Impact of Paraffin Composition on the Interactions between Waxes, Asphaltenes, and Paraffin Inhibitors in a Light Crude Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Solution Preparation
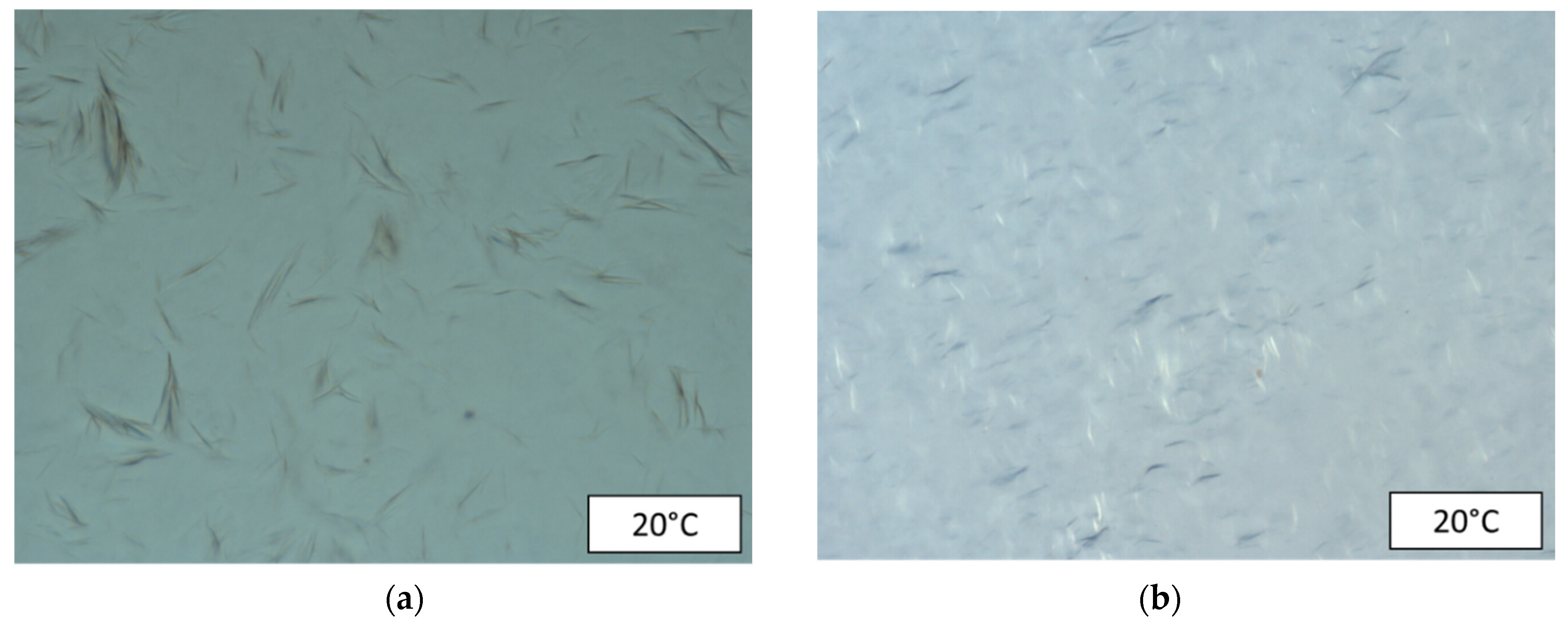
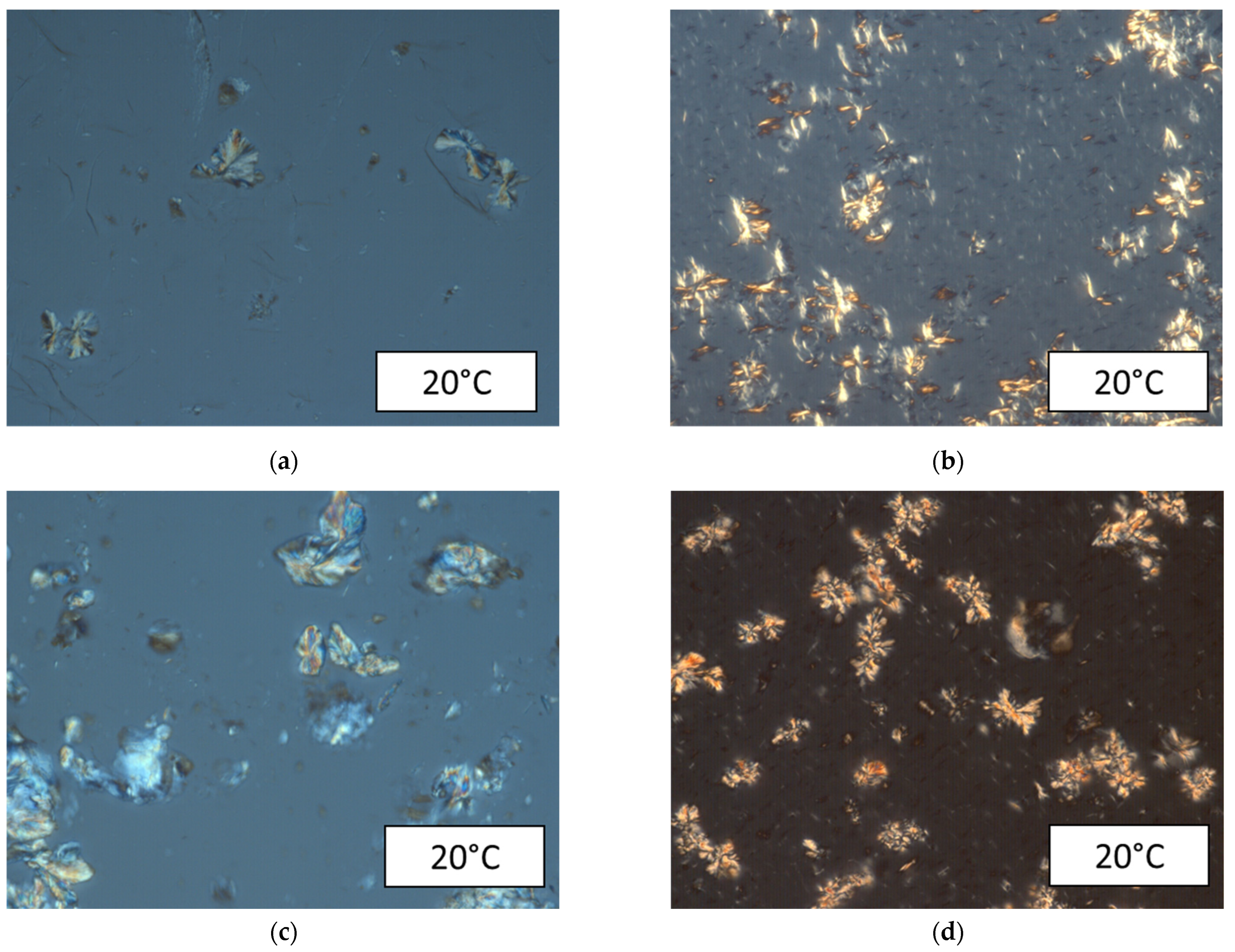
2.2.2. Cross-Polarized Microscopy (CPM)
2.2.3. Viscosity Measurement
3. Results and Discussion
3.1. Rheological Characterization and Microstructure of Oil Containing Wax
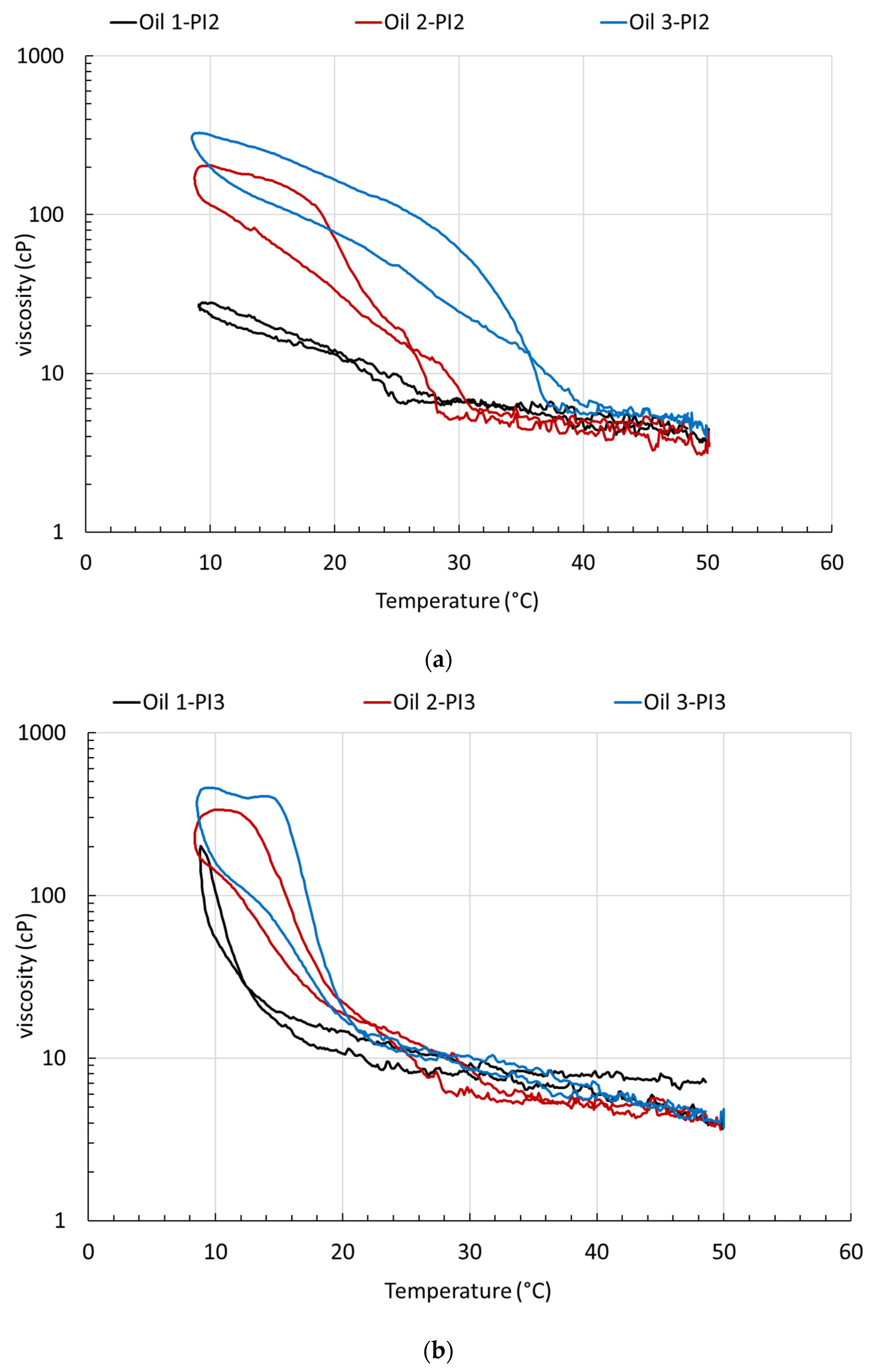
3.2. Rheological Characterization and Microstructure of Oil Containing Wax, Treated with PI
3.3. Rheological Characterization and Microstructure of Oil Containing Wax and Asphaltenes
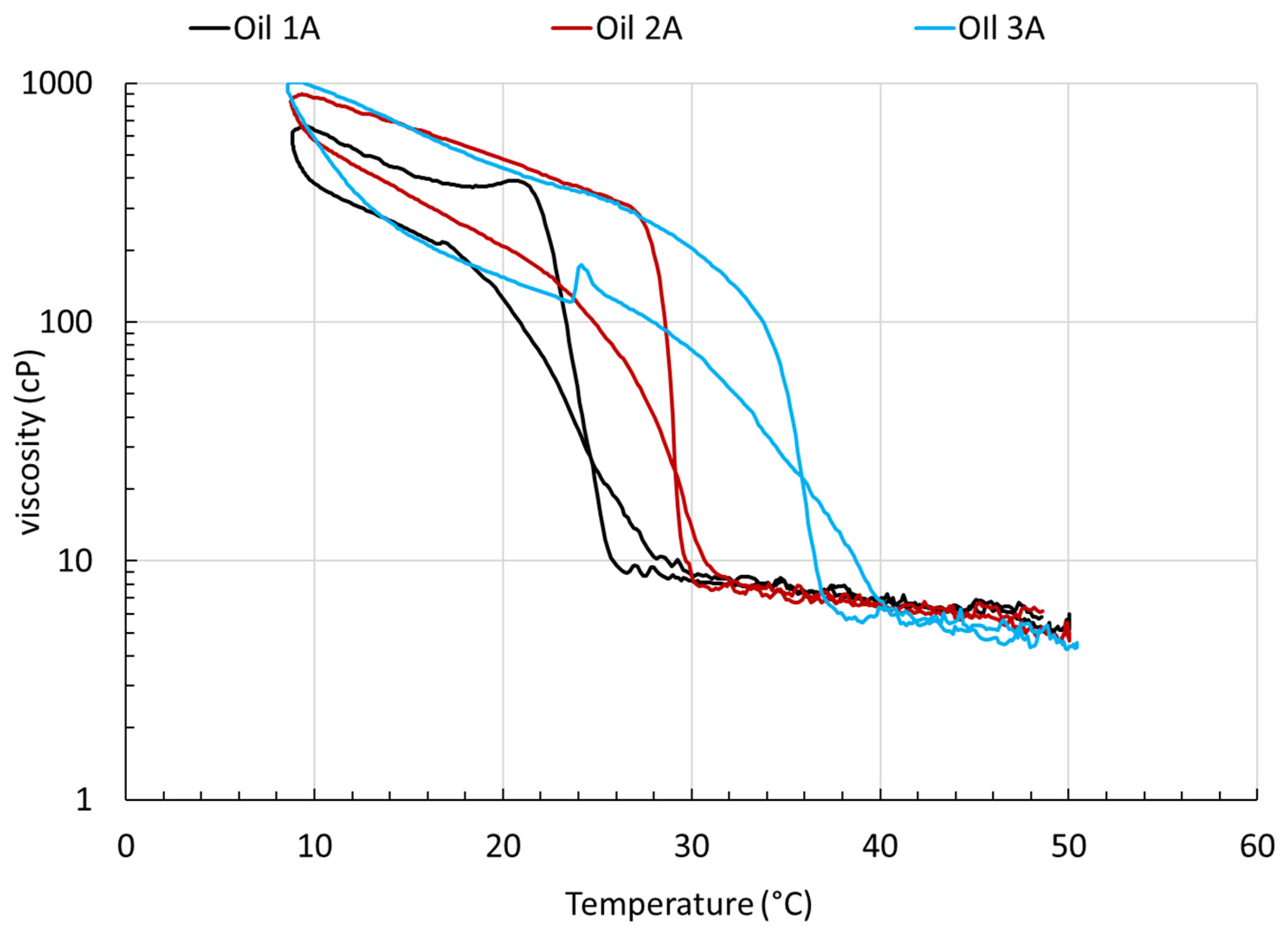
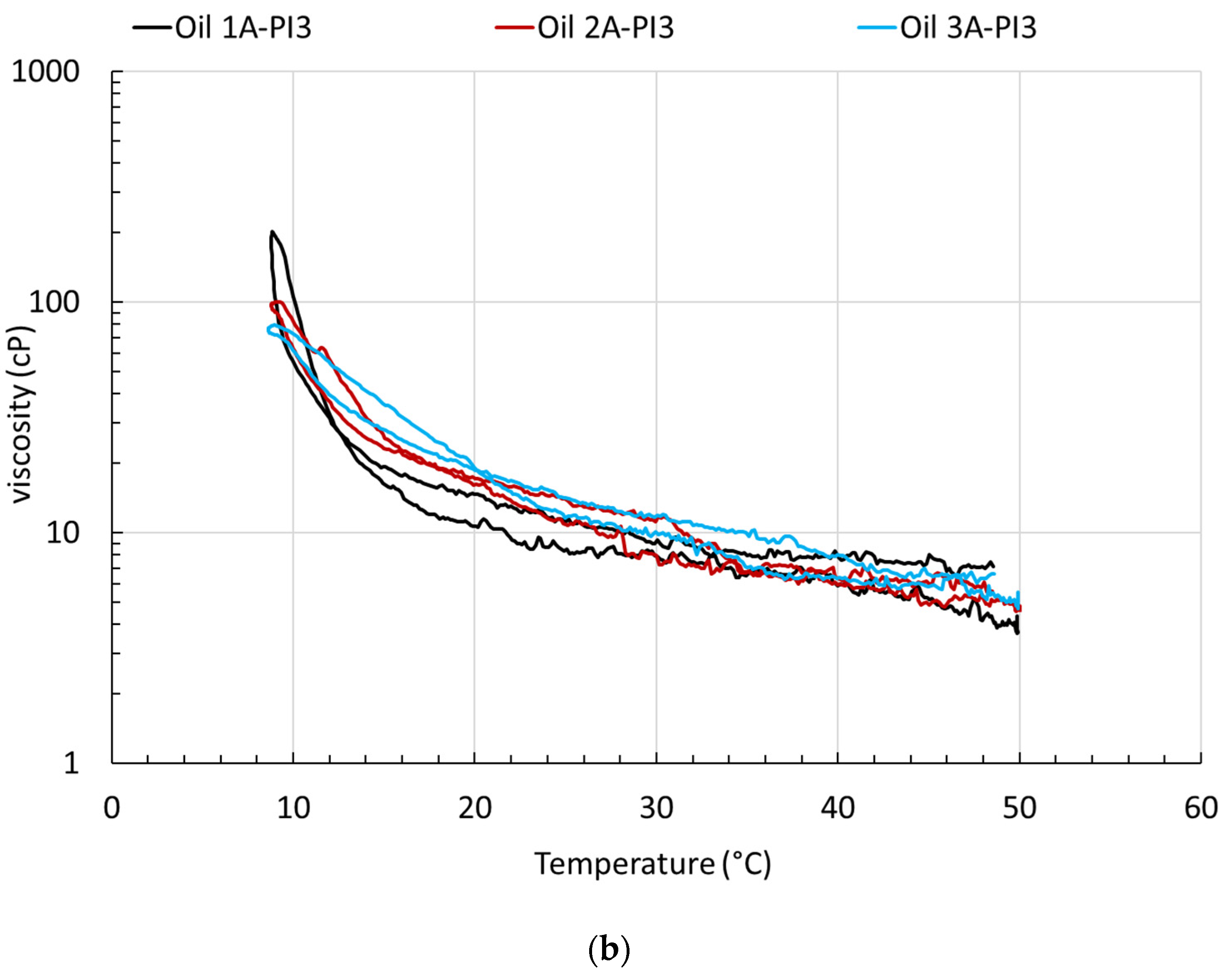
4. Rheological Characterization and Microstructure of Oil Containing Wax and Asphaltenes, Treated with PI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.; Zheng, S.; Fogler, H.S. Wax Deposition: Experimental Characterizations, Theoretical Modeling, and Field