2. Materials and Methods
Sodium dodecylsulfate for biochemistry (99%) was acquired from Acros (Geel, Belgium), sodium tetradecylsulfate from Aldrich (Darmstadt, Germany) (99%), and sodium hexadecylsulfate from Alfa Aesar (Karlsruhe, Germany) (99%). Hematite was synthesized by heating an acidified 0.02 M FeCl
3 solution at 100 °C for 1 d, according to the classical recipe by Matijević [
21]. The powder was removed from solution by centrifugation, washed with water, and freeze-dried. Its specific surface area (determined by means of BET method, Gemini V, Micromeritics, Norcross, USA) was 26 m
2/g, the particle diameter determined via TEM (transmission electron microscopy) was 50 nm, and the pristine IEP was at pH 9. Alu C alumina (100 m
2/g, 20 nm, pH 9) and P25 titania (50 m
2/g, 30 nm, pH 6.5, respectively) were acquired from Degussa-Evonik. All commercial chemicals were used as obtained. The dispersions of the powders (1:10,000 by mass) in 10
−3 M NaCl were prepared and stored in plastic test-tubes to avoid contact with glass. Storage of dispersions of metal oxides in glass is well known to induce a shift in their IEP to low pH due to specific adsorption of silicate anions. The pH was adjusted with NaOH or HCl solutions, and measured just before