Effect of Acyl Chain Length on Hydrophobized Cashew Gum Self-Assembling Nanoparticles: Colloidal Properties and Amphotericin B Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Acylated Cashew Gum
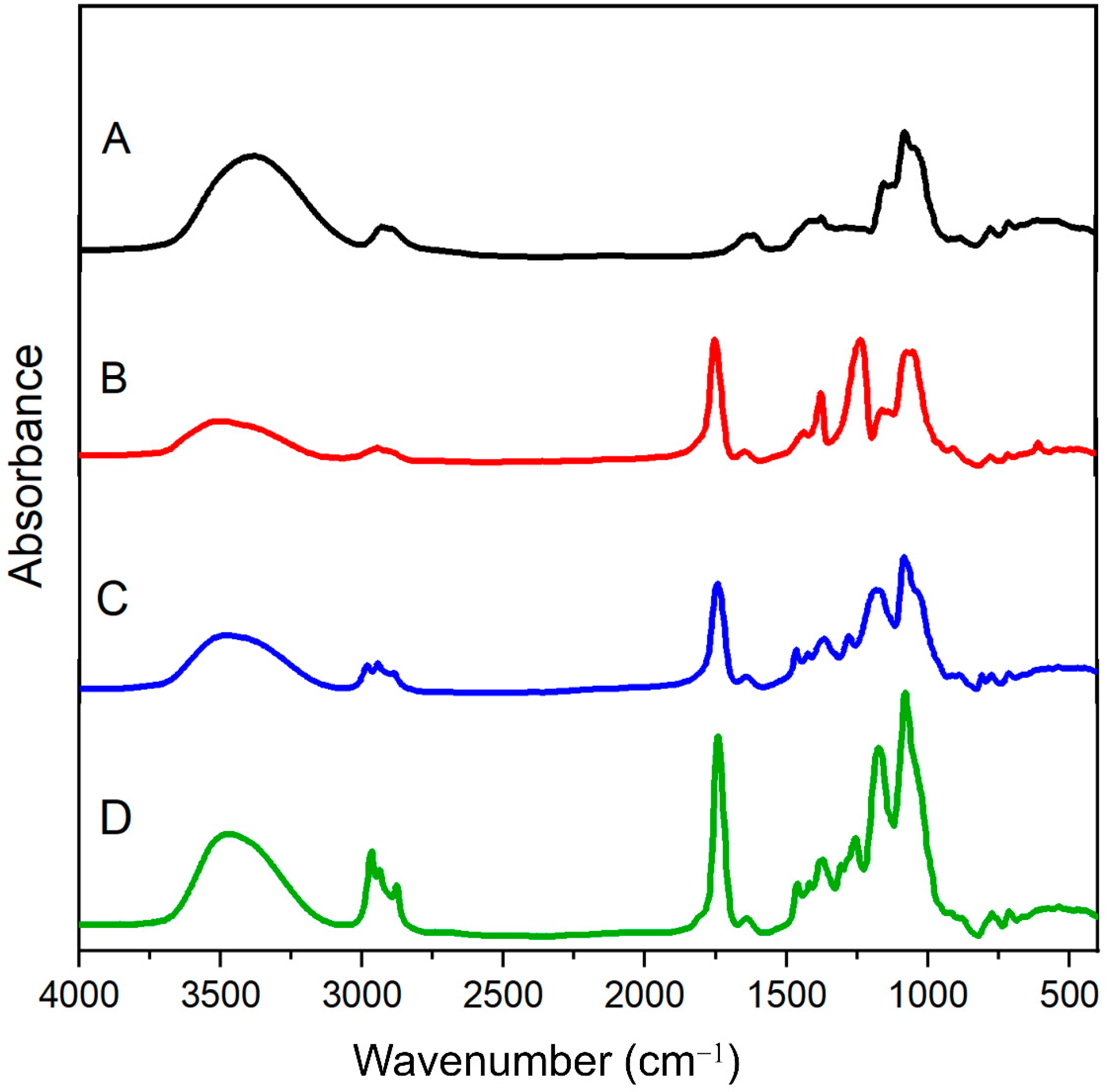
2.3. FT-IR Spectroscopy
2.4. Nuclear Magnetic Resonance
2.5. Acyl Content and Degree of Substitution (DS)
2.6. Preparation of Cashew Gum Acylated Nanoparticles
2.7. Amphotericin B Encapsulation
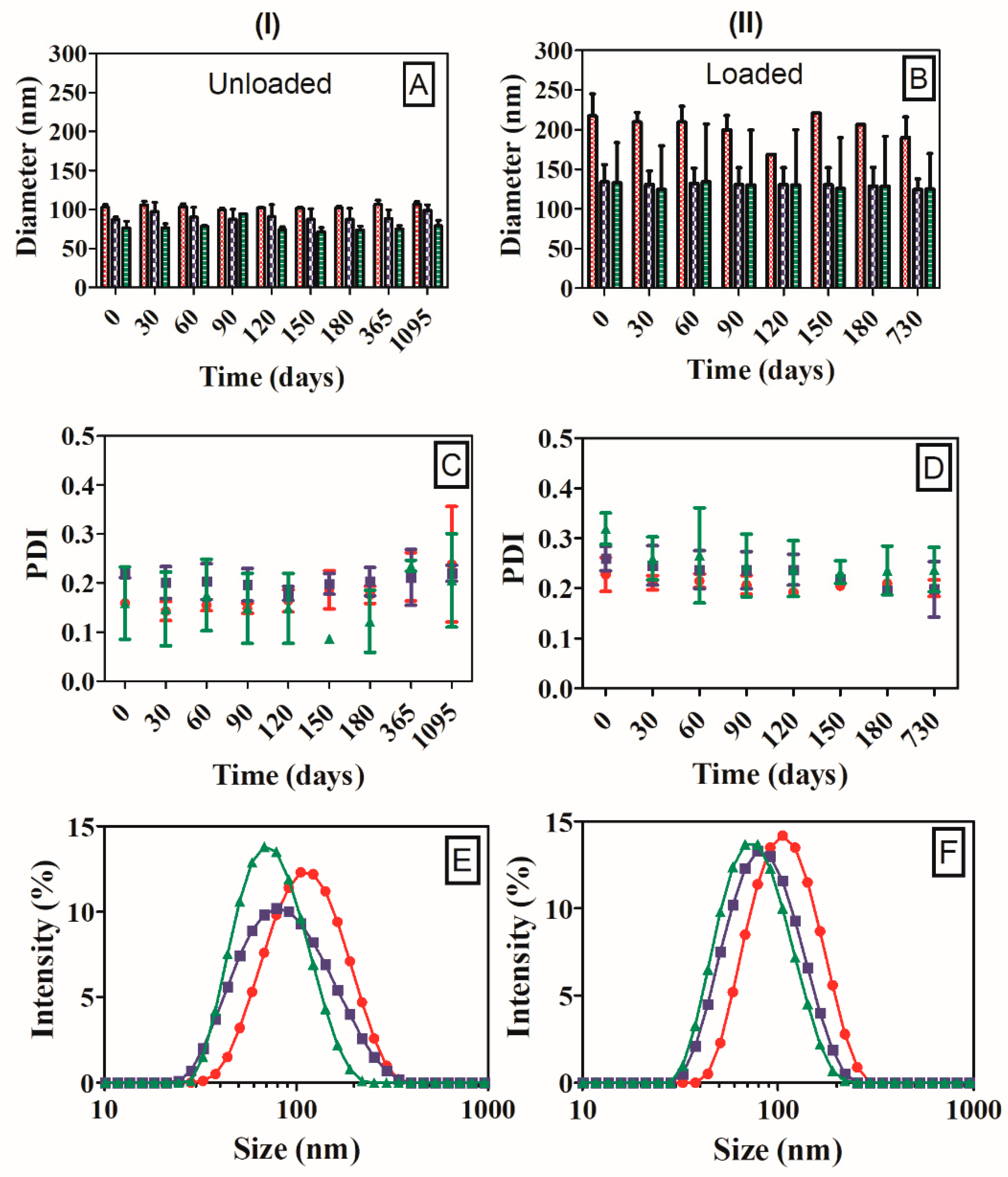
2.8. Dynamic Light Scattering
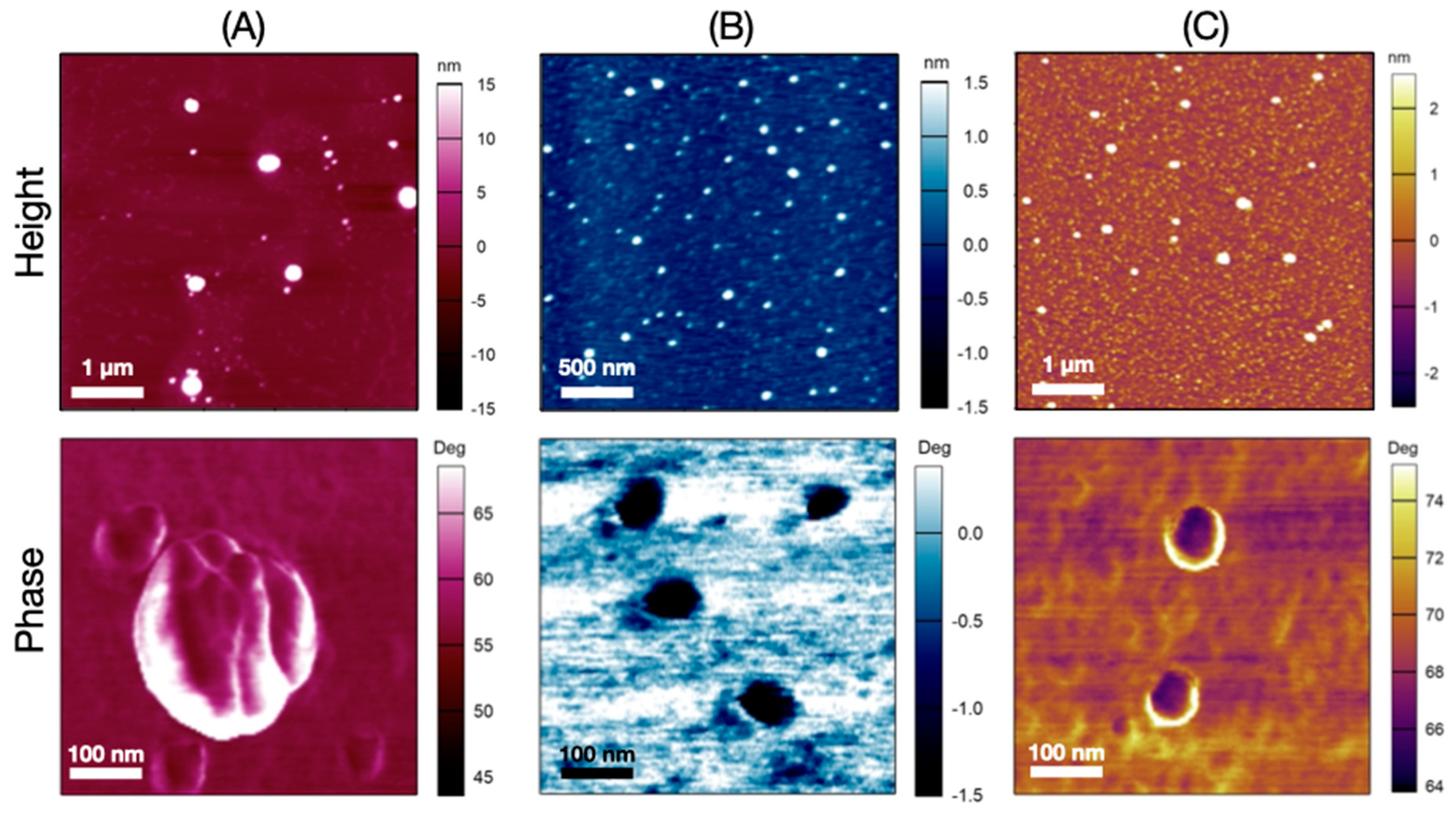
2.9. Morphology
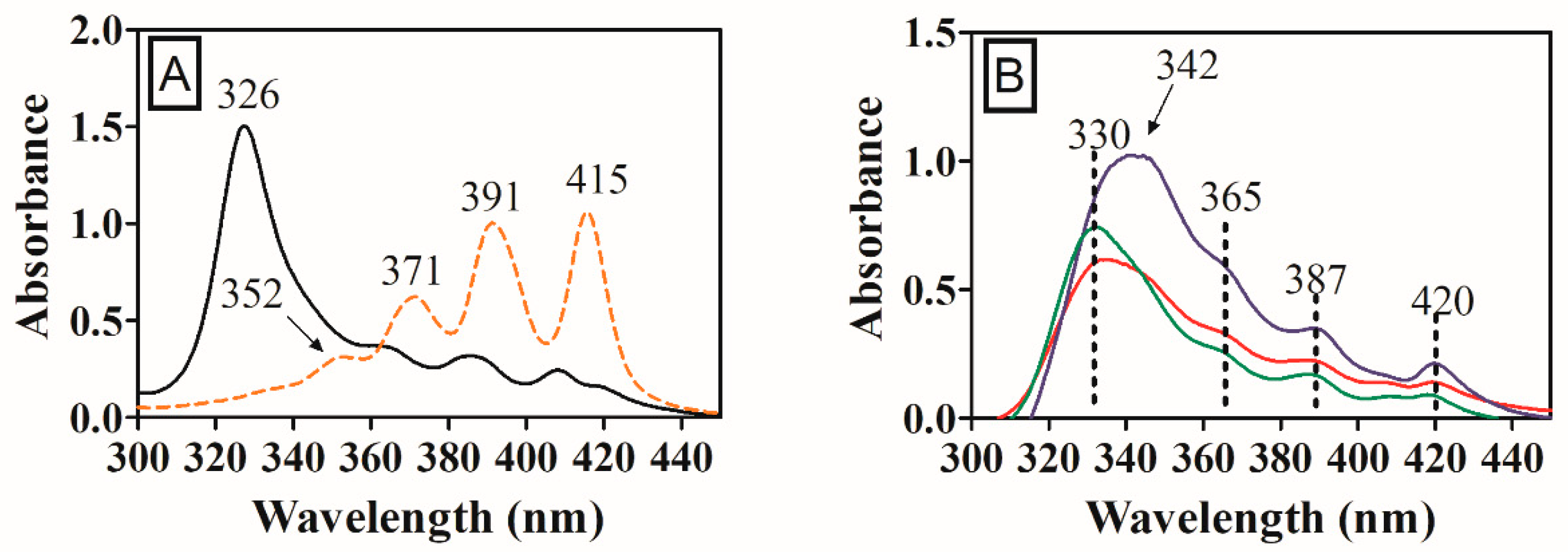
2.10. State of AmB Aggregation
2.11. Storage Stability
2.12. In Vitro Drug Release
2.13. In Vitro Antifungal Assay—Broth Microdilution Method
2.14. Cytotoxicity Assay
2.15. Statistical Analysis
3. Results and Discussion
3.1. Acylated Cashew Gum
3.2. FTIR Analysis
3.3. NMR Spectroscopy
3.4. Characterization of Nanoparticles
3.5. Colloidal Storage Stability of Nanoparticles
3.6. Morphology
3.7. Aggregated States of AmB in Acylated Nanoparticles
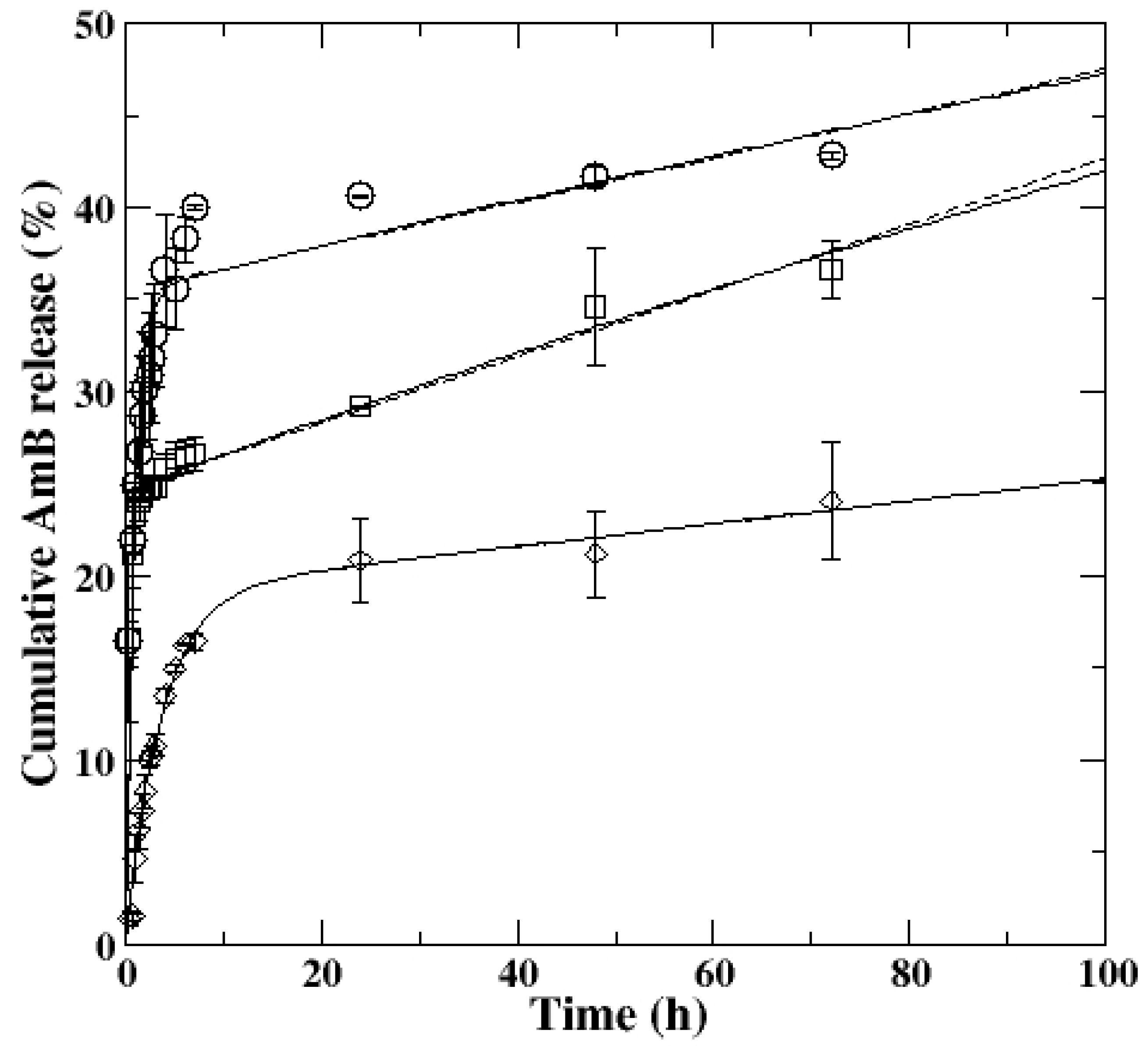
3.8. AmB Encapsulation and In Vitro Release
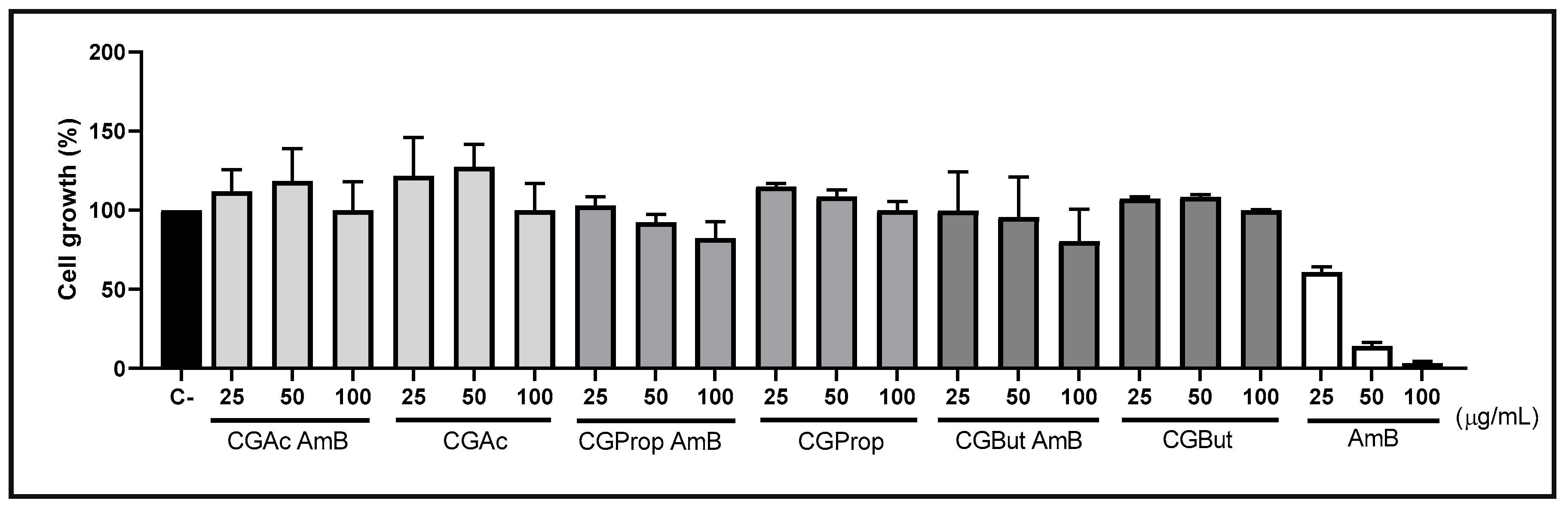
3.9. Cytotoxicity Assay
3.10. In Vitro Antifungal Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubey, K.M.; Brenner, J.S. Nanomedicine to fight infectious disease. Adv. Drug Deliv. Rev. 2021, 179, 113996. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Kumar, P.; Kumar, S.; Rajana, V.K.; Kant, V.; Prasad, R.S.; Mohan, U.; Mandal, D. Current status of nanoscale drug delivery and the future of nano-vaccine development for leishmaniasis—A review. Biomed. Pharmacother. 2021, 141, 111920. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C. Drug resistance in visceral leishmaniasis. J. Biomed. Biotechnol. 2010, 2010, 617521. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, L.R.; Fernandes, F.R.; Costa, D.F.; Frézard, F.; Afonso, L.C.C.; Ferreira, L.A.M. Nanoemulsions loaded with amphotericin B: A new approach for the treatment of leishmaniasis. Eur. J. Pharm. Sci. 2015, 70, 125–131. [Google Scholar] [CrossRef]
- Liu, Y.C.; Han, Y.; Fang, T.; Cheng, S.M.; Hu, X.Y.; Song, L.J.; Shen, H.; Dong, H.Q.; Jiang, Y.Y.; An, M.M. Turning weakness into strenght: Albumin nanoparticle-redirected amphotericin B biodistribution for reducing nephrotoxicity and enhancing antifungal activity. J. Control. Release 2020, 324, 657–668. [Google Scholar] [CrossRef]
- Gedda, M.R.; Madhukar, P.; Shukla, A.; Mudavath, S.L.; Srivastava, O.N.; Singh, O.P.; Sundar, S. Nanodiagnostics in leishmaniasis: A new frontiers for early elimination. Rev. Nanomed. Nanobiotechnol. 2020, 13, e1675. [Google Scholar] [CrossRef]
- Saqib, M.; Bhatti, A.S.A.; Ahmad, N.M.; Ahmed, N.; Shahnaz, G.; Lebaz, N.; Elaissari, A. Amphotericin B Loaded Polymeric Nanoparticles for Treatment of Leishmania Infections. J. Nanomater. 2020, 10, 1152. [Google Scholar] [CrossRef]
