Efficient Oil Removal of Polymer Flooding Produced Sewerage Using Super-Hydrophobic Mesh Filtration Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
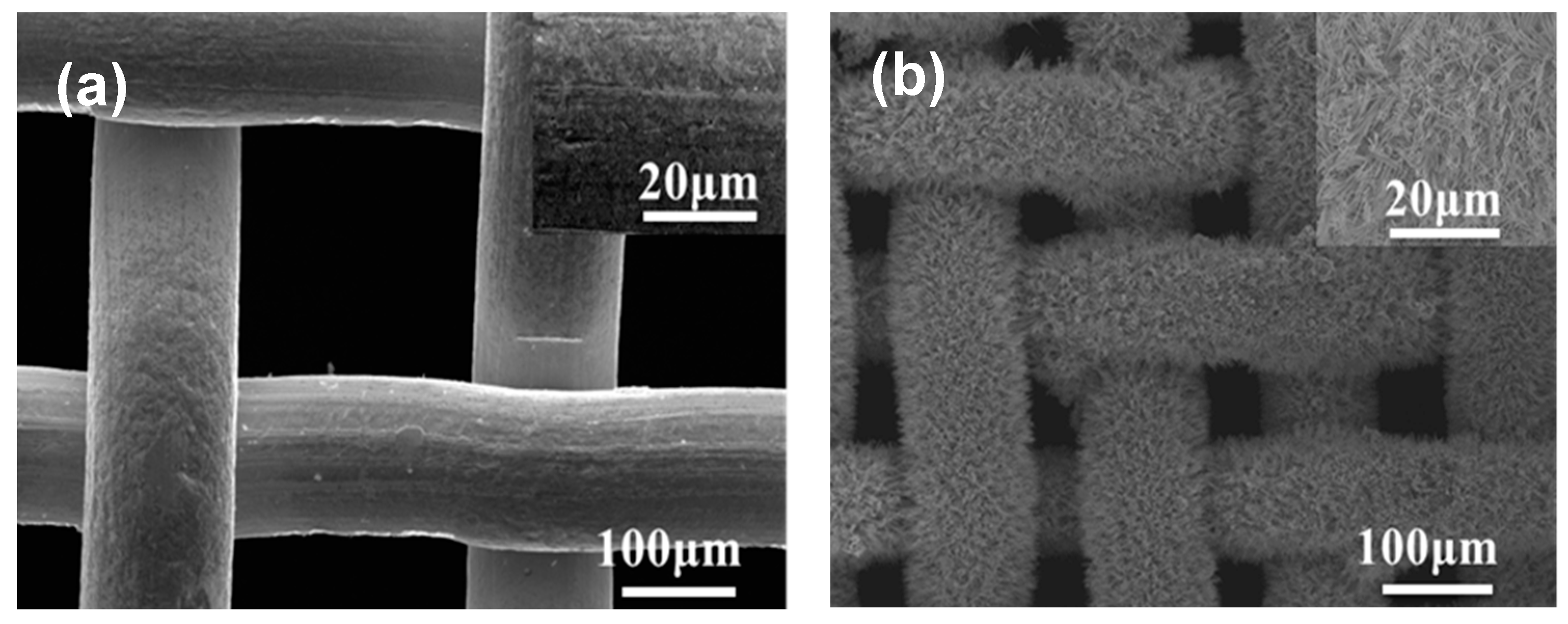
2.2.1. Fabrication of the Super-Hydrophobic Copper Mesh
2.2.2. Scanning Electron Microscope (SEM)
2.2.3. Contact Angle (CA) Measurement
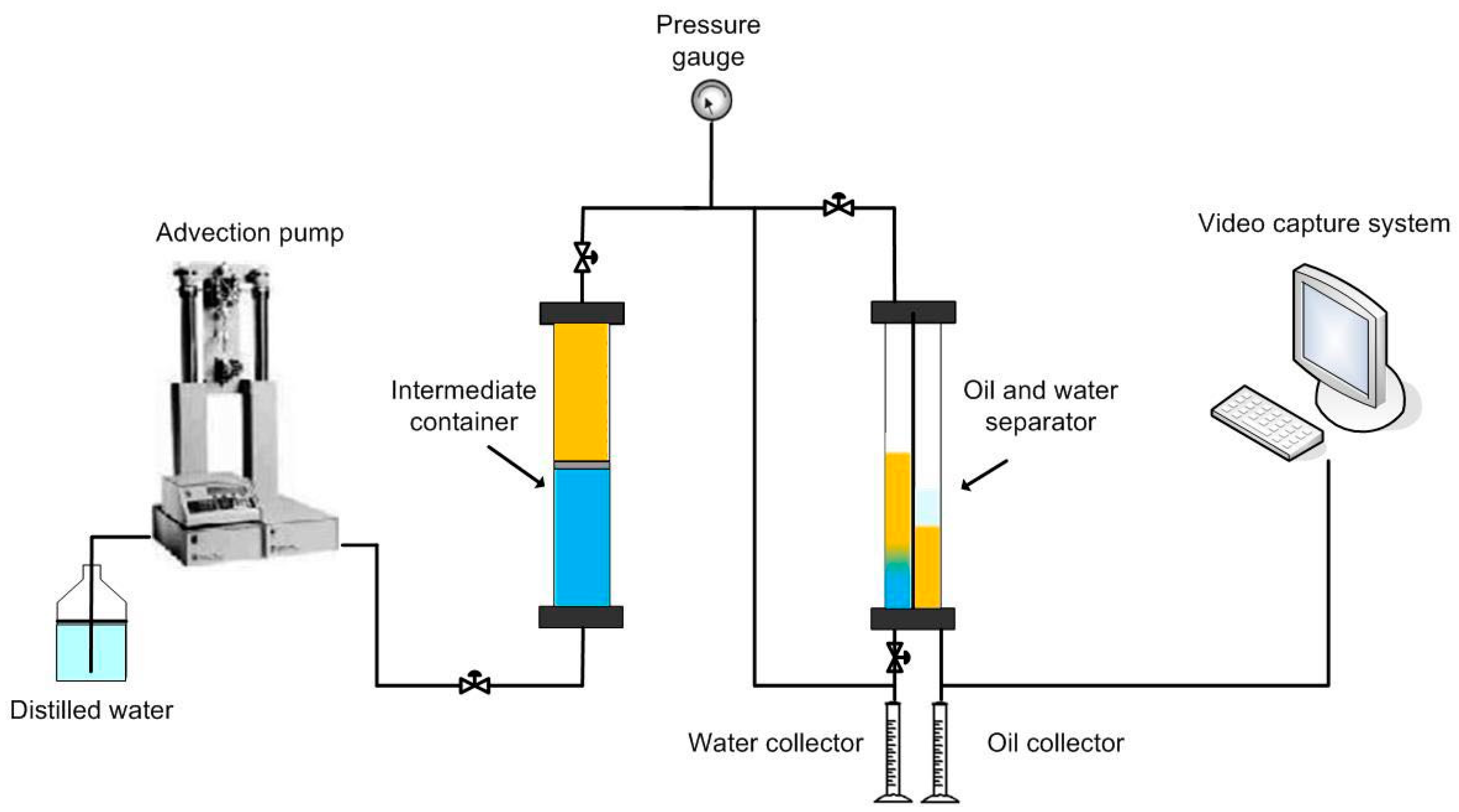
2.2.4. Separation Set Up and Separation Efficiency Measurement
3. Results and Discussion
3.1. Surface Characterization
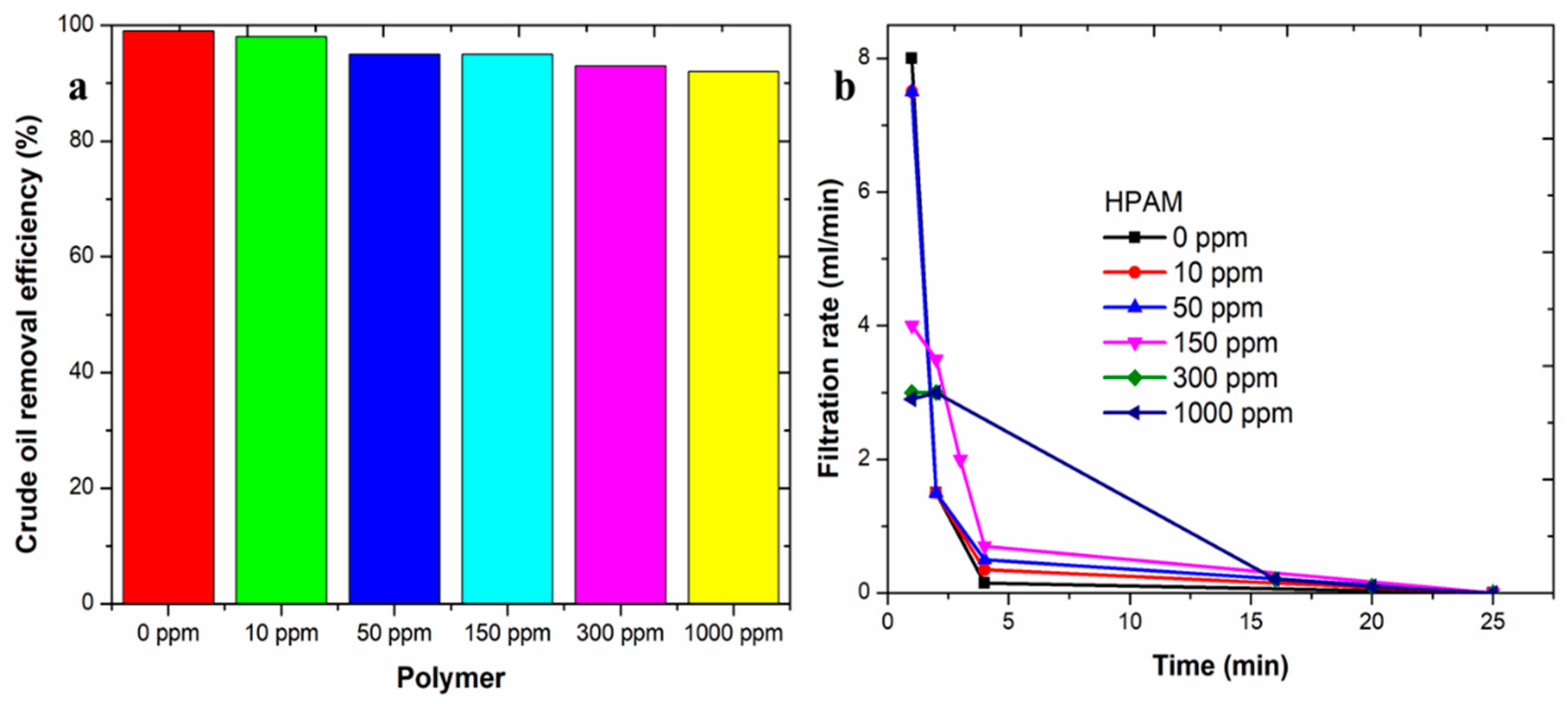
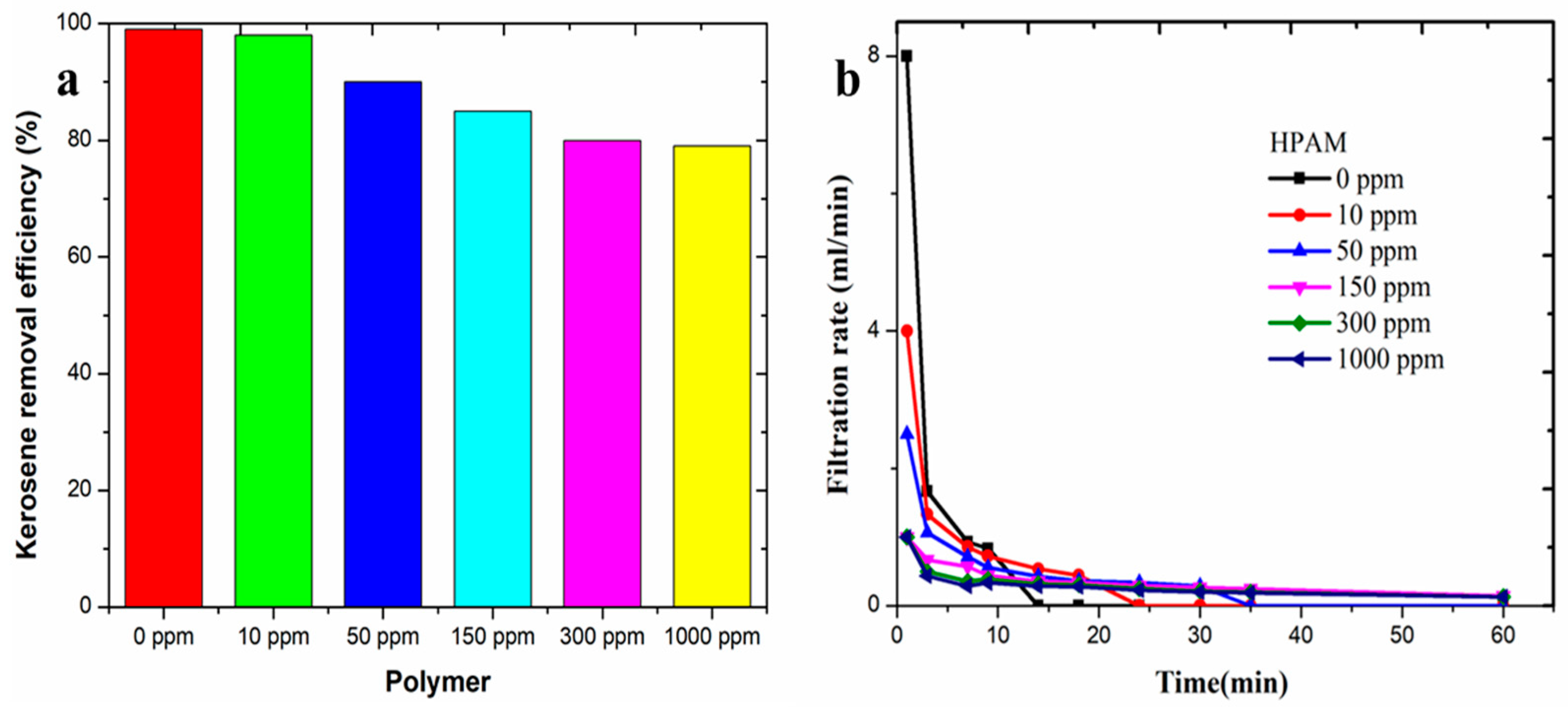
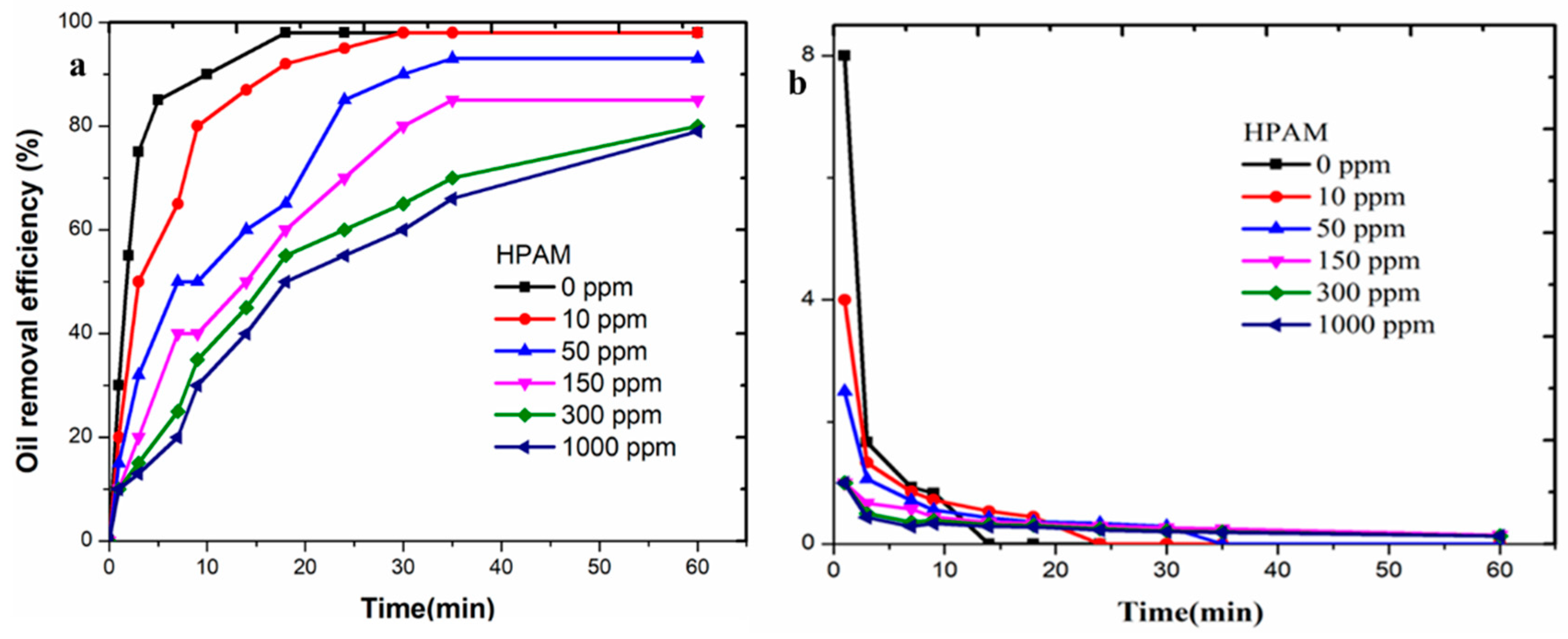
3.2. Separation Rules of Polymer-Containing Sewerage
3.3. Effect of Oil/Water Volume Ratio
