Alginate Films Encapsulating Lemongrass Essential Oil as Affected by Spray Calcium Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation and Characterization
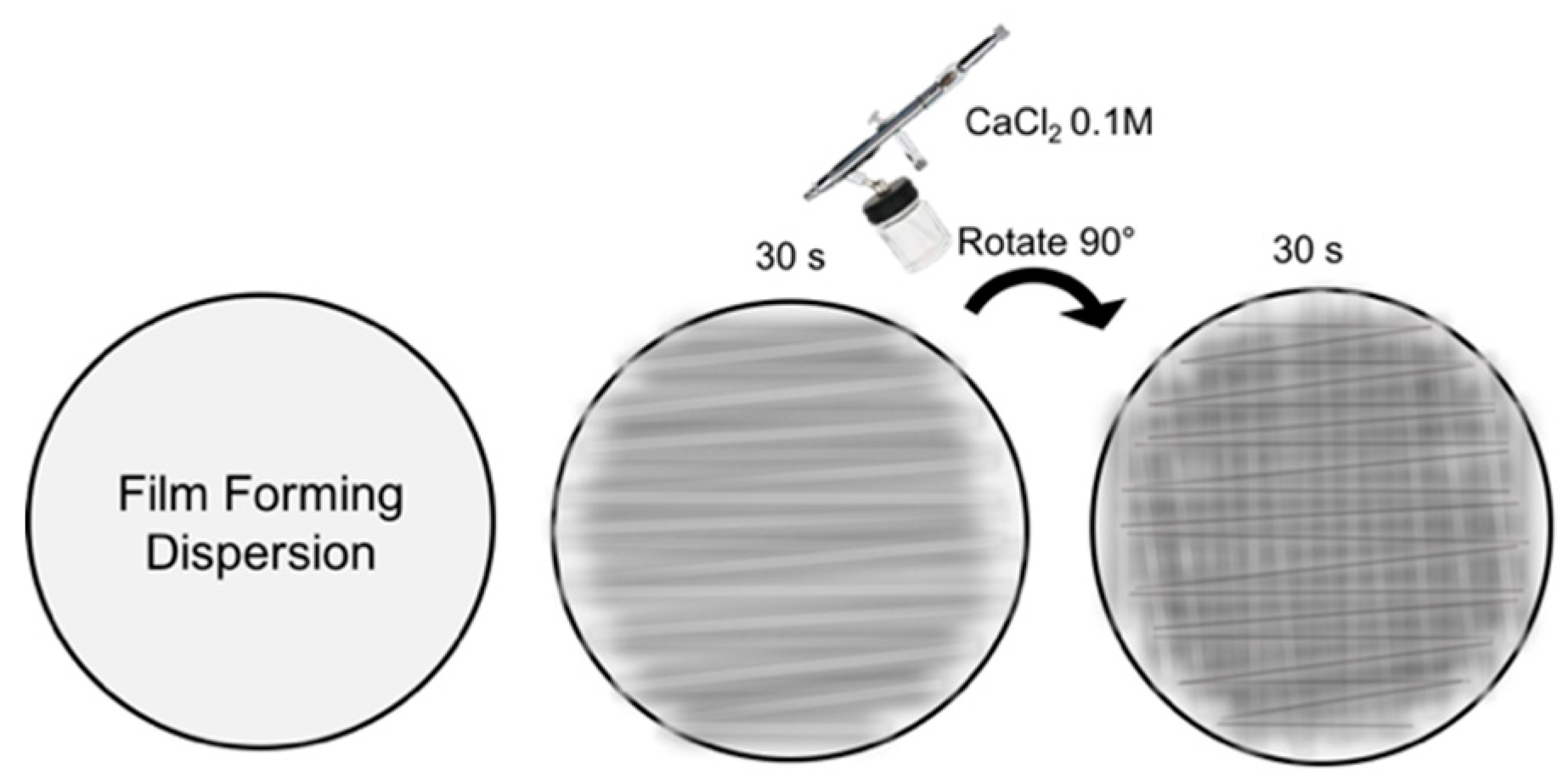
2.3. Film Preparation
2.4. Film Characterization
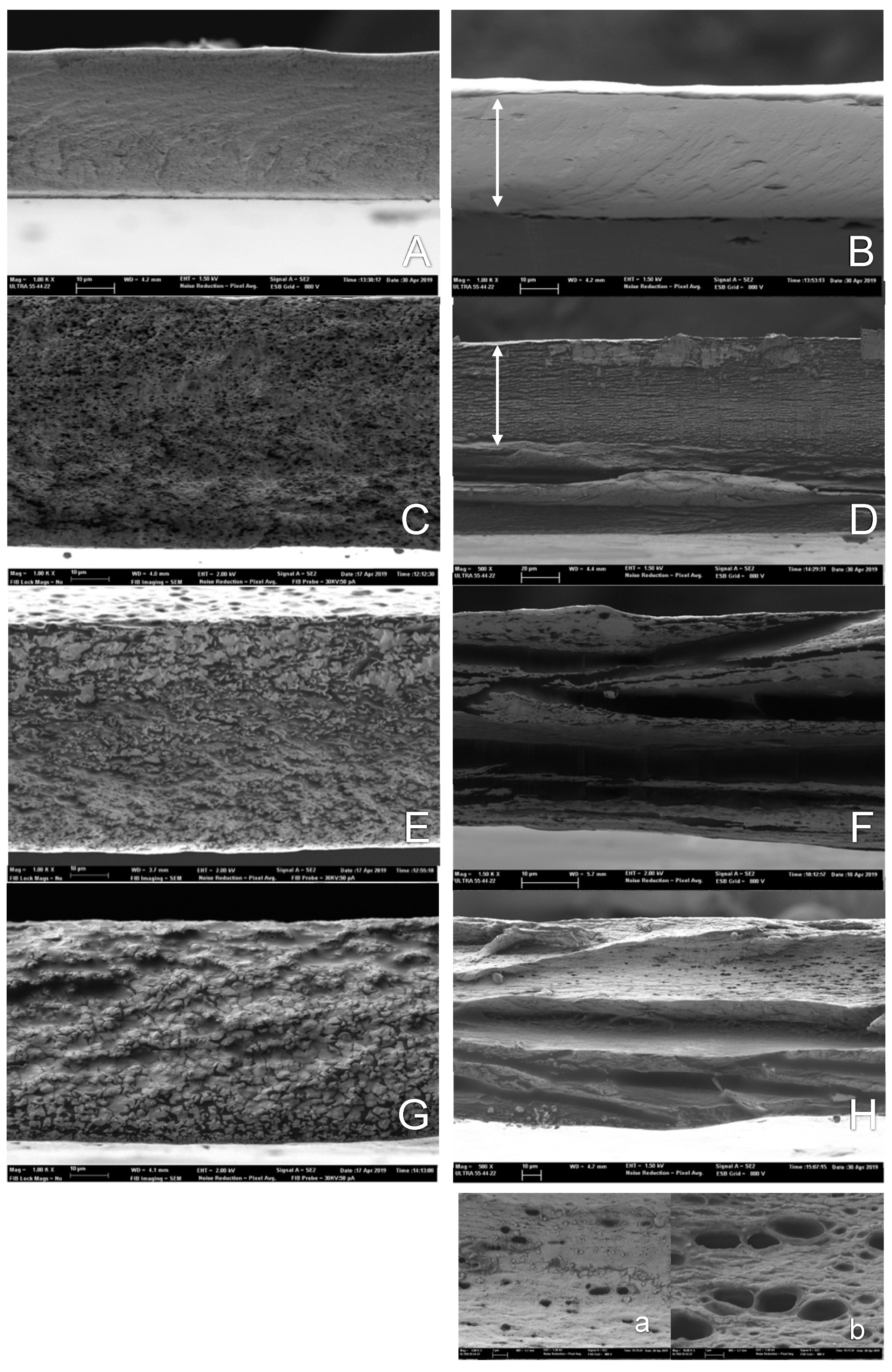
2.4.1. Film Microstructure
2.4.2. Film Thickness
2.4.3. Mechanical Properties
2.4.4. Water Vapor Permeability
2.4.5. Moisture Content and Solubility
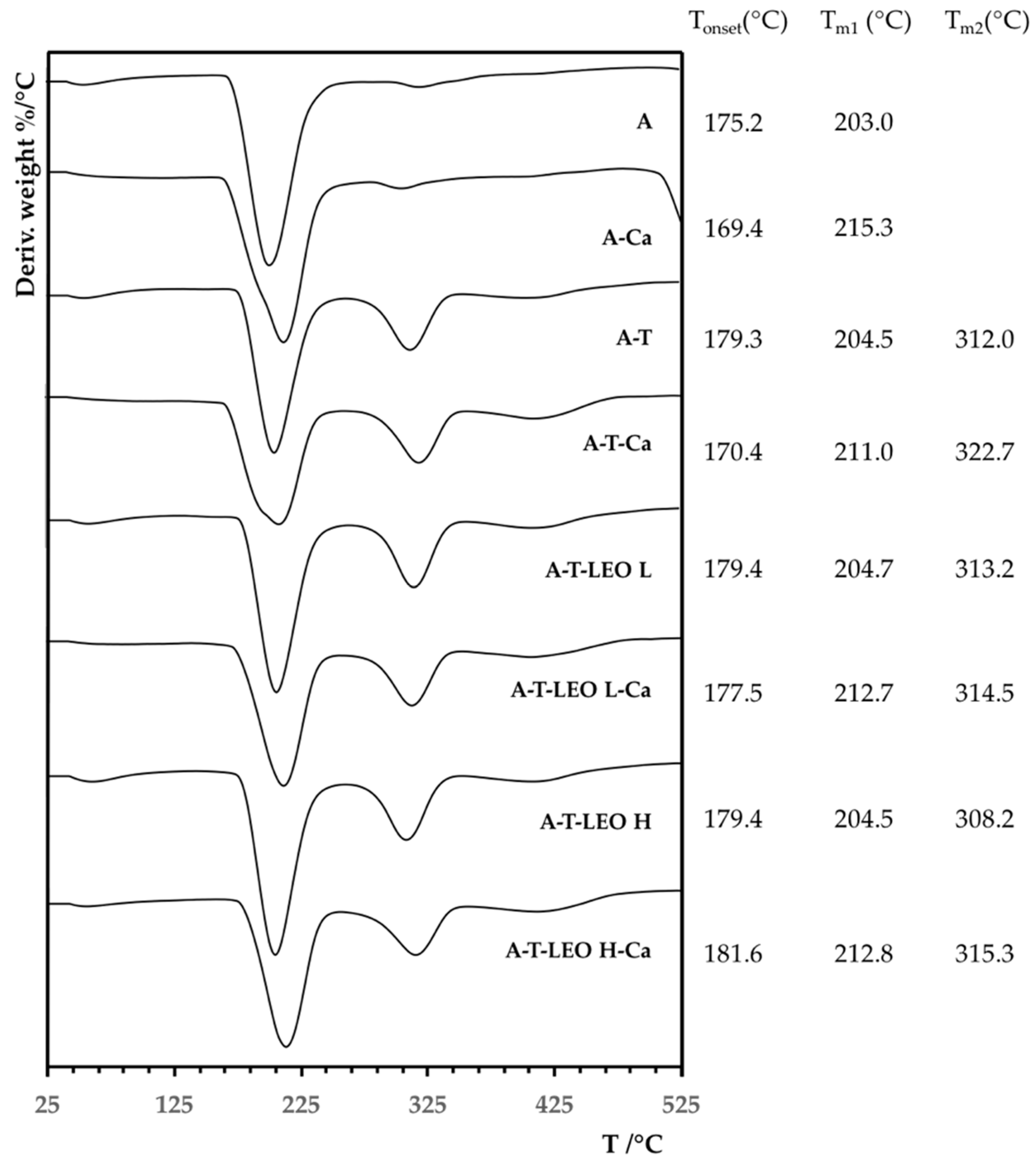
2.4.6. Thermal Analysis
2.4.7. Optical Properties
2.5. Statistical Analysis
3. Results and Discussion
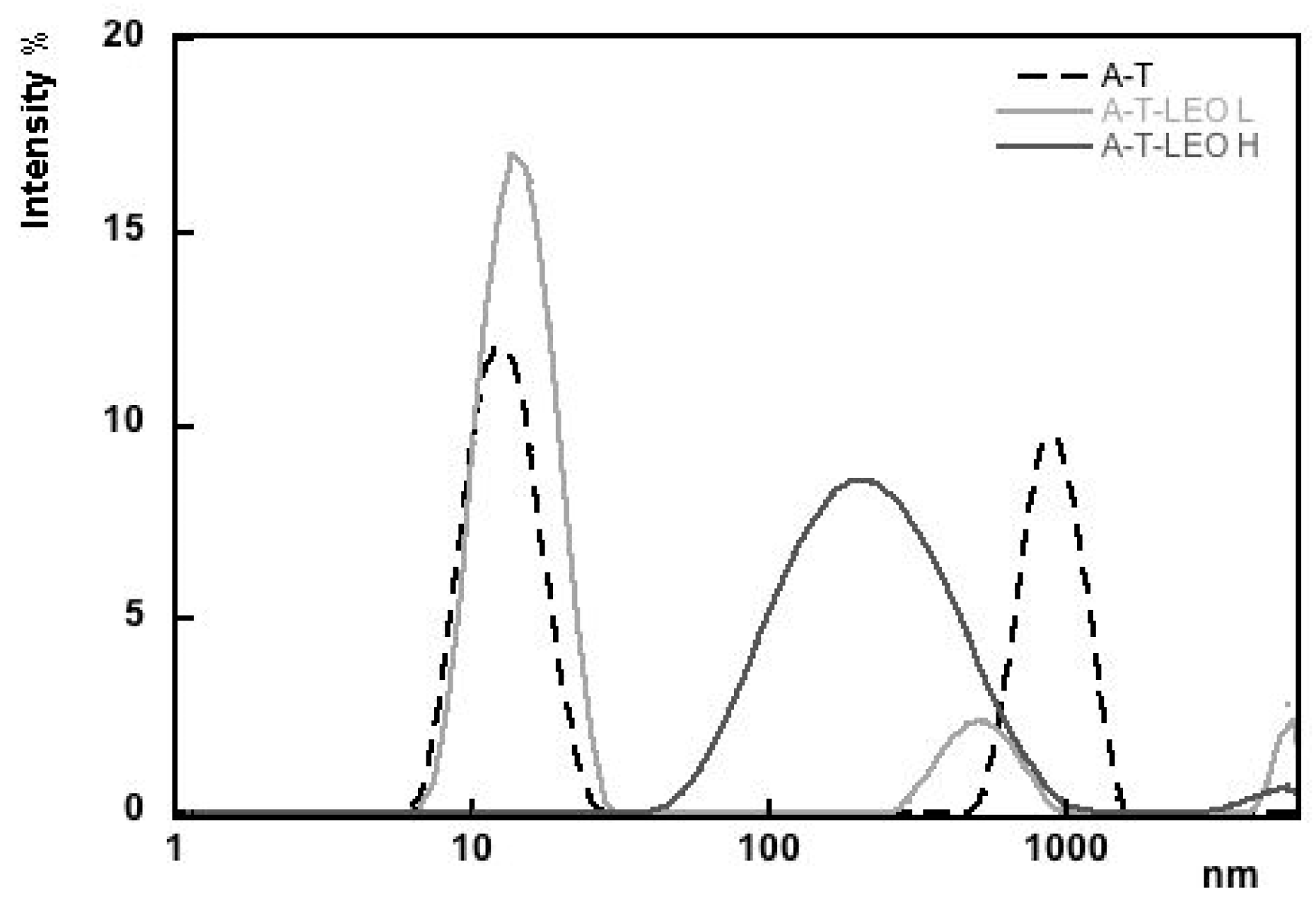
3.1. Alginate Emulsions
3.2. Film Properties
3.2.1. Microstructure
3.2.2. Moisture Content and Solubility
3.2.3. Thickness
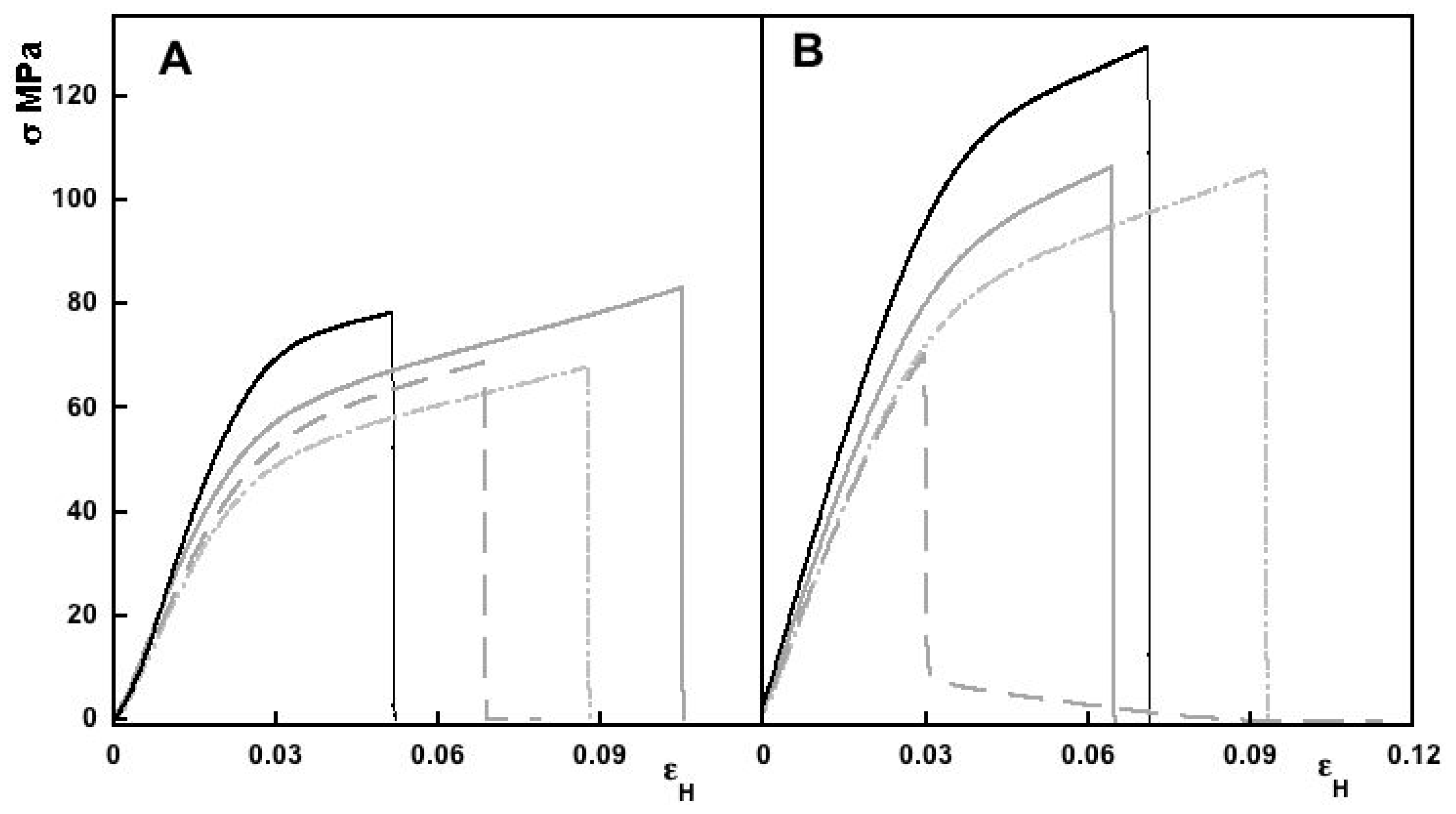
3.2.4. Mechanical Properties
3.2.5. Barrier Properties
3.2.6. Optical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rossi, M.; Passeri, D.; Sinibaldi, A.; Angjellari, M.; Tamburri, E.; Sorbo, A.; Carata, E.; Dini, L. Nanotechnology for Food Packaging and Food Quality Assessment. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 82, pp. 149–204. [Google Scholar]
- Risch, S.J. New Developments in Packaging Materials; ACS Publications: Washington, DC, USA, 2000. [Google Scholar]
