Nucleation, Coalescence, and Thin-Film Growth of Triflate-Based Ionic Liquids on ITO, Ag, and Au Surfaces
Abstract
:1. Introduction
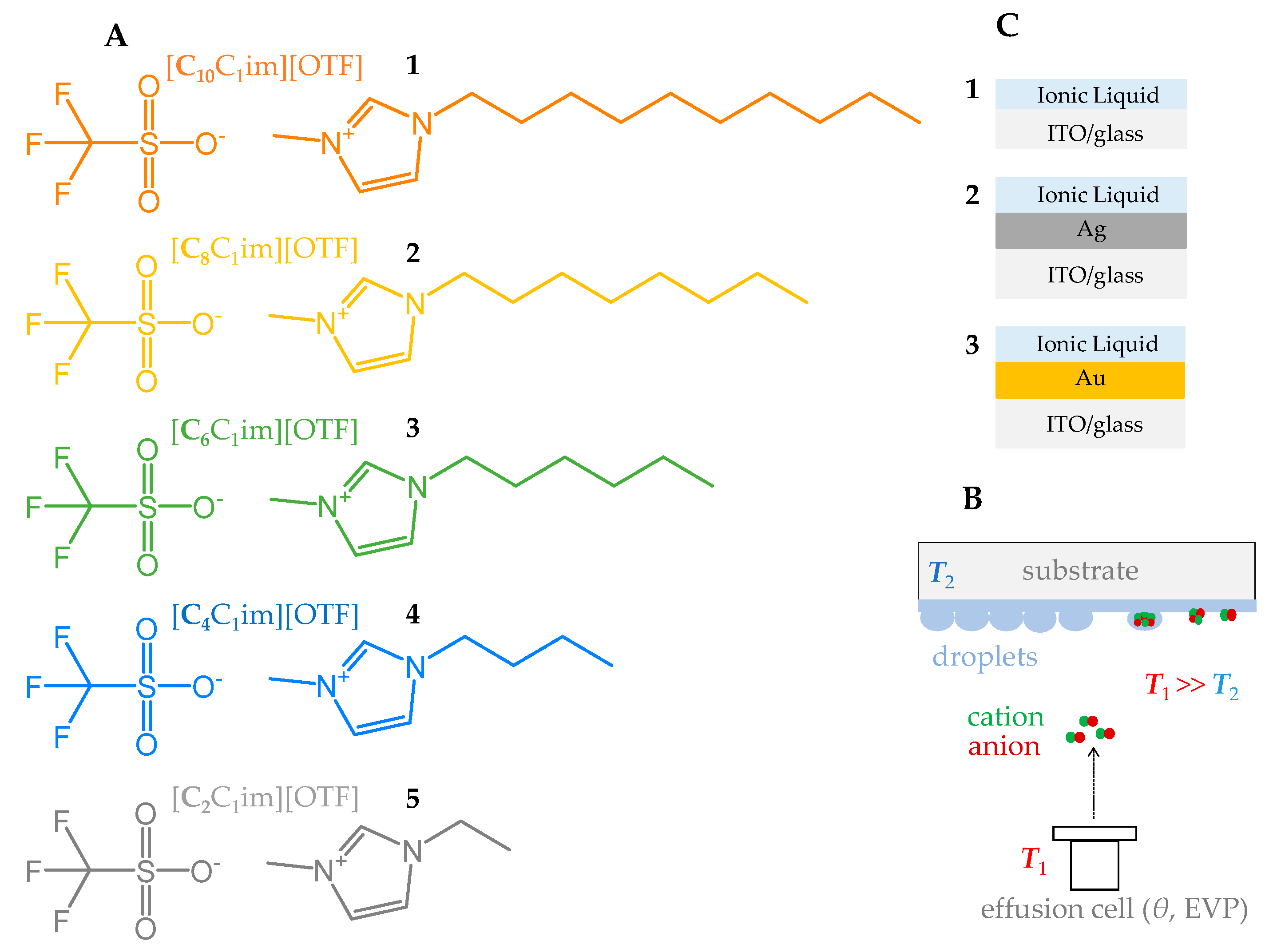
2. Materials and Methods
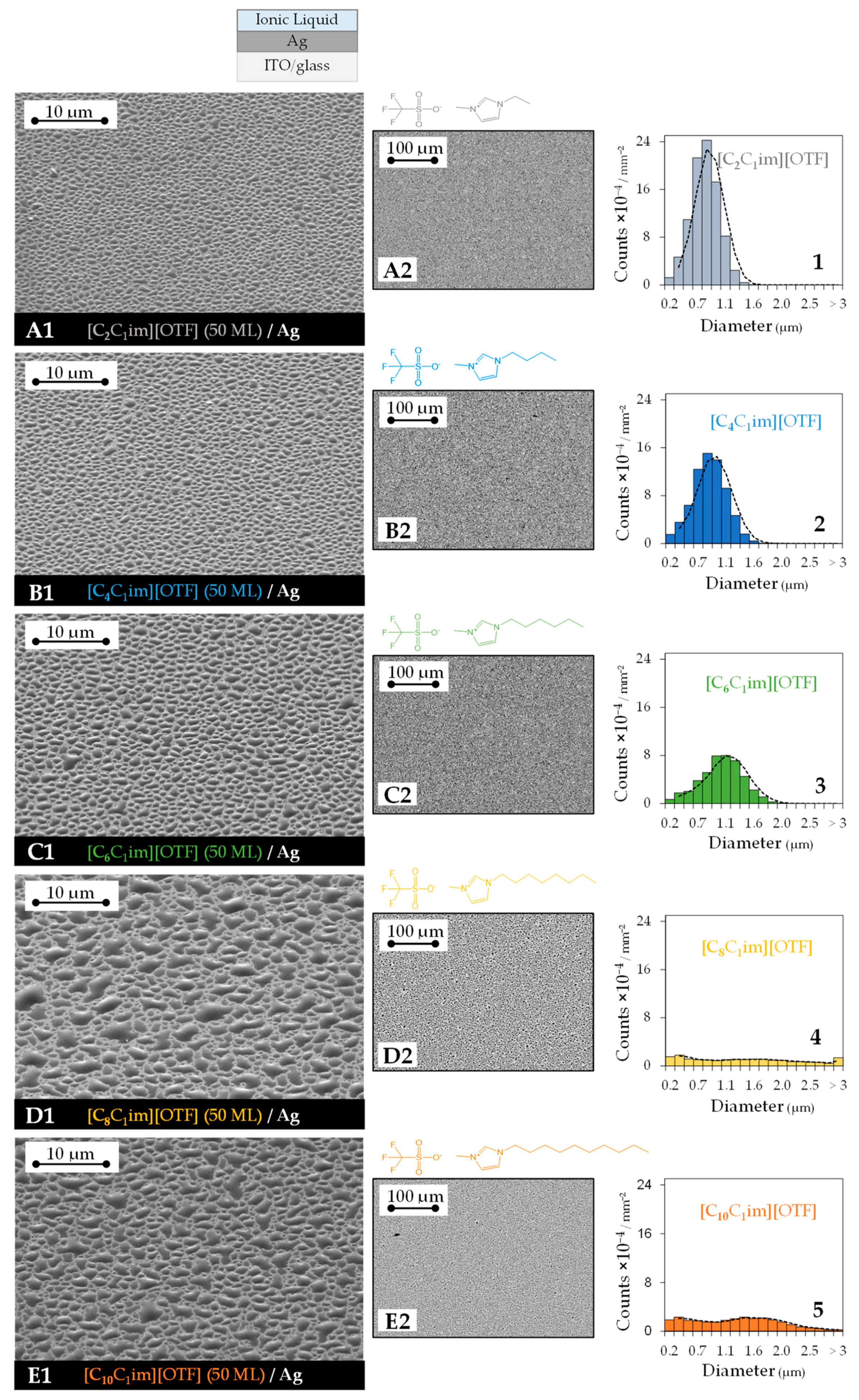
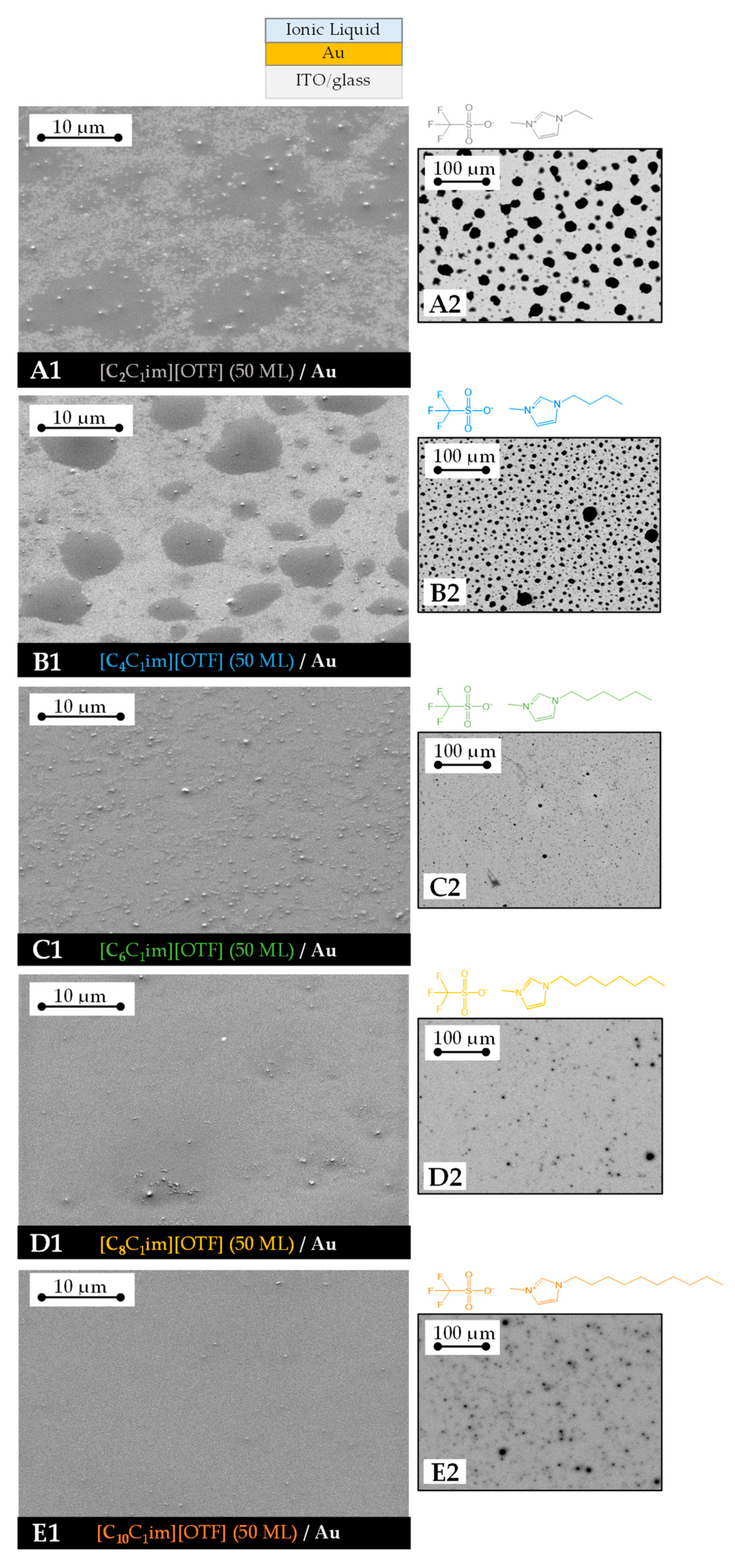
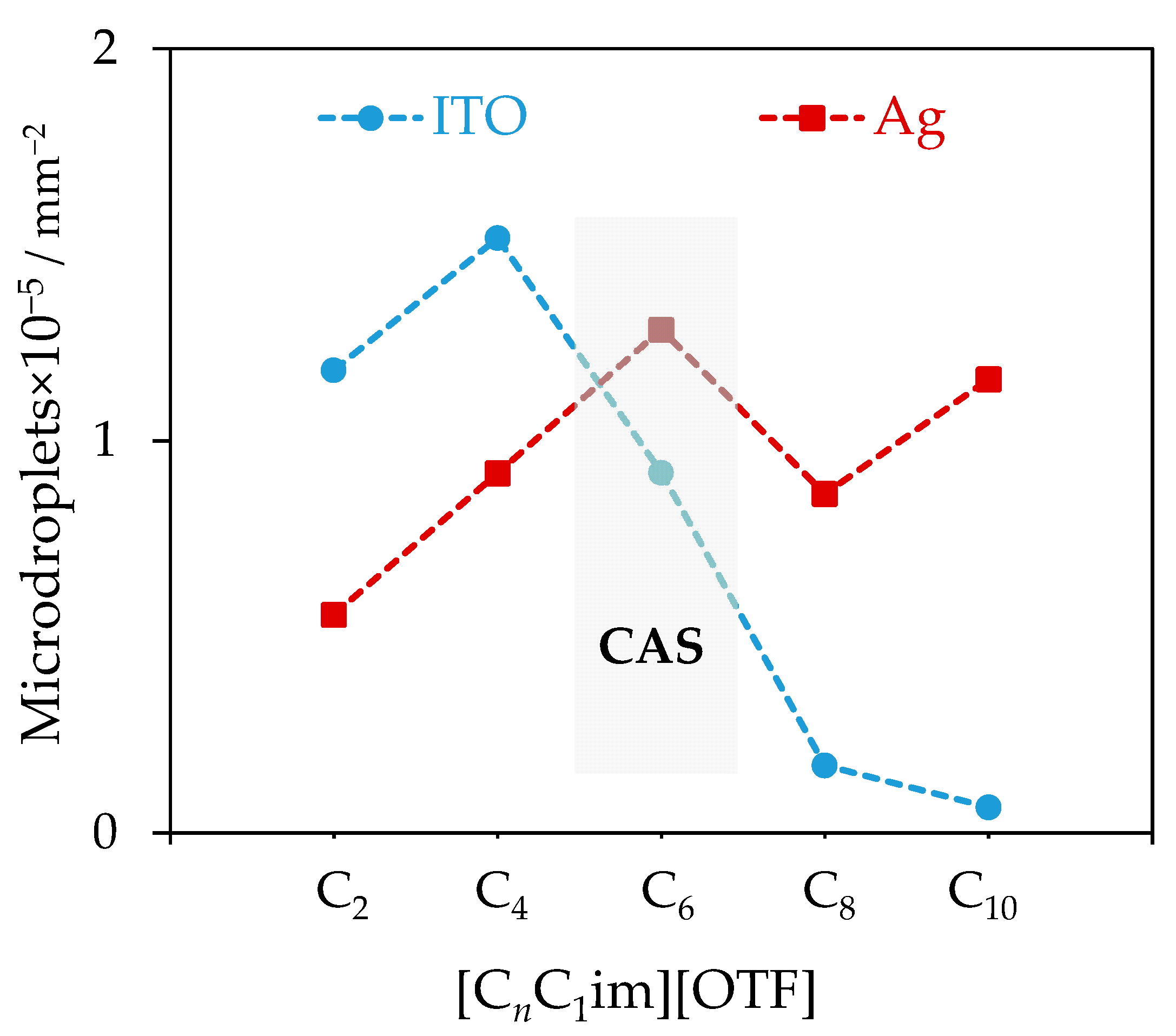
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, R.; Warr, G.G. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [PubMed]
- Canongia Lopes, J.N.A.; Pádua, A.A.H. Nanostructural Organization in Ionic Liquids. J. Phys. Chem. B 2006, 110, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Del Pópolo, M.G.; Voth, G.A. On the Structure and Dynamics of Ionic Liquids. J. Phys. Chem. B 2004, 108, 1744–1752. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.E. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Weingärtner, H. Understanding Ionic Liquids at the Molecular Level: Facts, Problems, and Controversies. Angew. Chem. Int. Ed. 2008, 47, 654–670. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic Liquids in Analytical Chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef]
- Wishart, J.F.; Castner, E.W., Jr. The Physical Chemistry of Ionic Liquids. J. Phys. Chem. B 2007, 111, 4639–4640. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical Properties of Ionic Liquids: Database and Evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Werner, S.; Haumann, M.; Wasserscheid, P. Ionic Liquids in Chemical Engineering. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible Ionic Liquids: Fundamental Behaviours and Applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H. Ionic Liquids for Electrochemical Energy Storage Devices Applications. J. Mater. Sci. Technol. 2019, 35, 674–686. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef] [PubMed]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The Distillation and Volatility of Ionic Liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- Esperança, J.M.S.S.; Canongia Lopes, J.N.; Tariq, M.; Santos, L.M.N.B.F.; Magee, J.W.; Rebelo, L.P.N. Volatility of Aprotic Ionic Liquids—A Review. J. Chem. Eng. Data 2010, 55, 3–12. [Google Scholar] [CrossRef]
