Polysaccharides-Based Injectable Hydrogels: Preparation, Characteristics, and Biomedical Applications
Abstract
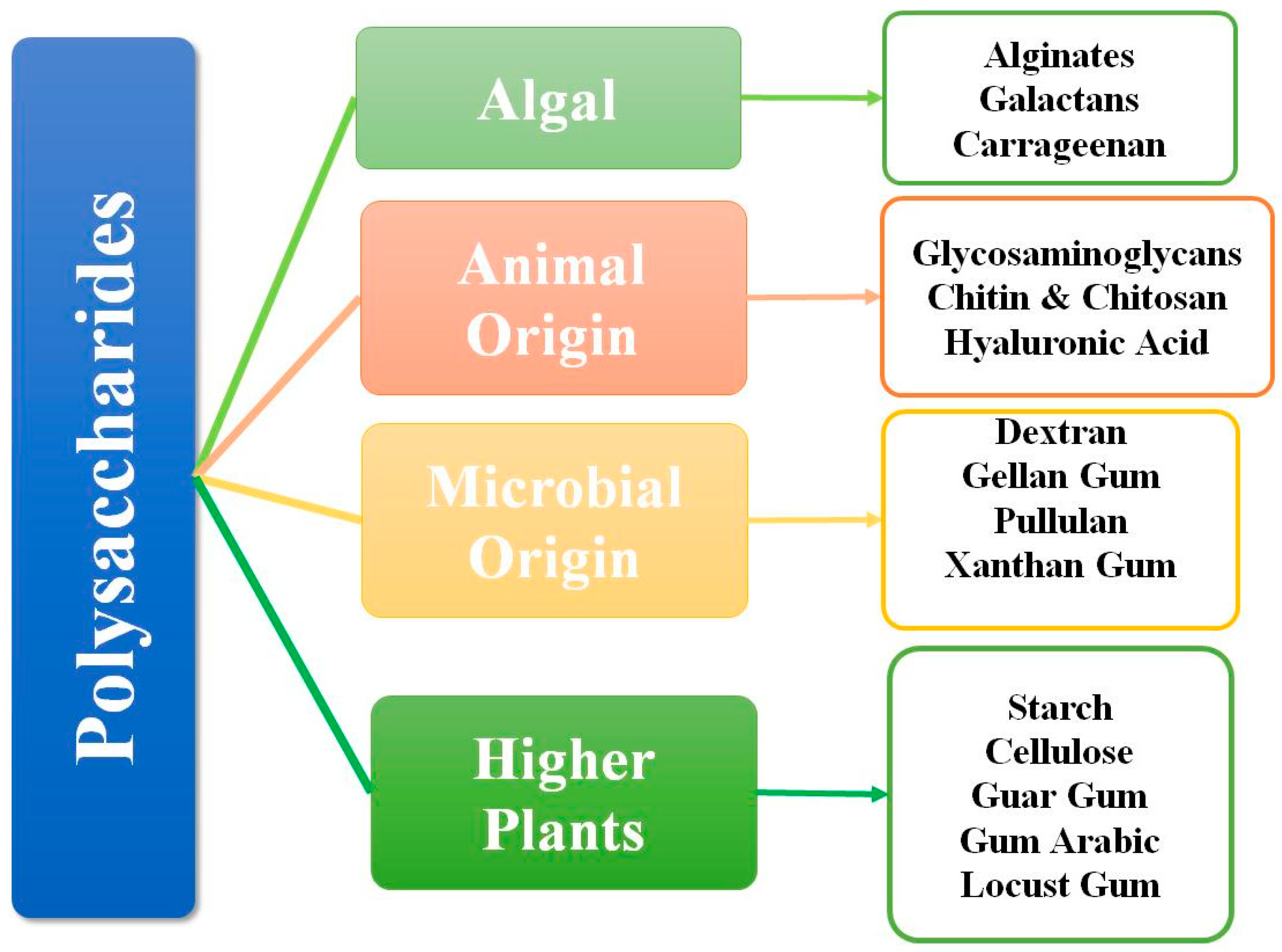
1. Introduction
2. Factors That Determine the Injectability of Hydrogels
3. Preparation Techniques and Mechanism
3.1. Physical Crosslinking
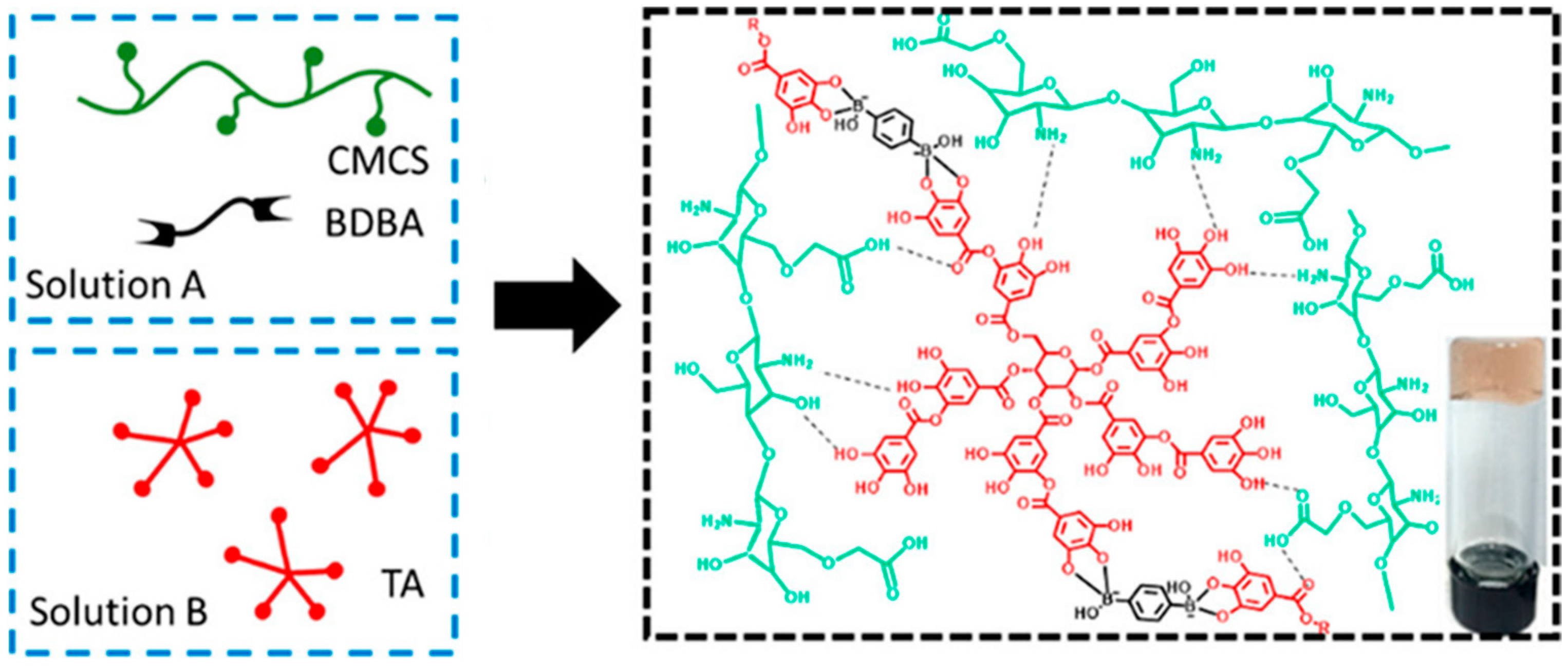
3.1.1. H-Bonding
3.1.2. Hydrophobic Interactions
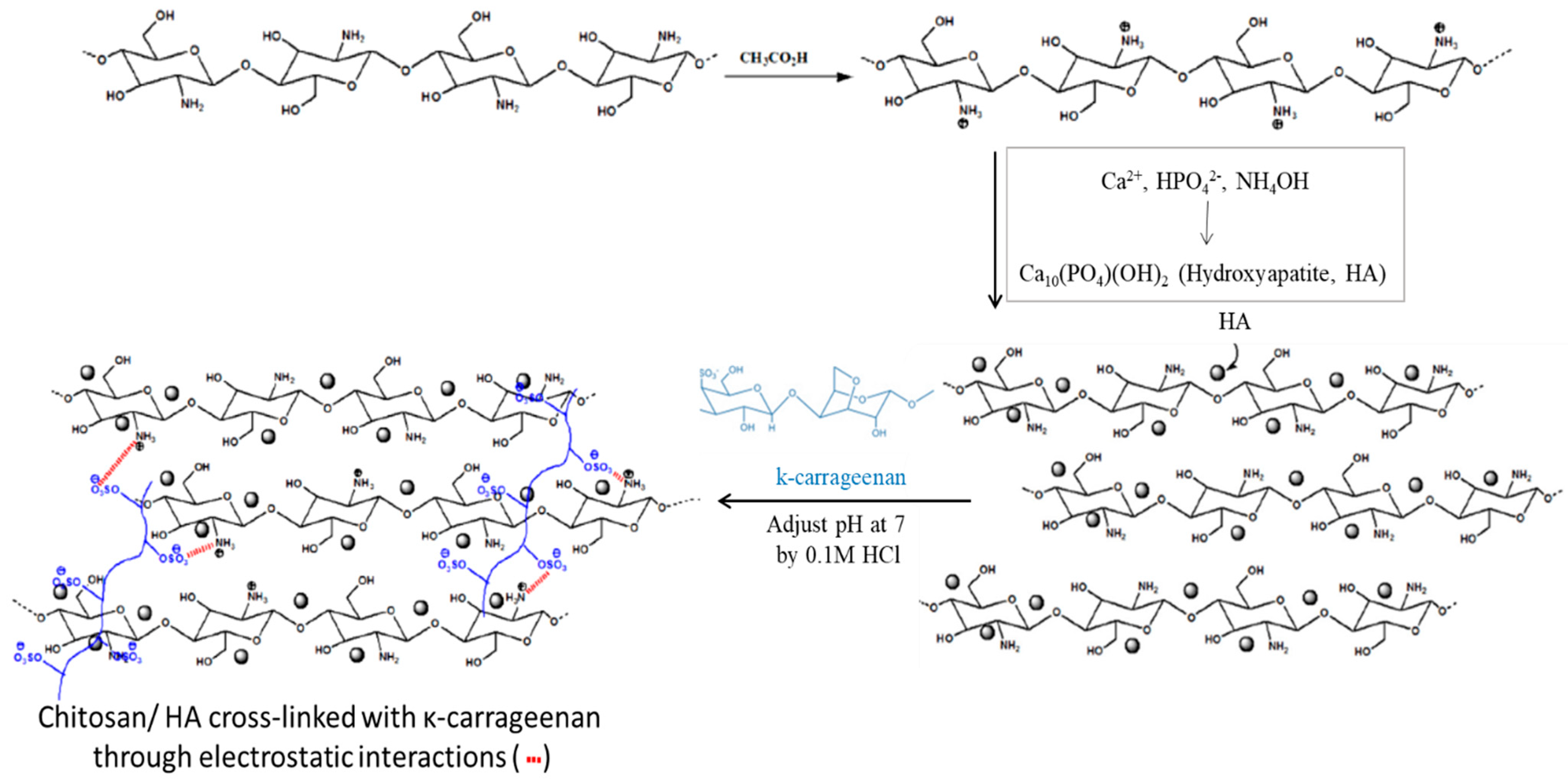
3.1.3. Ionic Interactions
3.1.4. Host–Guest Interaction (Inclusion Mechanism)
3.2. Chemical Crosslinking
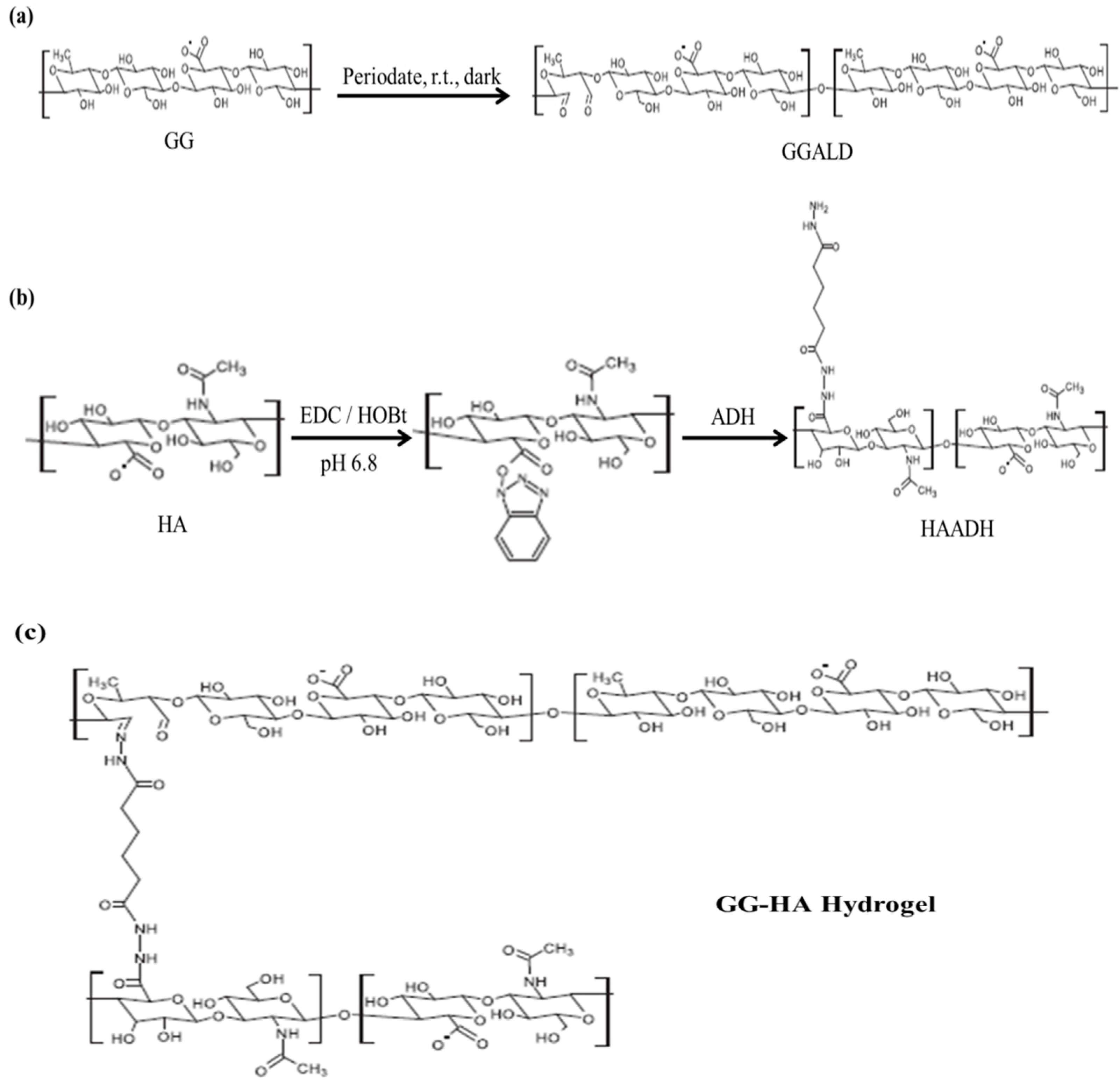
3.2.1. Schiff Base Coupling Reactions
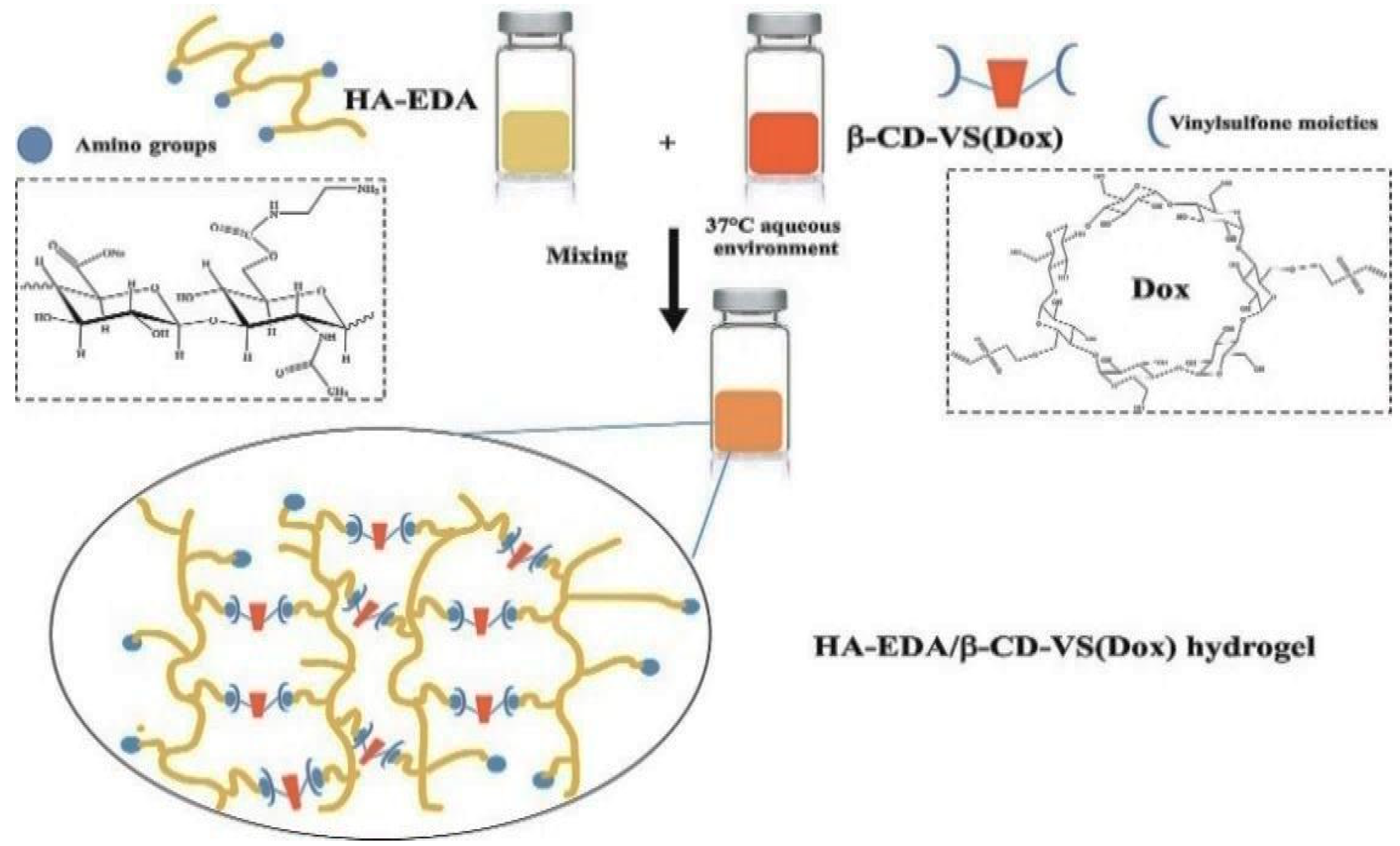
3.2.2. Michael-Type Addition Reactions
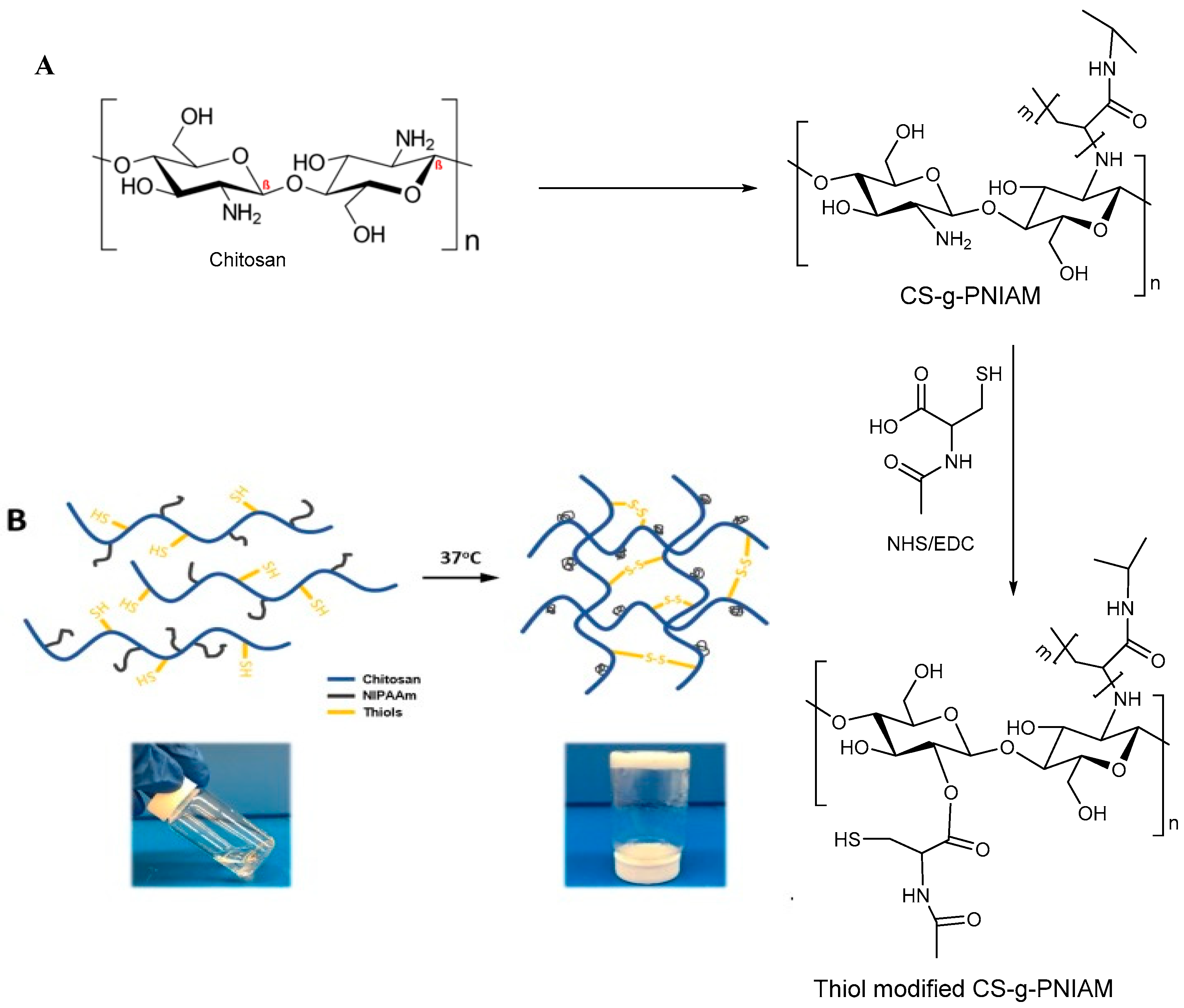
3.2.3. Disulfide Bridge
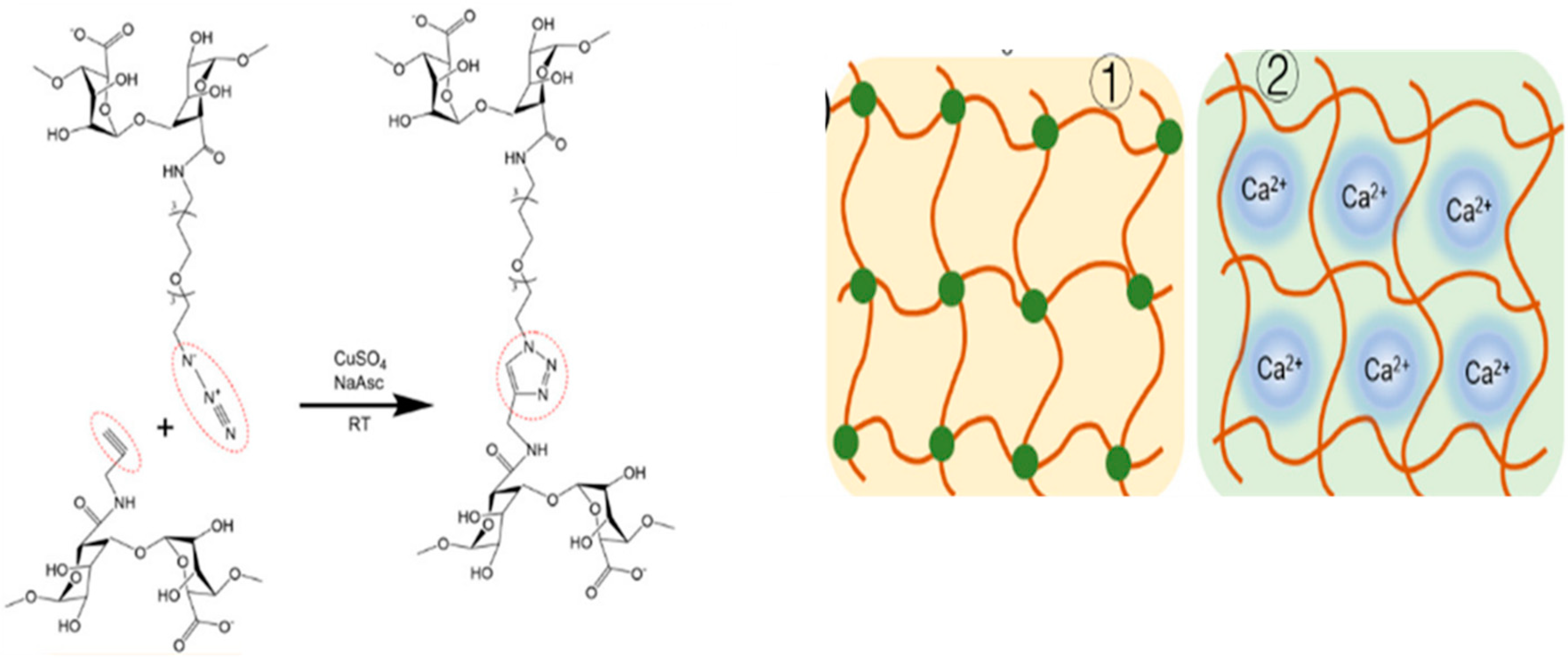
3.2.4. Crosslinking via Click Chemistry
Alkyne–Azide
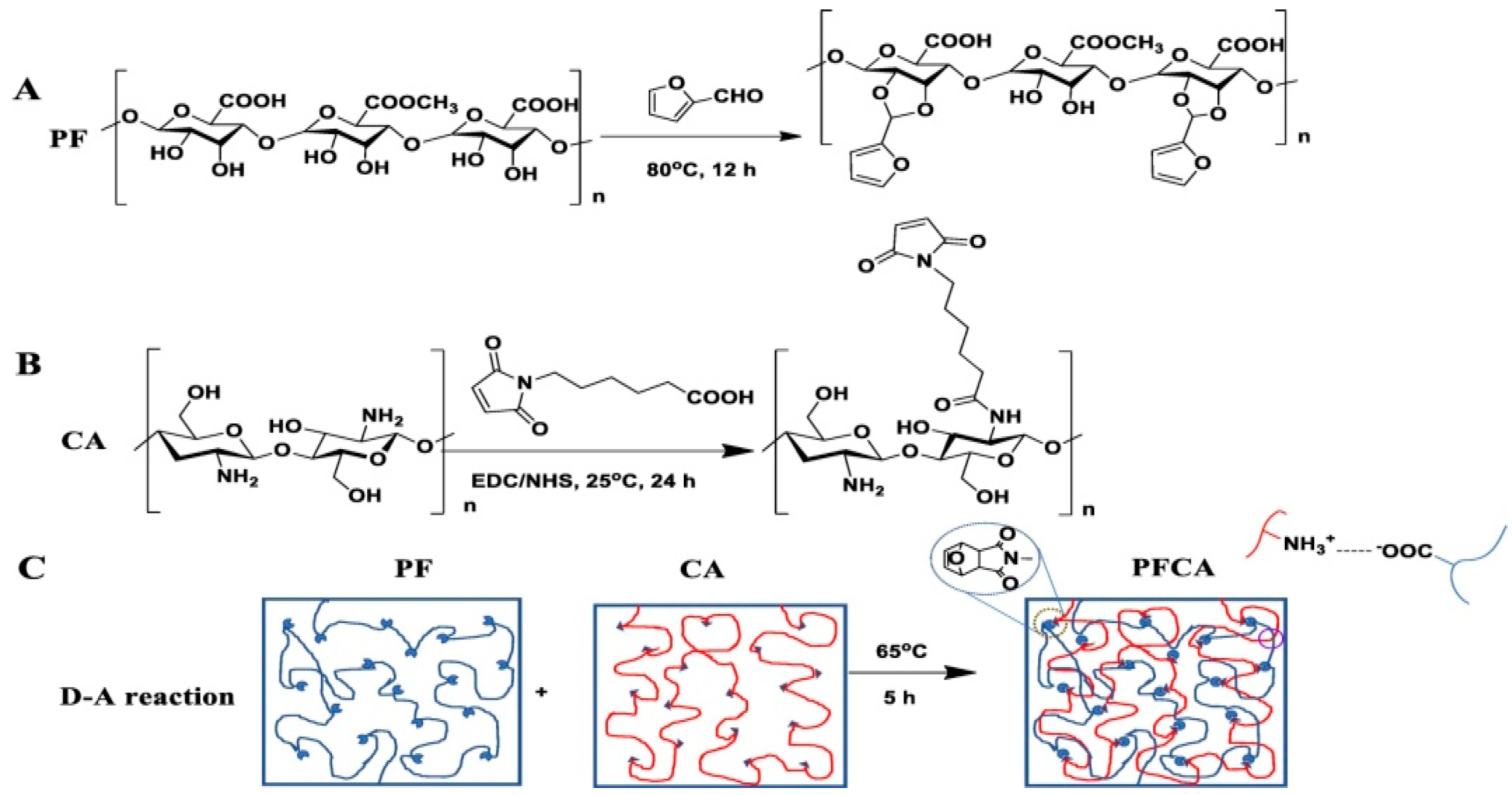
Diels–Alder (DA)
Cycloaddition
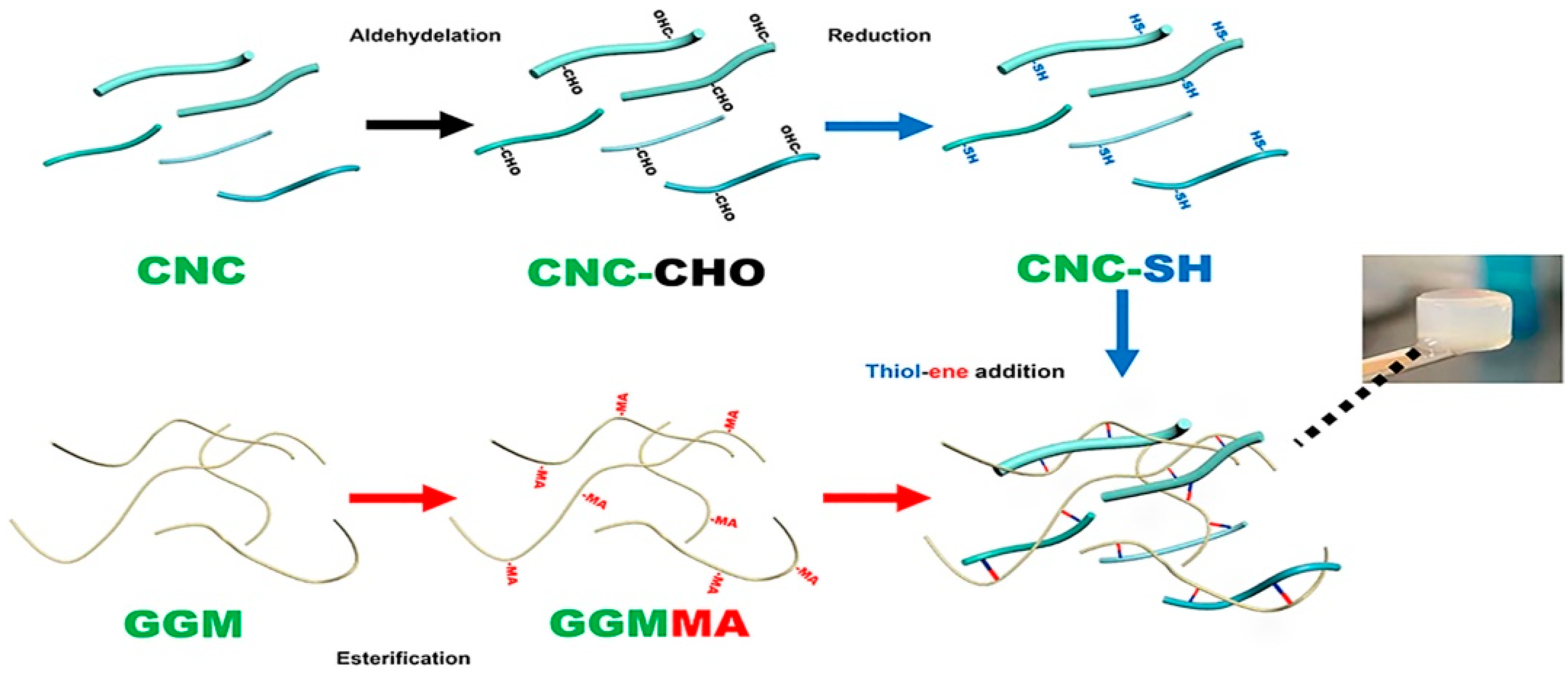
Thiolene Addition
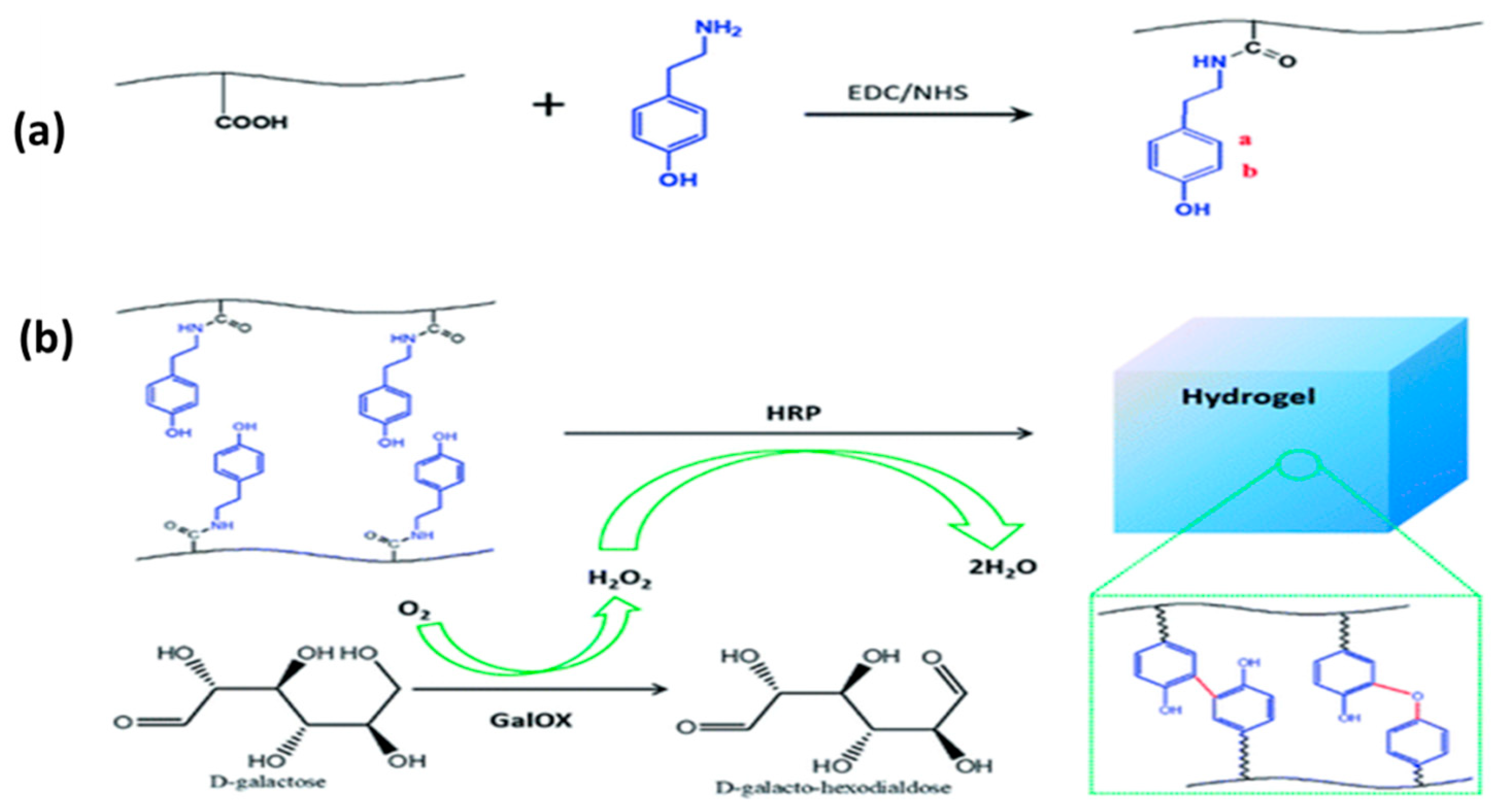
3.3. Enzyme-Mediated Reaction
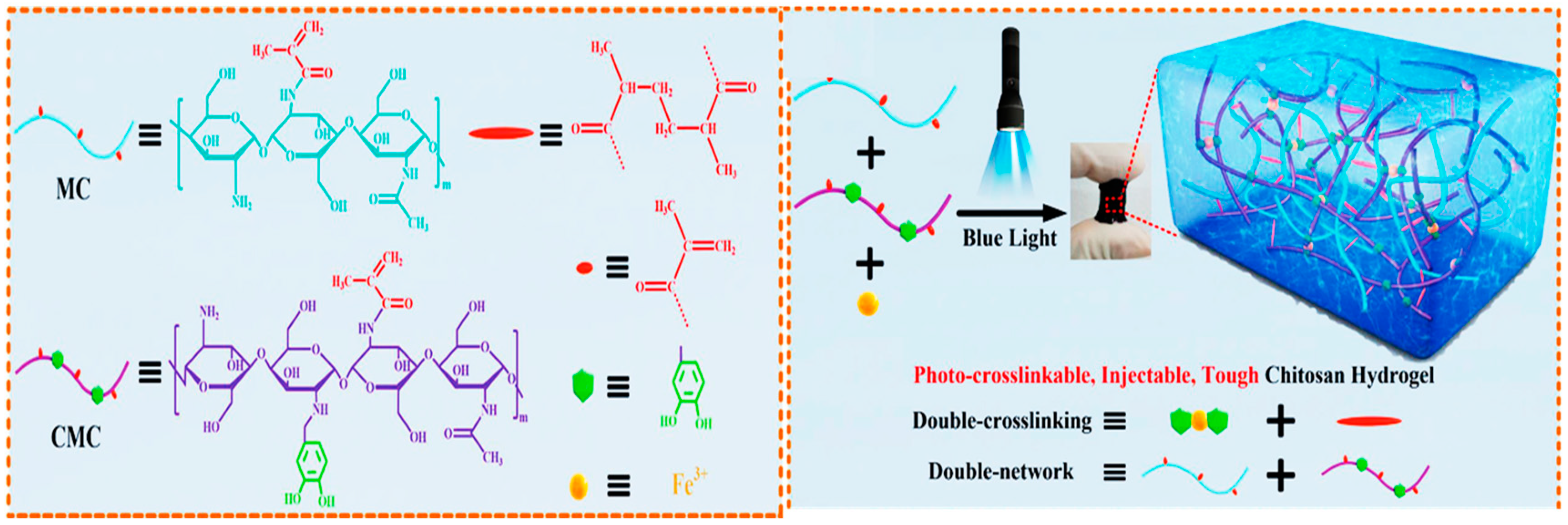
3.4. Photo Initiation
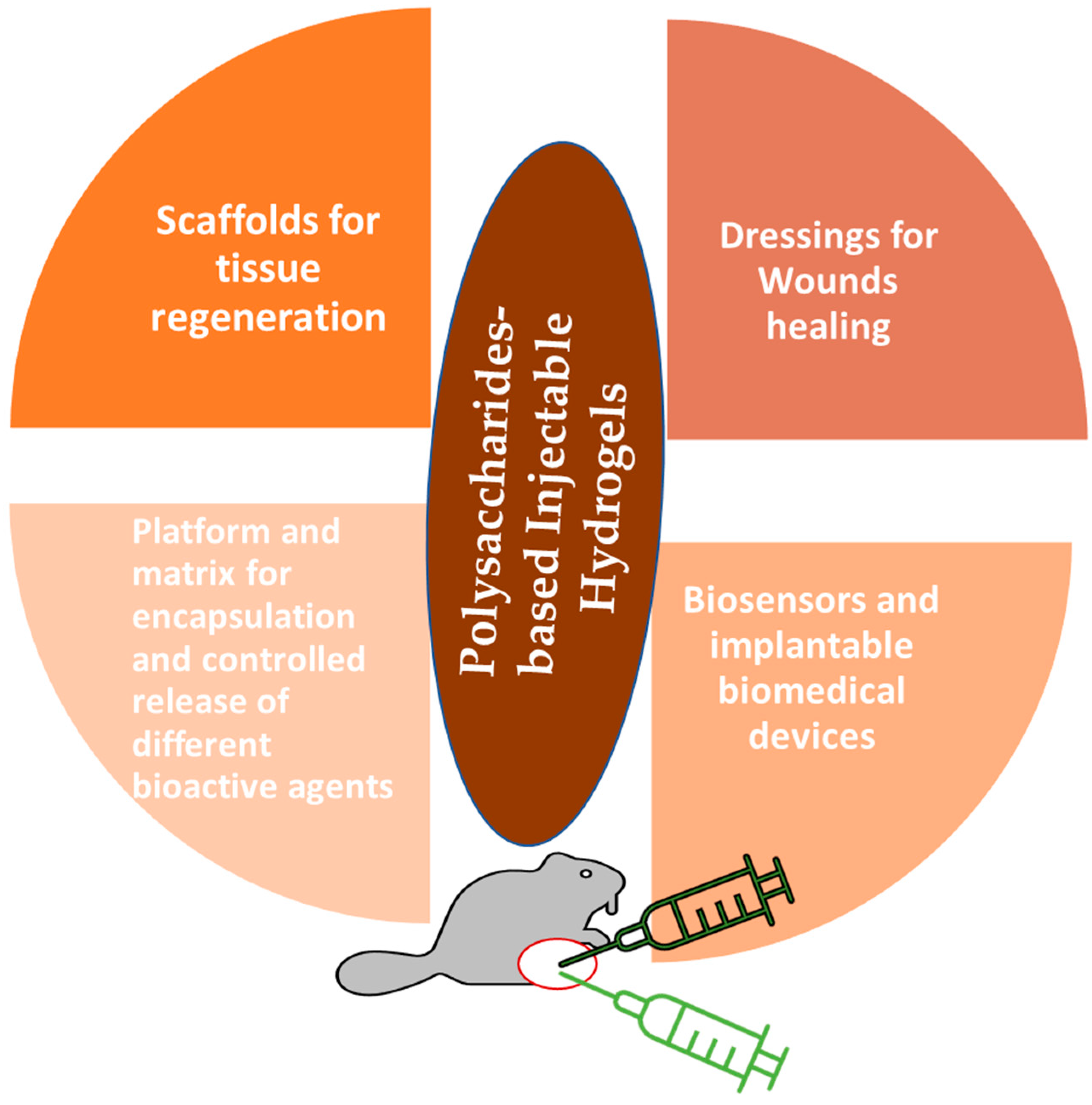
4. Biomedical Applications of Polysaccharides-Based Injectable Hydrogels (PSIHs)
4.1. Delivery of Chemotherapeutics
4.1.1. Delivery of Antibiotic
4.1.2. Protein and Growth Factors Delivery
4.1.3. Encapsulation of Cells
4.2. Regenerative Medicine
4.2.1. Tissue Regeneration
4.2.2. Wound Healing
4.3. Biosensors and Implantable Biomedical Devices
5. Status and Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; classification, chemical properties, and future perspective applications in fields of pharmacology and biological medicine (A review of current applications and upcoming potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Sharma, M.; Aliabadi, H.M.; El-Meligy, M.G.; El-Zaity, A.K.; Nageib, Z.A.; Tiwari, R.K. Synthesis, characterization, and in vitro cytotoxicity of fatty acyl-CGKRK-chitosan oligosaccharides conjugates for siRNA delivery. Int. J. Biol. Macromol. 2018, 112, 694–702. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Sajid, M.I.; Parang, K.; Tiwari, R.K. Synthesis, characterization, and cytotoxicity evaluation of dextran-myristoyl-ECGKRK peptide conjugate. Int. J. Biol. Macromol. 2021, 191, 1204–1211. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; El-Sakhawy, M.; Hesemann, P.; Brun, N.; Kamel, S. Rational design of novel water-soluble ampholytic cellulose derivatives. Int. J. Biol. Macromol. 2018, 114, 363–372. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Salama, A.; Guarino, V. Coupling of 3-Aminopropyl Sulfonic Acid to Cellulose Nanofibers for Efficient Removal of Cationic Dyes. Materials 2022, 15, 6964. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; El-Sakhawy, M.; Brun, N.; Hesemann, P.; Kamel, S. New approach for immobilization of 3-aminopropyltrimethoxysilane and TiO2 nanoparticles into cellulose for BJ1 skin cells proliferation. Carbohydr. Polym. 2018, 199, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Karthikeyan, C. Natural Polysaccharides: Structural Features and Properties. Polysaccharide Carriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–17. [Google Scholar]
- Turky, G.; Moussa, M.A.; Hasanin, M.; El-Sayed, N.S.; Kamel, S. Carboxymethyl cellulose-based hydrogel: Dielectric study, antimicrobial activity and biocompatibility. Arab. J. Sci. Eng. 2021, 46, 17–30. [Google Scholar] [CrossRef]
- EL-Sayed, N.S.; El-Ziaty, A.; El-Meligy, M.G.; Nagieb, Z.A. Syntheses of New Antimicrobial Cellulose Materials Based 2-((2-aminoethyl) amino)-4-aryl-6-indolylnicotinonitriles. Egypt. J. Chem. 2017, 60, 465–477. [Google Scholar]
- El-Sayed, N.S.; Moussa, M.A.; Kamel, S.; Turky, G. Development of electrical conducting nanocomposite based on carboxymethyl cellulose hydrogel/silver nanoparticles@ polypyrrole. Synth. Met. 2019, 250, 104–114. [Google Scholar] [CrossRef]
- Mellati, A.; Akhtari, J. Injectable hydrogels: A review of injectability mechanisms and biomedical applications. Res. Mol. Med. (RMM) 2019, 250, 104–114. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Awad, H.; El-Sayed, G.M.; Nagieb, Z.A.; Kamel, S. Synthesis and characterization of biocompatible hydrogel based on hydroxyethyl cellulose-g-poly (hydroxyethyl methacrylate). Polym. Bull. 2020, 77, 6333–6347. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; Al Kiey, S.A.; Darwish, A.; Turky, G.; Kamel, S. High performance hydrogel electrodes based on sodium alginate-g-poly (AM-c o-ECA-co-AMPS for supercapacitor application. Int. J. Biol. Macromol. 2022, 218, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Bai, X.; Lü, S.; Liu, H.; Cao, Z.; Ning, P.; Wang, Z.; Gao, C.; Ni, B.; Ma, D.; Liu, M. Polysaccharides based injectable hydrogel compositing bio-glass for cranial bone repair. Carbohydr. Polym. 2017, 175, 557–564. [Google Scholar] [CrossRef]