Practices; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Chouparova, E.; Lanzirotti, A.; Feng, H.; Jones, K.W.; Marinkovic, N.; Whitson, C.; Philp, P. Characterization of Petroleum Deposits Formed in a Producing Well by Synchrotron Radiation-Based Microanalyses. Energy Fuels 2004, 18, 1199–1212. [Google Scholar] [CrossRef]
- Speight, J.G. Wax Deposition and Fouling. In Fouling in Refineries; Elsevier: Amsterdam, The Netherlands, 2015; pp. 155–173. [Google Scholar]
- Chi, Y.; Yang, J.; Sarica, C.; Daraboina, N. A Critical Review of Controlling Paraffin Deposition in Production Lines Using Chemicals. Energy Fuels 2019, 33, 2797–2809. [Google Scholar] [CrossRef]
- Bansal, R.; Ravishankar, B.; Sharma, S.S.; Afzal, K. Dynamic Simulation for Optimising Pigging Frequency for Dewaxing. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 28–30 March 2012. [Google Scholar]
- Atta, A.; Al-Shafy, H.; Ismail, E. Influence of ethylene acrylic alkyl ester copolymer wax dispersants on the rhological behavior of Egyptian crude oil. J. Dispers. Sci. Technol. 2011, 32, 1296–1305. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, X.; Ma, J.; Zhang, B. Investigation into a pour point depressant for Shengli crude oil. Ind. Eng. Chem. Res. 2012, 51, 11605–11612. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El-Hamouly, S.H.; Khidr, T.T.; El-Ghazawy, R.A.; Higazy, S.A. Preparation the Esters of Oleic Acid-Maleic Anhydride Copolymer and Their Evaluation as Flow Improvers for Waxy Crude Oil. J. Dispers. Sci. Technol. 2013, 34, 1585–1596. [Google Scholar] [CrossRef]
- Wang, K.-S.; Wu, C.-H.; Creek, J.L.; Shuler, P.J.; Tang, Y. Evaluation of Effects of Selected Wax Inhibitors on Paraffin Deposition. Pet. Sci. Technol. 2003, 21, 369–379. [Google Scholar] [CrossRef]
- Soldi, R.A.; Oliveira, A.R.S.; Barbosa, R.V.; César-Oliveira, M.A.F. Polymethacrylates: Pour point depressants in diesel oil. Eur. Polym. J. 2007, 43, 3671–3678. [Google Scholar] [CrossRef]
