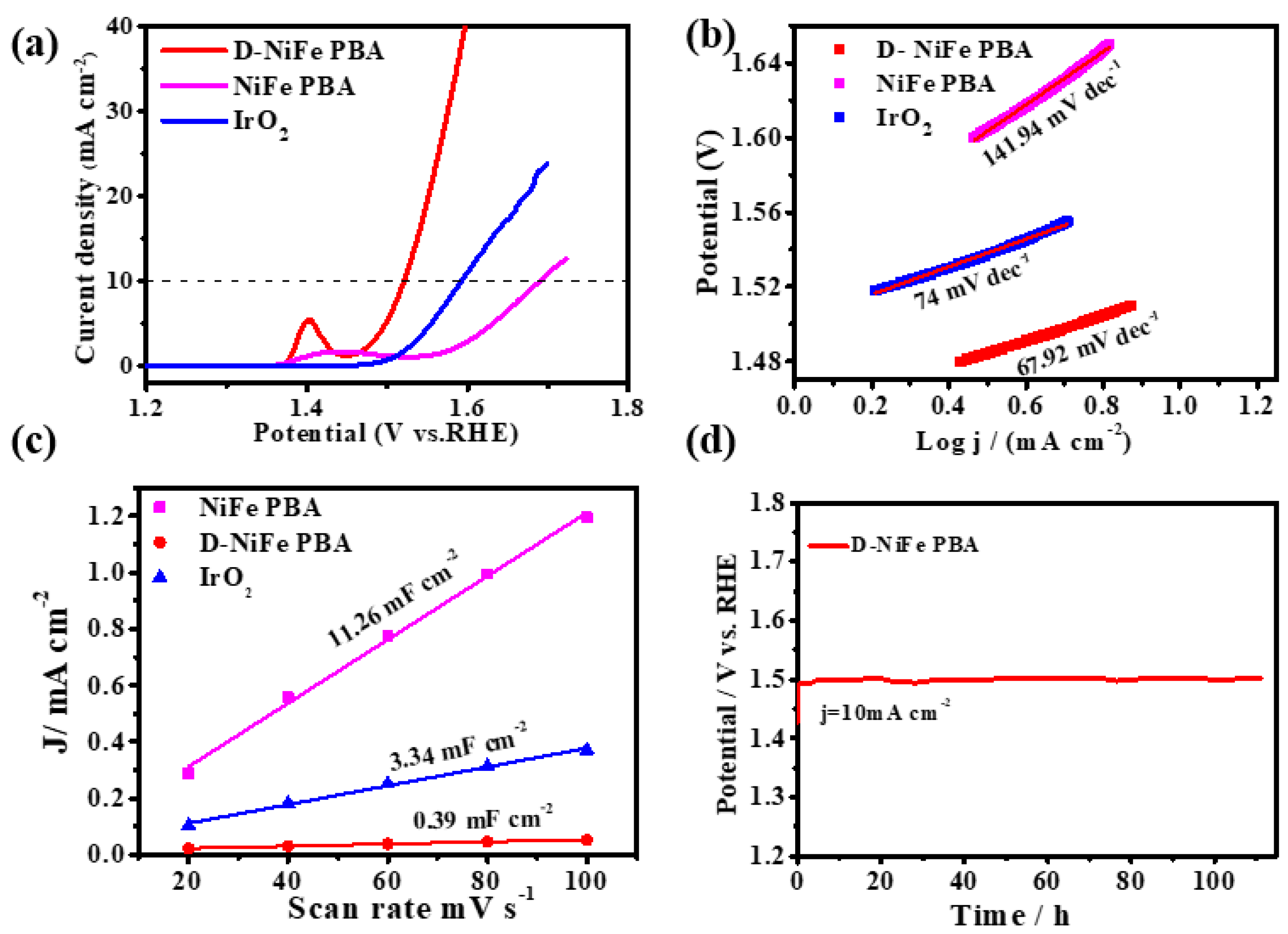
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, T.I.; Rajeshkhanna, G.; Pan, U.N.; Kshetri, T.; Lin, H.; Kim, N.H.; Lee, J.H. Alkaline Water Splitting Enhancement by MOF-Derived Fe-Co-Oxide/Co@NC-mNS Heterostructure: Boosting OER and HER through Defect Engineering and In Situ Oxidation. Small 2021, 17, 2101312. [Google Scholar] [CrossRef]
- Asnavandi, M.; Yin, Y.; Li, Y.; Sun, C.; Zhao, C. Promoting Oxygen Evolution Reactions through Introduction of Oxygen Vacancies to Benchmark NiFe-OOH Catalysts. ACS Energy Lett. 2018, 3, 1515–1520. [Google Scholar] [CrossRef]
- Jo, S.; Kwon, J.; Choi, S.; Lu, T.; Byeun, Y.; Han, H.; Song, T. Engineering [Fe(CN)6]3− vacancy via free-chelating agents in Prussian blue analogues on reduced graphene oxide for efficient oxygen evolution reaction. Appl. Surf. Sci. 2022, 574, 151620. [Google Scholar] [CrossRef]
- Jo, Y.; Cho, S.; Seo, J.; Ahmed, A.T.A.; Lee, C.H.; Seok, J.H.; Hou, B.; Patil, S.A.; Park, Y.; Shrestha, N.K.; et al. Experimental and Theoretical Insights into the Borohydride-Based Reduction-Induced Metal Interdiffusion in Fe-Oxide@NiCo2O4 for Enhanced Oxygen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 53725–53735. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, J.; Lou, X.W.D. Construction of single-crystalline Prussian blue analog hollow nanostructures with tailorable topologies. Chem 2018, 4, 1967–1982. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Dong, P.; Li, J.; Feng, L.; Huang, J.; Wang, C. Boosting the activity of Prussian-blue analogue as efficient electrocatalyst for water and urea oxidation. Sci. Rep. 2019, 9, 15965. [Google Scholar] [CrossRef]
- Jiang, M.; Fan, X.; Cao, S.; Wang, Z.; Yang, Z.; Zhang, W. Thermally activated carbon–nitrogen vacancies in double-shelled NiFe Prussian blue analogue nanocages for enhanced electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 12734–12745. [Google Scholar] [CrossRef]
- Xuan, C.; Zhang, J.; Wang, J.; Wang, D. Rational design and engineering of nanomaterials derived from prussian blue and its analogs for electrochemical water splitting. Chem. Asian J. 2020, 15, 958–972. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, B.; Zhao, D.; Wang, H.; Selomulya, C. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 2017, 15, 26–55. [Google Scholar] [CrossRef]
- Li, W.J.; Han, C.; Cheng, G.; Chou, S.L.; Liu, H.K.; Dou, S.X. Chemical properties, structural properties, and energy storage applications of Prussian blue analogues. Small 2019, 15, 1900470. [Google Scholar] [CrossRef]
