Bentonite Nanoparticle Stability and the Effect of Fulvic Acids: Experiments and Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Colloid Preparation
2.2. Coagulation Studies
2.3. Surface Potential Determination
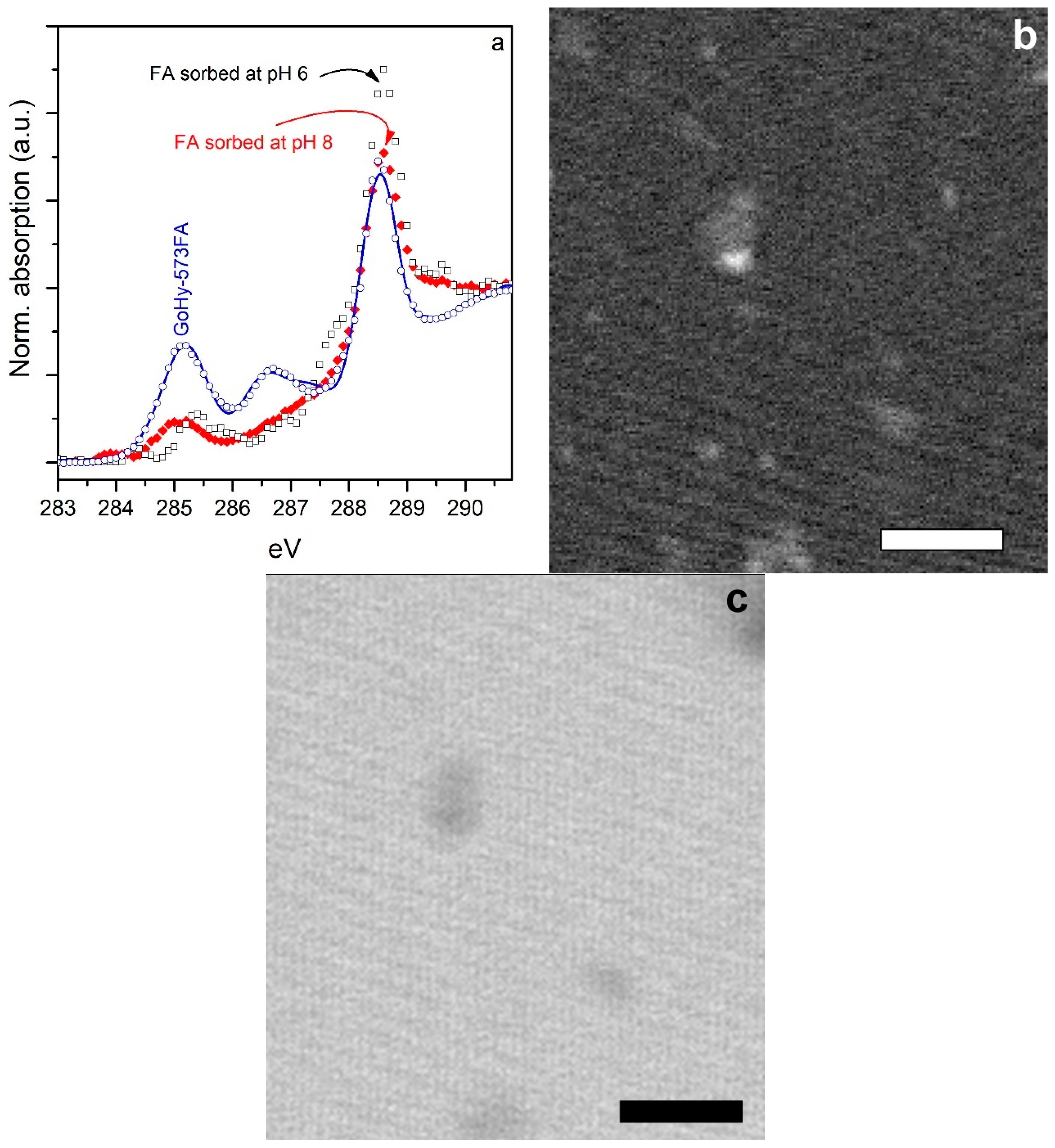
2.4. Scanning Transmission X-Ray Microscopy (STXM) Studies
3. Results and Discussion
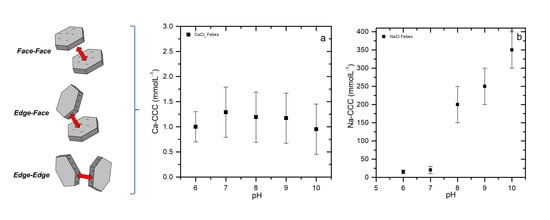
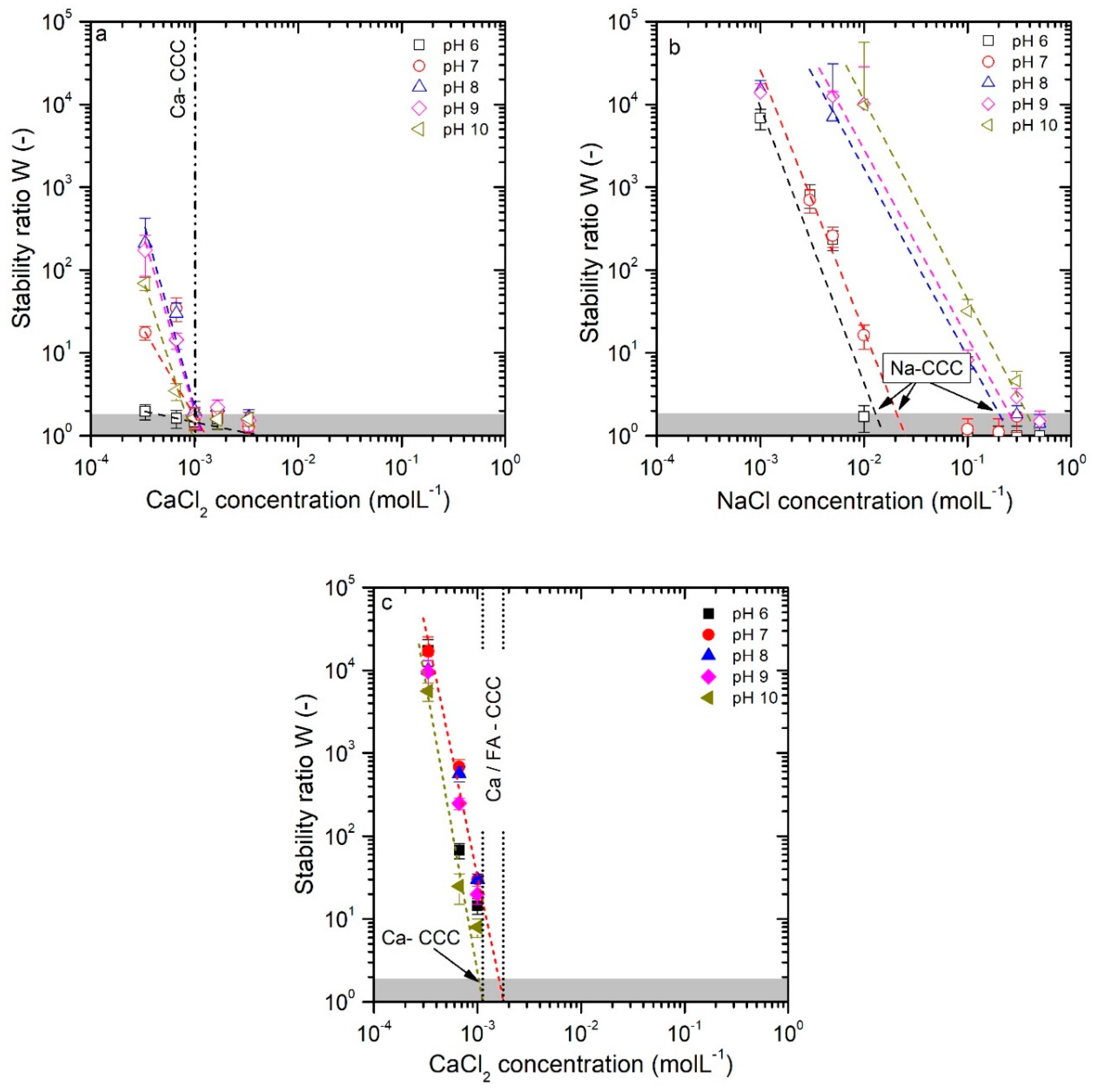
3.1. Colloid Stability
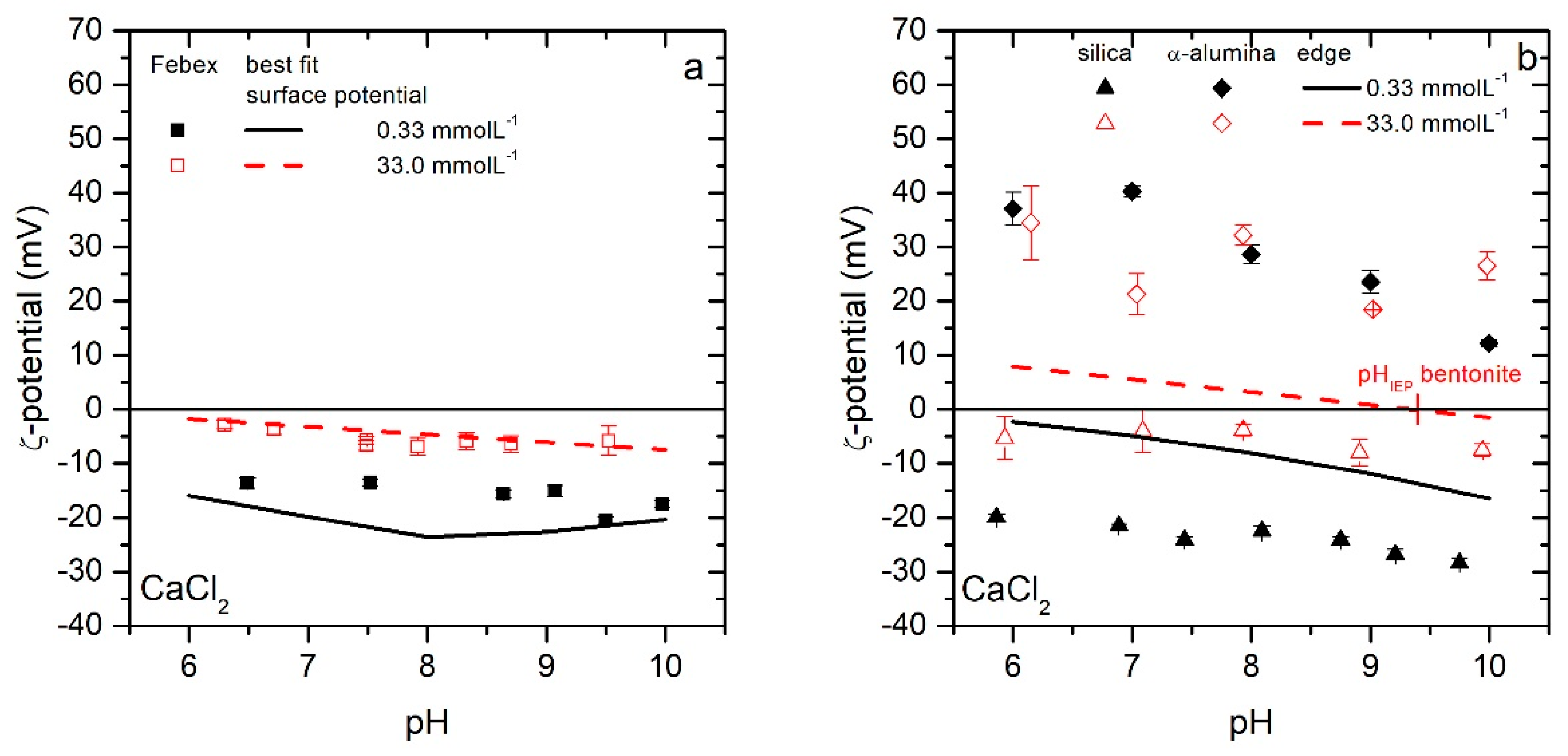
3.2. Surface Electrokinetic Potential Estimation
3.2.1. CaCl2 System
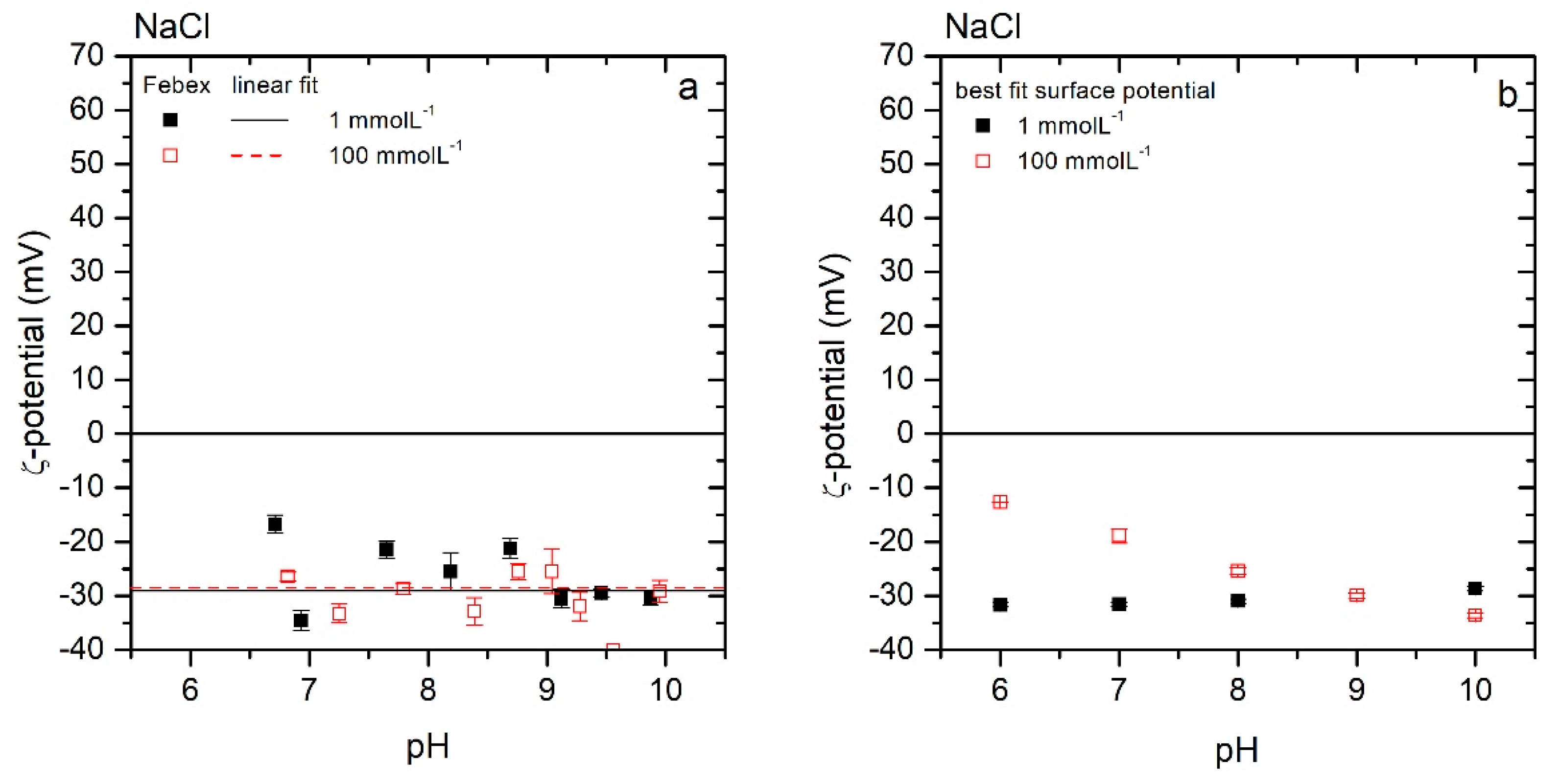
3.2.2. NaCl System
