Line Patterns and Fractured Coatings in Deposited Colloidal Hydrochar on Glass Substrates after Evaporation of Water
Abstract
:1. Introduction
2. Experiments and Characterizations
2.1. Preparation of Hydrochar
2.2. Means Used for the Evaporation of Water
2.3. Electron Microscopy
2.4. Dynamic Light Scattering (DLS)
3. Results and Discussion
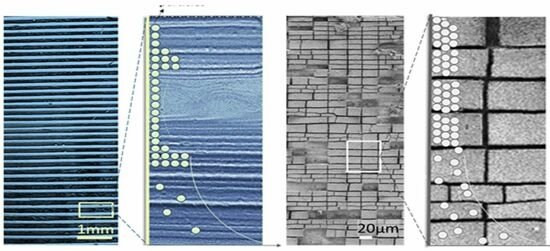
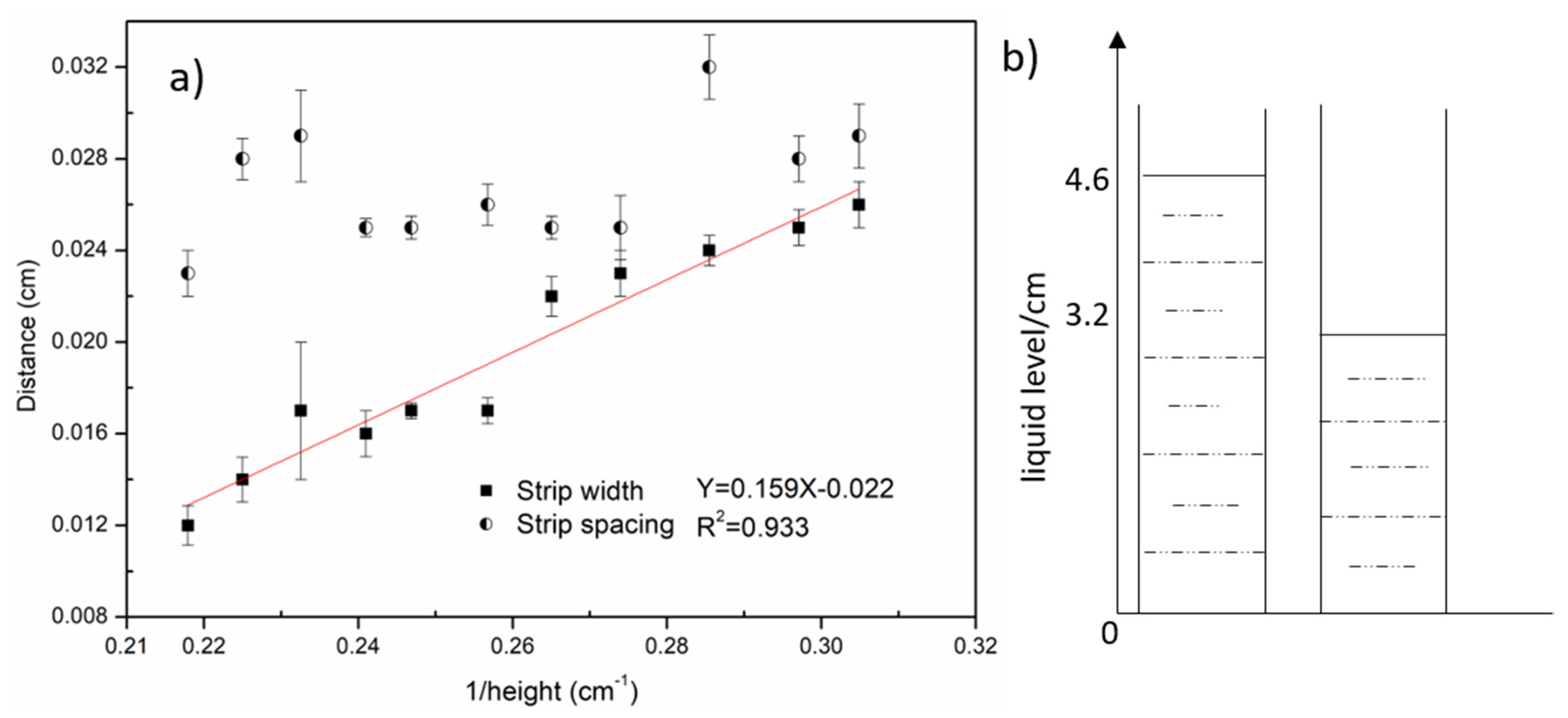
3.1. Lines or Rings of Deposited Colloidal Hydrochar on Glass Formed Due to the Evaporation of Water
3.2. Mud-Like Patterns and Cracked Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohara, P.C.; Heath, J.R.; Gelbart, W.M. Self-Assembly of Submicrometer Rings of Particles from Solutions of Nanoparticles. Angew. Chem. Int. Ed. Engl. 1997, 36, 1078–1080. [Google Scholar] [CrossRef]
- Xia, Y.; Gates, B.; Yin, Y.; Lu, Y. Monodispersed Colloidal Spheres: Old Materials with New Applications. Adv. Mater. 2000, 12, 693–713. [Google Scholar] [CrossRef]
- Murray, C.B.; Sun, S.; Gaschler, W.; Doyle, H.; Betley, T.A.; Kagan, C.R. Colloidal Synthesis of Nanocrystals and Nanocrystal Superlattices. IBM J. Res. Dev. 2001, 45, 47–56. [Google Scholar] [CrossRef]
- Xia, Y.; Nguyen, T.D.; Yang, M.; Lee, B.; Santos, A.; Podsiadlo, P.; Tang, Z.; Glotzer, S.C.; Kotov, N.A. Self-Assembly of Self-Limiting Monodisperse Supraparticles from Polydisperse Nanoparticles. Nat. Nanotechnol. 2011, 6, 580–587. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.F.; Colvin, V.L. A Lost-Wax Approach to Monodisperse Colloids and Their Crystals. Science 2001, 291, 453–457. [Google Scholar] [CrossRef]
- van Dommelen, R.; Fanzio, P.; Sasso, L. Surface Self-Assembly of Colloidal Crystals for Micro- and Nano-Patterning. Adv. Colloid Interface Sci. 2018, 251, 97–114. [Google Scholar] [CrossRef]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and Characterization of Monodisperse Nanocrystals and Close-Packed Nanocrystal Assemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef] [Green Version]
- Weissman, J.M.; Sunkara, H.B.; Tse, A.S.; Asher, S.A. Thermally Switchable Periodicities and Diffraction from Mesoscopically Ordered Materials. Science 1996, 274, 959–963. [Google Scholar] [CrossRef] [Green Version]
- Holtz, J.H.; Asher, S.A. Polymerized Colloidal Crystal Hydrogel Films as Intelligent Chemical Sensing Materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef]
- Lee, K.; Asher, S.A. Photonic Crystal Chemical Sensors: PH and Ionic Strength. J. Am. Chem. Soc. 2000, 122, 9534–9537. [Google Scholar] [CrossRef]
- Asher, S.A.; Alexeev, V.L.; Goponenko, A.V.; Sharma, A.C.; Lednev, I.K.; Wilcox, C.S.; Finegold, D.N. Photonic Crystal Carbohydrate Sensors: Low Ionic Strength Sugar Sensing. J. Am. Chem. Soc. 2003, 125, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.M. Photonic Band Gap Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-94-009-1665-4. [Google Scholar]
- Watanabe, S.; Inukai, K.; Mizuta, S.; Miyahara, M.T. Mechanism for Stripe Pattern Formation on Hydrophilic Surfaces by Using Convective Self-Assembly. Langmuir 2009, 25, 7287–7295. [Google Scholar] [CrossRef] [PubMed]