- Zhou, W.-Y.; Sun, R.; Li, S.-S.; Guo, Y.; Shen, W.; Wang, J.; Deepak, F.L.; Li, Y.; Wang, Z. Engineering surface electron and active site at electrochemical sensing interface of CN vacancy-mediated Prussian blue analogue for analysis of heavy metal ions. Appl. Surf. Sci. 2021, 564, 150131. [Google Scholar] [CrossRef]
- Hou, J.; Tang, Z.; Wei, K.; Lai, Q.; Liang, Y. Surface reconstruction of Ni doped Co–Fe Prussian blue analogues for enhanced oxygen evolution. Catal. Sci. Technol. 2021, 11, 1110–1115. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Liu, J.D.; Chen, Y.; Liu, X.K.; Liu, W.; Ma, T.; Li, Y.; Zheng, X.S.; Yao, T.; et al. Unconventional CN vacancies suppress iron-leaching in Prussian blue analogue pre-catalyst for boosted oxygen evolution catalysis. Nat. Commun. 2019, 10, 2799. [Google Scholar] [CrossRef]
- Lai, C.; Li, H.; Sheng, Y.; Zhou, M.; Wang, W.; Gong, M.; Jiang, K. 3D Spatial Combination of CN Vacancy-Mediated NiFe-PBA with N-Doped Carbon Nanofibers Network toward Free-Standing Bifunctional Electrode for Zn-Air Batteries. Adv. Sci. 2022, 9, 2105925. [Google Scholar] [CrossRef]
- Chi, H.; Liu, J.; Zhang, X.; Xue, X.; Zhang, D.; Lin, X.; Huang, P.; Sun, L.; Xiong, J.; Cai, P.; et al. Synergetic defects boost charge separation in CN for enhanced photocatalytic water splitting. J. Mater. Chem. C 2020, 8, 9366–9372. [Google Scholar] [CrossRef]
- Xiang, K.; Wu, D.; Fan, Y.; You, W.; Zhang, D.; Luo, J.L.; Fu, X.Z. Enhancing bifunctional electrodes of oxygen vacancy abundant ZnCo2O4 nanosheets for supercapacitor and oxygen evolution. Chem. Eng. J. 2021, 425, 130583. [Google Scholar] [CrossRef]
- Yan, K.L.; Shang, X.; Liu, Z.Z.; Dong, B.; Lu, S.S.; Chi, J.Q.; Liu, C.G. A facile method for reduced CoFe2O4 nanosheets with rich oxygen vacancies for efficient oxygen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 24150–24158. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.T.; Zheng, Y.Q. Co3O4 nanosheet arrays treated by defect engineering for enhanced electrocatalytic water oxidation. Int. J. Hydrogen Energy 2018, 43, 2009–2017. [Google Scholar] [CrossRef]
- Chen, Z.; Fei, B.; Hou, M.; Yan, X.; Chen, M.; Qing, H.; Wu, R. Ultrathin Prussian blue analogue nanosheet arrays with open bimetal centers for efficient overall water splitting. Nano Energy 2020, 68, 104371. [Google Scholar] [CrossRef]