3.2.3. Surface Potentials
3.3. DLVO Model Predictions
3.3.1. Variation of Surface Electrokinetic Potential (WDLVO, best fit)
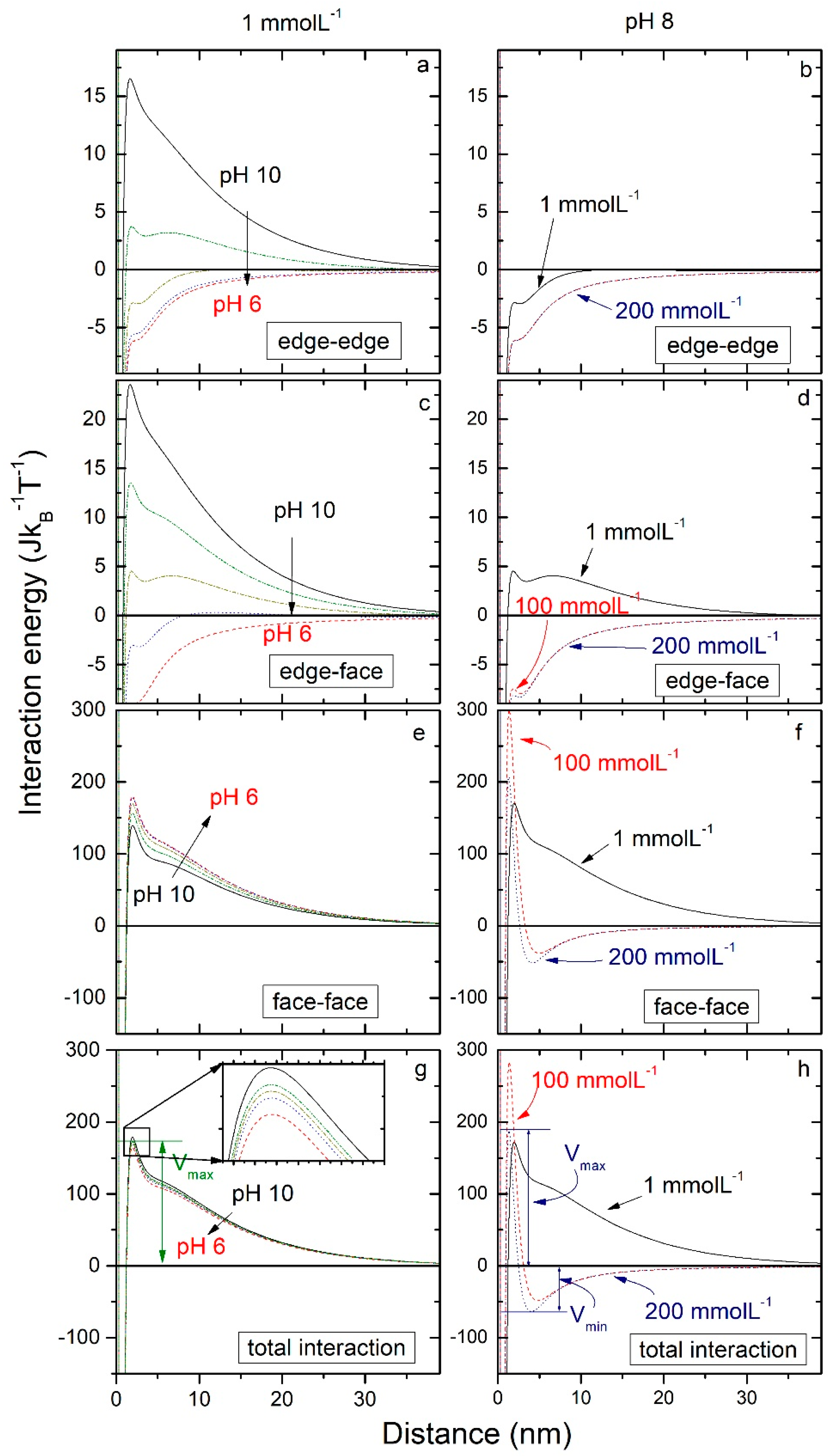
3.3.2. Interaction Energy
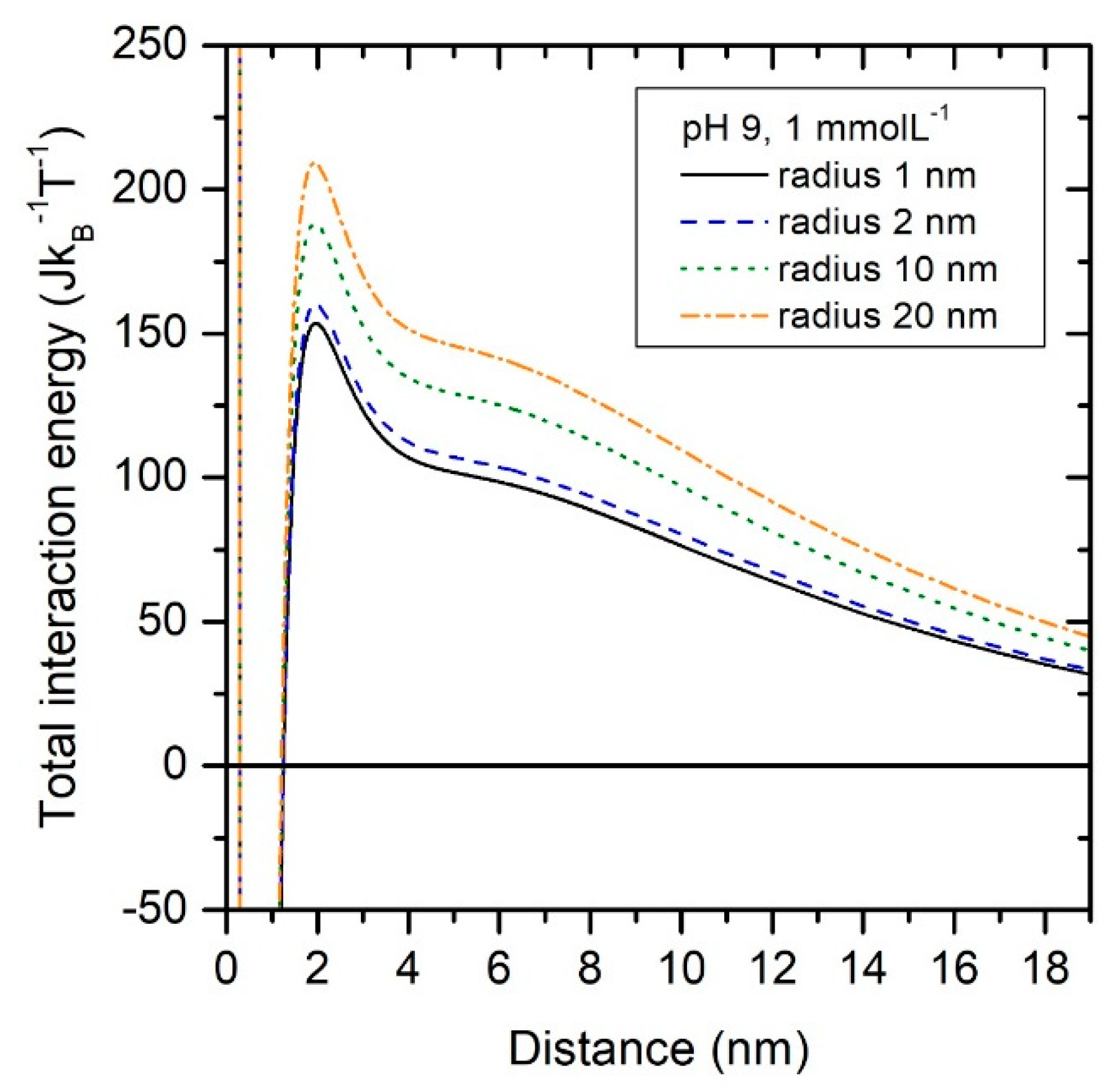
3.3.3. Particle Geometry Assumption
3.3.4. Energy Barrier Minima
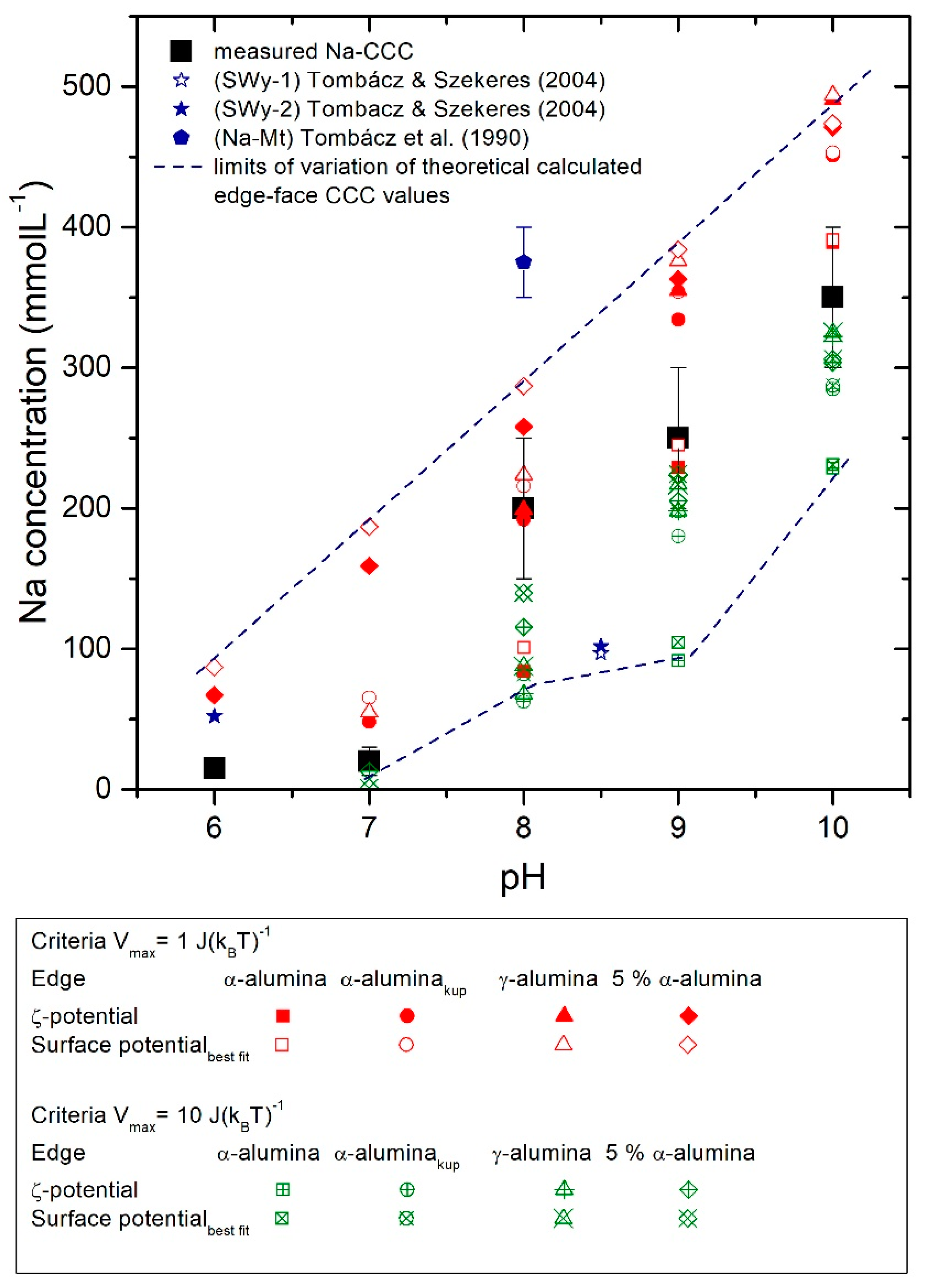
3.3.5. Fit of CCC over Vmax
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Symbols
| Symbol | Entity | Name |
| ε0 | C V−1 m−1 | Dielectric permittivity of vacuum |
| εr | Relative permittivity of water at 25 °C | |
| ĸ | m−1 | Reciprocal double layer thickness |