- Shit, S.C.; Shah, P.M. Edible polymers: Challenges and opportunities. J. Polym. 2014, 2014. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; Volume 9. [Google Scholar]
- Donhowe, I.G.; Fennema, O. Edible films and coatings: Characteristics, formation, definitions, and testing methods. Edible Coatings Films Improv. Food Qual. 1994, 1–24. [Google Scholar]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Cofelice, M.; Cuomo, F.; Lopez, F. Rheological Properties of Alginate–Essential Oil Nanodispersions. Colloids Interfaces 2018, 2, 48. [Google Scholar] [CrossRef]
- Cuomo, F.; Lopez, F.; Ceglie, A.; Maiuro, L.; Miguel, M.G.; Lindman, B. pH-responsive liposome-templated polyelectrolyte nanocapsules. Soft Matter 2012, 8, 4415–4420. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Lopez, F. Rheological Characterization of Hydrogels from Alginate-Based Nanodispersion. Polymers 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Grumezescu, A.; Holban, A.; Florin, I.; D’Autilia, F.; Carzino, R.; Bianchini, P.; Athanassiou, A. Polylactic acid—lemongrass essential oil nanocapsules with antimicrobial properties. Pharmaceuticals 2016, 9, 42. [Google Scholar] [CrossRef]
- Mbili, N.C.; Opara, U.L.; Lennox, C.L.; Vries, F.A. Citrus and lemongrass essential oils inhibit Botrytis cinerea on ‘Golden Delicious’,‘Pink Lady’and ‘Granny Smith’apples. J. Plant Dis. Prot. 2017, 124, 499–511. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Adzahan, N.M. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Boil. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality Control of Fresh-Cut Apples after Coating Application. Foods 2019, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Sullca, C.; Jiménez, M.; Jiménez, A.; Atarés, L.; Vargas, M.; Chiralt, A. Influence of liposome encapsulated essential oils on properties of chitosan films. Polym. Int. 2016, 65, 979–987. [Google Scholar] [CrossRef]
- ASTM. Standard test method for tensile properties of thin plastic sheeting. Annu. Book Am. Stand. Test. Methods 2001, 162–170. [Google Scholar]
- ASTM. Standard test methods for water vapour transmission of materials. Annu. Book ASTM 1995, 406–413. [Google Scholar]
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Hutchings, J.B. Instrumental specification. In Food Colour and Appearance; Springer: New York, NY, USA, 1999; pp. 199–237. [Google Scholar]