- Tétreault, N.; Míguez, H.; Ozin, G.A. Silicon Inverse Opal—A Platform for Photonic Bandgap Research. Adv. Mater. 2004, 16, 1471–1476. [Google Scholar] [CrossRef]
- Wang, X.; Hao, W.; Zhang, P.; Szego, A.E.; Svensson, G.; Hedin, N. Macroscopic Rods from Assembled Colloidal Particles of Hydrothermally Carbonized Glucose and Their Use as Templates for Silicon Carbide and Tricopper Silicide. J. Colloid Interface Sci. 2021, 602, 480–489. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the Coffee-Ring Effect by Shape-Dependent Capillary Interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Contact Line Deposits in an Evaporating Drop. Phys. Rev. E 2000, 62, 756–765. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Larson, R.G. Evaporation of a Sessile Droplet on a Substrate. J. Phys. Chem. B 2002, 106, 1334–1344. [Google Scholar] [CrossRef]
- Adachi, E.; Dimitrov, A.S.; Nagayama, K. Stripe Patterns Formed on a Glass Surface during Droplet Evaporation. Langmuir 1995, 11, 1057–1060. [Google Scholar] [CrossRef]
- Xu, J.; Xia, J.; Lin, Z. Evaporation-Induced Self-Assembly of Nanoparticles from a Sphere-on-Flat Geometry. Angew. Chem. Int. Ed. 2007, 46, 1860–1863. [Google Scholar] [CrossRef]
- Lyushnin, A.V.; Golovin, A.A.; Pismen, L.M. Fingering Instability of Thin Evaporating Liquid Films. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2002, 65, 021602. [Google Scholar] [CrossRef]
- Giraldo, O.; Durand, J.P.; Ramanan, H.; Laubernds, K.; Suib, S.L.; Tsapatsis, M.; Brock, S.L.; Marquez, M. Dynamic Organization of Inorganic Nanoparticles into Periodic Micrometer-Scale Patterns. Angew. Chem. Int. Ed. 2003, 42, 2905–2909. [Google Scholar] [CrossRef]
- Huang, J.; Kim, F.; Tao, A.R.; Connor, S.; Yang, P. Spontaneous Formation of Nanoparticle Stripe Patterns through Dewetting. Nat. Mater. 2005, 4, 896–900. [Google Scholar] [CrossRef]
- Abkarian, M.; Nunes, J.; Stone, H.A. Colloidal Crystallization and Banding in a Cylindrical Geometry. J. Am. Chem. Soc. 2004, 126, 5978–5979. [Google Scholar] [CrossRef]
- Colosqui, C.E.; Morris, J.F.; Stone, H.A. Hydrodynamically Driven Colloidal Assembly in Dip Coating. Phys. Rev. Lett. 2013, 110, 188302. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, R.; Fang, X.; Attinger, D. Pattern Formation during the Evaporation of a Colloidal Nanoliter Drop: A Numerical and Experimental Study. New J. Phys. 2009, 11, 075020. [Google Scholar] [CrossRef]
- Okubo, T. 5-Drying Patterns of Dispersions and Solutions. In Colloidal Organization; Okubo, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 282–423. ISBN 978-0-12-802163-7. [Google Scholar]
- Volpe, M.; Messineo, A.; Mäkelä, M.; Barr, M.R.; Volpe, R.; Corrado, C.; Fiori, L. Reactivity of Cellulose during Hydrothermal Carbonization of Lignocellulosic Biomass. Fuel Processing Technol. 2020, 206, 106456. [Google Scholar] [CrossRef]