| kB | J K−1 | Boltzmann constant |
| rH | m | Hydrodynamic radius of colloid |
| r | Coagulation rate | |
| η | N s m−2 | Dynamic viscosity |
| T | K | Absolute temperature |
| μ | m2 V−1 s−1 | Electrophoretic mobility |
| ζ | V | Zeta-potential |
| D | m2 s−1 | Diffusion coefficient |
| C | g L−1 | Colloid concentration |
| OD | Optical density | |
| d | m | Sample thickness |
| µ(E) | Mass absorption coefficient | |
| I0(E) | Incident flux on the sample | |
| I(E) | Flux behind the sample | |
| δP | m | Particle thickness |
| SP | m2 | Particle surface area |
| ρ | kg m−3 | Density |
| W | Stability ratio | |
| V | J | Interaction energy |
References
- Sellin, P.; Leupin, O.X. The use of clay as an engineered barrier in radioactive-waste management-a review. CCM 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Dohrmann, R.; Kaufhold, S.; Lundqvist, B. The Role of Clays for Safe Storage of Nuclear Waste. In Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 677–710. [Google Scholar] [CrossRef]
- Pusch, R. Stability of bentonite gels in crystalline rock-Physical aspects. KBS Rep. TR 83. 1983. Available online: https://www.skb.se/publikation/3102/TR83-04webb.pdf (accessed on 20 March 2020).