- Overstreet, D.J.; Dutta, D.; Stabenfeldt, S.E.; Vernon, B.L. Injectable hydrogels. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 881–903. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- You, J.; Cao, J.; Zhao, Y.; Zhang, L.; Zhou, J.; Chen, Y. Improved mechanical properties and sustained release behavior of cationic cellulose nanocrystals reinforeced cationic cellulose injectable hydrogels. Biomacromolecules 2016, 17, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Nune, K.; Misra, R. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Roshangar, L.; Rad, J.S. Development of reinforced chitosan/pectin scaffold by using the cellulose nanocrystals as nanofillers: An injectable hydrogel for tissue engineering. Eur. Polym. J. 2020, 130, 109697. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Huang, Y.; Chang, G.; Cao, K.; Yang, J.; Zhang, R.; Sheng, X.; Ye, X. pH and redox dual stimuli-responsive injectable hydrogels based on carboxymethyl cellulose derivatives. Macromol. Res. 2016, 24, 602–608. [Google Scholar] [CrossRef]
- Sultana, T.; Van Hai, H.; Abueva, C.; Kang, H.J.; Lee, S.-Y.; Lee, B.-T. TEMPO oxidized nano-cellulose containing thermo-responsive injectable hydrogel for post-surgical peritoneal tissue adhesion prevention. Mater. Sci. Eng. C 2019, 102, 12–21. [Google Scholar] [CrossRef]
- Yang, J.-A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Li, Y.; Wang, N.; Liu, W. A nucleoside responsive diaminotriazine-based hydrogen bonding strengthened hydrogel. Mater. Lett. 2015, 142, 71–74. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Shi, J.M.; Chi, Y.H. Tannic acid physically cross-linked responsive hydrogel. Macromol. Chem. Phys. 2018, 219, 1800234. [Google Scholar] [CrossRef]
- Ma, M.; Zhong, Y.; Jiang, X. Thermosensitive and pH-responsive tannin-containing hydroxypropyl chitin hydrogel with long-lasting antibacterial activity for wound healing. Carbohydr. Polym. 2020, 236, 116096. [Google Scholar] [CrossRef]
- Geng, H.; Dai, Q.; Sun, H.; Zhuang, L.; Song, A.; Caruso, F.; Hao, J.; Cui, J. Injectable and sprayable polyphenol-based hydrogels for controlling hemostasis. ACS Appl. Bio Mater. 2020, 3, 1258–1266. [Google Scholar] [CrossRef]
- You, S.; Xiang, Y.; Qi, X.; Mao, R.; Cai, E.; Lan, Y.; Lu, H.; Shen, J.; Deng, H. Harnessing a biopolymer hydrogel reinforced by copper/tannic acid nanosheets for treating bacteria-infected diabetic wounds. Mater. Today Adv. 2022, 15, 100271. [Google Scholar] [CrossRef]
- Liu, C.; Yang, L.; Qiao, L.; Liu, C.; Zhang, M.; Jian, X. An injectable and self-healing novel chitosan hydrogel with low adamantane substitution degree. Polym. Int. 2019, 68, 1102–1112. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Leganés, J.; Sánchez-Migallón, A.; Merino, S.; Vázquez, E. Stimuli-responsive graphene-based hydrogel driven by disruption of triazine hydrophobic interactions. Nanoscale 2020, 12, 7072–7081. [Google Scholar] [CrossRef]
- Rizzo, F.; Kehr, N.S. Recent advances in injectable hydrogels for controlled and local drug delivery. Adv. Healthc. Mater. 2021, 10, 2001341. [Google Scholar] [CrossRef]
- Hsiao, M.-H.; Larsson, M.; Larsson, A.; Evenbratt, H.; Chen, Y.-Y.; Chen, Y.-Y.; Liu, D.M. Design and characterization of a novel amphiphilic chitosan nanocapsule-based thermo-gelling biogel with sustained in vivo release of the hydrophilic anti-epilepsy drug ethosuximide. J. Control. Release 2012, 161, 942–948. [Google Scholar] [CrossRef]
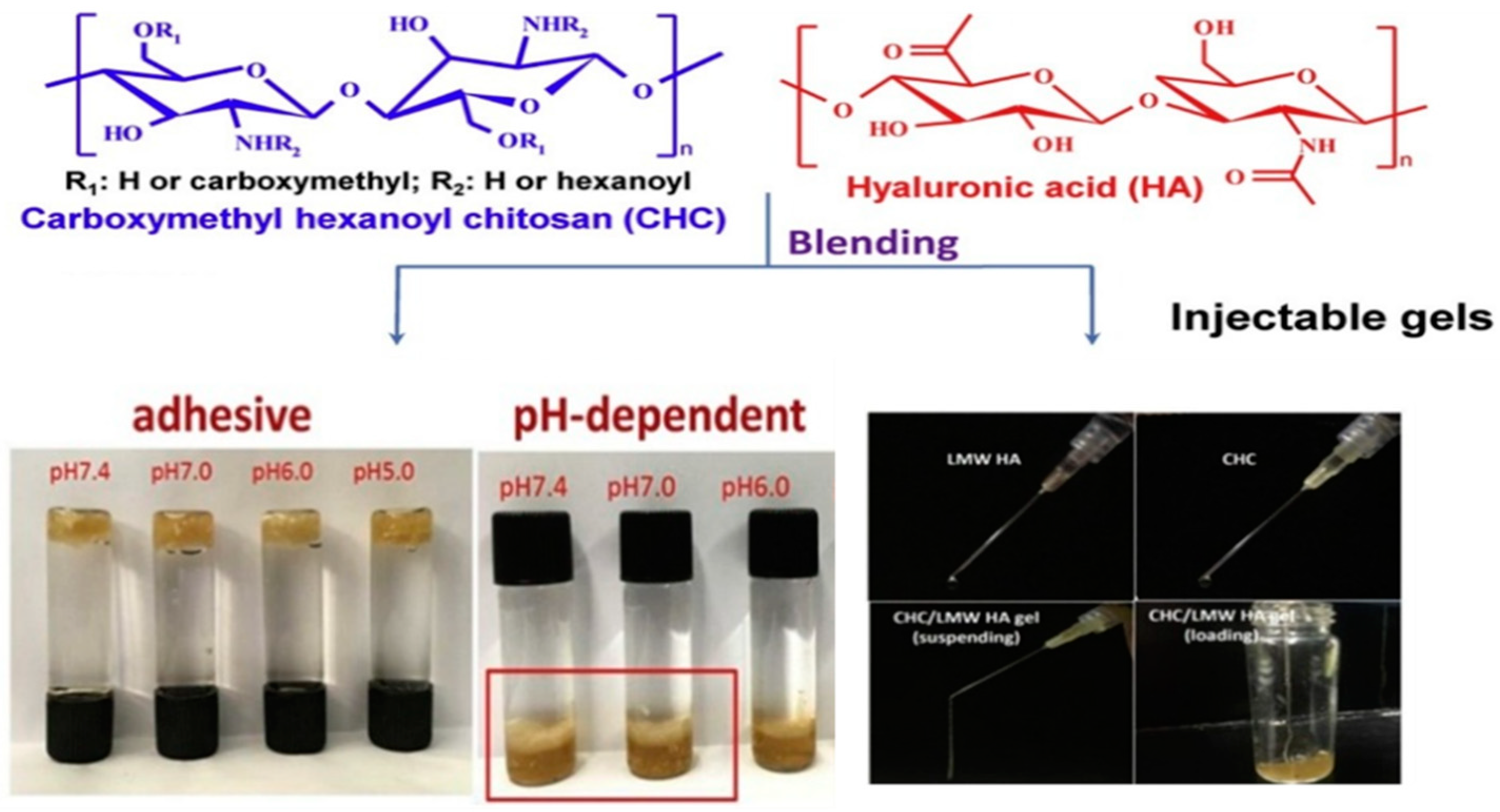
- Lu, K.-Y.; Lin, Y.-C.; Lu, H.-T.; Ho, Y.-C.; Weng, S.-C.; Tsai, M.-L.; Mi, F.-L. A novel injectable in situ forming gel based on carboxymethyl hexanoyl chitosan/hyaluronic acid polymer blending for sustained release of berberine. Carbohydr. Polym. 2019, 206, 664–673. [Google Scholar] [CrossRef]
- Geng, Z.; Ji, Y.; Yu, S.; Liu, Q.; Zhou, Z.; Guo, C.; Lu, D.; Pei, D. Preparation and characterization of a dual cross-linking injectable hydrogel based on sodium alginate and chitosan quaternary ammonium salt. Carbohydr. Res. 2021, 507, 108389. [Google Scholar] [CrossRef]