- Richter, A.R.; Carneiro, M.J.M.; Sousa, N.A.; Pinto, V.P.T.; Freire, R.S.; de Sousa, J.S.; Mendes, F.S.; Fontenelle, R.O.S.; Feitosa, J.P.A.; Paula, H.C.B.; et al. Self-assembling cashew gum-graft-polylactide copolymer nanoparticles as a potential amphotericin B delivery matrix. Int. J. Biol. Macromol. 2020, 152, 492–502. [Google Scholar] [CrossRef]
- Ariaga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale self-assembly for therapeutic drug loading for pH-triggeres drug delivery. Biomacromolecules 2020, 15, 524–532. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Wu, Y.; Dai, F.; Yuan, M.; Bai, Y.; Deng, H. Natural polysaccharide based self-assembled nanoparticles for biomedical applications—A review. Int. J. Biol. Macromol. 2021, 192, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef] [PubMed]
- de Paula, R.C.M.; Heatley, F.; Budd, P.M. Characterization of Anacardium occidentale exudate polysaccharide. Polym. Int. 1998, 45, 27–35. [Google Scholar] [CrossRef]
- Menestrina, J.M.; Iacomini, M.; Jones, C.; Gorin, P.A.J. Similarity of monosaccharide, oligosaccharide, and polysaccharide structure in gum from Anacardium occidentale. Phytochemistry 1998, 47, 715–721. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Souza, F.R.L.; Bezerra, J.M.N.A.; Oliveira, C.; Nadvorny, D.; Soares, M.F.R.; Nunes, L.C.C.; Silva-Filho, E.C.; Veiga, F.; Sobrinho, J.L.S. Gums’ based delivery systems: Review on cashew gum and its derivatives. Carbohydr. Polym. 2016, 147, 188–200. [Google Scholar] [CrossRef]