- Wnukowski, M.; Owczarek, P.; Niedźwiecki, Ł. Wet Torrefaction of Miscanthus–Characterization of Hydrochars in View of Handling, Storage and Combustion Properties. J. Ecol. Eng. 2015, 16, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Bach, Q.-V.; Tran, K.-Q.; Skreiberg, Ø.; Khalil, R.A.; Phan, A.N. Effects of Wet Torrefaction on Reactivity and Kinetics of Wood under Air Combustion Conditions. Fuel 2014, 137, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Xie, L.; Li, H.; Wang, Y. Sustainable and Scalable Production of Monodisperse and Highly Uniform Colloidal Carbonaceous Spheres Using Sodium Polyacrylate as the Dispersant. Chem. Commun. 2014, 50, 12633–12636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tao, S.; Miao, X.; Zhu, Y. A Green, Rapid, Scalable and Versatile Hydrothermal Strategy to Fabricate Monodisperse Carbon Spheres with Tunable Micrometer Size and Hierarchical Porosity. Chem. Eng. J. 2019, 372, 1164–1173. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Xiao, Y.; Zhu, Y.; Guan, Q.; Yuan, D.; Zhang, J. An Easy Catalyst-Free Hydrothermal Method to Prepare Monodisperse Carbon Microspheres on a Large Scale. J. Phys. Chem. C 2009, 113, 8455–8459. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Chen, L.; Huang, X. Monodispersed Hard Carbon Spherules with Uniform Nanopores. Carbon 2001, 39, 2211–2214. [Google Scholar] [CrossRef]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One Stage Olive Mill Waste Streams Valorisation via Hydrothermal Carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuel Bioprod. Bior. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.-H.; Antonietti, M.; Titirici, M.-M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of Hydrothermal Carbonization Conditions on the Formation of Hydrochars and Secondary Chars from the Organic Fraction of Municipal Solid Waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–400. ISBN 978-0-12-817909-3. [Google Scholar]
- Ragheb, R.; Nobbmann, U. Multiple Scattering Effects on Intercept, Size, Polydispersity Index, and Intensity for Parallel (VV) and Perpendicular (VH) Polarization Detection in Photon Correlation Spectroscopy. Sci. Rep. 2020, 10, 21768. [Google Scholar] [CrossRef]
- Damasceno, J.P.V.; Kubota, L.T. Colloidal Chemistry as a Guide to Design Intended Dispersions of Carbon Nanomaterials. Mater. Today Chem. 2021, 21, 100526. [Google Scholar] [CrossRef]
- Bodiguel, H.; Doumenc, F.; Guerrier, B. Stick−Slip Patterning at Low Capillary Numbers for an Evaporating Colloidal Suspension. Langmuir 2010, 26, 10758–10763. [Google Scholar] [CrossRef] [PubMed]
- Sawadaishi, T.; Shimomura, M. Two-Dimensional Patterns of Ultra-Fine Particles Prepared by Self-Organization. Colloids Surf. A Physicochem. Eng. Asp. 2005, 257–258, 71–74. [Google Scholar] [CrossRef]
- Sun, W.; Zeng, Q.; Yu, A. Interaction Forces between Carbon Nanospheres: A Molecular Dynamics Simulation Study. Chem. Eng. Sci. 2015, 121, 23–31. [Google Scholar] [CrossRef]
- Sun, W.; Zeng, Q.; Yu, A. Calculation of Noncontact Forces between Silica Nanospheres. Langmuir 2013, 29, 2175–2184. [Google Scholar] [CrossRef]
- Bergström, L. Hamaker Constants of Inorganic Materials. Adv. Colloid Interface Sci. 1997, 70, 125–169. [Google Scholar] [CrossRef]
- Titirici, M.-M. Sustainable Carbon Materials from Hydrothermal Processes; John Wiley & Sons: London, UK, 2013; ISBN 978-1-118-62220-9. [Google Scholar]