- Pettinelli, N.; Rodriguez-Llamazares, S.; Bouza, R.; Barral, L.; Feijoo-Bandin, S.; Lago, F. Carrageenan-based physically crosslinked injectable hydrogel for wound healing and tissue repairing applications. Int. J. Pharm. 2020, 589, 119828. [Google Scholar] [CrossRef]
- Chen, W.; Bu, Y.; Li, D.; Liu, C.; Chen, G.; Wan, X.; Li, N. High-strength, tough, and self-healing hydrogel based on carboxymethyl cellulose. Cellulose 2020, 27, 853–865. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Karimi, M.H.; Soltaniniya, M.; Massoumi, B. In vitro evaluation of sustained ciprofloxacin release from κ-carrageenan-crosslinked chitosan/hydroxyapatite hydrogel nanocomposites. Int. J. Biol. Macromol. 2019, 126, 443–453. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Y. Biomedical applications of supramolecular systems based on host–guest interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef]
- Dong, S.; Zheng, B.; Wang, F.; Huang, F. Supramolecular polymers constructed from macrocycle-based host–guest molecular recognition motifs. Acc. Chem. Res. 2014, 47, 1982–1994. [Google Scholar] [CrossRef]
- Loh, X.J. Supramolecular host–guest polymeric materials for biomedical applications. Mater. Horiz. 2014, 1, 185–195. [Google Scholar] [CrossRef]
- Almawash, S.; El Hamd, M.A.; Osman, S.K. Polymerized β-Cyclodextrin-Based Injectable Hydrogel for Sustained Release of 5-Fluorouracil/Methotrexate Mixture in Breast Cancer Management: In Vitro and In Vivo Analytical Validations. Pharmaceutics 2022, 14, 817. [Google Scholar] [CrossRef]
- Hu, Q.-D.; Tang, G.-P.; Chu, P.K. Cyclodextrin-based host–guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef]
- Okubo, M.; Iohara, D.; Anraku, M.; Higashi, T.; Uekama, K.; Hirayama, F. A thermoresponsive hydrophobically modified hydroxypropylmethylcellulose/cyclodextrin injectable hydrogel for the sustained release of drugs. Int. J. Pharm. 2020, 575, 118845. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeon, S.I.; Sim, S.B.; Byun, Y.; Ahn, C.-H. A supramolecular host-guest interaction-mediated injectable hydrogel system with enhanced stability and sustained protein release. Acta Biomater. 2021, 131, 286–301. [Google Scholar] [CrossRef]
- Lim, H.L.; Hwang, Y.; Kar, M.; Varghese, S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014, 2, 603–618. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Hashem, A.H.; Kamel, S. Preparation and characterization of Gum Arabic Schiff’s bases based on 9-aminoacridine with in vitro evaluation of their antimicrobial and antitumor potentiality. Carbohydr. Polym. 2022, 277, 118823. [Google Scholar] [CrossRef]
- Mi, F.-L.; Kuan, C.-Y.; Shyu, S.-S.; Lee, S.-T.; Chang, S.-F. The study of gelation kinetics and chain-relaxation properties of glutaraldehyde-cross-linked chitosan gel and their effects on microspheres preparation and drug release. Carbohydr. Polym. 2000, 41, 389–396. [Google Scholar] [CrossRef]
- Gupta, K.C.; Jabrail, F.H. Glutaraldehyde and glyoxal cross-linked chitosan microspheres for controlled delivery of centchroman. Carbohydr. Res. 2006, 341, 744–756. [Google Scholar] [CrossRef]
- Feng, X.; Li, D.; Han, J.; Zhuang, X.; Ding, J. Schiff base bond-linked polysaccharide–doxorubicin conjugate for upregulated cancer therapy. Mater. Sci. Eng. C 2017, 76, 1121–1128. [Google Scholar] [CrossRef]
- Su, H.; Zhang, W.; Wu, Y.; Han, X.; Liu, G.; Jia, Q.; Shan, S. Schiff base-containing dextran nanogel as pH-sensitive drug delivery system of doxorubicin: Synthesis and characterization. J. Biomater. Appl. 2018, 33, 170–181. [Google Scholar] [CrossRef]
- Millan, C.; Cavalli, E.; Groth, T.; Maniura-Weber, K.; Zenobi-Wong, M. Engineered microtissues formed by schiff base crosslinking restore the chondrogenic potential of aged mesenchymal stem cells. Adv. Healthc. Mater. 2015, 4, 1348–1358. [Google Scholar] [CrossRef]
- Thomas, J.; Sharma, A.; Panwar, V.; Chopra, V.; Ghosh, D. Polysaccharide-based hybrid self-healing hydrogel supports the paracrine response of mesenchymal stem cells. ACS Appl. Bio Mater. 2019, 2, 2013–2027. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.-X.; Mo, X.-M.; Pan, J.-F. In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S.-H. Hydrogels based on Schiff base linkages for biomedical applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef]