- Ravula, S.; Larm, N.E.; Mottaleb, M.A.; Heitz, M.P.; Baker, G.A. Vapor Pressure Mapping of Ionic Liquids and Low-Volatility Fluids Using Graded Isothermal Thermogravimetric Analysis. ChemEngineering 2019, 3, 42. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Lima, C.F.R.A.C.; Gomes, L.R.; Schroder, B.; Coutinho, J.A.P.; Marrucho, I.M.; Esperança, J.M.S.S.; Rebelo, L.P.N.; Shimizu, K.; Canongia Lopes, J.N.; et al. High-Accuracy Vapor Pressure Data of the Extended [CnC1im][Ntf2] Ionic Liquid Series: Trend Changes and Structural Shifts. J. Phys. Chem. B 2011, 115, 10919–10926. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Freire, M.G.; Fernandes, A.M.; Lopes-da-Silva, J.A.; Morgado, P.; Shimizu, K.; Filipe, E.J.M.; Canongia Lopes, J.N.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Cation Alkyl Side Chain Length and Symmetry Effects on the Surface Tension of Ionic Liquids. Langmuir 2014, 30, 6408–6418. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, L. Wettability by Ionic Liquids. Small 2016, 12, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Minea, A.A.; Murshed, S.M.S. A Review on Development of Ionic Liquid Based Nanofluids and their Heat Transfer Behavior. Renew. Sustain. Energy Rev. 2018, 91, 584–599. [Google Scholar] [CrossRef]
- Yebra, F.; Troncoso, J.; Romaní, K. Thermal Conductivity of Ionic Liquids under Pressure. Fluid Phase Equil. 2020, 515, 112573. [Google Scholar] [CrossRef]
- Bica, K.; Deetlefs, M.; Schroder, C.; Seddon, K.R. Polarisabilities of Alkylimidazolium Ionic Liquids. Phys. Chem. Chem. Phys. 2013, 15, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Dutta, C.K.; Rather, G.M. Exploring Physicochemical Aspects of N-alkylimidazolium Based Ionic Liquids. J. Mol. Liq. 2013, 181, 142–151. [Google Scholar] [CrossRef]