- Kumar, A.; Moin, A.; Shruthi, R.; Ahmed, A.; Shivakumar, H.G. Cashew Gum: A Versatile Hydrophyllic Polymer: A Review. Curr. Drug Ther. 2012, 7, 2–12. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ansari, M.T.; Sami, F.; Bera, H.; Hasnaim, M.S. Cashew gum in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 263–283. [Google Scholar]
- Dueramae, I.; Yoneyama, M.; Shinyashiki; Yagihara, N.; Kita, S.R. Self-assembly of acetylated dextran with various acetylation degrees in aqueous solutions: Studied by light scattering. Carbohydr. Polym. 2017, 159, 171–177. [Google Scholar] [CrossRef]
- Najafi, S.H.M.; Baghaie, M.; Ashori, A. Preparation and characterization of acetylated starch nanoparticles as drug carrier: Ciprofloxacin as a model. Int. J. Biol. Macromol. 2016, 87, 48–54. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Rocha, A.; Soares, R.M.D.; Fonseca, L.P.; da Silveira, N.P. Synthesis and characterization of acetylated amylose and development of inclusion complexes with rifampicin. Carbohydr. Polym. 2017, 157, 267–274. [Google Scholar] [CrossRef]
- Xie, J.H.; Zhang, F.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Xie, M.Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef]
- Pitombeira, N.A.O.; Veras Neto, J.G.; Silva, D.A.; Feitosa, J.P.A.; Paula, H.C.B.; de Paula, R.C.M. Self-assembled nanoparticles of acetylated cashew gum: Characterization and evaluation as potential drug carrier. Carbohydr. Polym. 