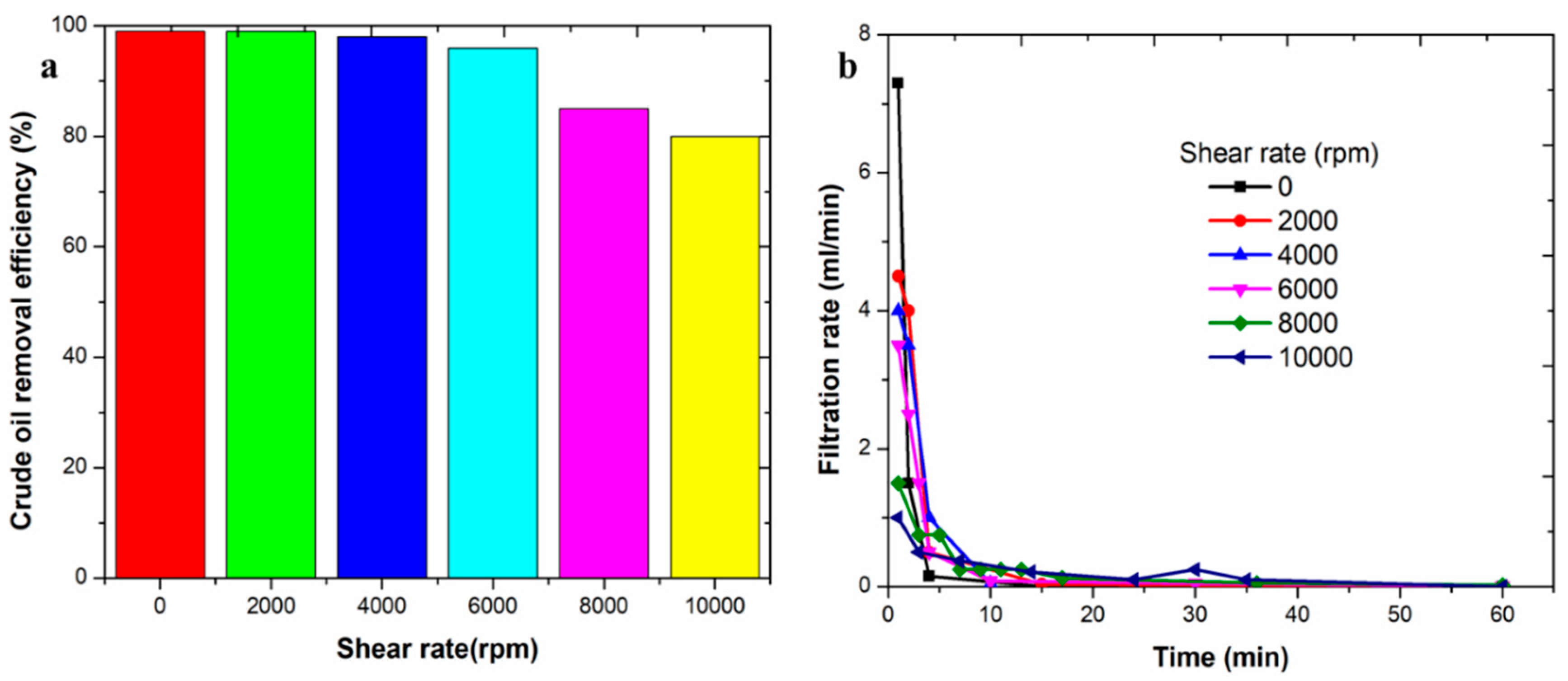
3.4. Effect of Shear Rate on Sewerage Treatment
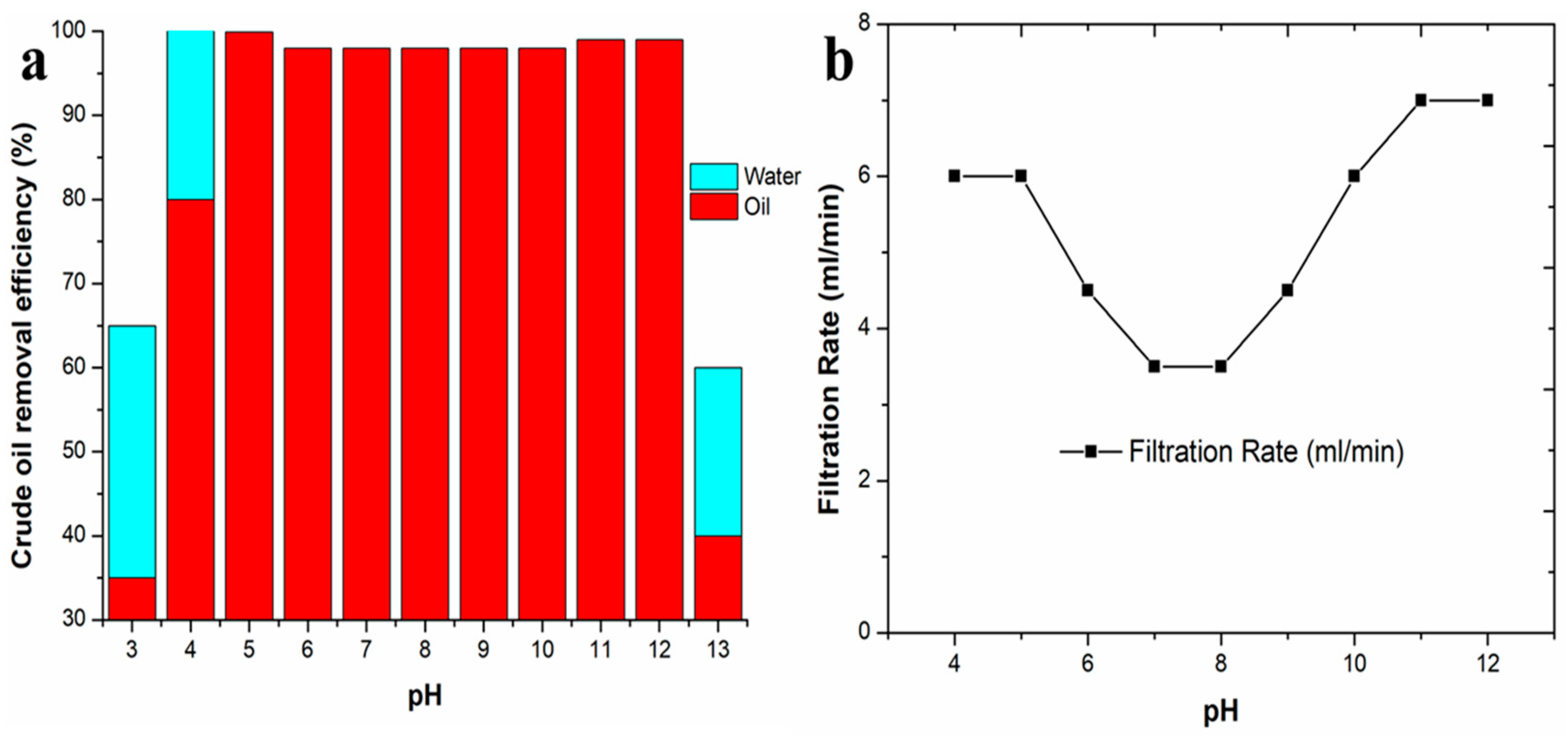
3.5. Effect of pH on Sewerage Treatment
3.6. Advantage of Super-Hydrophobic Mesh Separation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Shiran, B.S.; Skauge, A. Enhanced Oil Recovery (EOR) by Combined Low Salinity Water/Polymer Flooding. Energy Fuels 2013, 27, 1223–1235. [Google Scholar] [CrossRef]
- Bamzad, S.; Nourani, M.; Ramazani S.A., A.; Masihi, M. Experimental Investigation of Flooding Hydrolyzed–Sulfonated Polymers for EOR Process in a Carbonate Reservoir. Pet. Sci. Technol. 2014, 32, 1114–1122. [Google Scholar] [CrossRef]