- Fadeeva, Y.A.; Gruzdev, M.S.; Kudryakova, N.O.; Shmukler, L.E.; Safonova, L.P. Physico-Chemical Characterization of Alkyl-imidazolium Protic Ionic Liquids. J. Mol. Liq. 2020, 297, 111305. [Google Scholar] [CrossRef]
- Sanchora, P.; Pandey, D.K.; Kagdada, H.L.; Materny, A.; Singh, D.K. Impact of Alkyl Chain Length and Water on the Structure and Properties of 1-Alkyl-3-Methylimidazolium Chloride Ionic Liquids. Phys. Chem. Chem. Phys. 2020, 22, 17687–17704. [Google Scholar] [CrossRef]
- Singh, T.; Kumar, A. Aggregation Behavior of Ionic Liquids in Aqueous Solutions: Effect of Alkyl Chain Length, Cations, and Anions. J. Phys. Chem. B 2007, 111, 7843–7851. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Neves, C.M.S.S.; Freire, M.G.; Russina, O.; Triolo, A.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Alkylimidazolium Based Ionic Liquids: Impact of Cation Symmetry on Their Nanoscale Structural Organization. J. Phys. Chem. B 2013, 117, 10889–10897. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Mohammed, A.; Hines, L.G.; Xiao, D.; Martinez, O.J.; Bartsch, R.A.; Simon, S.L.; Russina, O.; Triolo, A.; Quitevis, E.L. Effect of Cation Symmetry on the Morphology and Physicochemical Properties of Imidazolium Ionic Liquids. J. Phys. Chem. B 2011, 115, 6572–6584. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Gurung, E.; Tamas, G.; Koh, Y.P.; Shadeck, M.; Simon, S.L.; Maroncelli, M.; Quitevis, E.L. Effect of Alkyl Chain Branching on Physicochemical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2016, 61, 1078–1091. [Google Scholar] [CrossRef]
- Shimizu, K.; Costa Gomes, M.F.; Pádua, A.A.H.; Rebelo, L.P.N.; Canongia Lopes, J.N. Three Commentaries on the Nano-segregated Structure of Ionic Liquids. Theochem 2010, 946, 70–76. [Google Scholar] [CrossRef]
- Abe, H.; Fukushima, R.; Onji, M.; Hirayama, K.; Kishimura, H.; Yoshimura, Y.; Ozawa, S. Two-Length Scale Description of Hydrophobic Room-Temperature Ionic Liquid–Alcohol Systems. J. Mol. Liq. 2016, 215, 417–422. [Google Scholar] [CrossRef]
