Abstract
This study presents the equilibrium surface tension (ST), critical micelle concentration (CMC) and the dilational viscoelasticity of sodium dodecylbenzene sulfonate (SDBS)-adsorbed layers in the presence of NaCl, KCl, LiCl, CaCl2 and MgCl2 at 0.001–0.1 M salt concentration. The ST and surface dilational viscoelasticity were determined using bubble-shape analysis technique. To capture the complete profile of dilational viscoelastic properties of SDBS-adsorbed layers, experiments were conducted within a wide range of SDBS concentrations at a fixed oscillating frequency of 0.01 Hz. Salts were found to lower the ST and induce micellar formation at all concentrations. However, the addition of salts increased dilational viscoelastic modulus only at a certain range of SDBS concentration (below 0.01–0.02 mM SDBS). Above this concentration range, salts decreased dilational viscoelasticity due to the domination of the induced molecular exchange dampening the ST gradient. The dilational viscoelasticity of the salts of interest were in the order CaCl2 > MgCl2 > KCl > NaCl > LiCl. The charge density of ions was found as the corresponding factor for the higher impact of divalent ions compared to monovalent ions, while the impact of monovalent ions was assigned to the degree of matching in water affinities, and thereby the tendency for ion-pairing between SDBS head groups and monovalent ions.
1. Introduction
The knowledge of interfacial dynamic properties of surfactant-adsorbed layers in the presence of inorganic electrolytes is essential for many industries dealing with foaming, emulsification and coating, such as pharmaceuticals [1], mining [2,3,4], environmental remediation [5,6], food [7,8] and biology [9,10], enhanced oil recovery [11] and gas well deliquification [12,13,14,15]. The stability of foam/emulsion is strongly influenced by surface properties of gas–fluid interfaces containing adsorbed surface-active compounds such as surfactants. Surface properties are imposed by the composition and structure of surface-active compounds at the interface and the inherent relaxation processes. Surface dilational rheology, demonstrating the dynamic surface response to variations in the surface area, while the shape is conserved, has been recognised as a powerful technique for investigating the dynamic surface behaviour of systems such as surfactant-adsorbed layers, the building block of foams, froths and emulsions [16].
The knowledge of dilational surface rheology provides insights into the conformation transitions of molecules at the interface, Marangoni flow and energy dissipation in the surface region, as well as foam/emulsion instability mechanisms such as liquid drainage, coalescence, and Ostwald ripening [17,18,19,20]. A higher dilational elastic modulus implies the ability of surfactant layers to prevent deformation via storing more energy. On the other hand, the flow resistance and molecules’ interaction during the oscillation could result in irreversible loss of energy of surfactant molecules, increasing the viscous modulus [21].
The dilatational interfacial rheology is conducted by altering the interfacial area and measuring the interfacial response to the change in the area [16,22]. The dynamic variation in interfacial tension is a consequence of relaxation processes within the adsorption layer, which are comprised of two main processes: (i) exchange of surfactant molecules between the adsorption layer and bulk solution, (ii) rearrangements of the molecules at the interface, changes of the orientation or conformation of the molecules and their aggregation.
Salts affect the surface activity of the surfactant, thus altering the dilational rheology of the interface [23]. Despite the numerous studies available on the effect of salt type on various physicochemical and interfacial properties of the surfactant solutions, few systematic investigations have focused on the effect of the type of added electrolytes on the dilational interfacial rheology of surfactant solutions [24,25,26,27]. Some have reported an increase in the dilational viscoelasticity [25,27,28], while others reported a decrease with the addition of salts [29,30]. The reason for this seemingly contradicting information is that most of the research that has been done in this field has focused only on a fixed surfactant concentration, while it is known that the surface viscoelasticity highly depends on the surfactant concentration. For instance, Ruwoldt et al. [27] investigated the surface shear viscoelasticity of 1.8 g/L lignosulfonate solutions in the presence of mono-, di- and trivalent cations. They showed that the addition of up to 50 mM NaCl, 3 mM CaCl2 and 1 mM AlCl3 increases the surface shear viscoelastic modulus, while a further increase in the salt concentration results in a decline in the viscoelasticity because of induced lignosulfonate precipitation. Sett et al. [28] investigated the addition of three monovalent salts (NaCl, KCl, and LiCl) to sodium dodecyl sulfate (SDS) solutions at 0.5 mM SDS. They reported that adding 11 mM salts to 0.5 mM SDS solutions results in a decline in the surface tension and an increase in the dilational elasticity modulus in the order of KCl > NaCl > LiCl. In another study, Vera et al. [26] investigated the effect of MgCl2, CaCl2, NaCl, NH4Cl, NaNO3, CH3COONa, or Na2SO4 on the surface properties of the SDBS (0.58 mM)-heptane-brine system at concentrations where the hydrophilic–lipophilic deviation is zero. They reported the order of SO42− CH3COO– Cl− NO3− for SDBS co-ions and Mg2+ Ca2+ Na+ NH4+ for SDBS counter-ions from highest to lowest rigidity (surface elasticity) [26].
All of the abovementioned studies on the effect of salts on surface viscoelasticity of surfactant-adsorbed layers are limited to a specific surfactant or salt concentration. As demonstrated in our recent work [16,31], a wide range of surfactant and salt concentrations need to be considered for provision of a clear image of the role of salts in surfactant solutions. In our previous work [31], we illustrated that adding NaCl (0.01–0.3 M) to the sodium dodecylbenzene sulfonate (SDBS) solutions can both increase and decrease the viscoelasticity depending on the surfactant and salt concentration. The contribution of NaCl to increase the viscoelastic modulus, observed at low SDBS concentrations, was attributed to the induced adsorption of SDBS layers at the interface. On the other hand, the increased molecular exchange between the bulk and the interface was argued to be responsible for the reduced viscoelastic modulus with the addition of salt to the SDBS solutions at relatively high surfactant concentrations [31].