- Garcia, M.d.C.; Carbognani, L.; Urbina, A.; Orea, M. Paraffin deposition in oil production. Oil composition and paraffin inhibitors activity. Pet. Sci. Technol. 1998, 16, 1001–1021. [Google Scholar] [CrossRef]
- M’barki, O.; Clements, J.; Salazar, L.; Machac, J.; Nguyen, Q.P. Effects of Structure and Asphaltenes on Paraffin Inhibitor Efficacy in a Light Crude Oil Model. Energy Fuels 2022, 36, 7531–7541. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, Y.; Sjöblom, J.; Li, C.; Paso, K.G. Polymeric Wax Inhibitors and Pour Point Depressants for Waxy Crude Oils: A Critical Review. J. Dispers. Sci. Technol. 2014, 36, 213–225. [Google Scholar] [CrossRef]
- Venkatesan, R.; Östlund, J.-A.; Chawla, H.; Wattana, P.; Nydén, M.; Fogler, H.S. The Effect of Asphaltenes on the Gelation of Waxy Oils. Energy Fuels 2003, 17, 1630–1640. [Google Scholar] [CrossRef]
- Kriz, P.; Andersen, S.I. Effect of asphaltenes on crude oil wax crystallization. Energy Fuels 2005, 19, 948–953. [Google Scholar] [CrossRef]