- Rodrigues, A.S.M.C.; Santos, L.M.N.B.F. Nanostructuration Effect on the Thermal Behavior of Ionic Liquids. ChemPhysChem 2016, 17, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.S.; Alves, A.; Bastos, M.; Santos, L.M.N.B.F. The Impact of the Cation Alkyl Chain Length on the Wettability of Alkylimidazolium-Based Ionic Liquids at the Nanoscale. Phys. Chem. Chem. Phys. 2022, 14, 13343–13355. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.S.; Mendes, A.; Santos, L.M.N.B.F. Morphology of Imidazolium-Based Ionic Liquids as Deposited by Vapor Deposition: Micro-/Nanodroplets and Thin Films. ChemPhysChem 2016, 17, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
- Rietzler, F.; May, B.; Steinruck, H.-P.; Maier, F. Switching Adsorption and Growth behavior of Ultrathin [C2C1Im][OTf] films on Au(111) by Pd deposition. Phys. Chem. Chem. Phys. 2016, 18, 25143–25150. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Coelho, A.F.S.M.G.; Mendes, A.; Santos, L.M.N.B.F. Nucleation and Growth of Microdroplets of Ionic Liquids Deposited by Physical Vapor Method onto Different Surfaces. Appl. Surf. Sci. 2018, 428, 242–249. [Google Scholar] [CrossRef]
- Campos, R.M.; Alves, A.C.P.M.; Lima, M.A.L.; Farinha, A.F.M.; Cardoso, J.P.S.; Mendes, A.; Costa, J.C.S.; Santos, L.M.N.B.F. Morphology, Structure, and Dynamics of Pentacene Thin Films and Their Nanocomposites with [C2C1im][NTf2] and [C2C1im][OTF] Ionic Liquids. ChemPhysChem 2020, 21, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Takeyama, Y.; Taniguchi, H.; Fukumoto, H.; Itoh, M.; Kumigashira, H.; Oshima, M.; Yamamoto, T.; Matsumoto, Y. Molecular Beam Deposition of Nanoscale Ionic Liquids in Ultrahigh Vacuum. ACS Nano 2010, 4, 5946–5952. [Google Scholar] [CrossRef] [PubMed]
- Meusel, M.; Lexow, M.; Gezmis, A.; Bayer, A.; Maier, F.; Steinruck, H.-P. Growth of Multilayers of Ionic Liquids on Au(111) Investigated by Atomic Force Microscopy in Ultrahigh Vacuum. Langmuir 2020, 36, 13670–13681. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Killian, M.; Gottfried, J.M.; Paape, N.; Wasserscheid, P.; Maier, F.; Steinruck, H.-P. Physical Vapor Deposition of [EMIM][Tf2N]: A New Approach to the Modification of Surface Properties with Ultrathin Ionic Liquid Films. ChemPhysChem 2008, 9, 2185–2190. [Google Scholar] [CrossRef]