2015, 117, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.F.L.; Nogueira, S.S.; Dourado, F.F.; Guimarães, M.A.; Pitombeira, N.A.O.; Gobbo, G.G.; Primo, F.L.; de Paula, R.C.M.; Feitosa, J.P.A.; Tedesco, A.C.; et al. Acetylated cashew gum-based nanoparticles for transdermal delivery of diclofenac diethyl amine. Carbohydr. Polym. 2016, 143, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.R.; Paula, H.C.B.; Abreu, F.O.M.; da Silva, R.B.C.; Sombra, F.M.; de Paula, R.C.M. Hydrophobization of cashew gum by acetylation mechanism and amphotericin B encapsulation. Int. J. Biol. Macromol. 2018, 108, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.D.; de Araújo, A.R.; Pitombeira, N.A.; Placido, A.; de Almeida, M.P.; Veras, L.M.C.; Delerue-Matos, C.; Lima, F.C.D.A.; Batagin Neto, A.; de Paula, R.C.M.; et al. Acetylated cashew gum-based nanoparticles for the incorporation of alkaloid epiisopiloturine. Int. J. Biol. Macromol. 2019, 128, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.L.; Oliveira, A.C.J.; Patriota, Y.B.C.; Ribeiro, A.J.; Veiga, F.; Sobrinho, F. Solvent-free synthesis of acetylated cashew gum for oral delivery system of insulin. Carbohydr. Polym. 2019, 207, 601–608. [Google Scholar] [CrossRef]
- Biswas, A.; Cheng, H.N.; Kim, S.; Albes, C.R.; Furtadi, R.F. Hydrophobic modification of cashew gum with alkenyl succinic anhydride. Polymers 2020, 12, 514. [Google Scholar] [CrossRef]