- Zhang, X.; Khan, I.U.; Huo, S.; Zhao, Y.; Liang, B.; Li, K.; Wang, H. In-situ integration of nickel-iron Prussian blue analog heterostructure on Ni foam by chemical corrosion and partial conversion for oxygen evolution reaction. Electrochim. Acta 2020, 363, 137211. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Zhang, J.; Luo, Y.; Chen, Y.; Xue, Y.; Yan, Y.; Jiao, Y.; Wang, G.; Wang, R. The activation of inert NiFe Prussian Blue analogues to boost oxygen evolution reaction activity. J Colloid Interface Sci. 2022, 607, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Y.; Malgras, V.; Ji, Q.; Jiang, D.; Qi, R.; Hu, M. Synthesis of monocrystalline nanoframes of prussian blue analogues by controlled preferential etching. Angew. Chem. Int. Ed. 2016, 55, 8228–8234. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, J.; Chen, Z.; Wang, R.; Fei, B.; Liu, H.; Guo, Y.; Wu, R. Unconventional bi-vacancies activating inert Prussian blue analogues nanocubes for efficient hydrogen evolution. Chem. Eng. J. 2021, 420, 127671. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Liu, J.; Xu, M.; Cheng, J.; Zhang, D.; Goodenough, J.B. A superior low-cost cathode for a Na-ion battery. Angew. Chem. Int. Ed. 2013, 152, 2018–2021. [Google Scholar] [CrossRef]
- Risset, O.N.; Knowles, E.S.; Ma, S.; Meisel, M.W.; Talham, D.R. RbjMk[Fe(CN)6]l (M = Co, Ni) Prussian Blue Analogue Hollow Nanocubes: A New Example of a Multilevel Pore System. Chem. Mater. 2012, 25, 42–47. [Google Scholar] [CrossRef]
- Han, L.; Yu, X.Y.; Lou, X.W. Formation of Prussian-Blue-Analog Nanocages via a Direct Etching Method and their Conversion into Ni-Co-Mixed Oxide for Enhanced Oxygen Evolution. Adv. Mater. 2016, 28, 4601–4605. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T.; Su, L.; Zhang, Y.; Dong, L.; Miao, X. In Situ Synthesis of Superhydrophilic Amorphous NiFe Prussian Blue Analogues for the Oxygen Evolution Reaction at a High Current Density. ACS Sustain. Chem. Eng. 2021, 9, 5693–5704. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.T.; Duan, J.J.; Yao, Y.Q.; Feng, J.J.; Wang, A.J. In-Situ construction of 3D hetero-structured sulfur-doped nanoflower-like FeNi LDH decorated with NiCo Prussian blue analogue cubes as efficient electrocatalysts for boosting oxygen evolution reaction. J. Colloid Interface Sci. 2022, 611, 205–214. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Zhang, C.; Zhang, S.; Jia, Y.; Dong, Y. An enzyme-free immunosensor for sensitive determination of procalcitonin using NiFe PBA nanocubes@ TB as the sensing matrix. Anal. Chim. Acta 2020, 1097, 169–175. [Google Scholar] [CrossRef]
- Yi, X.; He, X.; Yin, F.; Chen, B.; Li, G.; Yin, H. Amorphous Ni–Fe–Se hollow nanospheres electrodeposited on nickel foam as a highly active and bifunctional catalyst for alkaline water splitting. Dalton Trans. 2020, 20, 6764–6775. [Google Scholar] [CrossRef]