- Borghi, F.; Podestà, A. Ionic Liquids under Nanoscale Confinement. Adv. Phys. X 2020, 5, 1736949. [Google Scholar] [CrossRef] [Green Version]
- Richey, N.E.; Paula, C.; Bent, S.F. Understanding Chemical and Physical Mechanisms in Atomic Layer Deposition. J. Chem. Phys. 2020, 152, 040902. [Google Scholar] [CrossRef]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and Growth of Thin Films. Rep. Progr. Phys. 1984, 47, 399–459. [Google Scholar] [CrossRef]
- Venables, J.A.; Spiller, G.D.T. Nucleation and Growth of Thin Films. In Surface Mobilities on Solid Materials; Springer: Boston, MA, USA, 1983; pp. 341–404. [Google Scholar]
- Ratsch, C.; Venables, J.A. Nucleation Theory and the Early Stages of Thin Film Growth. J. Vac. Sci. Technol. A Vacuum Surfaces Films 2003, 21, S96–S109. [Google Scholar] [CrossRef]
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Adam, N.K. Use of the Term ‘Young’s Equation’ for Contact Angles. Nature 1957, 180, 809–810. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Giljean, S.; Bigerelle, M.; Anselme, K.; Haidara, H. New Insights on Contact Angle/Roughness Dependence on High Surface Energy Materials. Appl. Surf. Sci. 2011, 257, 9631–9638. [Google Scholar] [CrossRef]
- Foadi, F.; Vaez Allaei, S.M.; Palasantzas, G.; Mohammadizadeh, M.R. Roughness-Dependent Wetting Behavior of Vapor-Deposited Metallic Thin Films. Phys. Rev. E 2019, 100, 022804. [Google Scholar] [CrossRef]
- Weijs, J.H.; Marchand, A.; Andreotti, B.; Lohse, D.; Snoeijer, J.H. Origin of Line Tension for a Lennard-Jones Nanodroplet. Phys. Fluids 2011, 23, 022001. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Borg, M.K.; Reese, J.M.; Wen, D. A Critical Assessment of the Line Tension Determined by the Modified Young’s Equation. Phys. Fluids 2018, 30, 082003. [Google Scholar] [CrossRef]