- Ou, Y.; Tian, M. Advances in multifunctional chitosan-based self-healing hydrogels for biomedical application. J. Mater. Chem. B 2021, 9, 7955–7971. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-healing hyaluronic acid hydrogels based on dynamic Schiff base linkages as biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Wu, S.; Fei, S.; Li, B.; Yang, Y.; Yuan, H. Injectable nanofiber-polysaccharide self-healing hydrogels for wound healing. Mater. Sci. Eng. C 2021, 128, 112264. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.; Xu, R.; Wei, S.; Xiong, F.; Cui, W.; Li, B.; Xue, Y.; Xuan, H.; Yuan, H. A spray-filming, tissue-adhesive, and bioactive polysaccharide self-healing hydrogel for skin regeneration. Mater. Des. 2022, 217, 110669. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; Shirazi, A.N.; El-Meligy, M.G.; El-Ziaty, A.K.; Nagieb, Z.A.; Parang, K.; Tiwari, R.K. Design, synthesis, and evaluation of chitosan conjugated GGRGDSK peptides as a cancer cell-targeting molecular transporter. Int. J. Biol. Macromol. 2016, 87, 611–622. [Google Scholar] [CrossRef]
- Hu, H.; Li, Y.; Zhou, Q.; Ao, Y.; Yu, C.; Wan, Y.; Xu, H.; Li, Z.; Yang, X. Redox-sensitive hydroxyethyl starch–doxorubicin conjugate for tumor targeted drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 30833–30844. [Google Scholar] [CrossRef]
- Karvinen, J.; Koivisto, J.T.; Jönkkäri, I.; Kellomäki, M. The production of injectable hydrazone crosslinked gellan gum-hyaluronan-hydrogels with tunable mechanical and physical properties. J. Mech. Behav. Biomed. Mater. 2017, 71, 383–391. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Puleio, R.; Condorelli, L.; Collura, G.; Giammona, G. A hyaluronic acid/cyclodextrin based injectable hydrogel for local doxorubicin delivery to solid tumors. Int. J. Pharm. 2020, 589, 119879. [Google Scholar] [CrossRef]
- Heath, L.; Thielemans, W. Cellulose nanowhisker aerogels. Green Chem. 2010, 12, 1448–1453. [Google Scholar] [CrossRef]
- Li, F.; Ba, Q.; Niu, S.; Guo, Y.; Duan, Y.; Zhao, P.; Lin, C.; Sun, J. In-situ forming biodegradable glycol chitosan-based hydrogels: Synthesis, characterization, and chondrocyte culture. Mater. Sci. Eng. C 2012, 32, 2017–2025. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Huang, Q.; He, W.; Feng, Q.; Yu, B. A novel thermo-sensitive hydrogel based on thiolated chitosan/hydroxyapatite/beta-glycerophosphate. Carbohydr. Polym. 2014, 110, 62–69. [Google Scholar] [CrossRef] [PubMed]
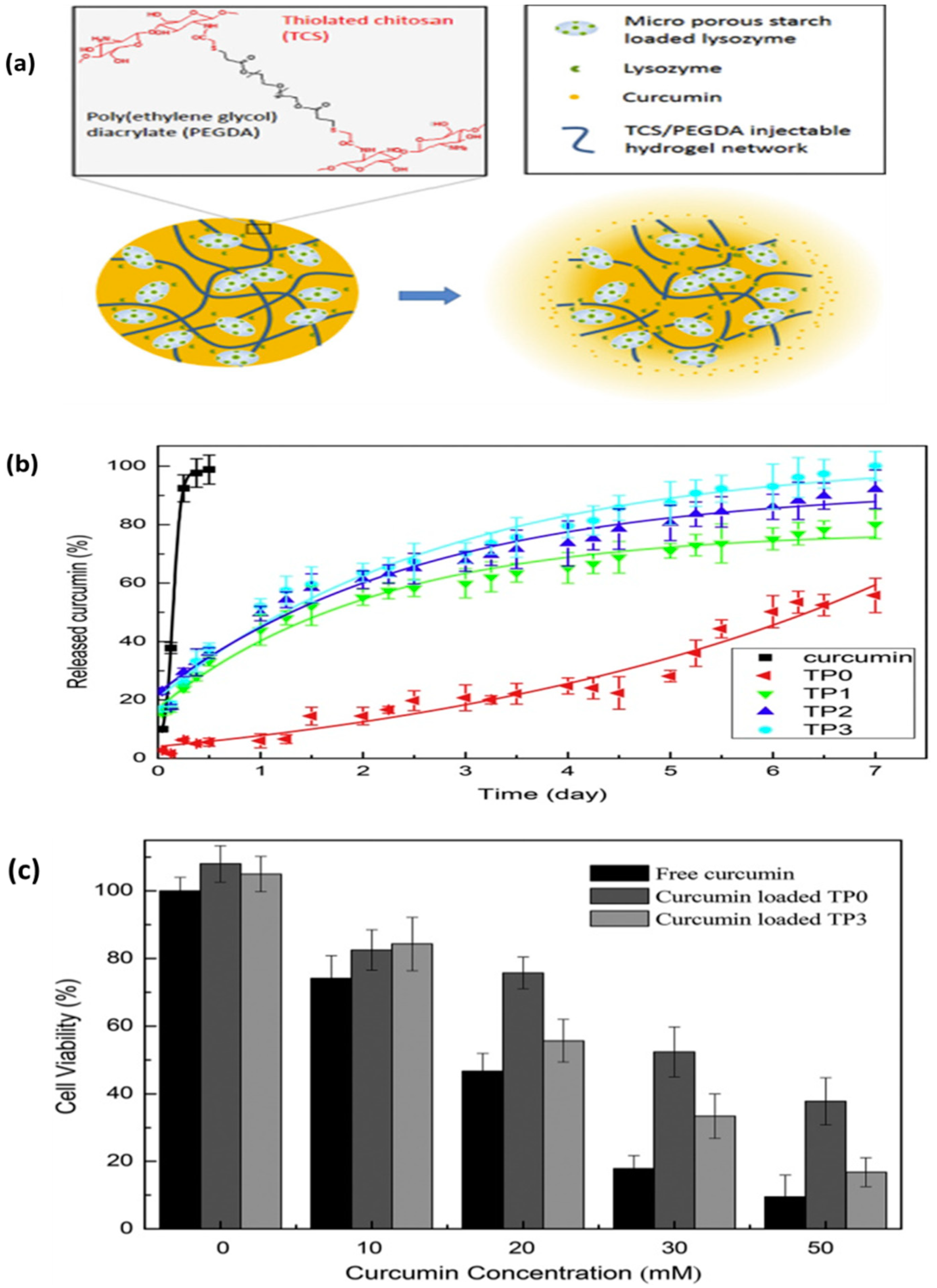
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated polymeric hydrogels for biomedical application: Cross-linking mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef]
- Wu, S.-W.; Liu, X.; Miller, A.L., II; Cheng, Y.-S.; Yeh, M.-L.; Lu, L. Strengthening injectable thermo-sensitive NIPAAm-g-chitosan hydrogels using chemical cross-linking of disulfide bonds as scaffolds for tissue engineering. Carbohydr. Polym. 2018, 192, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, X.; Yang, W.; He, K.; Ye, X. Injectable in situ cross-linking hyaluronic acid/carboxymethyl cellulose based hydrogels for drug release. J. Biomater. Sci. Polym. Ed. 2018, 29, 1643–1655. [Google Scholar] [CrossRef]
- Lallana, E.; Fernandez-Trillo, F.; Sousa-Herves, A.; Riguera, R.; Fernandez-Megia, E. Click chemistry with polymers, dendrimers, and hydrogels for drug delivery. Pharm. Res. 2012, 29, 902–921. [Google Scholar] [CrossRef]
- Anseth, K.S.; Klok, H.-A. Click chemistry in biomaterials, nanomedicine, and drug delivery. Biomacromolecules 2016, 17, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kusamori, K.; Nishikawa, M. Click chemistry as a tool for cell engineering and drug delivery. Molecules 2019, 24, 172. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Lee, D.; Lim, D.K.; Koo, H.; Kim, K. Copper-Free Click Chemistry: Applications in Drug Delivery, Cell Tracking, and Tissue Engineering. Adv. Mater. 2022, 34, 2107192. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.-D.; Wu, D.-Q.; Zhang, X.-Z.; Zhuo, R.-X. Synthesis of thermosensitive P (NIPAAm-co-HEMA)/cellulose hydrogels via “click” chemistry. Carbohydr. Polym. 2009, 77, 583–589. [Google Scholar] [CrossRef]
- Li, D.-Q.; Wang, S.-Y.; Meng, Y.-J.; Guo, Z.-W.; Cheng, M.-M.; Li, J. Fabrication of self-healing pectin/chitosan hybrid hydrogel via Diels-Alder reactions for drug delivery with high swelling property, pH-responsiveness, and cytocompatibility. Carbohydr. Polym. 2021, 268, 118244. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, J.Y.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. An injectable click-crosslinked hyaluronic acid hydrogel modified with a BMP-2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 2020, 117, 108–120. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, W.; Koppolu, R.; van Bochove, B.; Seppälä, J.; Hupa, L.; Willför, S.; Xu, C.; Wang, X. Injectable thiol-ene hydrogel of galactoglucomannan and cellulose nanocrystals in delivery of therapeutic inorganic ions with embedded bioactive glass nanoparticles. Carbohydr. Polym. 2022, 276, 118780. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.; Lee, S.W.; Ji, H.Y.; Lee, J.H.; Lee, D.S.; Park, T.G. Enzyme-mediated cross-linking of Pluronic copolymer micelles for injectable and in situ forming hydrogels. Acta Biomater. 2011, 7, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.S.M.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Guo, S.; Zhao, C.; Wang, L.; Ma, S.; Guan, F.; Yao, M. Dual-enzymatically cross-linked gelatin hydrogel promotes neural differentiation and neurotrophin secretion of bone marrow-derived mesenchymal stem cells for treatment of moderate traumatic brain injury. Int. J. Biol. Macromol. 2021, 187, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Saghati, S.; Khoshfetrat, A.B.; Tayefi Nasrabadi, H.; Roshangar, L.; Rahbarghazi, R. Fabrication of alginate-based hydrogel cross-linked via horseradish peroxidase for articular cartilage engineering. BMC Res. Notes 2021, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, J.; Yuan, Z.; Wang, Y.; Xi, Z.; Li, L.; Liu, Z.; Guo, X. A mussel-inspired carboxymethyl cellulose hydrogel with enhanced adhesiveness through enzymatic crosslinking. Colloids Surf. B Biointerfaces 2019, 179, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Zhang, T.; Zan, Y.; Ni, T.; Cao, Y.; Wang, J.; Liu, M.; Pei, R. Injectable hydrogels from enzyme-catalyzed crosslinking as BMSCs-laden scaffold for bone repair and regeneration. Mater. Sci. Eng. C 2019, 96, 841–849. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, D.; Ma, S.; Zhang, J.; Gao, F.; Guan, F.; Yao, M. Dual-enzymatically crosslinked and injectable hyaluronic acid hydrogels for potential application in tissue engineering. RSC Adv. 2020, 10, 2870–2876. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Ghorbani, M.; Roshangar, L.; Rad, J.S. Chemical gelling of hydrogels-based biological macromolecules for tissue engineering: Photo-and enzymatic-crosslinking methods. Int. J. Biol. Macromol. 2019, 139, 760–772. [Google Scholar] [CrossRef]
- Chu, W.; Nie, M.; Ke, X.; Luo, J.; Li, J. Recent advances in injectable dual crosslinking hydrogels for biomedical applications. Macromol. Biosci. 2021, 21, 2100109. [Google Scholar] [CrossRef]
- Lim, K.S.; Klotz, B.J.; Lindberg, G.C.; Melchels, F.P.; Hooper, G.J.; Malda, J.; Gawlitta, D.; Woodfield, T.B. Visible light cross-linking of gelatin hydrogels offers an enhanced cell microenvironment with improved light penetration depth. Macromol. Biosci. 2019, 19, 1900098. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, B.; Lei, M.; Lu, Z.; Liu, J.; Guo, B.; Yu, Y. Visible-Light-Mediated Nano-biomineralization of Customizable Tough Hydrogels for Biomimetic Tissue Engineering. ACS Nano 2022, 16, 4734–4745. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Liu, Y.; Zheng, P.; Gao, T.; Cao, Y.; Liu, X.; Yin, J.; Pei, R. In Situ Forming Cellulose Nanofibril-Reinforced Hyaluronic Acid Hydrogel for Cartilage Regeneration. Biomacromolecules 2021, 22, 5097–5107. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yang, K.; Fu, Y.V.; Xu, T.; Li, S.; Zhang, D.; Wang, L.N.; Lee, C.S. A novel double-crosslinking-double-network design for injectable hydrogels with enhanced tissue adhesion and antibacterial capability for wound treatment. Adv. Funct. Mater. 2020, 30, 1904156. [Google Scholar] [CrossRef]
- Jain, K.K. Current status and future prospects of drug delivery systems. Drug Deliv. Syst. 2014, 1141, 1–56. [Google Scholar]
- Jain, K.K. An overview of drug delivery systems. Drug Deliv. Syst. 2020, 2059, 1–54. [Google Scholar]
- Lim, S.; Park, J.; Shim, M.K.; Um, W.; Yoon, H.Y.; Ryu, J.H.; Lim, D.K.; Kim, K. Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 2019, 9, 7906. [Google Scholar] [CrossRef]
- Park, S.E.; El-Sayed, N.S.; Shamloo, K.; Lohan, S.; Kumar, S.; Sajid, M.I.; Tiwari, R.K. Targeted Delivery of Cabazitaxel Using Cyclic Cell-Penetrating Peptide and Biomarkers of Extracellular Matrix for Prostate and Breast Cancer Therapy. Bioconjugate Chem. 2021, 32, 1898–1914. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.I.; Mandal, D.; El-Sayed, N.S.; Lohan, S.; Moreno, J.; Tiwari, R.K. Oleyl Conjugated Histidine-Arginine Cell-Penetrating Peptides as Promising Agents for siRNA Delivery. Pharmaceutics 2022, 14, 881. [Google Scholar] [CrossRef] [PubMed]