- Oliveira, A.C.J.; Chaves, L.P.; Ribeiro, F.O.S.; Lima, L.R.M.; Oliveira, T.C.; García-Villén, F.; Viseras, C.; de Paula, R.C.M.; Rolim-Neto, P.J.; Hallwass, F.; et al. Microwave-initiated rapid synthesis of phthalated cashew gum for drug delivery systems. Carbohydr. Polym. 2021, 254, 117226. [Google Scholar] [CrossRef]
- Rodrigues, J.F.; de Paula, R.C.M.; Costa, S.M.O. Métodos de Isolamento de Gomas Naturais: Comparação Através da Goma do Cajueiro (Anacardium occidentale L). Polímeros Ciência Tecnol. 1993, 3, 31–36. [Google Scholar]
- Motozato, Y.; Ihara, H.; Tomoda, T.; Hirayama, C. Preparation and gel permeation chromatographic properties of pullulan spheres. J. Chromatogr. 1986, 355, 434–437. [Google Scholar] [CrossRef]
- Sánchez-Rivera, M.M.; Flores-Ramírez, I.; Zamudio-Flores, P.B.; Gonzalez-Soto, R.A.; Rodríguez-Ambríz, S.L.; Bello-Pérez, L.A. Acetylation of banana (Musa paradisiaca L.) and maize (Zea mays L.) starches using a microwave heating procedure and iodine as catalyst: Partial characterization. Starch/Staerke 2010, 62, 155–164. [Google Scholar] [CrossRef]
- Garg, S.; Jana, A.K. Characterization and evaluation of acylated starch with different acyl groups and degrees of substitution. Carbohydr. Polym. 2011, 83, 1623–1630. [Google Scholar] [CrossRef]
- Malvern. Zetasizer Nano Series. Malvern Instrument SZ User Manual. Dynamic Light Scattering DLS | Malvern Panalytical. Available online: https://www.malvernpanalytical.com/en/products/technology/light-scattering/dynamic-light-scattering (accessed on 1 October 2022).