- Kang, W.; Zhang, H.; Lu, Y.; Yang, H.; Zhu, T.; Zhang, X.; Chen, C.; Sarsenbekuly, B.; Besembaevna, O.Z. Study on the enhanced viscosity mechanism of the cyclodextrin polymer and betaine-type amphiphilic polymer inclusion complex. J. Mol. Liq. 2019, 296, 111792. [Google Scholar] [CrossRef]
- Rostami, A.; Meybodi, M.K.; Karimi, M.; Tatar, A.; Mohammadi, A.H. Efficient estimation of hydrolyzed polyacrylamide (HPAM) solution viscosity for enhanced oil recovery process by polymer flooding. Oil Gas Sci. Technol. Rev. d’IFP Energ. nouvelles 2018, 73, 22. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, X.; Guo, F. Micro-mechanisms of residual oil mobilization by viscoelastic fluids. Pet. Sci. 2008, 5, 56–61. [Google Scholar] [CrossRef]
- Lu, Y.; Meng, Z.; Gao, K.; Hou, J.; Wu, H.; Kang, W. Interaction of Amphiphilic Polymers with Medium-Chain Fatty Alcohols to Enhance Rheological Performance and Mobility Control Ability. Energy Fuels 2019, 33, 6273–6282. [Google Scholar] [CrossRef]
- Xuan, Y.; Ma, D.; Zhou, M.; Gao, M. Significance of polymer on emulsion stability in surfactant-polymer flooding. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Putatunda, S.; Bhattacharya, S.; Sen, D.; Bhattacharjee, C. A review on the application of different treatment processes for emulsified oily wastewater. Int. J. Environ. Sci. Technol. 2018, 16, 2525–2536. [Google Scholar] [CrossRef]