- Dawson, R.; Elliott, D.; Elliott, W. Jones KM: Data for Biochemical Research; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Rao, J.; McClements, D.J. Formation of flavor oil microemulsions, nanoemulsions and emulsions: Influence of composition and preparation method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar]
- Soares, J.d.P.; Santos, J.; Chierice, G.O.; Cavalheiro, E. Thermal behavior of alginic acid and its sodium salt. Eclética Quím. 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Hosseini, M.H.; Razavi, S.H.; Mousavi, S.M.A.; Yasaghi, S.A.S.; Hasansaraei, A.G. Improving antibacterial activity of edible films based on chitosan by incorporating thyme and clove essential oils and EDTA. J. Appl. Sci. 2008, 8, 2895–2900. [Google Scholar]
- Riquelme, N.; Herrera, M.L.; Matiacevich, S. Active films based on alginate containing lemongrass essential oil encapsulated: Effect of process and storage conditions. Food Bioprod. Process. 2017, 104, 94–103. [Google Scholar] [CrossRef]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Roselló, J.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control. 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Pavlath, A.; Gossett, C.; Camirand, W.; Robertson, G. Ionomeric films of alginic acid. J. Food Sci. 1999, 64, 61–63. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT-Food Sci. Technol. 2008, 41, 359–366. [Google Scholar] [CrossRef]
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.; Tylewicz, U.; Lotti, N. Characterization of Active Edible Films based on Citral Essential Oil, Alginate and Pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef] [PubMed]
- Liling, G.; Di, Z.; Jiachao, X.; Xin, G.; Xiaoting, F.; Qing, Z. Effects of ionic crosslinking on physical and mechanical properties of alginate mulching films. Carbohydr. Polym. 2016, 136, 259–265. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Atarés, L.; Pérez-Masiá, R.; Chiralt, A. The role of some antioxidants in the HPMC film properties and lipid protection in coated toasted almonds. J. Food Eng. 2011, 104, 649–656. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacte rial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- Rhim, J.-W. Physical and mechanical properties of water resistant sodium alginate films. LWT-Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Baek, S.-K.; Kim, S.; Song, K. Characterization of Ecklonia cava Alginate Films Containing Cinnamon Essential Oils. Int. J. Mol. Sci. 2018, 19, 3545. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Physical and antimicrobial properties of chitosan–tea tree essential oil composite films. J. Food Eng. 2010, 98, 443–452. [Google Scholar] [CrossRef]

| Sample | Composition | XA | XT80 | X CaCl2 | XLEO |
|---|---|---|---|---|---|
| A | Alginate | 1.00 | |||
| A-Ca | Alginate + CaCl2 | 0.92 | 0.081 | ||
| A-T | Alginate-Tween 80 | 0.77 | 0.23 | ||
| A-T-Ca | Alginate-Tween 80 + CaCl2 | 0.72 | 0.22 | 0.063 | |
| A-T-LEO L | Alginate-Tween 80-LEO Low | 0.71 | 0.21 | 0.072 | |
| A-T-LEO L-Ca | Alginate-Tween 80-LEO Low + CaCl2 | 0.67 | 0.20 | 0.059 | 0.067 |
| A-T-LEO H | Alginate-Tween 80-LEO High | 0.55 | 0.17 | 0.279 | |
| A-T-LEO H-Ca | Alginate-Tween 80-LEO High + CaCl2 | 0.53 | 0.16 | 0.046 | 0.266 |
| MC (g/100g) | S (%) | WVP *10−9 (g/msPa) | EM (MPa) | TS (MPa) | E (%) | Thickness (μm) | |
|---|---|---|---|---|---|---|---|
| A | 10.7 (0.4) a,b | 99 (0) c | 2.5 (0.2) a | 3200 (120) d | 80 (5) a | 5 (1) a | 50 (4) a |
| A-Ca | 11.3 (0.8) b | 58 (3.7) a | 3.1 (0.6) a,b | 3800 (200) e | 133 (16) b | 7 (2) a,b | 62 (7) b |
| A-T | 9.4 (0.2) a,b | 95 (2.7) c | 3.1 (0.5) a | 2500 (98) a,b,c | 82 (5) a | 11 (1) d | 61 (4) b |