- Patil, S.J.; Chodankar, N.R.; Hwang, S.K.; Rama Raju, G.S.; Huh, Y.S.; Han, Y.K. Fluorine Engineered Self-Supported Ultrathin 2D Nickel Hydroxide Nanosheets as Highly Robust and Stable Bifunctional Electrocatalysts for Oxygen Evolution and Urea Oxidation Reactions. Small 2022, 7, 2103326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, X.; Chen, G.; Shang, L.; Waterhouse, G.I.; Wu, L.Z.; Zhang, T. Ultrafine NiO nanosheets stabilized by TiO2 from monolayer NiTi-LDH precursors: An active water oxidation electrocatalyst. J. Am. Chem. Soc. 2016, 20, 6517–6524. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.H.; Lim, J.M.; Kim, H.; Kim, S. Conventional and photoinduced radioactive 137Cs removal by adsorption on FeFe, CoFe, and NiFe Prussian blue analogues. Chem. Eng. J. 2021, 405, 126568. [Google Scholar] [CrossRef]
- An, L.; Feng, J.; Zhang, Y.; Zhao, Y.; Si, R.; Wang, G.; Cheng, F.; Xi, P.; Sun, S. Controllable tuning of Fe-N nanosheets by Co substitution for enhanced oxygen evolution reaction. Nano Energy 2019, 57, 644–652. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, S.; Wang, H.; Zhong, Y.; Hu, Y. An efficient and stable Ni–Fe selenides/nitrogen-doped carbon nanotubes in situ-derived electrocatalyst for oxygen evolution reaction. J. Mater. Sci. 2020, 55, 13927–13937. [Google Scholar] [CrossRef]
- Tian, X.; Cheng, C.; Qian, L.; Zheng, B.; Yuan, H.; Xie, S.; Choi, M.M. Microwave-assisted non-aqueous homogenous precipitation of nanoball-like mesoporous α-Ni (OH)2 as a precursor for NiOx and its application as a pseudocapacitor. J. Mater. Chem. A 2012, 22, 8029–8035. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, S.; Hui, K.; Li, H.-F.; Liang, F.; Wu, X.-L.; Zhang, Q.; Zhou, W.; Chen, L.; Chen, F.J. [Fe(CN)6] vacancy-boosting oxygen evolution activity of Co-based Prussian blue analogues for hybrid sodium-air battery. Mater. Today Energy 2021, 20, 100572. [Google Scholar]
- Pan, Y.; Zhang, J.; Zhao, Z.; Shi, L.; Wu, B.; Zeng, L. Iron-doped metal-organic framework with enhanced oxygen evolution reaction activity for overall water splitting. Int. J. Hydrogen Energy 2021, 46, 34565–34573. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Chen, S.; Xie, F.; Riley, D.J. Anodic Transformation of a Core-Shell Prussian Blue Analogue to a Bifunctional Electrocatalyst for Water Splitting. Adv. Funct. Mater. 2021, 31, 2106835. [Google Scholar] [CrossRef]