- Frising, T.; Noik, C.; Dalmazzone, C. The Liquid/Liquid Sedimentation Process: From Droplet Coalescence to Technologically Enhanced Water/Oil Emulsion Gravity Separators: A Review. J. Dispers. Sci. Technol. 2006, 27, 1035–1057. [Google Scholar] [CrossRef]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An overview of oil–water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, X.; Lu, B.; Wu, B.; Lu, L.; Liu, J.; Peng, K. Acceleration of floc-water separation and floc reduction with magnetic nanoparticles during demulsification of complex waste cutting emulsions. J. Environ. Sci. 2020, 89, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Han, D.S.; Yiming, W.; Ahzi, S.; Abdel-Wahab, A.; Liu, Z. A windable and stretchable three-dimensional all-inorganic membrane for efficient oil/water separation. Sci. Rep. 2017, 7, 16081. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Jiao, D.; Wang, C.; Han, S.; Shen, Q.; Wang, J.; Han, X.; Hou, T.; Liu, J.; Zhang, Y. Ethanol-induced one-step fabrication of superhydrophobic-superoleophilic poly(vinylidene fluoride) membrane for efficient oil/water emulsions separation. J. Water Process. Eng. 2020, 34, 101121. [Google Scholar] [CrossRef]
- Padaki, M.; Murali, R.S.; Abdullah, M.; Misdan, N.; Moslehyani, A.; Kassim, M.; Hilal, N.; Ismail, A. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Azad, A.J. Preparation of blend polyethersulfone/cellulose acetate/polyethylene glycol asymmetric membranes for oil–water separation. J. Polym. Res. 2014, 21, 375. [Google Scholar] [CrossRef]