- Missana, T.; Alonso, Ú.; Turrero, M.J. Generation and stability of bentonite colloids at the bentonite/granite interface of a deep geological radioactive waste repository. J. Cont. Hydrol. 2003, 61, 17–31. [Google Scholar] [CrossRef]
- Baik, M.-H.; Cho, W.-J.; Hahn, P.-S. Erosion of bentonite particles at the interface of a compacted bentonite and a fractured granite. Eng. Geol. 2007, 91, 229–239. [Google Scholar] [CrossRef]
- Alonso, U.; Missana, T.; Fernández, A.M.; García-Gutiérrez, M. Erosion behaviour of raw bentonites under compacted and confined conditions: Relevance of smectite content and clay/water interactions. Appl. Geochem. 2018, 94, 11–20. [Google Scholar] [CrossRef]
- Huber, F.; Leone, D.; Trumm, M.; Moreno, L.; Neretnieks, I.; Wenka, A.; Schäfer, T. Impact of fracture geometry on bentonite erosion - a numerical study. Appl. Clay Sci. 2020. (Under Review). [Google Scholar]
- Schäfer, T.; Huber, F.; Seher, H.; Missana, T.; Alonso, U.; Kumke, M.; Eidner, S.; Claret, F.; Enzmann, F. Nanoparticles and their influence on radionuclide mobility in deep geological formations. Appl. Geochem. 2012, 27, 390–403. [Google Scholar] [CrossRef]
- Buddemeier, R.W.; Hunt, J.R. Transport of colloidal contaminants in groundwater: radionuclide migration at the Nevada test site. Appl. Geochem. 1988, 3, 535–548. [Google Scholar] [CrossRef]
- Kersting, A.B.; Efurd, D.W.; Finnegan, D.L.; Rokop, D.J.; Smith, D.K.; Thompson, J.L. Migration of plutonium in ground water at the Nevada Test Site. Nature 1999, 397, 56–59. [Google Scholar] [CrossRef]
- Vilks, P.; Miller, H.G.; Doern, D.C. Natural colloids and suspended particles in the Whiteshell Research Area, Manitoba, Canada, and their potential effect on radiocolloid formation. Appl. Geochem. 1991, 6, 565–574. [Google Scholar] [CrossRef]
- Möri, A.; Alexander, W.R.; Geckeis, H.; Hauser, W.; Schäfer, T.; Eikenberg, J.; Fierz, T.; Degueldre, C.; Missana, T. The colloid and radionuclide retardation experiment at the Grimsel Test Site: influence of bentonite colloids on radionuclide migration in a fractured rock. Colloids Surf. A 2003, 217, 33–47. [Google Scholar] [CrossRef]
- Geckeis, H.; Schäfer, T.; Hauser, W.; Rabung, T.; Missana, T.; Degueldre, C.; Möri, A.; Eikenberg, J.; Fierz, T.; Alexander, W.R. Results of the Colloid and Radionuclide Retention experiment (CRR) at the Grimsel Test Site (GTS), Switzerland -Impact of reaction kinetics and speciation on radionuclide migration. Radiochim. Acta 2004, 92, 765–774. [Google Scholar] [CrossRef]
- Huber, F.; Noseck, U.; Schäfer, T. Colloid/nanoparticle Formation and Mobility in the Context of Deep Geological Nuclear Waste Disposal. Project KOLLORADO-2; Final Report; KIT Scientific Publishing: Karlsruhe, Germany, 2014. [Google Scholar] [CrossRef]
- Quinto, F.; Golser, R.; Lagos, M.; Plaschke, M.; Schäfer, T.; Steier, P.; Geckeis, H. Accelerator Mass Spectrometry of Actinides in Ground- and Seawater: An Innovative Method Allowing for the Simultaneous Analysis of U, Np, Pu, Am, and Cm Isotopes below ppq Levels. Anal. Chem. 2015, 87, 5766–5773. [Google Scholar] [CrossRef] [PubMed]
- Quinto, F.; Blechschmidt, I.; Garcia Perez, C.; Geckeis, H.; Geyer, F.; Golser, R.; Huber, F.; Lagos, M.; Lanyon, B.; Plaschke, M.; et al. Multiactinide Analysis with Accelerator Mass Spectrometry for Ultratrace Determination in Small Samples: Application to an in Situ Radionuclide Tracer Test within the Colloid Formation and Migration Experiment at the Grimsel Test Site (Switzerland). Anal. Chem. 2017, 89, 7182–7189. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Holthoff, H.; Sticher, H. Influence of pH and Humic Acid on Coagulation Kinetics of Kaolinite: A Dynamic Light Scattering Study. J. Coll. Interf. Sci. 1998, 202, 95–103. [Google Scholar] [CrossRef]
- Missana, T.; Adell, A. On the Applicability of DLVO Theory to the Prediction of Clay Colloids Stability. J. Coll. Interf. Sci. 2000, 230, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Norrfors, K.K.; Bouby, M.; Heck, S.; Finck, N.; Marsac, R.; Schäfer, T.; Geckeis, H.; Wold, S. Montmorillonite colloids: I. Characterization and stability of dispersions with different size fractions. Appl. Clay Sci. 2015, 114, 179–189. [Google Scholar] [CrossRef]
- Novich, B.E.; Ring, T.A. Colloid Stability of Clays Using Photon Correlation Spectroscopy. CCM 1984, 32, 400–407. [Google Scholar] [CrossRef]
- Mayordomo, N.; Degueldre, C.; Alonso, U.; Missana, T. Size distribution of FEBEX bentonite colloids upon fast disaggregation in low-ionic strength water. Clay Miner. 2016, 51, 213–222. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; Fernández, A.M.; García-Gutiérrez, M. Analysis of the stability behaviour of colloids obtained from different smectite clays. Appl. Geochem. 2018, 92, 180–187. [Google Scholar] [CrossRef]
- Villar, M.V.; Martín, P.L.; Pelayo, M.; Ruiz, B.; Rivas, P.; Alonso, E.; Lloret, A.; Pintado, X.; Gens, A.; Linares, J.; et al. FEBEX Bentonite: Origin, Properties and Fabrication of Blocks; ENRESA: Madrid, Spain, 1998. [Google Scholar]
- Lanyon, G.W.; Gaus, I. Grimsel Test Site Investigation Phase VI: Main outcomes and review of the FEBEX In Situ Test (GTS) and Mock-up after 15 years of operation, NAGRA technical report NTB 15-04, NAGRA: Wettingen, Switzerland. 2016. Available online: https://www.nagra.ch/data/documents/database/dokumente/$default/Default%20Folder/Publikationen/NTBs%202014%20-%202015/e_ntb15-04.pdf (accessed on 20 March 2020).