In this paper, we aim to elaborate on the effect of adding different types and concentrations of salts on the surface properties of SDBS surfactant solutions at a wide range of surfactant concentrations. To the best of our knowledge, there is no such study showing the salt effect on the surface rheology of ionic surfactants [16]. Here, the effect of adding 0.001–0.1 M monovalent and divalent salts including NaCl, KCl, LiCl, CaCl2, and MgCl2 on the surface dilational rheology of anionic SDBS solutions is investigated. The equilibrium surface tension and dilational viscoelasticity within a wide range of concentrations of salts and SDBS are determined.
2. Materials and Methods
The technical-grade sodium dodecylbenzene sulfonate (SDBS) with a purity of more than 99% supplied by Sigma Aldrich, was used as the anionic surfactant. Sodium chloride (NaCl), potassium chloride (KCl), lithium chloride (LiCl), magnesium chloride (MgCl2), and calcium chloride (CaCl2) were supplied by Sigma Aldrich with a purity of more than 99% and are used as electrolytes. All salts were further purified by progressively being heated in a furnace up to 500 °C, remaining at this temperature for 2 h and then slowly cooled down to room temperature. The solutions of salt and surfactant were prepared using Milli-Q water.
The dynamic surface tension and the surface dilational viscoelasticity were measured using a drop profile analyser PAT-1 (SINTERFACE Technologies, Berlin, Germany). For measuring the surface dilational rheology, a sinusoidal signal is applied to a pendant bubble of air at a controlled frequency and amplitude. The recommended frequency and amplitude range for this device are 0.01–0.1 Hz and 8–10% of the surface area, respectively [22]. In our previous study, we showed that a low frequency of surface perturbations between 0.01 and 0.1 Hz is appropriate for investigating the surface dilational rheology of SDBS solutions in the absence and presence of 0.01–0.3 M NaCl [31].
The dilational viscoelasticity modulus is determined from the ratio of the change in the surface tension to the relative variation in the surface area, as below:
where is the dilational modulus, and and represent the change in the surface tension and the relative surface area. For harmonic perturbations with small amplitudes, the viscoelastic modulus is a complex quantity as . The real part represents the elasticity of the surface (the ability to store energy), while the imaginary part represents the viscosity (the ability to dissipate energy); represents the phase angle which reflects the ratio of the viscous modulus over the elasticity modulus.
3. Results and Discussions
3.1. Equilibrium Surface Tension (ST)
The equilibrium ST at different SDBS concentrations in the presence of different concentrations of salts including NaCl, KCl, LiCl, CaCl2 and MgCl2 is shown in Figure 1. The general trend shows a continuous decline in ST with increasing SDBS concentration until the critical micelle concentration (CMC) is reached, where the interface becomes saturated, and ST remains almost constant. The CMC of SDBS solution in the absence of any salt is found to be around 2.9 mM, which is in line with other reported values for the CMC of SDBS [26,32].
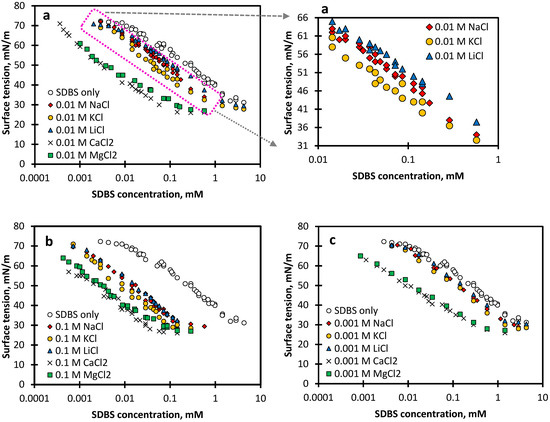
Figure 1.
Surface tension as a function of SDBS concentration in the presence of different salt concentrations: (a) 0.01 M salt, (b) 0.1 M salt, and (c) 0.001 M salt.
As expected at all surfactant concentrations, the addition of 0.001–0.1 M of all salts was found to lower the equilibrium ST: the higher the salt concentration, the lower the ST. This is attributed to the salts (counter-ions) screening out the electrostatic repulsion between neighbouring anionic surfactant molecules, resulting in a denser surfactant layer at the air–water interface. In addition, the presence of electrolytes forces out the polar head groups of the SDBS molecules from water to the air–water interface, advancing the formation of micelles in the surfactant solution [16,31]. This is known as the salting-out effect, resulting in a lower CMC, as reported in Figure 2a.
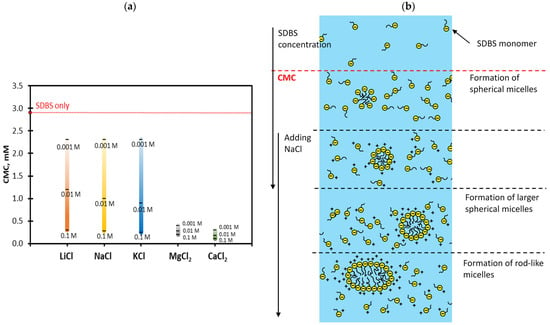
Figure 2.
(a) CMC of SDBS in the presence of different salt concentrations. (b) Schematic representation of SDBS micellization in the absence and presence of salts; adapted with permission from [31].
It should be highlighted that salts not only promote the growth rate of micelles (reduces the CMC), but they also increase the size and shape of micelles [32,33]. Sood and Aggarwal [32] showed that, for SDBS and salt solutions at a concentration twice the observed CMC of the mixed system, the hydrodynamic diameter of the micelles increased drastically by approximately 25 nm and 43 nm with the addition of 10 mM NaCl and MgCl2, respectively. The associated change in the packing parameter Pm defined as Pm = υ/(A0l) (υ and l represent the volume and length of the hydrophobic chain of the micelle and A0 is the micellar surface area per head group) also confirmed the change in the shape of the micelles from spherical to cylindrical at higher salt concentrations. This was consistent with an earlier study of Cheng and Gulari in 1982, showing the prolate ellipsoid or the rod shape micellar aggregates using quasielastic light scattering [33], as depicted in Figure 2b.