| A-T-Ca | 10.1 (0.3) a,b | 76 (0) a,b,c | 2.8 (0.8) a | 2600 (400) b,c | 77 (27) a | 5 (3) a,b | 89 (16) c |
| A-T-LEO L | 9.6 (1.1) a,b | 88 (2.4) b,c | 2.9 (0.6) a,b | 2000 (150) a | 70 (7) a | 9 (3) b,c,d | 66 (6) b |
| A-T-LEO L-Ca | 9.1 (0.6) a,b | 65 (7.3) a,b | 3.6 (0.5) b | 2800 (130) c,d | 122 (18) b | 11 (3) c,d | 83 (12) c |
| A-T-LEO H | 8.9 (1.0) a | 98 (2.7) c | 2.9 (0.4) a | 2400 (90) a,b | 70 (7) a | 7 (2) a,b,c | 64 (6) b |
| A-T-LEO H-Ca | 8.8 (0.9) a | 75 (11.4) a,b,c | 3.1 (0.4) a,b | 2600 (140) a,b,c | 72 (16) a | 4 (2) a | 91 (16) c |
| Sample | L* | Cab* | hab* | Ti 450 nm | WI | ΔE |
|---|---|---|---|---|---|---|
| A | 83.6 (0.9) d | 9.0 (0.6) a,b,c | 89.3 (0.9) a,b | 0.842 (0.003) f | 81 (1) d | - |
| A-Ca | 80.0 (1.6) b,c | 8.4 (1.0) a,b | 89.8 (1.8) a,b | 0.838 (0.006) e,f | 78 (1) b,c | 3.8 (1.6) a |
| A-T | 82.0 (1.3) c,d | 10.7 (1.0) c | 89.3 (0.8) a,b | 0.826 (0.008) d,e | 79 (1) c,d | 2.7 (0.8) a |
| A-T-Ca | 77.6 (2.1) a,b | 7.1 (0.8) a | 94.5 (2.3) c | 0.798 (0.012) b,c | 76(2) b,c | 6.9 (1.7) c |
| A-T-LEO L | 79.6 (1.6) b,c | 12.9 (0.7) d | 88.0 (0.9) a | 0.811 (0.007) c,d | 76 (1) b | 5.8 (0.8) b,c |
| A-T-LEO L-Ca | 78.7 (4.1) a,b,c | 10.1 (1.9) b,c | 91.0 (2.1) b | 0.782 (0.015) b | 76 (3) b | 4.2 (1.5) a,b |