- Oh, K.; Deo, M. Characteristics of wax gel formation in the presence of asphaltenes. Energy Fuels 2009, 23, 1289–1293. [Google Scholar] [CrossRef]
- Tinsley, J.F.; Jahnke, J.P.; Dettman, H.D.; Prud’home, R.K. Waxy gels with asphaltenes 1: Characterization of precipitation, gelation, yield stress, and morphology. Energy Fuels 2009, 23, 2056–2064. [Google Scholar] [CrossRef]
- Alcazar-Vara, L.A.; Garcia-Martinez, J.A.; Buenrostro-Gonzalez, E. Effect of asphaltenes on equilibrium and rheological properties of waxy model systems. Fuel 2012, 93, 200–212. [Google Scholar] [CrossRef]
- Wu, Y.; Ni, G.; Yang, F.; Li, C.; Dong, G. Modified Maleic Anhydride Co-polymers as Pour-Point Depressants and Their Effects on Waxy Crude Oil Rheology. Energy Fuels 2012, 26, 995–1001. [Google Scholar] [CrossRef]
- Soni, H.P.; Kiranbala; Bharambe, D.P. Performance-Based Designing of Wax Crystal Growth Inhibitors. Energy Fuels 2008, 22, 3930–3938. [Google Scholar] [CrossRef]
- García, M.d.C. Crude Oil Wax Crystallization. The Effect of Heavy n-Paraffins and Flocculated Asphaltenes. Energy Fuels 2000, 14, 1043–1048. [Google Scholar] [CrossRef]