- Barwicz, J.; Christian, S.; Gruda, I. Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob. Agents Chemother. 1992, 36, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standart Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast, 3rd ed.; Approved Standard M27-A3; CSLI: Wayne, PA, USA, 2008. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sombra, F.M.; Richter, A.R.; de Araújo, A.R.; Ribeiro, F.O.S.; Mendes, J.F.S.; Fontenelle, R.O.S.; Silva, D.A.; Paula, H.C.B.; Feitosa, J.P.A.; Goycoolea, F.M.; et al. Nanocapsules of Sterculia striata acetylated polysaccharide as a potential monomeric amphotericin B delivery matrix. Int. J. Biol. Macromol. 2019, 130, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Sombra, F.M.; Richter, A.R.; de Araújo, A.R.; Ribeiro, F.O.S.; Mendes, J.F.S.; Fontenelle, R.O.S.; Silva, D.A.; Paula, H.C.B.; Feitosa, J.P.A.; Goycoolea, F.M.; et al. Development of amphotericin B-loaded propionate Sterculia striata polysaccharide nanocarrier. Int. J. Biol. Macromol. 2020, 146, 1133–1144. [Google Scholar] [CrossRef]
- Cunha, P.L.R.; Maciel, J.S.; Sierakowski, M.R.; de Paula, R.C.M.; Feitosa, J.P.A. Oxidation of cashew tree gum exudate polysaccharide with TEMPO reagent. J. Braz. Chem. Soc. 2007, 18, 85–92. [Google Scholar] [CrossRef]
- Silva, D.A.; de Paula, R.C.M.; Feitosa, J.P.A.; de Brito, A.C.F.; Maciel, J.S.; Paula, H.C.B. Carboxymethylation of cashew tree exudate polysaccharide. Carbohydr. Polym. 2004, 58, 163–171. [Google Scholar] [CrossRef]
- Moura Neto, E.; Maciel, J.S.M.; Cunha, P.L.R.; de Paula, R.C.M.; Feitosa, J.P.A. Preparation and characterization of a chemically sulfated cashew gum polysaccharide. J. Braz. Chem. Soc. 2011, 22, 1953–1960. [Google Scholar] [CrossRef]
- Kemas, C.U.; Ngwuluka, N.C.; Ochekpe, N.A.; Nep, E.I. Starch-based xerogels: Effect of acetylation on Physicochemical and rheological properties. Int. J. Biol. Macromol. 2017, 98, 94–102. [Google Scholar] [CrossRef]
- Niu, B.; Shao, P.; Chen, H.J.; Sun, P.L. Structural and physiochemical characterization of novel hydrophobic packaging films based on pullulan derivatives for fruits preservation. Carbohydr. Polym. 2019, 208, 276–284. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Gao, X.; Meng, X.; Yi, Y. Synthesis and Characterization of Inulin Butyrate Ester, and Evaluation of Its Antioxidant Activity and in Vitro Effect on SCFA Production. Starch/Staerke 2020, 72, 1900323. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS end zeta—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sedlarik, V. Amphiphilic chitosan-grafted-functionalized polylactic acid based nanoparticles as a delivery system for doxorubicin and temozolomide co-therapy. Int. J. Pharm. 2014, 474, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Brasili, F.; Capocefalo, A.; Palmieri, D.; CApitano, F.; Chiessi, E.; Paradossi, G.; Bordi, F.; Domenici, F. Assembling patchy plasmonic nanoparticles with aggregation-dependent antibacterial activity. J. Colloid Interface Sci. 2020, 580, 419–428. [Google Scholar] [CrossRef]
- Sennato, S.; Truzzolillo, D.; Bordi, F. Aggregation and stability of polyelectrolyte-decorated liposome complexes in water-salt media. Soft Matter 2012, 8, 9384–9395. [Google Scholar] [CrossRef]
- Hierrezuelo, J.; Sadeghpour, A.; Szilagyi, I.; Vaccaro, A.; Borkovec, M. Electrostatic stabilization of charged colloidal particles with adsorbed polyelectrolytes of opposite charge. Langmuir 2010, 26, 15109–15111. [Google Scholar] [CrossRef]
- Kouchakzadeh, H.; Shojaosadati, S.A.; Maghsoudi, A.; Farahani, E.V. Optimization of PEGylation conditions for BSA nanoparticles using response surface methodology. Aaps Pharmscitech 2010, 11, 1206–1211. [Google Scholar] [CrossRef]
- Adair, J.H.; Suvaci, E.; Sindel, J. Surface and Colloid Chemistry. In Encyclopedia of Materials: Science and Technology; Buschow, R.W., Cahn, M.C., Flemings, B., Ilschner, E.J., Kramer, S., Mahajan, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1–10. [Google Scholar] [CrossRef]
- Tancrède, P.; Barwicz, J.; Jutras, S.; Gruda, I. The effect of surfactants on the aggregation state of amphotericin B. Biochim. Biophys. Acta. 1990, 1030, 289–295. [Google Scholar] [CrossRef]
- Casa, D.M.; Carraro, T.C.; Camargo, L.E.; Dalmolin, L.F.; Khalil, N.M.; Mainardes, R.M. Poly(L-lactide) nanoparticles reduce amphotericin B cytotoxicity and maintain its in vitro antifungal activity. J. Nanosci. Nanotechnol. 2015, 15, 848–854. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, X.; Voo, Z.X.; Yap, S.S.L.; Yang, C.; Gao, S.; Liu, S.; Venkataraman, S.; Khara, S.J.; Yang, Y.Y.; et al. Biodegradable functional polycarbonate micelles for controlled release of amphotericin B. Acta Biomater. 2016, 46, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bolard, J.; Seigneuret, M.; Boudet, G. Interaction between phospholipid bilayer membranes and the polyene antibiotic amphotericin b lipid state and cholesterol content dependence. Biochim. Biophys. Acta. 1980, 599, 280–293. [Google Scholar] [CrossRef]