- Nasrolahi Shirazi, A.; Salem El-Sayed, N.; Kumar Tiwari, R.; Tavakoli, K.; Parang, K. Cyclic peptide containing hydrophobic and positively charged residues as a drug delivery system for curcumin. Curr. Drug Deliv. 2016, 13, 409–417. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Shirazi, A.N.; Sajid, M.I.; Park, S.E.; Parang, K.; Tiwari, R.K. Synthesis and antiproliferative activities of conjugates of paclitaxel and camptothecin with a cyclic cell-penetrating peptide. Molecules 2019, 24, 1427. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Spizzirri, U.G.; Curcio, M.; Nicoletta, F.P.; Iemma, F. Injectable hydrogels for cancer therapy over the last decade. Pharmaceutics 2019, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of multiresponsive and dynamic chitosan-based hydrogels for controlled release of bioactive molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef]
- Yan, X.; Wang, F.; Zheng, B.; Huang, F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 2012, 41, 6042–6065. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Choi, B.; Kim, S.; Lin, B.; Li, K.; Bezouglaia, O.; Kim, J.; Evseenko, D.; Aghaloo, T.; Lee, M. Visible-light-initiated hydrogels preserving cartilage extracellular signaling for inducing chondrogenesis of mesenchymal stem cells. Acta Biomater. 2015, 12, 30–41. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Z.; Li, F.; Yang, Z.M.; Chen, Y.M.; Zrinyi, M.; Osada, Y. Synthesis of a morphology controllable Fe3O4 nanoparticle/hydrogel magnetic nanocomposite inspired by magnetotactic bacteria and its application in H2O2 detection. Green Chem. 2014, 16, 1255–1261. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohydr. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Minhas, M.U.; Ahmad, M.; Sohail, M. Self-assembled supramolecular thermoreversible β-cyclodextrin/ethylene glycol injectable hydrogels with difunctional Pluronic® 127 as controlled delivery depot of curcumin. Development, characterization and in vitro evaluation. J. Biomater. Sci. Polym. Ed. 2018, 29, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liang, Y.; Zhang, J.; Bai, L.; Xu, M.; Han, Q.; Han, X.; Xiu, J.; Li, M.; Zhou, X. Synergistic enhancement of tendon-to-bone healing via anti-inflammatory and pro-differentiation effects caused by sustained release of Mg2+/curcumin from injectable self-healing hydrogels. Theranostics 2021, 11, 5911. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Li, Z.; Li, X.; Qiu, S.; Lei, J.; Li, D.; Mu, C.; Ge, L. Functionalization of an Injectable Self-Healing pH-Responsive Hydrogel by Incorporating a Curcumin/Polymerized β-Cyclodextrin Inclusion Complex for Selective Toxicity to Osteosarcoma. ACS Appl. Polym. Mater. 2022, 4, 1243–1254. [Google Scholar] [CrossRef]
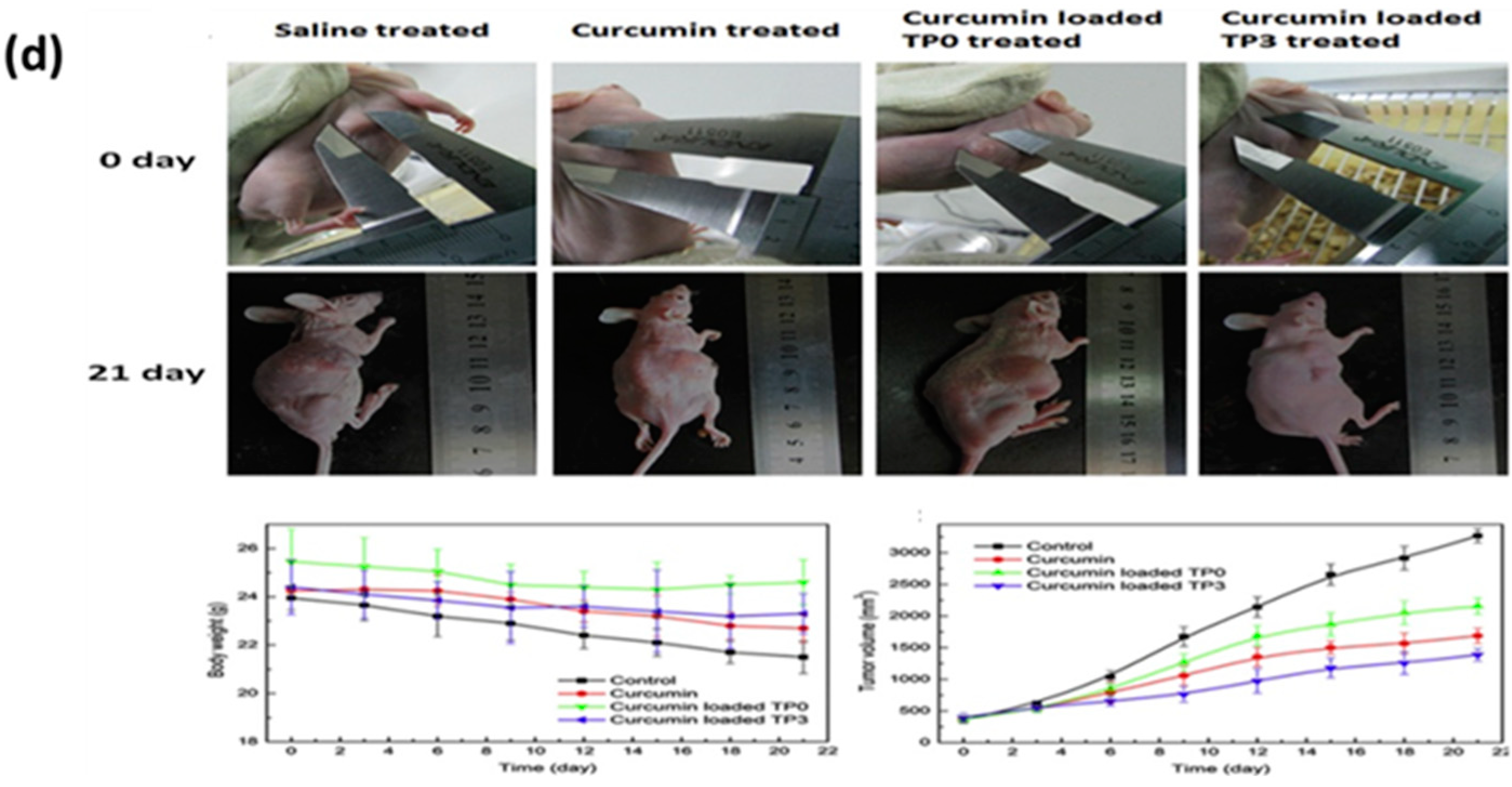
- Ning, P.; Lü, S.; Bai, X.; Wu, X.; Gao, C.; Wen, N.; Liu, M. High encapsulation and localized delivery of curcumin from an injectable hydrogel. Mater. Sci. Eng. C 2018, 83, 121–129. [Google Scholar] [CrossRef]
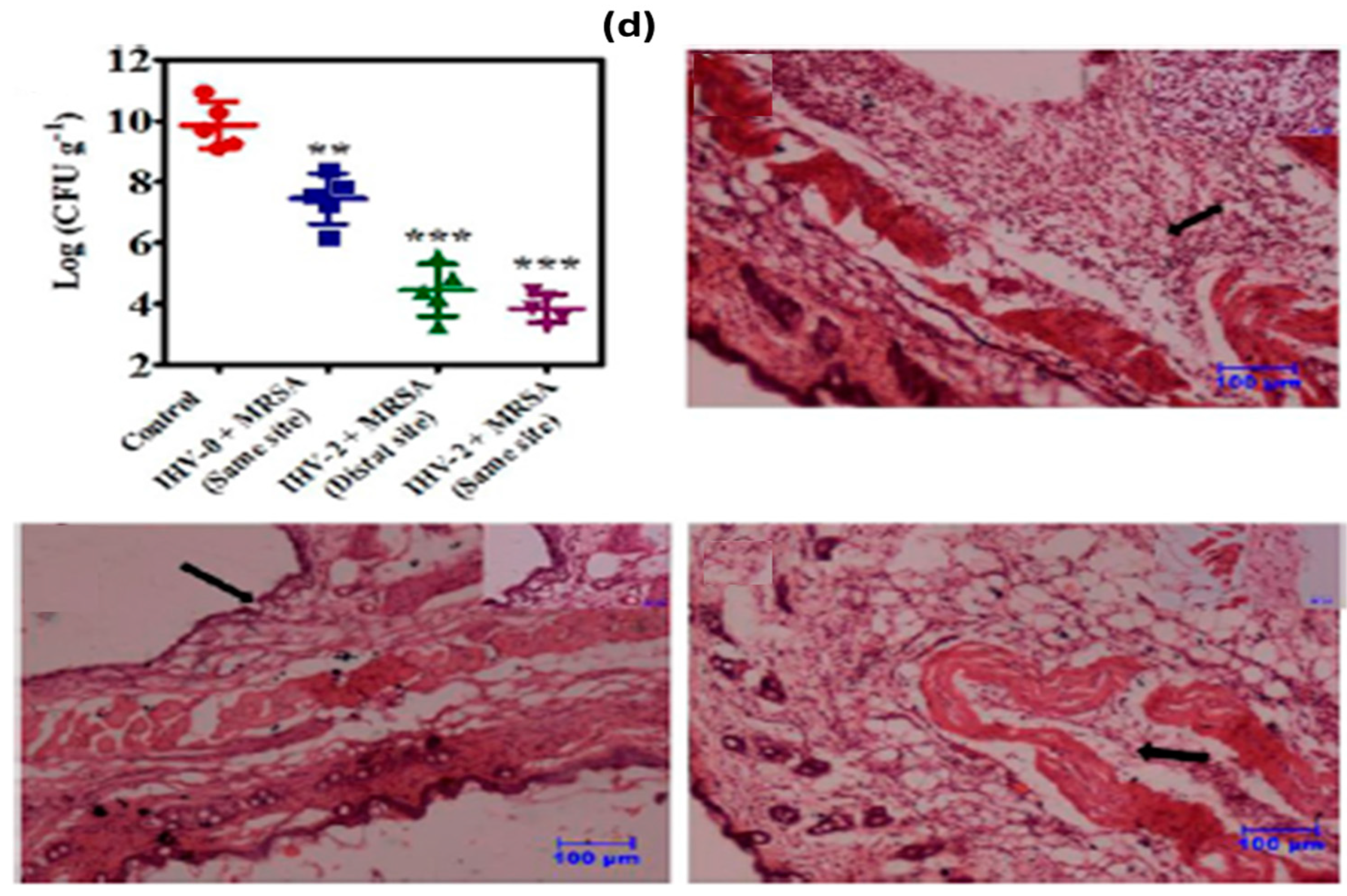
- Hoque, J.; Bhattacharjee, B.; Prakash, R.G.; Paramanandham, K.; Haldar, J. Dual function injectable hydrogel for controlled release of antibiotic and local antibacterial therapy. Biomacromolecules 2018, 19, 267–278. [Google Scholar] [CrossRef]
- Vulic, K.; Shoichet, M.S. Tunable growth factor delivery from injectable hydrogels for tissue engineering. J. Am. Chem. Soc. 2012, 134, 882–885. [Google Scholar] [CrossRef]
- Li, H.; Koenig, A.M.; Sloan, P.; Leipzig, N.D. In vivo assessment of guided neural stem cell differentiation in growth factor immobilized chitosan-based hydrogel scaffolds. Biomaterials 2014, 35, 9049–9057. [Google Scholar] [CrossRef]
- Schneider, M.C.; Chu, S.; Randolph, M.A.; Bryant, S.J. An in vitro and in vivo comparison of cartilage growth in chondrocyte-laden matrix metalloproteinase-sensitive poly (ethylene glycol) hydrogels with localized transforming growth factor β3. Acta Biomater. 2019, 93, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, T.; Chen, W.; Qin, H.; Chi, B.; Ye, Z. Injectable hydrogels based on the hyaluronic acid and poly (γ-glutamic acid) for controlled protein delivery. Carbohydr. Polym. 2018, 179, 100–109. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, G.; Chen, M.; Wang, P.; Li, Z.; Han, X.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. Redox and pH dual-responsive injectable hyaluronan hydrogels with shape-recovery and self-healing properties for protein and cell delivery. Carbohydr. Polym. 2020, 250, 116979. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.; Clouet, J.; Fragale, A.; Griveau, L.; Chédeville, C.; Véziers, J.; Weiss, P.; Le Bideau, J.; Guicheux, J.; Le Visage, C. Pullulan microbeads/Si-HPMC hydrogel injectable system for the sustained delivery of GDF-5 and TGF-β1: New insight into intervertebral disc regenerative medicine. Drug Deliv. 2017, 24, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef]