- Titirici, M.-M.; Thomas, A.; Antonietti, M. Replication and Coating of Silica Templates by Hydrothermal Carbonization. Adv. Funct. Mater. 2007, 17, 1010–1018. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black Perspectives for a Green Future: Hydrothermal Carbons for Environment Protection and Energy Storage. Energy Environ. Sci. 2012, 5, 6796. [Google Scholar] [CrossRef]
- Tolias, P. Lifshitz Calculations of Hamaker Constants for Fusion Relevant Materials. Fusion Eng. Des. 2018, 133, 110–116. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M. Chemistry and Materials Options of Sustainable Carbon Materials Made by Hydrothermal Carbonization. Chem. Soc. Rev. 2009, 39, 103–116. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem.-A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B. The Production of Carbon Materials by Hydrothermal Carbonization of Cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Mu, G. The Adsorption and Corrosion Inhibition of Anion Surfactants on Aluminium Surface in Hydrochloric Acid. Corros. Sci. 1999, 41, 1937–1944. [Google Scholar] [CrossRef]
- Mizev, A.; Trofimenko, A.; Schwabe, D.; Viviani, A. Instability of Marangoni Flow in the Presence of an Insoluble Surfactant. Experiments. Eur. Phys. J. Spec. Top. 2013, 219, 89–98. [Google Scholar] [CrossRef]
- Shanks, P.C.; Franses, E.I. Estimation of Micellization Parameters of Aqueous Sodium Dodecyl Sulfate from Conductivity Data. J. Phys. Chem. 1992, 96, 1794–1805. [Google Scholar] [CrossRef]
- Thongngam, M.; McClements, D.J. Influence of PH, Ionic Strength, and Temperature on Self-Association and Interactions of Sodium Dodecyl Sulfate in the Absence and Presence of Chitosan. Langmuir 2005, 21, 79–86. [Google Scholar] [CrossRef]
- Chatterjee, A.; Moulik, S.P.; Sanyal, S.K.; Mishra, B.K.; Puri, P.M. Thermodynamics of Micelle Formation of Ionic Surfactants: A Critical Assessment for Sodium Dodecyl Sulfate, Cetyl Pyridinium Chloride and Dioctyl Sulfosuccinate (Na Salt) by Microcalorimetric, Conductometric, and Tensiometric Measurements. J. Phys. Chem. B 2001, 105, 12823–12831. [Google Scholar] [CrossRef]
- Mukerjee, P.; Mysels, K.J. Critical Micelle Concentrations of Aqueous Surfactant Systems; US Government Printing Office: Washington, DC, USA, 1971. [Google Scholar]
- Paula, S.; Sues, W.; Tuchtenhagen, J.; Blume, A. Thermodynamics of Micelle Formation as a Function of Temperature: A High Sensitivity Titration Calorimetry Study. J. Phys. Chem. 1995, 99, 11742–11751. [Google Scholar] [CrossRef]
- Rezende, C.A.; Lee, L.-T.; Galembeck, F. Liquid Templating for Nanoparticle Organization into Complex Patterns. Langmuir 2007, 23, 2824–2828. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Pattern Formation in Binary Colloidal Assemblies: Hidden Symmetries in a Kaleidoscope of Structures. Langmuir 2018, 34, 7827–7843. [Google Scholar] [CrossRef]
- Shepidchenko, T.; Zhang, J.; Tang, X.; Liu, T.; Dong, Z.; Zheng, G.; Yang, L. Experimental Study of the Main Controlling Factors of Desiccation Crack Formation from Mud to Shale. J. Pet. Sci. Eng. 2020, 194, 107414. [Google Scholar] [CrossRef]
- Banerjee, S.; Kundu, A.; Eriksson, P.G.; Sinha, S. Investigation on the Possible Control of Grain Size on Development of ‘Mud Cracks’: A Preliminary Experimental Study of Recent Gangetic Alluvial Samples, West Bengal, India. Arab. J. Geosci. 2021, 14, 106. [Google Scholar] [CrossRef]