| Properties | Values |
|---|---|
| Viscosity at room temperature (mPa·s) | 50 |
| Density at room temperature (g·cm−3) | 0.81 |
| Saturate (%) | 73.1 |
| Aromatics (%) | 14.4 |
| Resin (%) | 6.2 |
| Asphaltene (%) | 7.3 |
| Shear Rate (rpm) | Liquid Viscosity (mPa·s) |
|---|---|
| 2000 | 42.1 |
| 6000 | 33.6 |
| 10,000 | 13.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Kang, X.; Yang, H.; Gebremariam, H.; Li, Z. Efficient Oil Removal of Polymer Flooding Produced Sewerage Using Super-Hydrophobic Mesh Filtration Method. Colloids Interfaces 2020, 4, 32. https://doi.org/10.3390/colloids4030032
Kang W, Kang X, Yang H, Gebremariam H, Li Z. Efficient Oil Removal of Polymer Flooding Produced Sewerage Using Super-Hydrophobic Mesh Filtration Method. Colloids and Interfaces. 2020; 4(3):32. https://doi.org/10.3390/colloids4030032
Chicago/Turabian StyleKang, Wanli, Xin Kang, Hongbin Yang, Hailu Gebremariam, and Zhe Li. 2020. "Efficient Oil Removal of Polymer Flooding Produced Sewerage Using Super-Hydrophobic Mesh Filtration Method" Colloids and Interfaces 4, no. 3: 32. https://doi.org/10.3390/colloids4030032
APA StyleKang, W., Kang, X., Yang, H., Gebremariam, H., & Li, Z. (2020). Efficient Oil Removal of Polymer Flooding Produced Sewerage Using Super-Hydrophobic Mesh Filtration Method. Colloids and Interfaces, 4(3), 32. https://doi.org/10.3390/colloids4030032