- Van Olphen, H. Clay Colloid Chemistry-For. Clay Technologists, Geologists and Soil Scientists, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Jasmund, K.; Lagaly, G. Tonminerale und Tone: Struktur, Eigenschaften, Anwendungen und Einsatz in Industrie und Umwelt; Steinkopf: Darmstadt, Germany, 1993. [Google Scholar]
- Meunier, A. Clays; Springer: Berlin, Germany, 2005; p. 472. [Google Scholar]
- Kaufhold, S.; Dohrmann, R. The variable charge of dioctahedral smectites. J. Coll. Interf. Sci. 2013, 390, 225–233. [Google Scholar] [CrossRef]
- Christidis, G.E.; Blum, A.E.; Eberl, D.D. Influence of layer charge and charge distribution of smectites on the flow behaviour and swelling of bentonites. Appl. Clay Sci. 2006, 34, 125–138. [Google Scholar] [CrossRef]
- Sun, L.; Ling, C.Y.; Lavikainen, L.P.; Hirvi, J.T.; Kasa, S.; Pakkanen, T.A. Influence of layer charge and charge location on the swelling pressure of dioctahedral smectites. Chem. Phys. 2016, 473, 40–45. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; Fernández, A.M.; García-Gutiérrez, M. Colloid properties of different smectite clays: significance for bentonite barrier erosion and radionuclide transport in radioactive waste repositories. Appl. Geochem. 2018, 97, 157–166. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Landau, L. Theory of the stability of strongly charged lyophobicsols and the adhesion of strongly charged particles in solutions of electrolytes. Acta Phys. URSS 1941, 14, 633–662. [Google Scholar]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the stability of lyophobic colloids; Elsevier: New York, NY, USA, 1948. [Google Scholar]
- Mahmood, T.; Amirtharajah, A.; Sturm, T.W.; Dennett, K.E. A micromechanics approach for attachment and detachment of asymmetric colloidal particles. Colloids Surf. A 2001, 177, 99–110. [Google Scholar] [CrossRef]
- Derjaguin, B. Untersuchungen über die Reibung und Adhäsion, IV. Colloid Polym. Sci. 1934, 69, 155–164. [Google Scholar] [CrossRef]
- Churaev, N.V.; Derjaguin, B.V. Inclusion of structural forces in the theory of stability of colloids and films. J. Coll. Interf. Sci. 1985, 103, 542–553. [Google Scholar] [CrossRef]
- Gregory, J. Approximate expressions for retarded van der Waals interaction. J. Coll. Interf. Sci. 1981, 83, 138–145. [Google Scholar] [CrossRef]
- Helmy, A.K.; Ferreiro, E.A. Flocculation of NH4-montmorillonite by electrolytes. J. Electroanal. Chem. 1974, 57, 103–112. [Google Scholar] [CrossRef]
- Williams, D.J.A.; Williams, K.P. Electrophoresis and zeta potential of kaolinite. J. Coll. Interf. Sci. 1978, 65, 79–87. [Google Scholar] [CrossRef]
- Cama, J.; Ganor, J.; Ayora, C.; Lasaga, C.A. Smectite dissolution kinetics at 80 °C and pH 8.8. Geochim. Et Cosmochim. Acta 2000, 64, 2701–2717. [Google Scholar] [CrossRef]
- Plaschke, M.; Schäfer, T.; Bundschuh, T.; Ngo Manh, T.; Knopp, R.; Geckeis, H.; Kim, J.I. Size Characterization of Bentonite Colloids by Different Methods. Anal. Chem. 2001, 73, 4338–4347. [Google Scholar] [CrossRef]
- Kumar, N.; Andersson, M.P.; van den Ende, D.; Mugele, F.; Siretanu, I. Probing the Surface Charge on the Basal Planes of Kaolinite Particles with High-Resolution Atomic Force Microscopy. Langmuir 2017, 33, 14226–14237. [Google Scholar] [CrossRef]
- Liu, J.; Sandaklie-Nikolova, L.; Wang, X.; Miller, J.D. Surface force measurements at kaolinite edge surfaces using atomic force microscopy. J. Coll. Interf. Sci. 2014, 420, 35–40. [Google Scholar] [CrossRef]
- Artinger, R.; Buckau, G.; Geyer, S.; Fritz, P.; Wolf, M.; Kim, J.I. Characterization of groundwater humic substances: influence of sedimentary organic carbon. Appl. Geochem. 2000, 15, 97–116. [Google Scholar] [CrossRef]
- Jacobsen, C.; Williams, S.; Anderson, E.; Browne, M.T.; Buckley, C.J.; Kern, D.; Kirz, J.; Rivers, M.; Zhang, X. Diffraction-limited imaging in a scanning transmission x-ray microscope. Opt. Commun. 1991, 86, 351–364. [Google Scholar] [CrossRef]
- Jacobsen, C.; Wirick, S.; Flynn, G.; Zimba, C. Soft X-ray spectroscopy from image sequences with sub-100 nm spatial resolution. J. Microsc. 2000, 197, 173–184. [Google Scholar] [CrossRef]
- Hitchcock, A.P.; Mancini, D.C. Bibliography of atomic and molecular inner-shell excitation studies. J. Electron. Spectrosc. Relat. Phenom. 1994, 67, 1–132. [Google Scholar] [CrossRef]
- Schäfer, T.; Buckau, G.; Artinger, R.; Kim, J.I.; Geyer, S.; Wolf, M.; Bleam, W.F.; Wirick, S.; Jacobsen, C. Origin and mobility of fulvic acids in the Gorleben aquifer system: implications from isotopic data and carbon/sulfur XANES. Org. Geochem. 2005, 36, 567–582. [Google Scholar] [CrossRef]
- Schäfer, T.; Hertkorn, N.; Artinger, R.; Claret, F.; Bauer, A. Functional group analysis of natural organic colloids and clay association kinetics using C(1s) spectromicroscopy. J. De Phys. Iv 2003, 104, 409–412. [Google Scholar] [CrossRef]
- SKB. Long-term safety for KBS-3 repository at Forsmark and Laxmar-a first evaluation; TR-06-09. SKB: Stockholm, Sweden, 2006. Available online: http://www.skb.com/publication/1192585/TR-06-09.pdf (accessed on 20 March 2020).
- SKB. Long-Term Safety for the Final Repository for Spent Nuclear Fuel at Forsmark, Main Report of the SR-Site Project; TR-11-01; SKB: Stockholm, Sweden, 2011; Volume II, pp. 279–552. Available online: https://skb.se/upload/publications/pdf/TR-11-01_vol2.pdf (accessed on 20 March 2020).