- Singh, K.B.; Tirumkudulu, M.S. Cracking in Drying Colloidal Films. Phys. Rev. Lett. 2007, 98, 218302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufresne, E.R.; Corwin, E.I.; Greenblatt, N.A.; Ashmore, J.; Wang, D.Y.; Dinsmore, A.D.; Cheng, J.X.; Xie, X.S.; Hutchinson, J.W.; Weitz, D.A. Flow and Fracture in Drying Nanoparticle Suspensions. Phys. Rev. Lett. 2003, 91, 224501. [Google Scholar] [CrossRef] [Green Version]
- Bohn, S.; Pauchard, L.; Couder, Y. Hierarchical Crack Pattern as Formed by Successive Domain Divisions. Phys. Rev. E 2005, 71, 046214. [Google Scholar] [CrossRef] [Green Version]
- Allain, C.; Limat, L. Regular Patterns of Cracks Formed by Directional Drying of a Collodial Suspension. Phys. Rev. Lett. 1995, 74, 2981–2984. [Google Scholar] [CrossRef]
- Bohn, S.; Platkiewicz, J.; Andreotti, B.; Adda-Bedia, M.; Couder, Y. Hierarchical Crack Pattern as Formed by Successive Domain Divisions. II. From Disordered to Deterministic Behavior. Phys. Rev. E 2005, 71, 046215. [Google Scholar] [CrossRef]


| Colloid Type | Dispersant | Substrate | Geometry/Method | Pattern Alignment vs. Drying Front | Reference |
|---|---|---|---|---|---|
| Polystyrene (PS) | Water | Borosilicate glass | Drop on flat | Parallel | Adachi et al. [21] |
| CdSe/ZnS quantum dots | Toluene | Silicon | Drop on flat | Parallel then orthogonal | Xu et al. [22] |
| PS | Ethanol etc. | Glass capillary | Standstill capillary | Parallel | Abkarian et al. [26] |
| Silica | Water | Glass plate | Standstill slide | Parallel | Bodiguel et al. [45] |
| Manganese oxide | Mixture of water and 2-butanol | Glass slide | Standstill slide | Parallel | Giraldo et al. [24] |
| Silica | Water and ethanol mixture | Solvophilic substrates | Dip coating | Parallel | Watanabe et al. [14] |
| Gold or silver nanoparticles | Water | SiO2/Si | Dip coating | Orthogonal | Huang et al. [25] |
| Spherical hydrochar particles | Water, water with SDS addition, water with pH adjusted, ethanol, or the mixture of water and ethanol | Glass vials, tubes, slides and two end opening cylinders, etc.; polypropylene tubes | Standstill substrates | Parallel, parallel then orthogonal or –like | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hedin, N. Line Patterns and Fractured Coatings in Deposited Colloidal Hydrochar on Glass Substrates after Evaporation of Water. Colloids Interfaces 2022, 6, 36. https://doi.org/10.3390/colloids6020036
Wang X, Hedin N. Line Patterns and Fractured Coatings in Deposited Colloidal Hydrochar on Glass Substrates after Evaporation of Water. Colloids and Interfaces. 2022; 6(2):36. https://doi.org/10.3390/colloids6020036
Chicago/Turabian StyleWang, Xia, and Niklas Hedin. 2022. "Line Patterns and Fractured Coatings in Deposited Colloidal Hydrochar on Glass Substrates after Evaporation of Water" Colloids and Interfaces 6, no. 2: 36. https://doi.org/10.3390/colloids6020036
APA StyleWang, X., & Hedin, N. (2022). Line Patterns and Fractured Coatings in Deposited Colloidal Hydrochar on Glass Substrates after Evaporation of Water. Colloids and Interfaces, 6(2), 36. https://doi.org/10.3390/colloids6020036