3.1.1. Effect of Counter-Ions
From Figure 1 and Figure 2, the ability of salts to lower ST and the CMC of SDBS solutions was found to follow Ca2+ > Mg2+ > K+ > Na+ > Li+. Salts of higher valency make the physicochemical environment less hydrophilic for surfactant molecules (due to the competition for water molecules), therefore having a more pronounced impact on the surface activity of dodecylbenzene sulfonate ions and causing micellization at lower surfactant concentrations [24,26,34]. Our results indicated that the observed trend among divalent (Ca2+ > Mg2+) and monovalent salts (K+ > Na+ > Li+) follows a reverse order of the Hofmeister series for cations. This reverse trend was also observed by other researchers [26,28,35,36,37].
If we order the monovalent salts according to their specific energy of adsorption on the air–water interface, it follows K+ > Na+ > Li+, which is also the reverse of the Hofmeister series [28,38]. This trend can be explained by applying the “law of matching water affinities (LMWA)” concept, originally formulated by Collins to elucidate the Hofmeister interactions. Ions were classified as kosmotropes, small ions with high charge density that bind tightly with water molecules and form a tight hydration shell. Unlike kosmotropes, chaotropes are large ions with low charge density which interact weakly with water molecules and form a loose hydration shell. Salts containing two oppositely charged kosmotropes form a strong ion pair due to strong electrostatic interactions. Salts containing two oppositely charged chaotropes also pair due to their loose hydration shells, despite their weak electrostatic interactions. However, salts comprising one kosmotropic and one chaotropic ion tends to remain apart in aqueous solutions, due to the opposite affinity of the ions to the interface [39].
The LMWA is therefore understood as a characterisation of the “degree of hydration”, claiming that counter-ions with similar hydration enthalpies have a higher tendency to form stable ion pairs [40]. Later, the Hofmeister series and LMWA were extended to colloidal systems such as surfactant solutions. It is reported that the interaction between the specific ion and the surfactant molecules can significantly affect the micellar formation and the shape and size of aggregates [32,41]. Kunz and coworkers [36,42,43] classified ionic surfactants as kosmotropes and chaotropes according to the nature of their head group. From kosmotropic to chaotropic, the order for anionic surfactant head groups is R-Carboxylate > R-Phosphate > R-Sulphate > R-Sulfonate. The same order for cationic surfactants is R-Dimethyl sulfonium > R-Trimethyl ammonium > R-Trimethyl phosphonium [36,42,43]. The LMWA concept has also been incorporated to explain counter-ion binding to surfactant head groups [36,42,43]. Figure 3 shows the ordering of anionic surfactant head groups and the respective counter-cations from kosmotropic to chaotropic proposed by Kunz and coworkers [36,43,44].
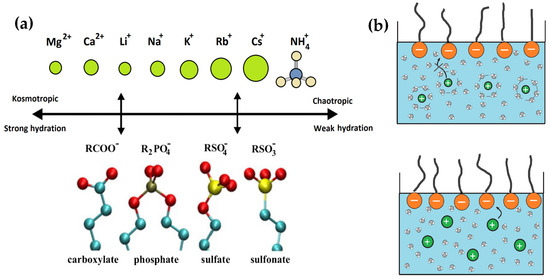
Figure 3.
(a) Ordering of anionic surfactant head groups and the counter-ions (cations) from kosmotrope to chaotrope. (b) Illustration of the cation–SDBS interactions that highlights the preferential interaction between surfactant head groups and counter-ions with strong hydration (top) and weak hydration (bottom).
According to LMWA, when ionic surfactants and counter-ions are both poorly hydrated (large ions, chaotropic) or strongly hydrated (small ions, kosmotropic), they form ion pairs [45].
In this sense, chaotropic surfactants (like SDBS) pair with K+ (being chaotropic) and promote the formation of micelles due to higher electrostatic interactions between the cations and the surfactant head groups, screening out the electrostatic repulsions between the surfactant head groups. However, the interaction between oppositely charged ions with a large difference in water affinity and charge density (i.e., kosmotrope-chaotrope ions) is not favourable. Therefore, in the case of Li+ (kosmotrope) and dodecylbenzene sulfonate (chaotrope), they stay apart because of the strong hydration of Li+ ions and therefore their propensity to stay in the bulk [36,39,46,47]. Consequently, there is less electrostatic interaction between Li+ and SDBS head groups, accommodating fewer surfactant molecules at the interface due to the higher repulsions present in the system compared with K+.
The same explanation applies to divalent cations Ca2+ and Mg2+. Ca2+ cation is less kosmotropic compared to Mg2+ (Figure 3), thereby having a relatively higher tendency to pair with SDBS and accommodating more surfactant molecules at the interface through the electrostatic interactions between the counter-ion and surfactant head group. Therefore, salts (counter-ions) promote the micellization of ionic surfactant through two mechanisms: (i) screening out the electrostatic repulsion between the surfactant head groups through the formation of an ion pair with the surfactant head group, and (ii) salting-out effect due to the formation of a tight hydration shell and not leaving enough water molecules for surfactant molecules to be hydrated.
Here, it is worth highlighting that, despite the high kosmotropic character of divalent cations such as Mg2+ and Ca2+, their impact on the adsorption rate and micellar formation of SDBS molecules is higher than monovalent salts due to having substantially higher charge density.