- Hetzel, F.; Doner, H.E. Some colloidal properties of Beidellite: comparison with low and high charge Montmorillonites. CCM 1993, 41, 453–460. [Google Scholar] [CrossRef]
- Séquaris, J.-M. Modeling the effects of Ca2+ and clay-associated organic carbon on the stability of colloids from topsoils. J. Coll. Interf. Sci. 2010, 343, 408–414. [Google Scholar] [CrossRef]
- Sellin, P.; Nyström, C.; Bailey, L.; Missana, T.; Schäfer, T.; Červinka, R.; Koskinen, K. ELBaR: Bentonite Erosion: Effects on the Long-term Performance of the Engineered Barrier and Radionuclide Transport. In Proceedings of the 8th EC Conference on the Management of Radioactive Waste Community Policy and Research on Disposal, EURADWASTE’13, Vilnius, Lithuania, 14–17 October 2013; pp. 293–300. [Google Scholar] [CrossRef]
- Lagaly, G.; Ziesmer, S. Colloid chemistry of clay minerals: the coagulation of montmorillonite dispersions. Adv. Colloid Interface Sci. 2003, 100, 105–128. [Google Scholar] [CrossRef]
- Chheda, P.; Grasso, D.; van Oss, C.J. Impact of ozone on stability of montmorillonite suspensions. J. Coll. Interf. Sci. 1992, 153, 226–236. [Google Scholar] [CrossRef]
- Delavernhe, L.; Steudel, A.; Darbha, G.K.; Schäfer, T.; Schuhmann, R.; Wöll, C.; Geckeis, H.; Emmerich, K. Influence of mineralogical and morphological properties on the cation exchange behavior of dioctahedral smectites. Colloids Surf. A 2015, 481, 591–599. [Google Scholar] [CrossRef]
- Mermut, A.R.; Lagaly, G. Baseline studies of the Clay Minerals Society Source Clays: Layer-charge determination and characteristics of those Minerals containing 2:1 Layers. CCM 2001, 49, 393–397. [Google Scholar] [CrossRef]
- Tombácz, E.; Szekeres, M. Colloidal behavior of aqueous montmorillonite suspensions: the specific role of pH in the presence of indifferent electrolytes. Appl. Clay Sci. 2004, 27, 75–94. [Google Scholar] [CrossRef]
- Tombácz, E.; Ábrahám, I.; Gilde, M.; Szántó, F. The pH-dependent colloidal stability of aqueous montmorillonite suspensions. Colloids Surf. 1990, 49, 71–80. [Google Scholar] [CrossRef]
- Liu, L.; Moreno, L.; Neretnieks, I. A Novel Approach to Determine the Critical Coagulation Concentration of a Colloidal Dispersion with Plate-like Particles. Langmuir 2009, 25, 688–697. [Google Scholar] [CrossRef]
- Bosbach, D.; Bouby, M.; Degueldre, C.; Filby, A.; Geckeis, H.; Götz, R.; Hauser, W.; Lützenkirchen, J.; Noseck, U.; Mihai, S.; et al. Colloid impact on radionuclide migration. In Annual Report 2008; Geckeis, H., Klenze, R., Eds.; Research Center Karlsruhe-Institute for Nuclear Wates Disposal (INE): Karlsruhe, Germany, 2009. [Google Scholar]
- Schäfer, T.; Chanudet, V.; Claret, F.; Filella, M. Spectromicroscopy Mapping of Colloidal/Particulate Organic Matter in Lake Brienz, Switzerland. Environ. Sci. Technol. 2007, 41, 7864–7869. [Google Scholar] [CrossRef]
- Schäfer, T.; Michel, P.; Claret, F.; Beetz, T.; Wirick, S.; Jacobsen, C. Radiation sensitivity of natural organic matter: Clay mineral association effects in the Callovo-Oxfordian argillite. J. Electron. Spectrosc. Relat. Phenom. 2009, 170, 49–56. [Google Scholar] [CrossRef]
- Naber, A.; Plaschke, M.; Rothe, J.; Hofmann, H.; Fanghänel, T. Scanning transmission X-ray and laser scanning luminescence microscopy of the carboxyl group and Eu(III) distribution in humic acid aggregates. J. Electron. Spectrosc. Relat. Phenom. 2006, 153, 71–74. [Google Scholar] [CrossRef]
- Christl, I.; Kretzschmar, R. C-1s NEXAFS Spectroscopy Reveals Chemical Fractionation of Humic Acid by Cation-Induced Coagulation. Environ. Sci. Technol. 2007, 41, 1915–1920. [Google Scholar] [CrossRef]
- Claret, F.; Schäfer, T.; Brevet, J.; Reiller, P.E. Fractionation of Suwannee River Fulvic Acid and Aldrich Humic Acid on alpha-Al2O3: Spectroscopic Evidence. Environ. Sci. Technol. 2008, 42, 8809–8815. [Google Scholar] [CrossRef]
- Stöhr, J. NEXAFS Spectroscopy; Springer: Berlin, Germany, 1996; Volume 25, p. 403. [Google Scholar]
- Dhez, O.; Ade, H.; Urquhart, S.G. Calibrated NEXAFS spectra of some common polymers. J. Electron. Spectrosc. Relat. Phenom. 2003, 128, 85–96. [Google Scholar] [CrossRef]
- Francis, J.T.; Hitchcock, A.P. Inner-shell spectroscopy of p-benzoquinone, hydroquinone, and phenol: distinguishing quinoid and benzenoid structures. J. Phys. Chem. 1992, 96, 6598–6610. [Google Scholar] [CrossRef]
- Kupcik, T.; Rabung, T.; Lützenkirchen, J.; Finck, N.; Geckeis, H.; Fanghänel, T. Macroscopic and spectroscopic investigations on Eu(III) and Cm(III) sorption onto bayerite (β-Al(OH)3) and corundum (α-Al2O3). J. Coll. Interf. Sci. 2016, 461, 215–224. [Google Scholar] [CrossRef]
- de Lint, W.B.S.; Benes, N.E.; Lyklema, J.; Bouwmeester, H.J.M.; van der Linde, A.J.; Wessling, M. Ion Adsorption Parameters Determined from Zeta Potential and Titration Data for a gamma-Alumina Nanofiltration Membrane. Langmuir 2003, 19, 5861–5868. [Google Scholar] [CrossRef]
- Franks, G.V.; Gan, Y. Charging behavior at the Alumina-Water interface and implications for ceramic processing. J. Am. Ceram. Soc. 2007, 90, 3373–3388. [Google Scholar] [CrossRef]
- Kosmulski, M. pH-dependent surface charging and points of zero charge II. Update. J. Coll. Interf. Sci. 2004, 275, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Missana, T.; Alonso, Ú.; García-Gutiérrez, M.; Mingarro, M. Role of bentonite colloids on europium and plutonium migration in a granite fracture. Appl. Geochem. 2008, 23, 1484–1497. [Google Scholar] [CrossRef]