- Divband, B.; Aghazadeh, M.; Al-Qaim, Z.H.; Samiei, M.; Hussein, F.H.; Shaabani, A.; Shahi, S.; Sedghi, R. Bioactive chitosan biguanidine-based injectable hydrogels as a novel BMP-2 and VEGF carrier for osteogenesis of dental pulp stem cells. Carbohydr. Polym. 2021, 273, 118589. [Google Scholar] [CrossRef]
- Ballios, B.G.; Cooke, M.J.; Donaldson, L.; Coles, B.L.; Morshead, C.M.; van der Kooy, D.; Shoichet, M.S. A hyaluronan-based injectable hydrogel improves the survival and integration of stem cell progeny following transplantation. Stem Cell Rep. 2015, 4, 1031–1045. [Google Scholar] [CrossRef]
- Sisso, A.M.; Boit, M.O.; DeForest, C.A. Self-healing injectable gelatin hydrogels for localized therapeutic cell delivery. J. Biomed. Mater. Res. Part A 2020, 108, 1112–1121. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Escobar Ivirico, J.L.; Kan, H.-M.; Shah, S.; Otsuka, T.; Bordett, R.; Barajaa, M.; Nagiah, N.; Pandey, R.; Nair, L.S. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc. Natl. Acad. Sci. USA 2022, 119, e2120968119. [Google Scholar] [CrossRef]
- Anamizu, M.; Tabata, Y. Design of injectable hydrogels of gelatin and alginate with ferric ions for cell transplantation. Acta Biomater. 2019, 100, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Hansrisuk, A.; Highley, C.B.; Caliari, S.R. Guest–host supramolecular assembly of injectable hydrogel nanofibers for cell encapsulation. ACS Biomater. Sci. Eng. 2021, 7, 4164–4174. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Cho, C.-S. Injectable hydrogels for regenerative medicine. Tissue Eng. Regen. Med. 2018, 15, 511–512. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2022. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Niu, D.; Wu, N.; Yun, W.; Wang, W.; Zhang, K.; Li, G.; Yan, S.; Xu, G. Mussel-inspired bisphosphonated injectable nanocomposite hydrogels with adhesive, self-healing, and osteogenic properties for bone regeneration. ACS Appl. Mater. Interfaces 2021, 13, 32673–32689. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Huang, J.; Zhao, M.; Wu, J. In situ formation of injectable hydrogels for chronic wound healing. J. Mater. Chem. B 2020, 8, 8768–8780. [Google Scholar] [CrossRef]
- Yan, C.; Altunbas, A.; Yucel, T.; Nagarkar, R.P.; Schneider, J.P.; Pochan, D.J. Injectable solid hydrogel: Mechanism of shear-thinning and immediate recovery of injectable β-hairpin peptide hydrogels. Soft Matter 2010, 6, 5143–5156. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Jee, J.-P.; Pangeni, R.; Jha, S.K.; Byun, Y.; Park, J.W. Preparation and in vivo evaluation of a topical hydrogel system incorporating highly skin-permeable growth factors, quercetin, and oxygen carriers for enhanced diabetic wound-healing therapy. Int. J. Nanomed. 2019, 14, 5449. [Google Scholar] [CrossRef]
- An, Z.; Zhang, L.; Liu, Y.; Zhao, H.; Zhang, Y.; Cao, Y.; Zhang, Y.; Pei, R. Injectable thioketal-containing hydrogel dressing accelerates skin wound healing with the incorporation of reactive oxygen species scavenging and growth factor release. Biomater. Sci. 2022, 10, 100–113. [Google Scholar] [CrossRef]
- Liu, X.; Jia, G. Modern wound dressing using polymers/biopolymers. J. Mater. Sci. Eng. 2018, 7, 3. [Google Scholar]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H. Recent progress of polysaccharide-based hydrogel interfaces for wound healing and tissue engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Shi, W.; Kong, Y.; Su, Y.; Kuss, M.A.; Jiang, X.; Li, X.; Xie, J.; Duan, B. Tannic acid-inspired, self-healing, and dual stimuli responsive dynamic hydrogel with potent antibacterial and anti-oxidative properties. J. Mater. Chem. B 2021, 9, 7182–7195. [Google Scholar] [CrossRef]
- Wang, Y.; Garcia, C.R.; Ding, Z.; Gabrilska, R.; Rumbaugh, K.P.; Wu, J.; Liu, Q.; Li, W. Adhesive, self-healing, and antibacterial chitosan hydrogels with tunable two-layer structures. ACS Sustain. Chem. Eng. 2020, 8, 18006–18014. [Google Scholar] [CrossRef]