- Deyko, A.; Cremer, T.; Rietzler, F.; Perkin, S.; Crowhurst, L.; Welton, T.; Steinruck, H.-P.; Maier, F. Interfacial Behavior of Thin Ionic Liquid Films on Mica. J. Phys. Chem. C 2013, 117, 5101–5111. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Rocha, R.M.; Vaz, I.C.M.; Torres, M.C.; Mendes, A.; Santos, L.M.N.B.F. Description and Test of a New Multilayer Thin Film Vapor Deposition Apparatus for Organic Semiconductor Materials. J. Chem. Eng. Data 2015, 60, 3776–3791. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Azevedo, J.; Araújo, J.P.; Santos, L.M.N.B.F.; Mendes, A. High Purity and Crystalline Thin Films of Methylammonium Lead Iodide Perovskites by a Vapor Deposition Approach. Thin Solid Films 2018, 664, 12–18. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Mendes, A.; Santos, L.M.N.B.F. Thin Film Deposition of Organic Hole Transporting Materials: Optical, Thermodynamic and Morphological Properties of Naphthyl-Substituted Benzidines. J. Mater. Sci. 2018, 53, 12974–12987. [Google Scholar] [CrossRef]
- Santos, L.M.N.B.F.; Lobo Ferreira, A.I.M.C.; Štejfa, V.; Rodrigues, A.S.M.C.; Rocha, M.A.A.; Torres, M.C.; Tavares, F.M.S.; Carpinteiro, F.S. Development of the Knudsen Effusion Methodology for Vapour Pressure Measurements of Low Volatile Liquids and Solids Based on a Quartz Crystal Microbalance. J. Chem. Thermodyn. 2018, 126, 171–186. [Google Scholar] [CrossRef]
- Swift, P. Adventitious Carbon—The Panacea for Energy Referencing? Surf. Interface Anal. 1982, 4, 47–51. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Foulston, R.; Gangopadhyay, S.; Chiutu, C.; Moriarty, P.; Jones, R.C. Mono- and Multilayer Adsorption of an Ionic Liquid on Au(110). Phys. Chem. Chem. Phys. 2012, 14, 6054–6066. [Google Scholar] [CrossRef]