- Lewis, J.A. Colloidal Processing of Ceramics. J. Am. Ceram. Soc. 2000, 83, 2341–2359. [Google Scholar] [CrossRef]
- Marmur, A. A kinetic theory approach to primary and secondary minimum coagulations and their combination. J. Coll. Interf. Sci. 1979, 72, 41–48. [Google Scholar] [CrossRef]
- Molina-Bolívar, J.A.; Galisteo-González, F.; Hidalgo-Álvarez, R. Repeptization Determined by Turbidity and Photon Correlation Spectroscopy Measurements: Particle Size Effects. J. Coll. Interf. Sci. 1997, 195, 289–298. [Google Scholar] [CrossRef][Green Version]
- Bagchi, P. Test for particle size dependence in the theory of slow flocculation at a secondary minimum. Colloid Polym. Sci. 1976, 254, 890–894. [Google Scholar] [CrossRef]
- Van de Ven, T.G.M. Colloid Hydrodynamics; Academic Press: London, UK, 1989. [Google Scholar]
- Litton, G.M.; Olson, T.M. Particle size effects on colloid deposition kinetics: evidence of secondary minimum deposition. Colloids Surf. A 1996, 107, 273–283. [Google Scholar] [CrossRef]
- Wan, J.; Tokunaga, T.K.; Saiz, E.; Larsen, J.T.; Zheng, Z.; Couture, R.A. Colloid Formation at Waste Plume Fronts. Environ. Sci. Technol. 2004, 38, 6066–6073. [Google Scholar] [CrossRef][Green Version]
- Hogg, R.; Yang, K.C. Secondary coagulation. J. Coll. Interf. Sci. 1976, 56, 573–576. [Google Scholar] [CrossRef]
- Secor, R.B.; Radke, C.J. Spillover of the diffuse double layer on montmorillonite particles. J. Coll. Interf. Sci. 1985, 103, 237–244. [Google Scholar] [CrossRef]
- Chang, F.-R.C.; Sposito, G. The Electrical Double Layer of a Disk-Shaped Clay Mineral Particle: Effects of Electrolyte Properties and Surface Charge Density. J. Coll. Interf. Sci. 1996, 178, 555–564. [Google Scholar] [CrossRef]
- Trefalt, G.; Behrens, S.H.; Borkovec, M. Charge Regulation in the Electrical Double Layer: Ion Adsorption and Surface Interactions. Langmuir 2016, 32, 380–400. [Google Scholar] [CrossRef]
- Vilks, P.; Bachinski, D.B. Characterization of organics in whiteshell research area groundwater and the implications for radionuclide transport. Appl. Geochem. 1996, 11, 387–402. [Google Scholar] [CrossRef]
- Degueldre, C.; Pfeiffer, H.R.; Alexander, W.; Wernli, B.; Bruetsch, R. Colloid properties in granitic groundwater systems. I: Sampling and characterisation. Appl. Geochem. 1996, 11, 677–685. [Google Scholar] [CrossRef]
- Schäfer, T.; Geckeis, H.; Bouby, M.; Fanghänel, T. U, Th, Eu and colloid mobility in a granite fracture under near-natural flow conditions. Radiochim. Acta 2004, 92, 731–737. [Google Scholar] [CrossRef]
- Tullborg, E.-L.; Gustafsson, E. 14C in bicarbonate and dissolved organics—a useful tracer? Appl. Geochem. 1999, 14, 927–938. [Google Scholar] [CrossRef]
- Kienzler, B.; Vejmelka, P.; Römer, J.; Fanghänel, E.; Jansson, M.; Eriksen, T.E.; Wikberg, P. Swedish-German actinide migration experiment at ÄSPÖ hard rock laboratory. J. Cont. Hydrol. 2003, 61, 219–233. [Google Scholar] [CrossRef]



| Concentration (mmol·L−1) | pH | Wmeasured | WDLVO | WDLVO, best fit | Wmeasured FA |
|---|---|---|---|---|---|
| 0.33 | 6 | 2 ± 0.4 | 2 | 2 | 17,373 |
| 0.33 | 7 | 18 ± 3.0 | 6 | 18 | 16,950 |
| 0.33 | 8 | 211 ± 20 | 19 | 210 | 10,000 |
| 0.33 | 9 | 173 ± 89 | 66 | 174 | 9596 |
| 0.33 | 10 | 69 ± 12 | 283 | 68 | 5614 |
| 33 | 6 | 1 ± 0.2 | 1 | 1 | n.m. |
| 33 | 7 | 1 ± 0.2 | 1 | 1 | n.m. |
| 33 | 8 | 1 ± 0.2 | 1 | 1 | n.m. |
| 33 | 9 | 1 ± 0.2 | 1 | 1 | n.m. |
| 33 | 10 | 1 ± 0.2 | 1 | 1 | n.m. |
| Concentration (mmol·L−1) | pH | Wmeasured | WDLVO α-Al2O3 | WDLVO α-Al2O3 Kup | WDLVO γ-Al2O3 | WDLVO 5% α-Al2O3 | WDLVO, best fit |
|---|---|---|---|---|---|---|---|
| 1 | 6 | 6720 ± 1877 | 572 | 687 | 619 | 1202 | 6717 ± 210 |
| 1 | 7 | 9810 ± 220 | 784 | 1102 | 1057 | 1913 | 9918 ± 367 |
| 1 | 8 | 12,900 ± 4138 | 1303 | 2306 | 2407 | 3513 | 13,028 ± 449 |
| 1 | 9 | 16,000 ± 3856 | 2898 | 6120 | 7219 | 7700 | 16,124 ± 454 |
| 1 | 10 | 19,100 ± 4604 | 9622 | 16,840 | 25,077 | 20,522 | 19,236 ± 567 |
| 100 | 6 | 1 ± 0.2 | 9 | 9 | 9 | 9 | 1 ± 0.01 |
| 100 | 7 | 2 ± 0.3 | 9 | 9 | 9 | 9 | 2 ± 0.01 |
| 100 | 8 | 5 ± 0.4 | 9 | 9 | 9 | 9 | 5 ± 1 |
| 100 | 9 | 13 ± 1 | 9 | 9 | 9 | 9 | 12 ± 1 |
| 100 | 10 | 34 ± 5 | 10 | 10 | 10 | 10 | 31 ± 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seher, H.; Geckeis, H.; Fanghänel, T.; Schäfer, T. Bentonite Nanoparticle Stability and the Effect of Fulvic Acids: Experiments and Modelling. Colloids Interfaces 2020, 4, 16. https://doi.org/10.3390/colloids4020016
Seher H, Geckeis H, Fanghänel T, Schäfer T. Bentonite Nanoparticle Stability and the Effect of Fulvic Acids: Experiments and Modelling. Colloids and Interfaces. 2020; 4(2):16. https://doi.org/10.3390/colloids4020016
Chicago/Turabian StyleSeher, Holger, Horst Geckeis, Thomas Fanghänel, and Thorsten Schäfer. 2020. "Bentonite Nanoparticle Stability and the Effect of Fulvic Acids: Experiments and Modelling" Colloids and Interfaces 4, no. 2: 16. https://doi.org/10.3390/colloids4020016
APA StyleSeher, H., Geckeis, H., Fanghänel, T., & Schäfer, T. (2020). Bentonite Nanoparticle Stability and the Effect of Fulvic Acids: Experiments and Modelling. Colloids and Interfaces, 4(2), 16. https://doi.org/10.3390/colloids4020016