3.2. Interfacial Rheology
3.2.1. Effect of Surfactant Concentration
The profile of the interfacial dilational elasticity and viscosity moduli as a function of SDBS concentration in the presence of different concentrations of salts including NaCl, KCl, LiCl, CaCl2 and MgCl2 at a fixed frequency of 0.01 Hz is shown in Figure 4.
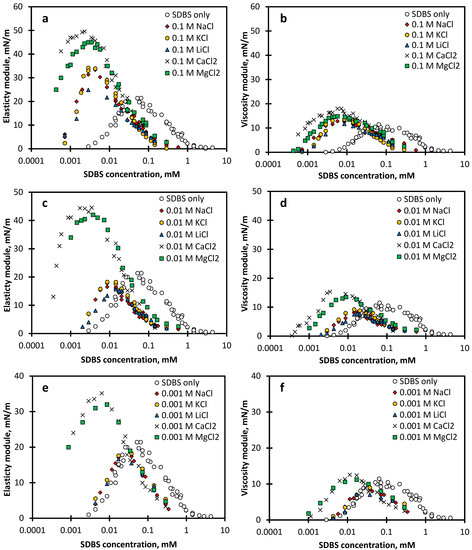
Figure 4.
(a,c,e) Elasticity and (b,d,f) viscosity moduli of SDBS solutions in the presence of different salt concentrations as a function of SDBS concentration at the dilational frequency of 0.01 Hz. (a,b) 0.1 M salt, (c,d) 0.01 M salt, and (e,f) 0.001 M salt.
The general trend of the data shows an ascending behaviour in the surface elasticity and viscosity with increasing surfactant concentration, reaching a local maximum followed by a decrease with a further increase of the SDBS concentration. As alluded to in the introduction, Section 1, the surface dilational viscoelastic behaviour in the presence of surface-active substances is defined by the surfactant relaxation processes on the gas–liquid interface and in the bulk of the liquid. In this study, because the chemical structure of SDBS molecules and counter-ions is very simple, relaxation caused by conformational changes is unlikely and the surfactant exchange between the bulk and the surface remains the only main relaxation process affecting the surface rheology.
With increasing the SDBS bulk concentration, the surface coverage, and the molecular exchange increase. The stimulated surface coverage enhances the surface viscoelasticity, while the induced molecular exchange decreases the viscoelasticity. At low SDBS concentrations, the molecular exchange is relatively less significant, thus the surface elasticity increases with the bulk concentration due to the increase of surface concentration. At higher surfactant concentrations, the diffuse surfactant molecular exchange decreases the significance of the surface tension gradient imposed by the oscillating interface via the fast supply of surfactant to the depleted area and fast surface relaxation, balancing the surface pressure at the interface. Therefore, the counter effect of these two opposing phenomena results in a bell shape behaviour of the viscoelasticity moduli [16,22,48,49]. We refer to the corresponding surfactant concentration where viscoelastic properties peak as the transition concentration [16,31].
Here, it is worth noting that the location of the transition concentration in surfactant solutions with no other additives is dependent on two factors: (I) the type of the surfactant [49,50], and (II) the frequency of the surface oscillations; the higher frequency shifts the maximum E to a higher surfactant concentration [51].
3.2.2. Effect of Adding Salts
As shown in Figure 4, similar to the surfactant-only solutions, dilational viscoelasticity of surfactant-adsorbed layers in the presence of salts shows a bell-shape behaviour; increasing the viscoelasticity with the surfactant concentration up to a maximum, followed by a drop in the dilational viscoelasticity with further addition of surfactant. The addition of all salts is found to shift the transition concentration to a lower SDBS concentration. The extent of the shift in the transition concentration and viscoelastic moduli depends on the type and concentration of the salt (counter-ion). As alluded to earlier, counter-ions of salts enhance the surfactant adsorption by screening out the electrostatic repulsion between the surfactant head groups. The reduced repulsion enhances the surfactant concentration at the interface. Once an oscillation (perturbation) is applied to the gas–liquid interface, the interface experiences a greater ST gradient due to the alteration of the coverage/arrangement in more packed surfactant molecules at the interface. This results in a higher surface viscoelastic modulus, which is defined as Equation (1). Similar to the surfactant-only adsorbed later, a further increase in the surfactant concentration leads to a decline in E due to the fast relaxation process as the result of the compensation for the depleted surfactant molecules. Salts can promote the mobility of surfactant molecules between the bulk and interface, resulting in the dilational viscoelasticity going through a local maximum at a much lower transition concentration relative to the SDBS-only solution.
The local maxima in the elastic modulus (E’max) and viscous modulus (E”max) shown in Figure 4 provide us with useful insights into the effect of electrolytes on the surface rheology of SDBS solutions. It is seen that the addition of salts results in increasing the E’ and E” at low SDBS concentration, compared with the SDBS-only solution. The results also show an increase in the peak value of the E’ and E data, E’max and E”max, compared with the SDBS-only solution (with the exception for low concentration of monovalent salts) [16]. Interestingly, for monovalent salts, we observed a mild decline in the E’max and E”max with the addition of 0.001–0.01 M monovalent salts, followed by a pronounced increase in E’max and E”max with further addition of the salt concentration to 0.1 M. This seemingly anomalous decrease indicates that there is a mild increase in the adsorption rate compared to the induced diffuse exchange rate at very low monovalent salt concentrations, leading to a smaller change in ST during the surface compression/expansion. In the presence of 0.001–0.01 M monovalent salts, the electrical double-layer repulsion is not completely suppressed and there is still a degree of repulsion between SDBS molecules [31]. Elevated molecular exchange as a result of the increase in the surfactant concentration was found to reduce the ST gradient over the expansion/compression of the interface for SDBS solutions with 0.001–0.01 M monovalent salts. Comparing the ST gradient of solutions at which the maximal values of E’ are reported shows that the SDBS-only solution had a surface tension change of ±2.9 mN/m over surface area oscillation, while the corresponding change in ST for the SDBS solution in the presence of 0.01 M LiCl, NaCl and KCl was ±1.8 mN/m, ±1.9 mN/m and ±2.1 mN/m, respectively. This explains a mild decline in and values for SDBS solutions with a low concentration of monovalent salts compared with the SDBS-only solutions.