- Garcia, M.C.; Carbognani, L.; Urbina, A.; Orea, M. Correlation Between Oil Composition and Paraffin Inhibitors Activity. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 27–30 September 1998. [Google Scholar]

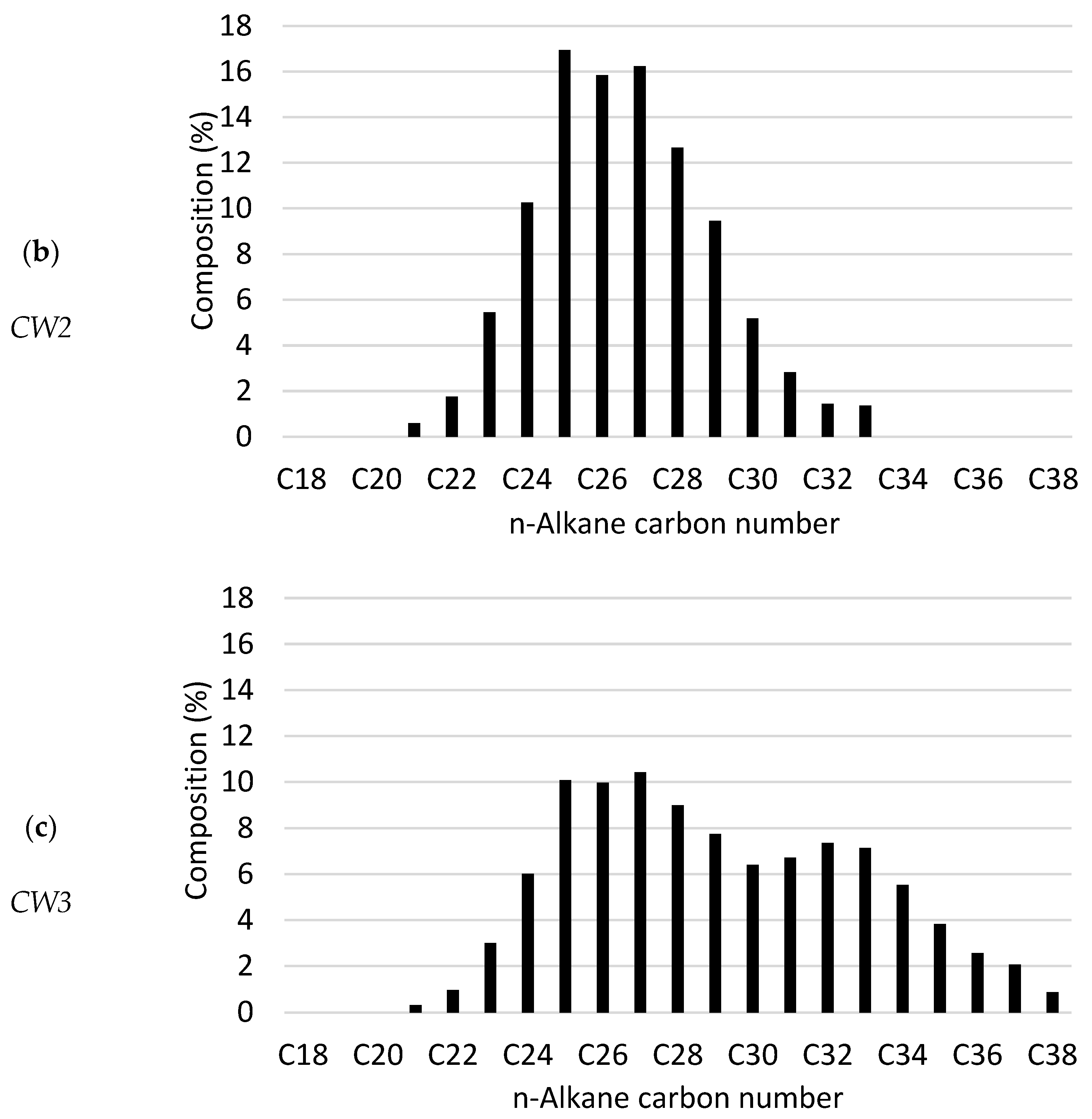




| Commercial Wax | Properties (Carbon No.) | |||||||
| CW1 | CW2 | CW3 | ||||||
| Wax Blend | Wt. % | MR (°C) | Wt. % | MR (°C) | Wt. % | MR (°C) | Avg. | Std. Dev. |
| W1 | 45 | 42–44 | 55 | 53–58 | --- | 65+ | 24.2 | 3.3 |
| W2 | --- | 100 | --- | 26.6 | 2.3 | |||
| W3 | --- | 55 | 45 | 29.1 | 3.8 | |||
| PI | Side-Chain Carbon No. Distribution | ||
| Range 1 | Avg. 2 | Density 3 | |
| PI2 | 18–26 | 21.1 ± 2.2 | 1.6 |
| PI3 | 18–22 | 20.7 ± 1.7 | 1.2 |
| Oil | Wax | PI | Oil | Wax | PI |
| No Asphaltenes | 1 wt. % Asphaltenes | ||||
| 1 1-PI2 1-PI3 | CW1 | --- PI2 PI3 | 1A 1A-PI2 1A-PI3 | CW1 | --- PI2 PI3 |
| 2 2-PI2 2-PI3 | CW2 | --- PI2 PI3 | 2A 2A-PI2 2A-PI3 | CW2 | --- PI2 PI3 |