| A-T-LEO H | 77.4 (1.5) a,b | 17.1 (1.6) e | 89.4 (1.4) a,b | 0.810 (0.007) c,d | 72 (1) a | 10.5 (0.9) d |
| A-T-LEO H-Ca | 75.3 (2.0) a | 15.1 (1.7) e | 90 (1.6) a,b | 0.763 (0.010) a | 71 (1) a | 10.6 (1.0) d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofelice, M.; Cuomo, F.; Chiralt, A. Alginate Films Encapsulating Lemongrass Essential Oil as Affected by Spray Calcium Application. Colloids Interfaces 2019, 3, 58. https://doi.org/10.3390/colloids3030058
Cofelice M, Cuomo F, Chiralt A. Alginate Films Encapsulating Lemongrass Essential Oil as Affected by Spray Calcium Application. Colloids and Interfaces. 2019; 3(3):58. https://doi.org/10.3390/colloids3030058
Chicago/Turabian StyleCofelice, Martina, Francesca Cuomo, and Amparo Chiralt. 2019. "Alginate Films Encapsulating Lemongrass Essential Oil as Affected by Spray Calcium Application" Colloids and Interfaces 3, no. 3: 58. https://doi.org/10.3390/colloids3030058
APA StyleCofelice, M., Cuomo, F., & Chiralt, A. (2019). Alginate Films Encapsulating Lemongrass Essential Oil as Affected by Spray Calcium Application. Colloids and Interfaces, 3(3), 58. https://doi.org/10.3390/colloids3030058