Figure 5 exhibits the maximum surface viscoelasticity data as a function of the normalised transition concentration, normalised with respect to the CMC of the SDBS solution in the presence of salt. One can see that the transition concentration, the concentration corresponding to Emax, for all SDBS solutions in the absence and presence of different salts falls within the normalised SDBS concentration of 0.01–0.02. This indicates that irrespective of the type and concentration of salts, the surface viscoelasticity of the combined SDBS and salt solutions peaks when the SDBS concentration is at 0.01–0.02 of the CMC of the solution. In other words, in the presence of higher salt concentrations or salts with a higher salting-out effect (i.e., lower CMC), a less amount of SDBS is required to observe the highest dilational viscoelasticity. The effect of oscillation frequency within the range of interest (0.01–0.1 Hz) on the transition concentration was found to be negligible.
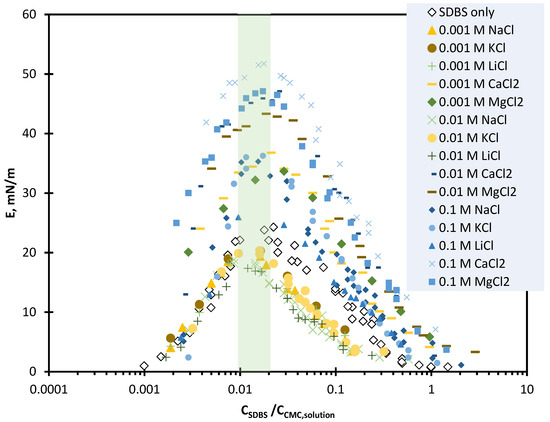
Figure 5.
Dilational viscoelastic modulus of SDBS solutions in the presence of different salt concentrations as a function of the ratio of SDBS concentration to CMC of the solution at the dilational frequency of 0.01 Hz. The light blue area highlights the range of where the elasticity is maximum.
Comparing the dilational viscoelastic properties of combined SDBS and salt solutions with SDBS-only solutions at a fixed surfactant concentration, the data show that, depending on the surfactant concentration, salts may increase or decrease the dilational viscoelasticity of surfactant-adsorbed layers. Below a certain surfactant concentration, salts improve the dilational viscoelastic properties. We refer to this concentration as the intersection concentration. Figure 6 shows the change in the viscoelasticity modulus in the presence of different salts with respect to the SDBS-only solution. The data show that, irrespective of the salt concentration and type, the intersection concentration for monovalent and divalent cations is around 0.02 mM and 0.03 mM, respectively. Figure 6 makes it clear that the contribution of monovalent salts to induced dilational viscoelasticity is more pronounced in the range of a 0.004–0.01 mM SDBS concentration, increasing the dilational viscoelasticity modulus to more than 31 mN/m for 0.1 M KCl. This range shifts to 0.0005–0.006 mM SDBS concentration for selected divalent salts, increasing the viscoelasticity modulus to more than 49 mN/m for 0.1 M CaCl2 (Figure 6f).
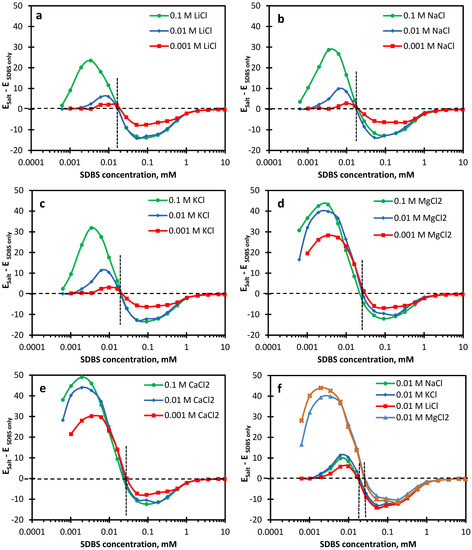
Figure 6.
The change in the viscoelasticity modulus of SDBS solutions with the addition of salts as a function of SDBS concentration. (a) 0.001−0.1 M LiCl, (b) 0.001−0.1 M NaCl, (c) 0.001−0.1 M KCl, (d) 0.001−0.1 M MgCl2, (e) 0.001−0.1 M CaCl2, and (f) 0.01 M of different salts.
3.2.3. Effect of Surface Pressure
As the dilational modulus is closely related to the change in the surface tension during oscillation (Equation (1)), the representation of the viscoelasticity modulus based on surface pressure could provide a better means to detect the impact of salts on the dilational viscoelastic behaviour of the surface layer of different mixtures [17,52]. Figure 7 shows the viscoelastic moduli and phase angle of SDBS and salt solutions as a function of surface pressure. One can easily distinguish the E for different types of salts at relatively high SDBS concentrations (more than the intersection concentration), while the data presented as a function of the SDBS concentration (Figure 4) better illustrate this effect at relatively low SDBS concentrations. Figure 7 also shows a distinct E profile for divalent cations compared to monovalent cations, which is more pronounced at lower salt concentrations. At a fixed surface pressure above ~10 mN/m, divalent salts show higher viscoelasticity over the salt concentration of 0.001–0.1 M, compared with the SDBS-only, while for monovalent salts, only 0.1 M salt concentration was able to establish intermolecular interactions which give rise to the viscoelastic moduli of SDBS solutions.