- Zou, H.; Liu, X.; Wang, K.; Duan, Y.; Wang, C.; Zhang, B.; Zhou, K.; Yu, D.; Gan, L.-Y.; Zhou, X. Constructing highly active Co sites in Prussian blue analogues for boosting electrocatalytic water oxidation. Chem. Commun. 2021, 57, 8011–8014. [Google Scholar] [CrossRef]
- Sun, W.; Wei, Z.; Qi, J.; Kang, L.; Li, J.; Xie, J.; Tang, B.; Xie, Y. Rapid and Scalable Synthesis of Prussian Blue Analogue Nanocubes for Electrocatalytic Water Oxidation. Chin. J. Chem. 2021, 39, 2347–2353. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.; Chen, G.; Shang, L.; Shi, R.; Kang, X.; Zhang, T. Ni3FeN nanoparticles derived from ultrathin NiFe-layered double hydroxide nanosheets: An efficient overall water splitting electrocatalyst. Adv. Energy Mater. 2016, 6, 1502585. [Google Scholar] [CrossRef]
- Karkera, G.; Sarkar, T.; Bharadwaj, M.D.; Prakash, A.S. Design and development of efficient bifunctional catalysts by tuning the electronic properties of cobalt-manganese tungstate for oxygen reduction and evolution reactions. ChemCatChem 2017, 9, 3681–3690. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Y.; Liu, X.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. Facile synthesis of electrospun MFe2O4 (M=Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale 2015, 7, 8920–8930. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.; Gao, S.; Su, J.; Lun, Z.; Xia, G.; Chen, Q. Tuning electronic structures of nonprecious ternary alloys encapsulated in graphene layers for optimizing overall water splitting activity. ACS Catal. 2017, 7, 469–479. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wu, W.; Wang, Z.; Xie, G.; Guo, X. Boosting Water Oxidation Activity via Carbon–Nitrogen Vacancies in NiFe Prussian Blue Analogue Electrocatalysts. Colloids Interfaces 2023, 7, 14. https://doi.org/10.3390/colloids7010014
Zhang M, Wu W, Wang Z, Xie G, Guo X. Boosting Water Oxidation Activity via Carbon–Nitrogen Vacancies in NiFe Prussian Blue Analogue Electrocatalysts. Colloids and Interfaces. 2023; 7(1):14. https://doi.org/10.3390/colloids7010014
Chicago/Turabian StyleZhang, Meng, Wenjie Wu, Zhen Wang, Gang Xie, and Xiaohui Guo. 2023. "Boosting Water Oxidation Activity via Carbon–Nitrogen Vacancies in NiFe Prussian Blue Analogue Electrocatalysts" Colloids and Interfaces 7, no. 1: 14. https://doi.org/10.3390/colloids7010014
APA StyleZhang, M., Wu, W., Wang, Z., Xie, G., & Guo, X. (2023). Boosting Water Oxidation Activity via Carbon–Nitrogen Vacancies in NiFe Prussian Blue Analogue Electrocatalysts. Colloids and Interfaces, 7(1), 14. https://doi.org/10.3390/colloids7010014