- Uhl, B.; Huang, H.; Alwast, D.; Buchner, F.; Behm, R.J. Interaction of Ionic Liquids with Noble Metal Surfaces: Structure Formation and Stability of [OMIM][TFSA] and [EMIM][TFSA] on Au(111) and Ag(111). Phys. Chem. Chem. Phys. 2015, 17, 23816–23832. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Kurnia, K.A.; Sousa, F.L.; Silva, N.J.O.; Lopes da Silva, J.A.; Coutinho, J.A.P.; Freire, M.G. Contact Angles and Wettability of Ionic Liquids on Polar and non-Polar Surfaces. Phys. Chem. Chem. Phys. 2015, 17, 31653–31661. [Google Scholar] [CrossRef] [PubMed]
- Delcheva, I.; Beattie, D.A.; Ralston, J.; Krasowska, M. Dynamic Wetting of Imidazolium-Based Ionic Liquids on Gold and Glass. Phys. Chem. Chem. Phys. 2018, 20, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Delcheva, I.; Ralston, J.; Beattie, D.A.; Krasowska, M. Static and Dynamic Wetting Behaviour of Ionic Liquids. Adv. Colloid Interface Sci. 2015, 222, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Carrera, G.V.S.M.; Afonso, C.A.M.; Branco, L.C. Interfacial Properties, Densities, and Contact Angles of Task Specific Ionic Liquids. J. Chem. Eng. Data 2010, 55, 609–615. [Google Scholar] [CrossRef]
- Lexow, M.; Maier, F.; Steinruck, H.-P. Ultrathin Ionic Liquid Films on Metal Surfaces: Adsorption, Growth, Stability and Exchange Phenomena. Adv. Phys. X 2020, 5, 1761266. [Google Scholar] [CrossRef]
- Lexow, M.; Talwar, T.; Heller, B.S.-J.; May, B.; Bhuin, R.G.; Maier, F.; Steinruck, H.-P. Time-Dependent Changes in the Growth of Ultrathin Ionic Liquid Films on Ag(111). Phys. Chem. Chem. Phys. 2018, 20, 12929–12938. [Google Scholar] [CrossRef]
- Cremer, T.; Stark, M.; Deyko, A.; Steinruck, H.-P.; Maier, F. Liquid/Solid Interface of Ultrathin Ionic Liquid Films: [C1C1Im][Tf2N] and [C8C1Im][Tf2N] on Au(111). Langmuir 2011, 27, 3662–3671. [Google Scholar] [CrossRef]
- Meusel, M.; Lexow, M.; Gezmis, A.; Schötz, S.; Wagner, M.; Bayer, A.; Maier, F.; Steinruck, H.-P. Atomic Force and Scanning Tunneling Microscopy of Ordered Ionic Liquid Wetting Layers from 110 K up to Room Temperature. ACS Nano 2020, 14, 9000–9010. [Google Scholar] [CrossRef]