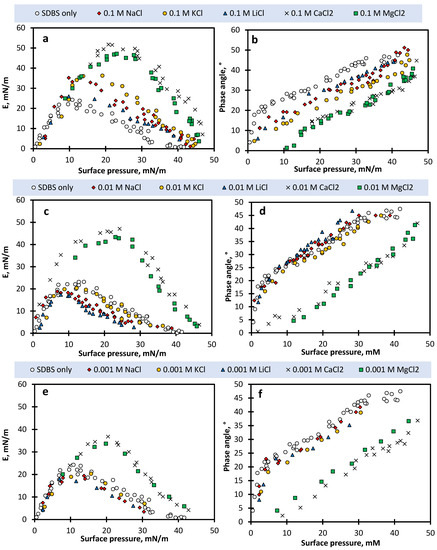
Figure 7.
(a,c,e) Dilational viscoelastic modulus and (b,d,f) the associated phase angle of SDBS solutions in the presence of different salt concentrations as a function of surface pressure at a dilational frequency of 0.01 Hz. (a,b) 0.1 M salt, (c,d) 0.01 M salt, and (e,f) 0.001 M salt.
3.2.4. Effect of Different Ions
This section focuses on the effect of ion type (ion-specific effect) on the dilational viscoelastic behaviour of surfactant-adsorbed layers. The results shown in the previous sections for the surface dilations rheology of SDBS in the presence of salts indicate that, at a fixed SDBS concentration, salts enhance the dilational viscoelasticity following the order CaCl2 > MgCl2 > KCl > NaCl > LiCl, from highest to lowest E. This order is consistent with our observations for the equilibrium ST, similarly, explained via (i) reduced electrostatic repulsion between the surfactant head groups because of the electrostatic interaction of counter-ions of salts with the surfactant head groups; and (ii) the salting-out effect of these salts, a reflection of the degree of water affinity of the salt and surfactant. Our results for surface dilational viscoelasticity show a more pronounced impact of divalent salts due to their higher charge density compared to the monovalent salts. A similar observation was observed by Ruwoldt et al. [27] for lignosulfonate solutions in the presence of NaCl, CaCl2 and AlCl3. They observed a higher profile of the surface shear rheology with the addition of salts in the order monovalent < divalent < trivalent, consistent with the charge density and salting-out ability of the ions.
Among each group of alkali metals (monovalent salts), the effect of salt type on the surface viscoelasticity is dominated by the latter effect, the degree of matching in water affinities. As discussed in Section 3.1.1, the electrostatic attraction between the SDBS head groups and the counter-ions with matched water affinities is stronger, promoting the surface concentration of the surfactant and thus a more pronounced change in the surface tension and the viscoelasticity modulus upon the applied surface area perturbation.
4. Conclusions
This report demonstrated the effect of the salt type, valency, and concentration of NaCl, KCl, NaCl, CaCl2 and MgCl2 on the surface tension (ST) and dilational viscoelasticity (E) of SDBS-adsorbed layers within a wide range of both salt and surfactant concentration.
The addition and increase in the concentration of all salts were found to lower the ST and the CMC within the entire range of SDBS concentrations. However, salts enhanced the dilational viscoelasticity compared with the SDBS-only solution only at a certain range of SDBS concentrations, below a surfactant concentration, referred to as, “intersection concentration”, at which the viscoelasticity of SDBS-only and mixed SDBS and salt layers intersect. Above the intersection concentration, the addition and increase in the salt concentration (both mono- and divalent salts) decreased the viscoelasticity compared to SDBS-only solutions. For a particular salt at a fixed concentration, dilational viscoelasticity increased with an increase in the SDBS concentration, followed by a sharp drop with further addition of SDBS, presenting a bell-shape trend.
For all salts, the viscoelasticity modulus peaked at the SDBS concentration of , where represents the CMC of the mixed salt and SDBS solution. This concentration was found to be relatively insensitive to the oscillation frequency within 0.01–0.1 Hz.
The impact of different salts on the dilational viscoelasticity was found to be in the order of CaCl2 > MgCl2 > KCl > NaCl > LiCl, consistent with the decrease in the surface tension and increase in the micellar formation. The divalent salts have a more pronounced effect compared to monovalent salts due to their higher charge density. Within each salt group, the salts’ relative impact was consistent with the matching degree in water affinities of SDBS and salts.