- Yang, R.; Liu, X.; Ren, Y.; Xue, W.; Liu, S.; Wang, P.; Zhao, M.; Xu, H.; Chi, B. Injectable adaptive self-healing hyaluronic acid/poly (γ-glutamic acid) hydrogel for cutaneous wound healing. Acta Biomater. 2021, 127, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, C.; Cheng, C.; Shi, C.; Sun, M.; Hu, H.; Shi, T.; Chen, X.; He, X.; Zheng, X. Bioactive Injectable Hydrogel Dressings for Bacteria-Infected Diabetic Wound Healing: A “Pull–Push” Approach. ACS Appl. Mater. Interfaces 2022, 14, 26404–26417. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.; Wang, Y.; Shi, R.; Zhang, L. Wearable, healable, and adhesive epidermal sensors assembled from mussel-inspired conductive hybrid hydrogel framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y. Flexible, stretchable sensors for wearable health monitoring: Sensing mechanisms, materials, fabrication strategies and features. Sensors 2018, 18, 645. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, N.; Wang, L.; Tan, Y.; Liu, C.; Xia, Y.; Nie, Z.; Sui, K. Supramolecular nanofibrillar hydrogels as highly stretchable, elastic and sensitive ionic sensors. Mater. Horiz. 2019, 6, 326–333. [Google Scholar] [CrossRef]
- Liu, Y.; He, K.; Chen, G.; Leow, W.R.; Chen, X. Nature-inspired structural materials for flexible electronic devices. Chem. Rev. 2017, 117, 12893–12941. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Duan, L.; Gao, G. Ultra-stretchable wearable strain sensors based on skin-inspired adhesive, tough and conductive hydrogels. Chem. Eng. J. 2019, 365, 10–19. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Liu, M. High performance strain sensors based on chitosan/carbon black composite sponges. Mater. Des. 2018, 141, 276–285. [Google Scholar] [CrossRef]
- Kanoun, O.; Müller, C.; Benchirouf, A.; Sanli, A.; Dinh, T.N.; Al-Hamry, A.; Bu, L.; Gerlach, C.; Bouhamed, A. Flexible carbon nanotube films for high performance strain sensors. Sensors 2014, 14, 10042–10071. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, J.; Li, Y.; Shi, G. High-performance strain sensors with fish-scale-like graphene-sensing layers for full-range detection of human motions. ACS Nano 2016, 10, 7901–7906. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Shen, B.; Mo, J.; Tang, F.; Feng, J. Highly stretchable and self-healing double network hydrogel based on polysaccharide and polyzwitterion for wearable electric skin. Polymer 2020, 194, 122381. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhang, H.; Chen, J.; Li, B.; Fu, S. Synergy coordination of cellulose-based dialdehyde and carboxyl with Fe3+ recoverable conductive self-healing hydrogel for sensor. Mater. Sci. Eng. C 2021, 125, 112094. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; He, P.; Liu, K.; Ni, Y.; Ouyang, X.; Chen, L.; Huang, L.; Wang, H.; Tan, Y. Mussel-inspired nanocomposite hydrogel-based electrodes with reusable and injectable properties for human electrophysiological signals detection. ACS Sustain. Chem. Eng. 2019, 7, 7918–7925. [Google Scholar] [CrossRef]
- Fan, L.; He, Z.; Peng, X.; Xie, J.; Su, F.; Wei, D.-X.; Zheng, Y.; Yao, D. Injectable, Intrinsically Antibacterial Conductive Hydrogels with Self-Healing and pH Stimulus Responsiveness for Epidermal Sensors and Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 53541–53552. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, W.; Sun, P.; Shi, L.; Liu, Y.; Zhang, X.; Zhang, L.; Han, W.; Chen, P. Skin-Inspired Packaging of Injectable Hydrogel Sensors Enabled by Photopolymerizable and Swellable Hydrogels toward Sustainable Electronics. ACS Sustain. Chem. Eng. 2022, 20, 6657–6666. [Google Scholar] [CrossRef]





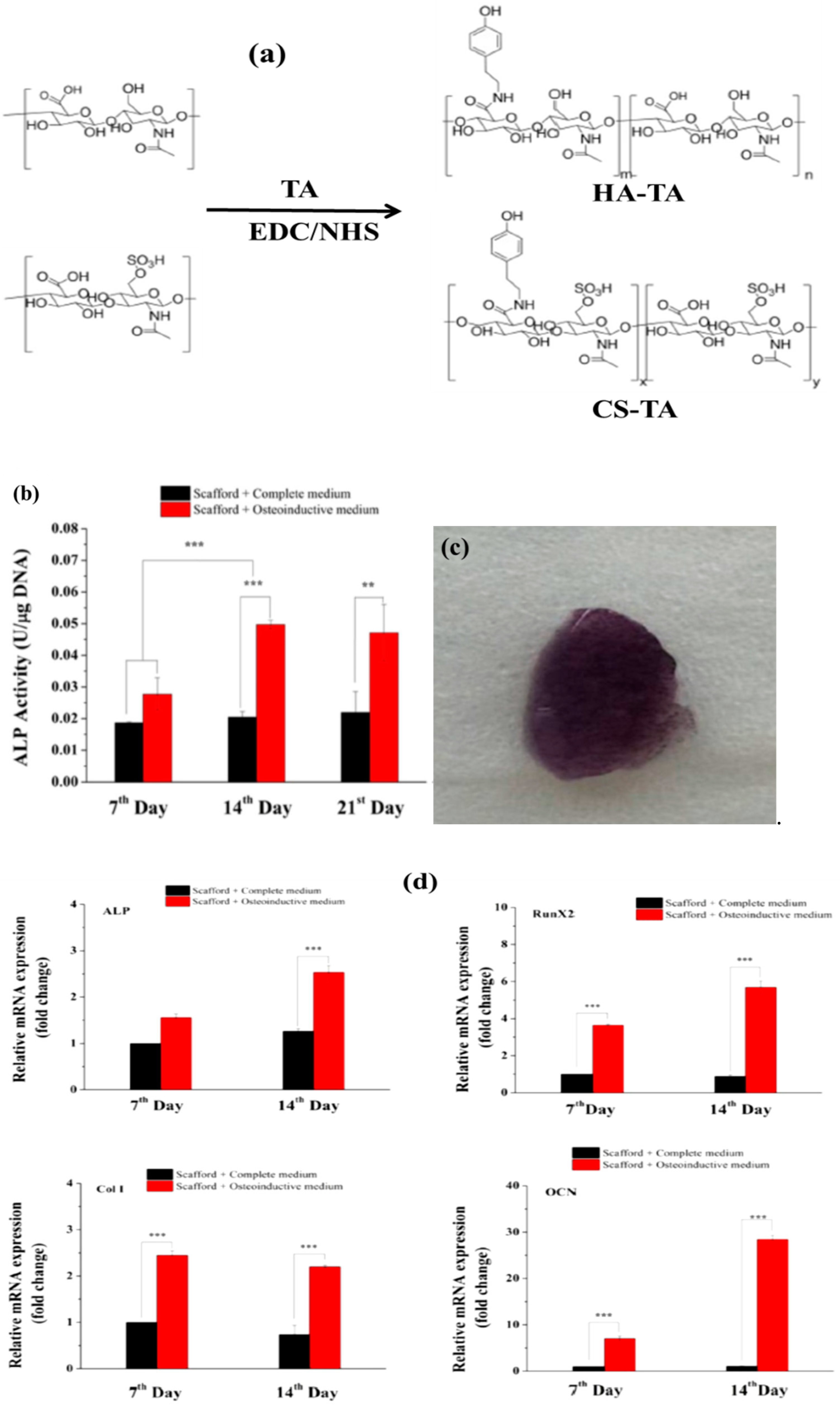

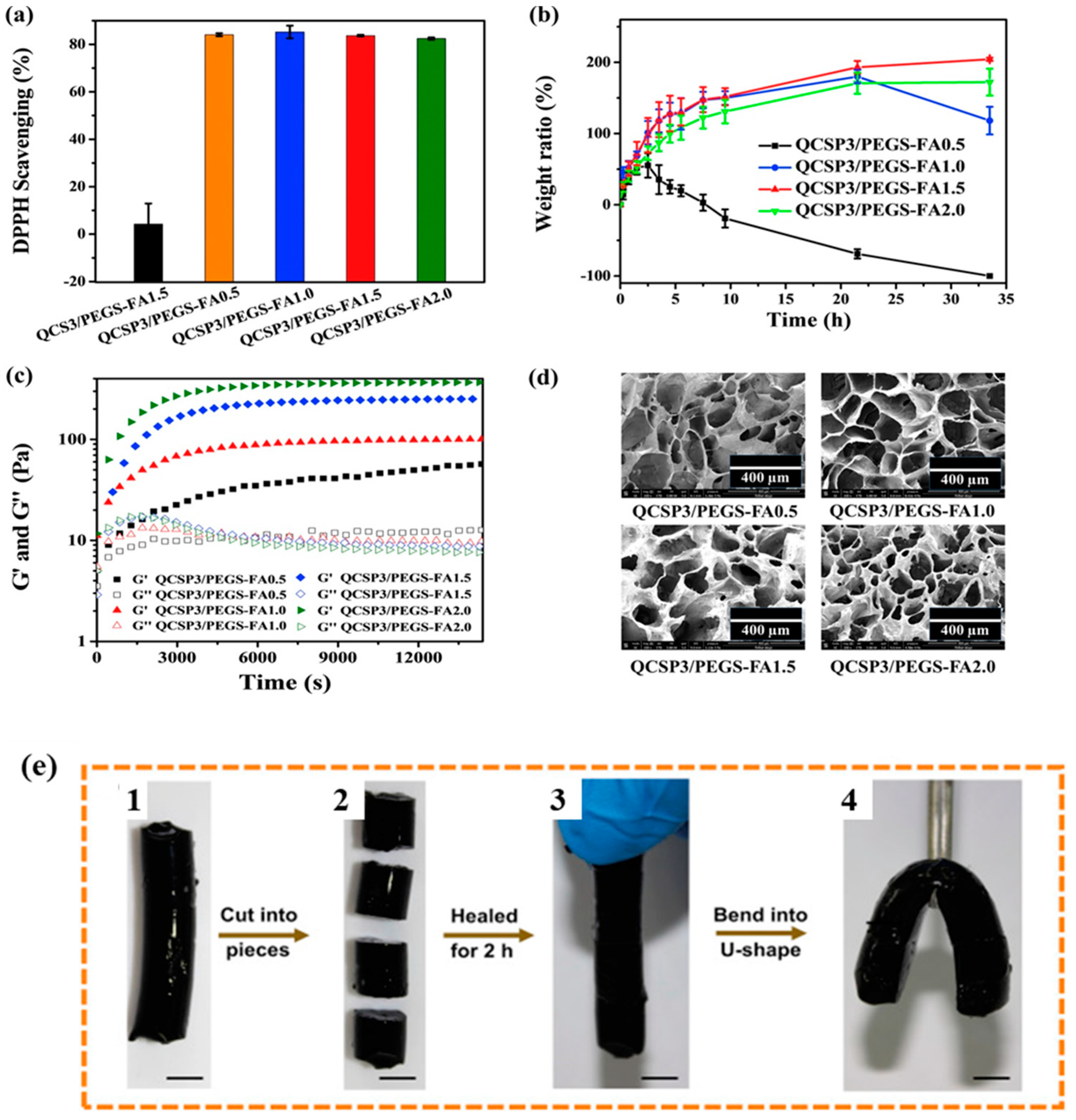

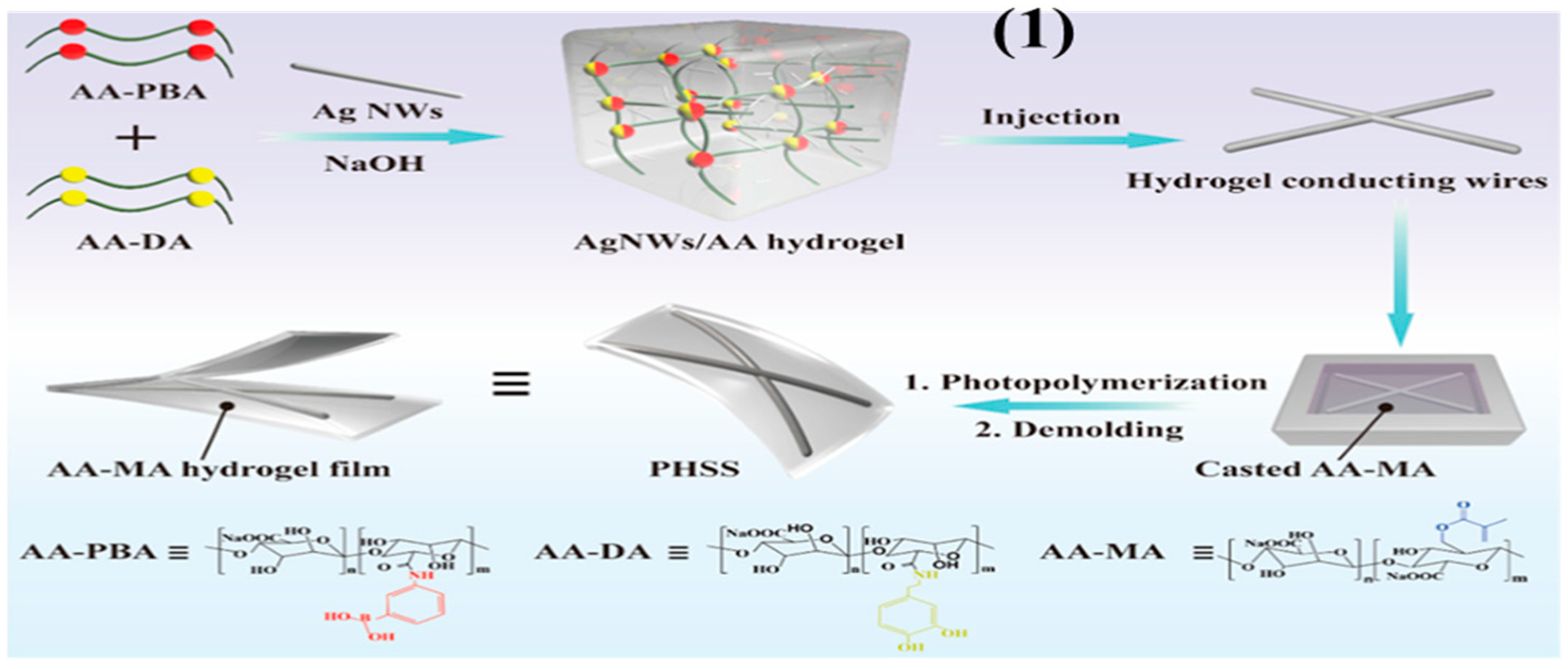
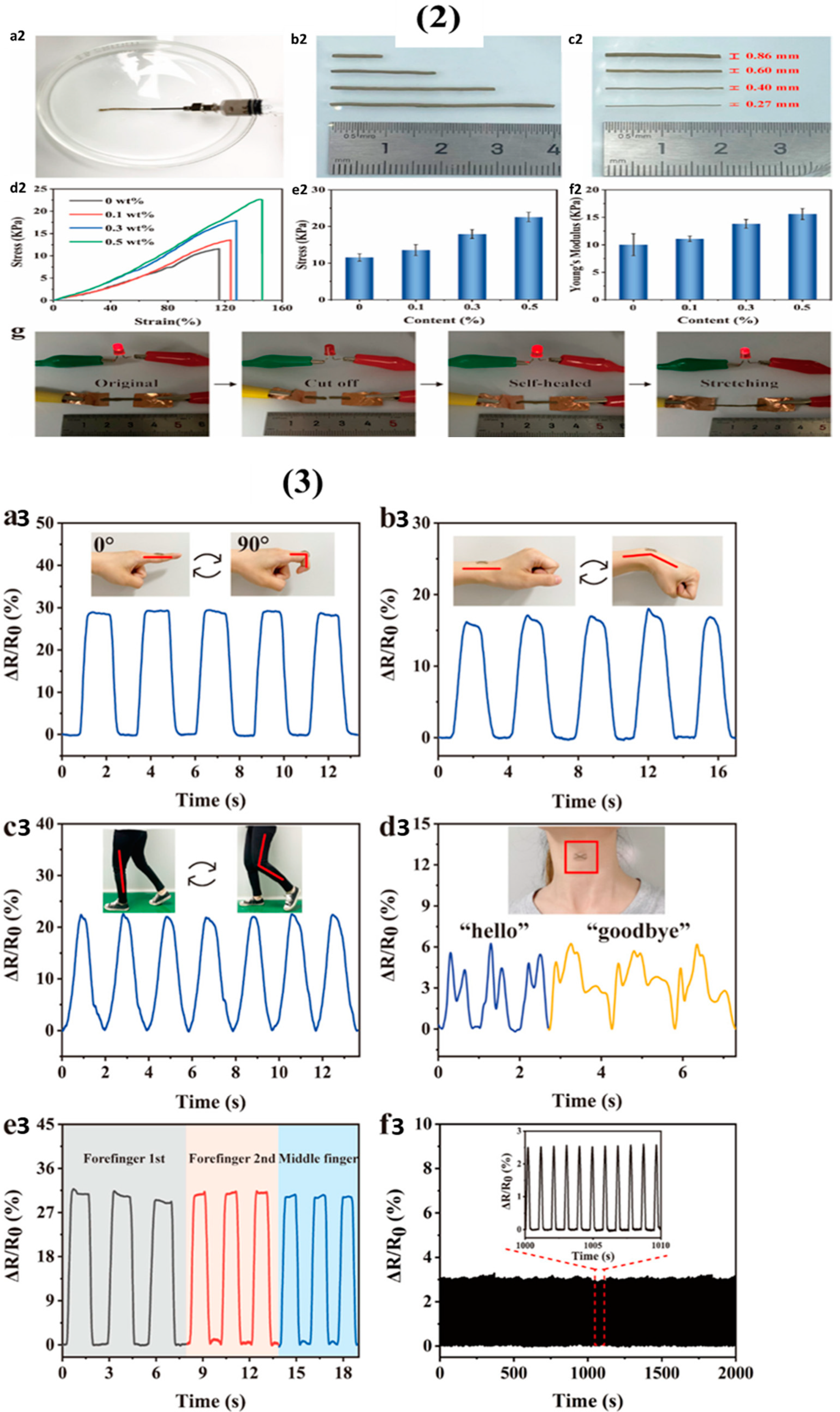
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, N.S.; Kamel, S. Polysaccharides-Based Injectable Hydrogels: Preparation, Characteristics, and Biomedical Applications. Colloids Interfaces 2022, 6, 78. https://doi.org/10.3390/colloids6040078
El-Sayed NS, Kamel S. Polysaccharides-Based Injectable Hydrogels: Preparation, Characteristics, and Biomedical Applications. Colloids and Interfaces. 2022; 6(4):78. https://doi.org/10.3390/colloids6040078
Chicago/Turabian StyleEl-Sayed, Naglaa Salem, and Samir Kamel. 2022. "Polysaccharides-Based Injectable Hydrogels: Preparation, Characteristics, and Biomedical Applications" Colloids and Interfaces 6, no. 4: 78. https://doi.org/10.3390/colloids6040078
APA StyleEl-Sayed, N. S., & Kamel, S. (2022). Polysaccharides-Based Injectable Hydrogels: Preparation, Characteristics, and Biomedical Applications. Colloids and Interfaces, 6(4), 78. https://doi.org/10.3390/colloids6040078