- Jha, K.C.; Liu, H.; Bockstaller, M.R.; Heinz, H. Facet Recognition and Molecular Ordering of Ionic Liquids on Metal Surfaces. J. Phys. Chem. C 2013, 117, 25969–25981. [Google Scholar] [CrossRef]
- Atkin, R.; El Abedin, S.Z.; Hayes, R.; Gasparotto, L.H.S.; Borisenko, N.; Endres, F. AFM and STM Studies on the Surface Interaction of [BMP]TFSA and [EMIm]TFSA Ionic Liquids with Au(111). J. Phys. Chem. C 2009, 113, 13266–13272. [Google Scholar] [CrossRef]
- Kamalakannan, S.; Prakash, M.; Chambaud, G.; Hochlaf, M. Adsorption of Hydrophobic and Hydrophilic Ionic Liquids at the Au(111) Surface. ACS Omega 2018, 3, 18039–18051. [Google Scholar] [CrossRef] [PubMed]
- Borghi, F.; Mirigliano, M.; Lenardi, C.; Milani, P.; Podestà, A. Nanostructure Determines the Wettability of Gold Surfaces by Ionic Liquid Ultrathin Films. Front. Chem. 2021, 9, 619432. [Google Scholar] [PubMed]
- Hessey, S.G.; Jones, R.G. On the Evaporation, Bonding, and Adsorbate Capture of an Ionic Liquid on Au(111). Chem. Sci. 2013, 4, 2519–2529. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, M.S.M.; Santos, L.M.N.B.F.; Costa, J.C.S. Nucleation, Coalescence, and Thin-Film Growth of Triflate-Based Ionic Liquids on ITO, Ag, and Au Surfaces. Colloids Interfaces 2022, 6, 46. https://doi.org/10.3390/colloids6030046
Teixeira MSM, Santos LMNBF, Costa JCS. Nucleation, Coalescence, and Thin-Film Growth of Triflate-Based Ionic Liquids on ITO, Ag, and Au Surfaces. Colloids and Interfaces. 2022; 6(3):46. https://doi.org/10.3390/colloids6030046
Chicago/Turabian StyleTeixeira, Mariana S. M., Luís M. N. B. F. Santos, and José C. S. Costa. 2022. "Nucleation, Coalescence, and Thin-Film Growth of Triflate-Based Ionic Liquids on ITO, Ag, and Au Surfaces" Colloids and Interfaces 6, no. 3: 46. https://doi.org/10.3390/colloids6030046
APA StyleTeixeira, M. S. M., Santos, L. M. N. B. F., & Costa, J. C. S. (2022). Nucleation, Coalescence, and Thin-Film Growth of Triflate-Based Ionic Liquids on ITO, Ag, and Au Surfaces. Colloids and Interfaces, 6(3), 46. https://doi.org/10.3390/colloids6030046