Author Contributions
Conceptualization, P.A. and M.F.; methodology, P.A. and M.F.; investigation, P.A.; writing—original draft preparation, P.A.; writing—review and editing, M.F.; project administration, M.F.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Australian Research Council’s Linkage Projects funding scheme grant number LP170100659.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Le Guenic, S.; Chaveriat, L.; Lequart, V.; Joly, N.; Martin, P. Renewable surfactants for biochemical applications and nanotechnology. J. Surfactants Deterg. 2019, 22, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Wang, C.; Sun, W.; Gao, Y.; Kowalczuk, P.B. Froth flotation of fluorite: A review. Adv. Colloid Interface Sci. 2021, 290, 102382. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Firouzi, M.; Albijanic, B.; Celik, M.S. A review on determination of particle–bubble encounter using analytical, experimental and numerical methods. Miner. Eng. 2018, 122, 296–311. [Google Scholar] [CrossRef]
- Amani, P.; Miller, R.; Javadi, A.; Firouzi, M. Pickering foams and parameters influencing their characteristics. Adv. Colloid Interface Sci. 2022, 301, 102606. [Google Scholar] [CrossRef]
- Buckley, T.; Xu, X.; Rudolph, V.; Firouzi, M.; Shukla, P. Review of foam fractionation as a water treatment technology. Sep. Sci. Technol. 2021, 57, 1–30. [Google Scholar] [CrossRef]
- Buckley, T.; Karanam, K.; Xu, X.; Shukla, P.; Firouzi, M.; Rudolph, V. Effect of mono-and di-valent cations on PFAS removal from water using foam fractionation–A modelling and experimental study. Sep. Purif. Technol. 2022, 286, 120508. [Google Scholar] [CrossRef]
- Murray, B.S. Recent developments in food foams. Curr. Opin. Colloid Interface Sci. 2020, 50, 101394. [Google Scholar] [CrossRef]
- Dickinson, E. Advances in food emulsions and foams: Reflections on research in the neo-Pickering era. Curr. Opin. Food Sci. 2020, 33, 52–60. [Google Scholar] [CrossRef]
- Ravera, F.; Miller, R.; Zuo, Y.Y.; Noskov, B.A.; Bykov, A.G.; Kovalchuk, V.I.; Loglio, G.; Javadi, A.; Liggieri, L. Methods and models to investigate the physicochemical functionality of pulmonary surfactant. Curr. Opin. Colloid Interface Sci. 2021, 55, 101467. [Google Scholar] [CrossRef]
- Bykov, A.; Milyaeva, O.Y.; Isakov, N.; Michailov, A.; Loglio, G.; Miller, R.; Noskov, B. Dynamic properties of adsorption layers of pulmonary surfactants. Influence of matter exchange with bulk phase. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125851. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. The use of surfactants in enhanced oil recovery: A review of recent advances. Energy Rep. 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Amani, P.; Hurter, S.; Rudolph, V.; Firouzi, M. Comparison of flow dynamics of air-water flows with foam flows in vertical pipes. Exp. Therm. Fluid Sci. 2020, 119, 110216. [Google Scholar] [CrossRef]
- Amani, P.; Firouzi, M. Effect of salt and particles on the hydrodynamics of foam flows in relation to foam static characteristics. Chem. Eng. Sci. 2022, 254, 117611. [Google Scholar] [CrossRef]
- Amani, P.; Firouzi, M. Foam flow characterisation in a vertical annulus. In Proceedings of the Chemeca 2021: Advance, Disrupt and Sustain, Barton, ACT, Australia, 27–28 September 2021. [Google Scholar]
- Amani, P.; Rudolph, V.; Hurter, S.; Firouzi, M. Sustainable dewatering of unconventional gas wells using engineered multiphase flow dynamics. Fuel 2022, 324, 124675. [Google Scholar] [CrossRef]
- Firouzi, M.; Kovalchuk, V.I.; Loglio, G.; Miller, R. Salt effects on the dilational viscoelasticity of surfactant adsorption layers. Curr. Opin. Colloid Interface Sci. 2022, 57, 101538. [Google Scholar] [CrossRef]
- Kovtun, A.; Kartashynska, E.; Vollhardt, D. Adsorption and viscoelastic properties of chitosan lactate at the liquid-gas interface. JCIS Open 2021, 1, 100001. [Google Scholar] [CrossRef]
- Fruhner, H.; Wantke, K.-D.; Lunkenheimer, K. Relationship between surface dilational properties and foam stability. Colloids Surf. A Physicochem. Eng. Asp. 2000, 162, 193–202. [Google Scholar] [CrossRef]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. Effects of surface rheology and surface potential on foam stability. Colloids Surf. A Physicochem. Eng. Asp. 2016, 488, 70–81. [Google Scholar] [CrossRef]
- Benjamins, J.; Lucassen-Reynders, E. Surface dilational rheology of proteins adsorbed at air/water and oil/water interfaces. Stud. Interface Sci. 1998, 7, 341–384. [Google Scholar]
- Zhang, Q.; Li, Y.; Cao, L.; Li, L.; Huang, K.; Li, W.; Yang, C. Dilational Viscoelastic Properties of Water–Fuel Interfaces in Single and Binary Surfactant Systems. Energy Fuels 2019, 33, 9055–9066. [Google Scholar] [CrossRef]
- Mucic, N.; Javadi, A.; Kovalchuk, N.; Aksenenko, E.; Miller, R. Dynamics of interfacial layers—Experimental feasibilities of adsorption kinetics and dilational rheology. Adv. Colloid Interface Sci. 2011, 168, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Amani, P.; Karakashev, S.I.; Grozev, N.A.; Simeonova, S.S.; Miller, R.; Rudolph, V.; Firouzi, M. Effect of selected monovalent salts on surfactant stabilized foams. Adv. Colloid Interface Sci. 2021, 295, 102490. [Google Scholar] [CrossRef] [PubMed]
- Patra, N.; Ray, D.; Aswal, V.K.; Ghosh, S. Exploring physicochemical interactions of different salts with sodium N-dodecanoyl sarcosinate in aqueous solution. ACS Omega 2018, 3, 9256–9266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fainerman, V.; Lylyk, S.; Aksenenko, E.; Kovalchuk, N.; Kovalchuk, V.; Petkov, J.; Miller, R. Effect of water hardness on surface tension and dilational visco-elasticity of sodium dodecyl sulphate solutions. J. Colloid Interface Sci. 2012, 377, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.E.; Salazar-Rodríguez, F.; Marquez, R.; Forgiarini, A.M. How the influence of different salts on interfacial properties of surfactant–oil–water systems at optimum formulation matches the Hofmeister series ranking. J. Surfactants Deterg. 2020, 23, 603–615. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Simon, S.; Øye, G. Viscoelastic properties of interfacial lignosulfonate films and the effect of added electrolytes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125478. [Google Scholar] [CrossRef]