- Song, Z.M.; Wen, Y.; Deng, P.Z.; Teng, F.F.; Zhou, F.L.; Xu, H.M.; Feng, S.J.; Zhu, L.; Feng, R.L. Linolenic acid-modified methoxy poly (ethylene glycol)-oligochitosan conjugate micelles for encapsulation of amphotericin B. Carbohydr. Polym. 2019, 205, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.B.; Carter, K.C.; Baillie, A.J. Comparison of the efficacies of various formulations of amphotericin B against murine visceral leishmaniasis. Antimicrob. Agents Chemother. 1997, 41, 2089–2092. [Google Scholar] [CrossRef][Green Version]
- Tiyaboonchai, W.; Limpeanchob, N. Formulation and characterization of amphotericin B-chitosan-dextran sulfate nanoparticles. Int. J. Pharm. 2007, 329, 142–149. [Google Scholar] [CrossRef]
- Gilani, K.; Moazeni, E.; Ramezanli, T.; Amini, M.; Fazeli, M.R.; Jamalifar, H. Development of respirable nanomicelle carriers for delivery of amphotericin B by jet nebulization. J. Pharma. Sci. 2011, 100, 252–259. [Google Scholar] [CrossRef]
- Imran, M.; Shah, M.R.; Ullah, F.; Ullah, S.; Elhissi, A.M.A.; Nawaz, W.; Ahmad, F.; Sadiq, A.; Ali, I. Sugar-based novel niosomal nanocarrier system for enhanced oral bioavailability of levofloxacin. Drug Deliv. 2016, 23, 3653–3664. [Google Scholar] [CrossRef]
- Italia, J.L.; Yahya, M.M.; Singh, D.; Kumar, M.N.V.R. Biodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity compared to intravenous fungizone®. Pharm. Res. 2009, 26, 1324–1331. [Google Scholar] [CrossRef]
- Krishnan, R.A.; Pant, T.; Sankaranarayan, S.; Stenberg, J.; Jain, R.; Dandekar, P. Protective nature of low molecular weight chitosan in a chitosan–Amphotericin B nanocomplex—A physicochemical study. Mater. Sci. Eng. C 2018, 93, 472–482. [Google Scholar] [CrossRef]
- Zeng, L.; An, L.; Wu, X. Modeling drug-carrier interaction in the drug release from nanocarriers. J. Drug. Deliv. 2011, 2011, 370308. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Domenici, F.; Giantulli, S.; Brasili, F.; D’Errico, C.; Tsaoili, G.; Tortorella, E.; Bordi, F.; Morrone, S.; Palocci, C.; et al. PLGA based particles as “drug reservoir” for antitumor drug delivery: Characterization and cytotoxicity studies. Colloids Surf. B. 2019, 180, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Agardan, N.B.; Yilmaz, S.; Onurdag, F.K.; Celebi, N. Development of effective AnB/AmB-αCD complex double loaded liposomes using a factorial design for systemic fungal infection treatment. J. Liposome. Res. 2021, 31, 177–188. [Google Scholar] [CrossRef] [PubMed]


| Sample | % acyl Groups | DS | Yield (%) |
|---|---|---|---|
| CGAc | 30.7 ± 0.9 | 1.65 ± 0.07 | 49.1 ± 5.1 |
| CGProp | 33.6 ± 3.7 | 1.29 ± 0.19 | 53.1 ± 3.9 |
| CGBut | 41.2 ± 1.3 | 1.12 ± 0.10 | 65.9 ± 3.5 |
| Acyl Derivatives | Model Parameters | ||||
|---|---|---|---|---|---|
| Ks (h−1) | Kon (h−1) | Koff (h−1) | ΔG (10−21 J) | R2 | |
| CGAc AmB | 1.18 | 0.0037 | 0.0020 | −2.63 | 0.9917 |
| CGProp AmB | 3.13 | 0.0080 | 0.0026 | −4.81 | 0.9995 |
| CGBut AmB | 0.28 | 0.0037 | 0.0008 | −6.19 | 0.9996 |
| Samples | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| C. albicans | |||||
| LABMIC 0103 | LABMIC 0104 | LABMIC 0105 | LABMIC 0106 | ATCC 90028 | |
| CGAc AmB | 1 | 1 | 1 | 1 | 1 |
| CGProp AmB | 1 | 1 | 1 | 1 | 2 |
| CGBut AmB | 2 | 2 | 2 | 2 | 4 |
| Free AmB | 2 | 1 | 1 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, A.R.; Veras-Neto, J.G.; Sousa, J.S.; Mendes, J.F.S.; Fontenelle, R.O.S.; Silva, S.A.N.M.; Marinho-Filho, J.D.B.; Araújo, A.J.; Feitosa, J.P.A.; Paula, H.C.B.; et al. Effect of Acyl Chain Length on Hydrophobized Cashew Gum Self-Assembling Nanoparticles: Colloidal Properties and Amphotericin B Delivery. Colloids Interfaces 2022, 6, 65. https://doi.org/10.3390/colloids6040065
Richter AR, Veras-Neto JG, Sousa JS, Mendes JFS, Fontenelle ROS, Silva SANM, Marinho-Filho JDB, Araújo AJ, Feitosa JPA, Paula HCB, et al. Effect of Acyl Chain Length on Hydrophobized Cashew Gum Self-Assembling Nanoparticles: Colloidal Properties and Amphotericin B Delivery. Colloids and Interfaces. 2022; 6(4):65. https://doi.org/10.3390/colloids6040065
Chicago/Turabian StyleRichter, Ana R., José G. Veras-Neto, Jeanlex S. Sousa, Josilayne F. S. Mendes, Raquel O. S. Fontenelle, Stéphanie A. N. M. Silva, José D. B. Marinho-Filho, Ana J. Araújo, Judith P. A. Feitosa, Haroldo C. B. Paula, and et al. 2022. "Effect of Acyl Chain Length on Hydrophobized Cashew Gum Self-Assembling Nanoparticles: Colloidal Properties and Amphotericin B Delivery" Colloids and Interfaces 6, no. 4: 65. https://doi.org/10.3390/colloids6040065
APA StyleRichter, A. R., Veras-Neto, J. G., Sousa, J. S., Mendes, J. F. S., Fontenelle, R. O. S., Silva, S. A. N. M., Marinho-Filho, J. D. B., Araújo, A. J., Feitosa, J. P. A., Paula, H. C. B., Goycoolea, F. M., & Paula, R. C. M. d. (2022). Effect of Acyl Chain Length on Hydrophobized Cashew Gum Self-Assembling Nanoparticles: Colloidal Properties and Amphotericin B Delivery. Colloids and Interfaces, 6(4), 65. https://doi.org/10.3390/colloids6040065