| 3 3-PI2 3-PI3 | CW3 | --- PI2 PI3 | 3A 3A-PI2 3A-PI2 | CW3 | --- PI2 PI3 |
| Oil | WAT (°C) | μmax (cP) | Reduction (%) | WAT (°C) | μmax (cP) | Reduction (%) | |
| Wax composition CW1 | |||||||
| Oil 1 | 27.5 | 834 | - | Oil 1A Oil 1A-PI2 Oil 1A-PI3 | 25 | 655 | 21.5 |
| Oil 1-PI2 | 26 | 28 | 96.6 | not defined | 36 | 95.7 | |
| Oil 1-PI3 | 17 | 202 | 75.8 | not defined | 202 | 75.8 | |
| Wax composition CW2 | |||||||
| Oil 2 | 30 | 788 | - | Oil 2A Oil 2A-PI2 Oil 2A-PI3 | 30 | 900 | −14.2 |
| Oil 2-PI2 | 28 | 204 | 74.1 | not defined | 117 | 85.2 | |
| Oil 2-PI3 | 28 | 337 | 57.2 | not defined | 100 | 87.3 | |
| Wax composition CW3 | |||||||
| Oil 3 | 38.5 | 991 | - | Oil 3A Oil 3A-PI2 Oil 3A-PI3 | 36 | 1010 | −1.9 |
| Oil 3-PI2 | 36 | 327 | 67.0 | not defined | 37 | 96.3 | |
| Oil 3-PI3 | 17 | 458 | 53.8 | not defined | 80 | 91.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M'barki, O.; Clements, J.; Salazar, L.; Machac, J., Jr.; Nguyen, Q.P. Impact of Paraffin Composition on the Interactions between Waxes, Asphaltenes, and Paraffin Inhibitors in a Light Crude Oil. Colloids Interfaces 2023, 7, 13. https://doi.org/10.3390/colloids7010013
M'barki O, Clements J, Salazar L, Machac J Jr., Nguyen QP. Impact of Paraffin Composition on the Interactions between Waxes, Asphaltenes, and Paraffin Inhibitors in a Light Crude Oil. Colloids and Interfaces. 2023; 7(1):13. https://doi.org/10.3390/colloids7010013
Chicago/Turabian StyleM'barki, Oualid, John Clements, Luis Salazar, James Machac, Jr., and Quoc P. Nguyen. 2023. "Impact of Paraffin Composition on the Interactions between Waxes, Asphaltenes, and Paraffin Inhibitors in a Light Crude Oil" Colloids and Interfaces 7, no. 1: 13. https://doi.org/10.3390/colloids7010013
APA StyleM'barki, O., Clements, J., Salazar, L., Machac, J., Jr., & Nguyen, Q. P. (2023). Impact of Paraffin Composition on the Interactions between Waxes, Asphaltenes, and Paraffin Inhibitors in a Light Crude Oil. Colloids and Interfaces, 7(1), 13. https://doi.org/10.3390/colloids7010013