- Sett, S.; Karakashev, S.I.; Smoukov, S.K.; Yarin, A.L. Ion-specific effects in foams. Adv. Colloid Interface Sci. 2015, 225, 98–113. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.-C.; Gong, Q.-T.; Luo, L.; Zhang, L.; Zhao, S.; Yong-Yu, J. Effect of Electrolyte on the Interfacial Dilational Properties of Sodium 4, 5-Diheptyl-2-propylbenzene Sulfonate at the Oil–Water Interface. J. Dispers. Sci. Technol. 2009, 30, 217–221. [Google Scholar] [CrossRef]
- Shu, N.-K.; Xu, Z.-C.; Gong, Q.-T.; Ma, W.-J.; Zhang, L.; Zhang, L. Effect of electrolyte on the surface dilational rheological properties of branched cationic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127170. [Google Scholar] [CrossRef]
- Amani, P.; Miller, R.; Ata, S.; Hurter, S.; Rudolph, V.; Firouzi, M. Dynamics of interfacial layers for sodium dodecylbenzene sulfonate solutions at different salinities. J. Ind. Eng. Chem. 2020, 92, 174–183. [Google Scholar] [CrossRef]
- Sood, A.K.; Aggarwal, M. Evaluation of micellar properties of sodium dodecylbenzene sulphonate in the presence of some salts. J. Chem. Sci. 2018, 130, 39. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.C.; Gulari, E. Micellization and intermicellar interactions in aqueous sodium dodecyl benzene sulfonate solutions. J. Colloid Interface Sci. 1982, 90, 410–423. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Han, P.; Sun, G.; Pan, F.; Li, B.; Wang, J.; Lv, C. Surface dilational rheology, foam, and core flow properties of alpha olefin sulfonate. J. Surfactants Deterg. 2017, 20, 35–45. [Google Scholar] [CrossRef]
- Lu, J.R.; Marrocco, A.; Su, T.J.; Thomas, R.K.; Penfold, J. Adsorption of dodecyl sulfate surfactants with monovalent metal counterions at the air-water interface studied by neutron reflection and surface tension. J. Colloid Interface Sci. 1993, 158, 303–316. [Google Scholar] [CrossRef]
- Vlachy, N.; Jagoda-Cwiklik, B.; Vácha, R.; Touraud, D.; Jungwirth, P.; Kunz, W. Hofmeister series and specific interactions of charged headgroups with aqueous ions. Adv. Colloid Interface Sci. 2009, 146, 42–47. [Google Scholar] [CrossRef]
- Moreira, L.; Firoozabadi, A. Molecular thermodynamic modeling of specific ion effects on micellization of ionic surfactants. Langmuir 2010, 26, 15177–15191. [Google Scholar] [CrossRef]
- Slavchov, R.I.; Karakashev, S.I.; Ivanov, I.B. Ionic surfactants and ion-specific effects: Adsorption, micellization, thin liquid films. In Surfactant Science and Technology: Retrospects and Prospects; CRC Press: Boca Raton, FL, USA, 2014; Volume 528. [Google Scholar]
- Collins, K.D.; Neilson, G.W.; Enderby, J.E. Ions in water: Characterizing the forces that control chemical processes and biological structure. Biophys. Chem. 2007, 128, 95–104. [Google Scholar] [CrossRef]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.-S.; Yang, N.; Xu, G.-Y.; Jia, Y.-F.; Liu, Q.; Nan, Y.-Q. Specific ion effects on the micellization of aqueous mixed cationic/anionic surfactant systems with various counterions. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 161–173. [Google Scholar] [CrossRef]
- Vlachy, N.; Drechsler, M.; Touraud, D.; Kunz, W. Anion specificity influencing morphology in catanionic surfactant mixtures with an excess of cationic surfactant. Comptes Rendus Chim. 2009, 12, 30–37. [Google Scholar] [CrossRef]
- Kunz, W. Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Interface Sci. 2010, 15, 34–39. [Google Scholar] [CrossRef]
- Vlachy, N.; Drechsler, M.; Verbavatz, J.-M.; Touraud, D.; Kunz, W. Role of the surfactant headgroup on the counterion specificity in the micelle-to-vesicle transition through salt addition. J. Colloid Interface Sci. 2008, 319, 542–548. [Google Scholar] [CrossRef]
- Firouzi, M.; Howes, T.; Nguyen, A.V. A quantitative review of the transition salt concentration for inhibiting bubble coalescence. Adv. Colloid Interface Sci. 2015, 222, 305–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharker, K.K.; Nazrul Islam, M.; Das, S. Interactions of some Hofmeister cations with sodium dodecyl Sulfate in aqueous solution. J. Surfactants Deterg. 2019, 22, 249–258. [Google Scholar] [CrossRef]
- Collins, K.D. Why continuum electrostatics theories cannot explain biological structure, polyelectrolytes or ionic strength effects in ion–protein interactions. Biophys. Chem. 2012, 167, 43–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fainerman, V.; Lylyk, S.; Aksenenko, E.; Petkov, J.; Yorke, J.; Miller, R. Surface tension isotherms, adsorption dynamics and dilational visco-elasticity of sodium dodecyl sulphate solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 8–15. [Google Scholar] [CrossRef]
- Monroy, F.; Kahn, J.G.; Langevin, D. Dilational viscoelasticity of surfactant monolayers. Colloids Surf. A Physicochem. Eng. Asp. 1998, 143, 251–260. [Google Scholar] [CrossRef]
- Lai, L.; Mei, P.; Wu, X.-M.; Cheng, L.; Ren, Z.-H.; Liu, Y. Interfacial dynamic properties and dilational rheology of sulfonate gemini surfactant and its mixtures with quaternary ammonium bromides at the air–water interface. J. Surfactants Deterg. 2017, 20, 565–576. [Google Scholar] [CrossRef]
- Stubenrauch, C.; Miller, R. Stability of Foam Films and Surface Rheology: An Oscillating Bubble Study at Low Frequencies. J. Phys. Chem. B 2004, 108, 6412–6421. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Q.; Liu, T.; Zhao, M. Dynamic surface pressure and dilatational viscoelasticity of sodium caseinate/xanthan gum mixtures at the oil–water interface. Food Hydrocoll. 2011, 25, 921–927. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).