the electrokinetic measurement. The electrophoretic mobility and particle size were measured by means of a Malvern ZetaSizer Nano ZS at 25 °C. Two series of measurements were performed: directly after preparation of the dispersion, and after aging the dispersion for a few days (cf.
Table S1).
3. Results and Discussion
The electrokinetic behavior of metal oxides in fresh dispersions containing different amounts of anionic surfactants was presented and discussed in detail in our previous paper [
20]. In
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8, we show the electrokinetic curves in dispersions aged from one to eight days.
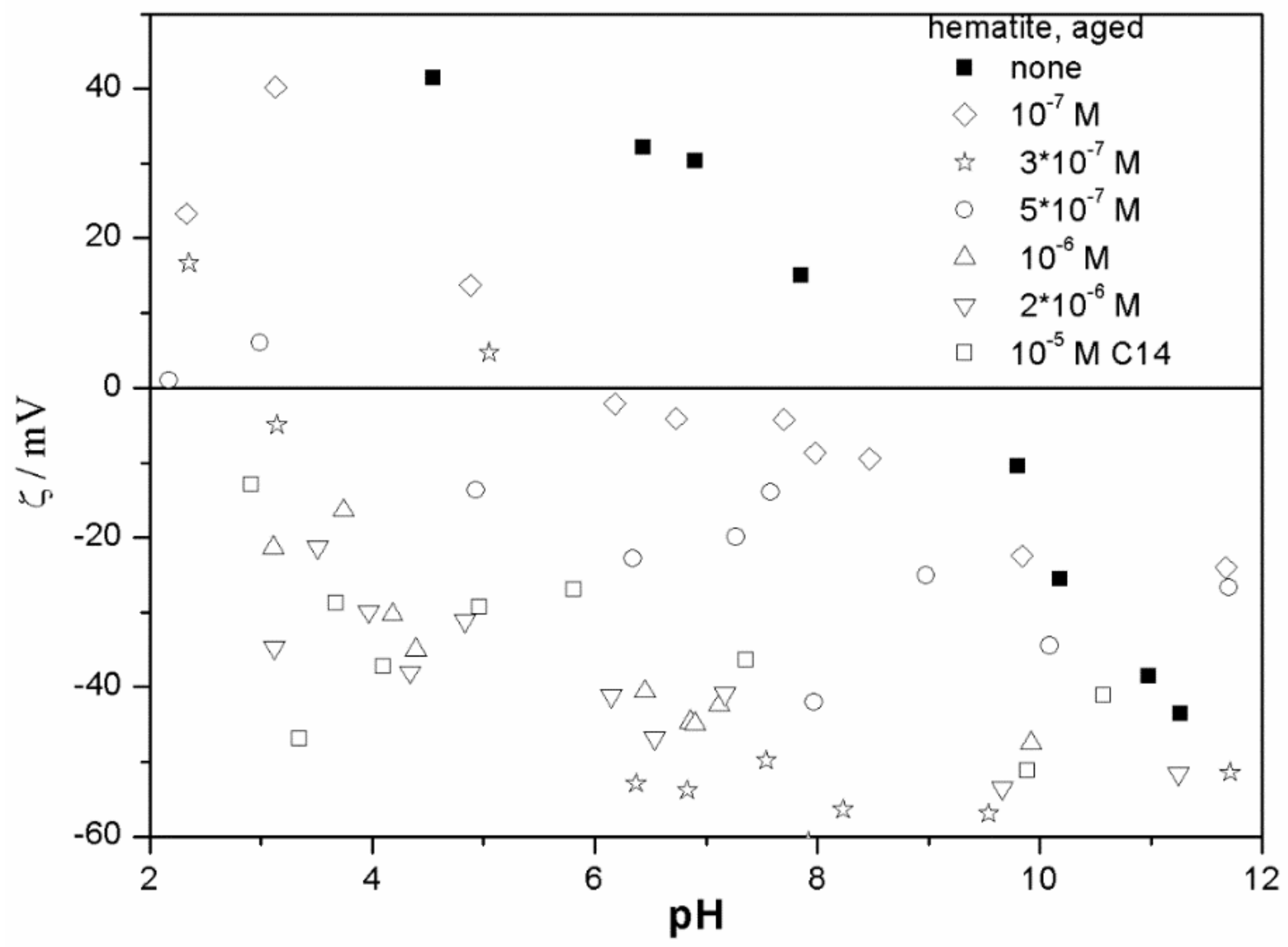
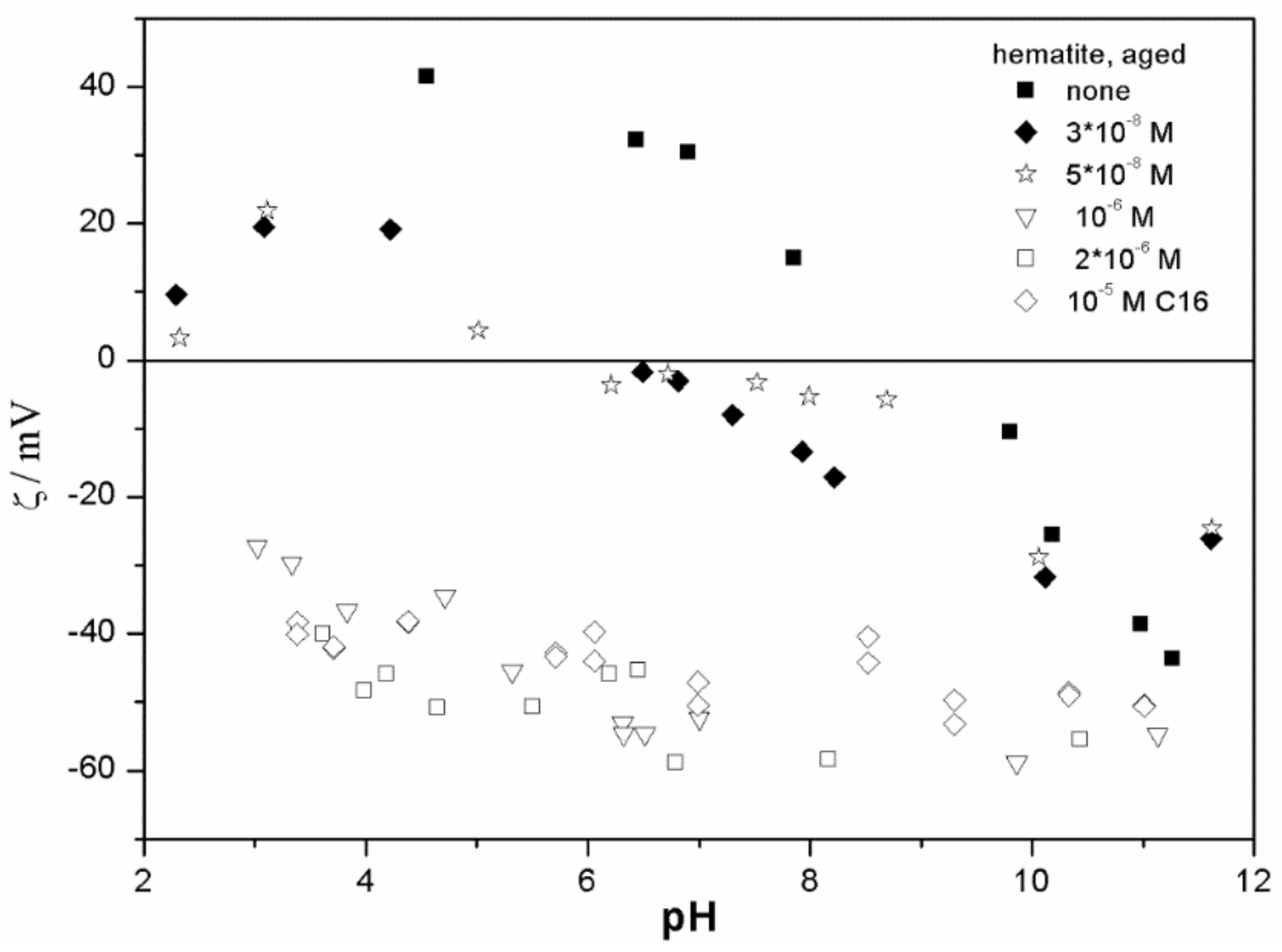
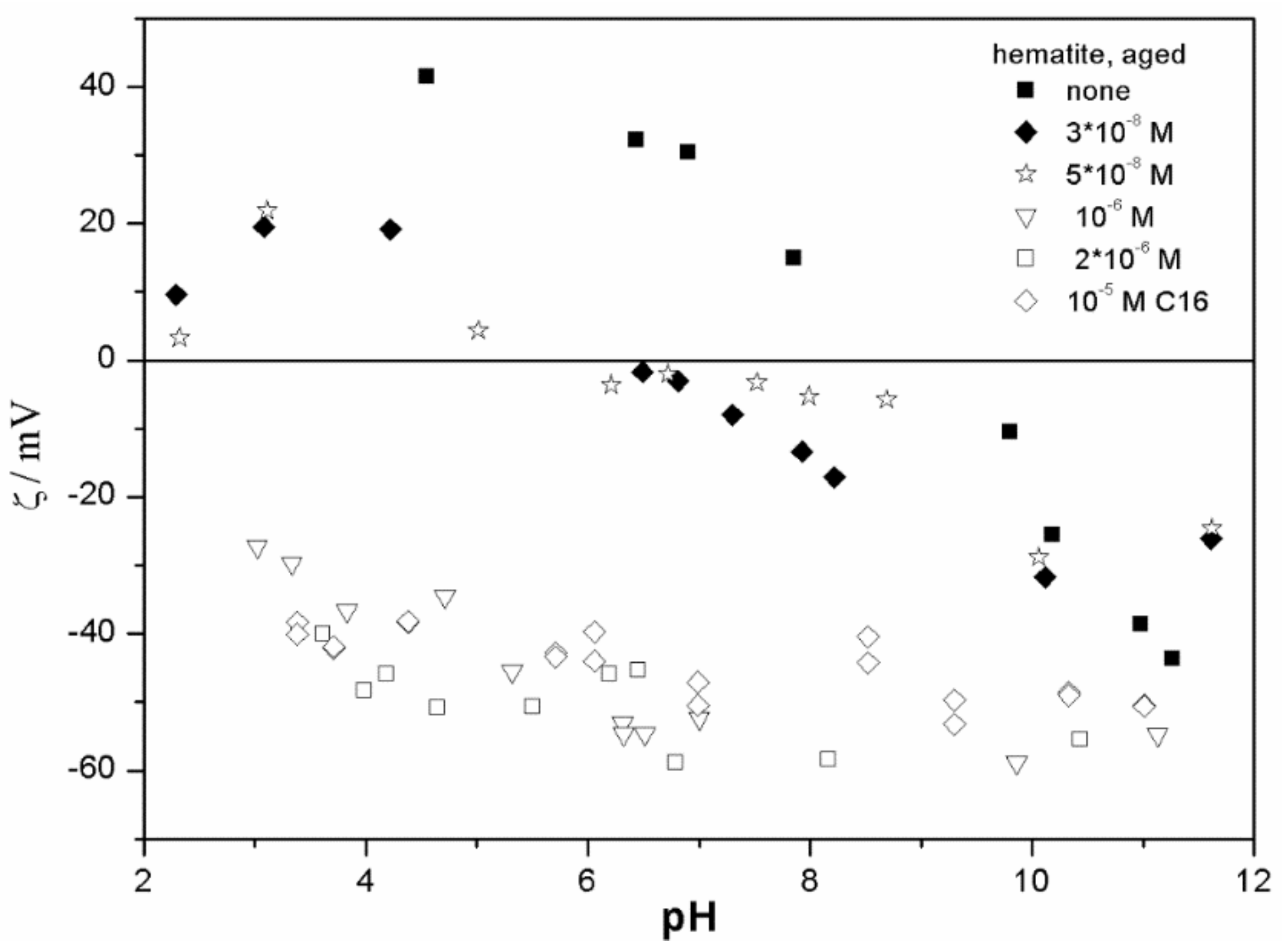
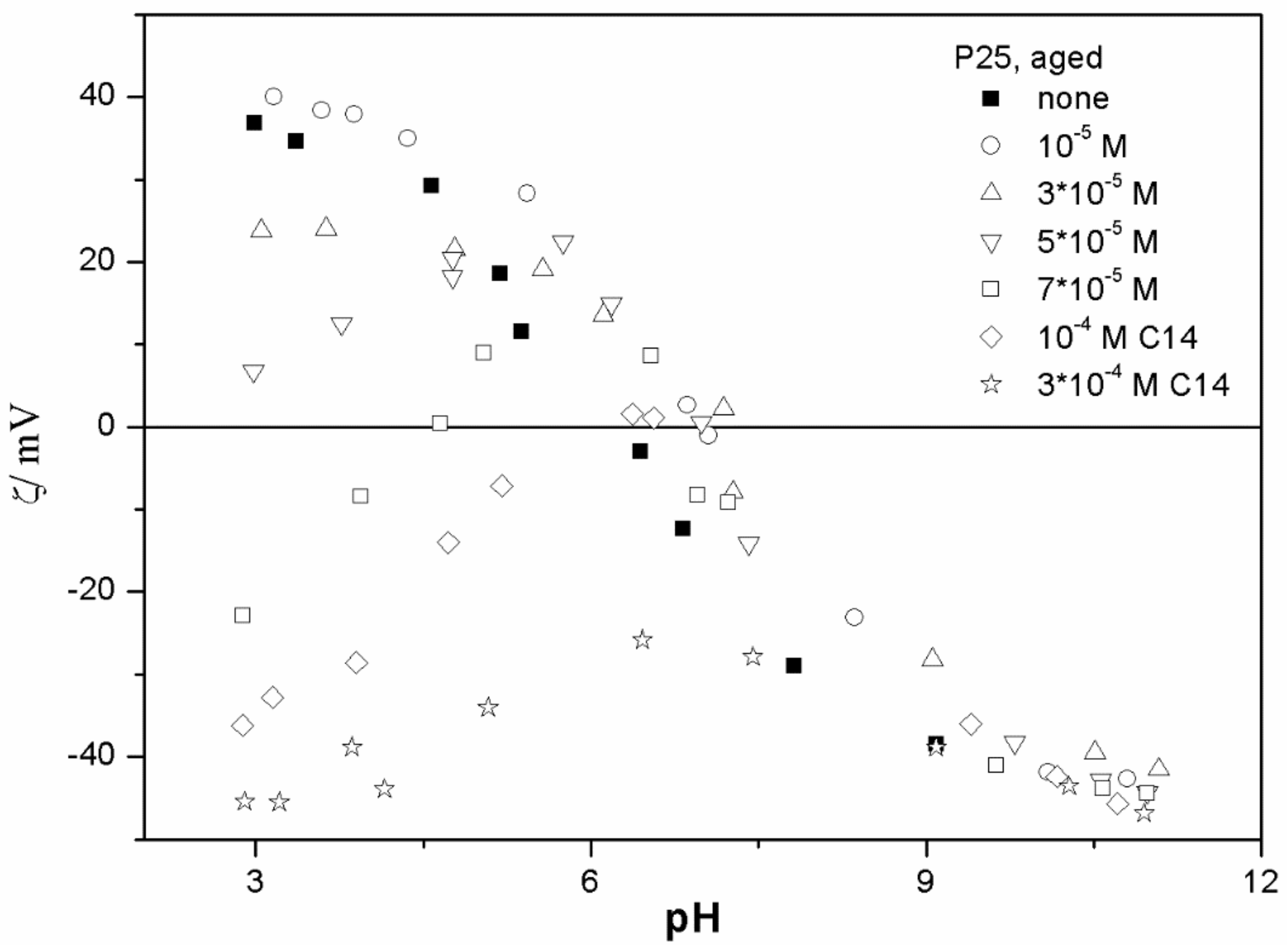
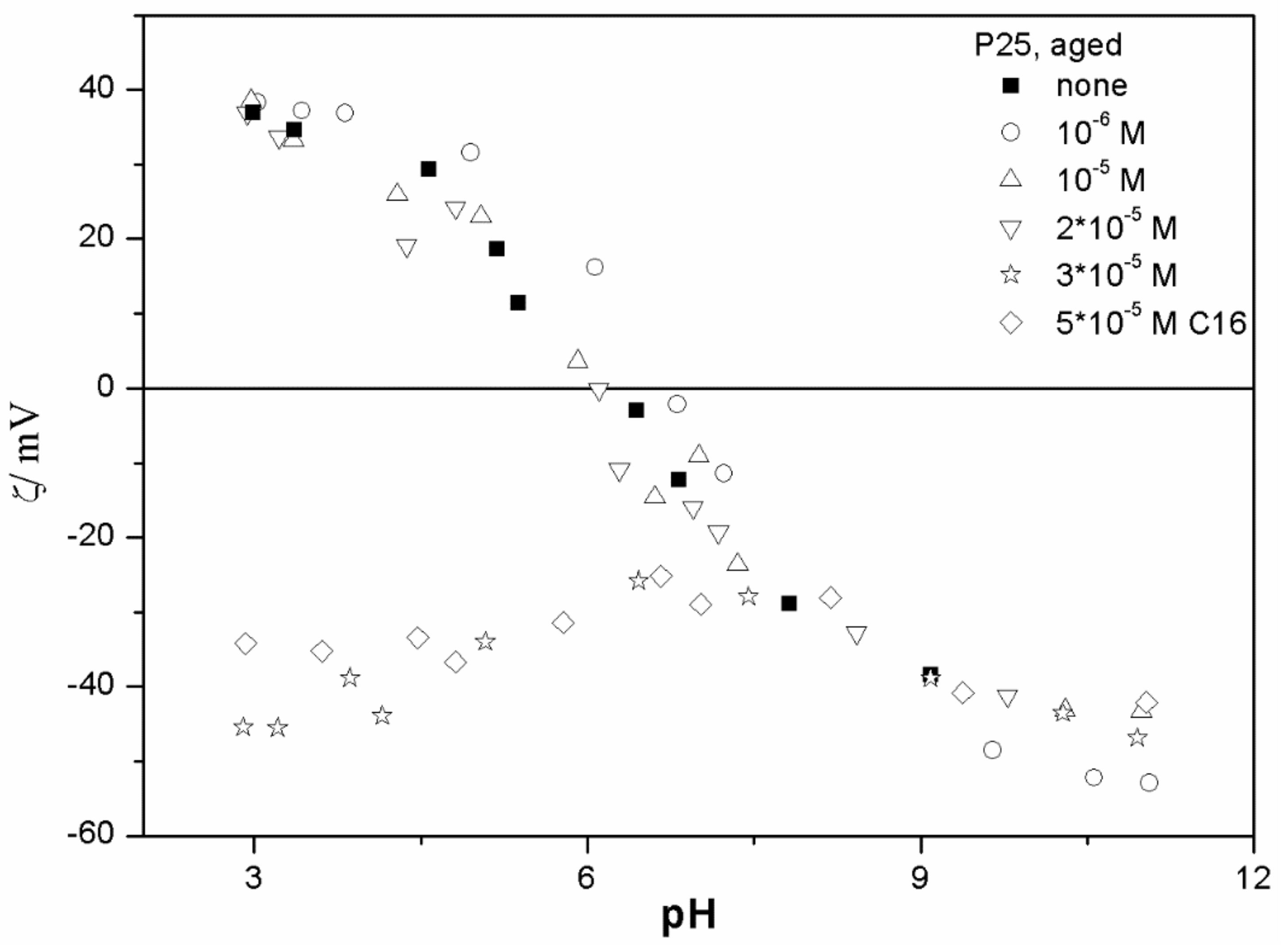
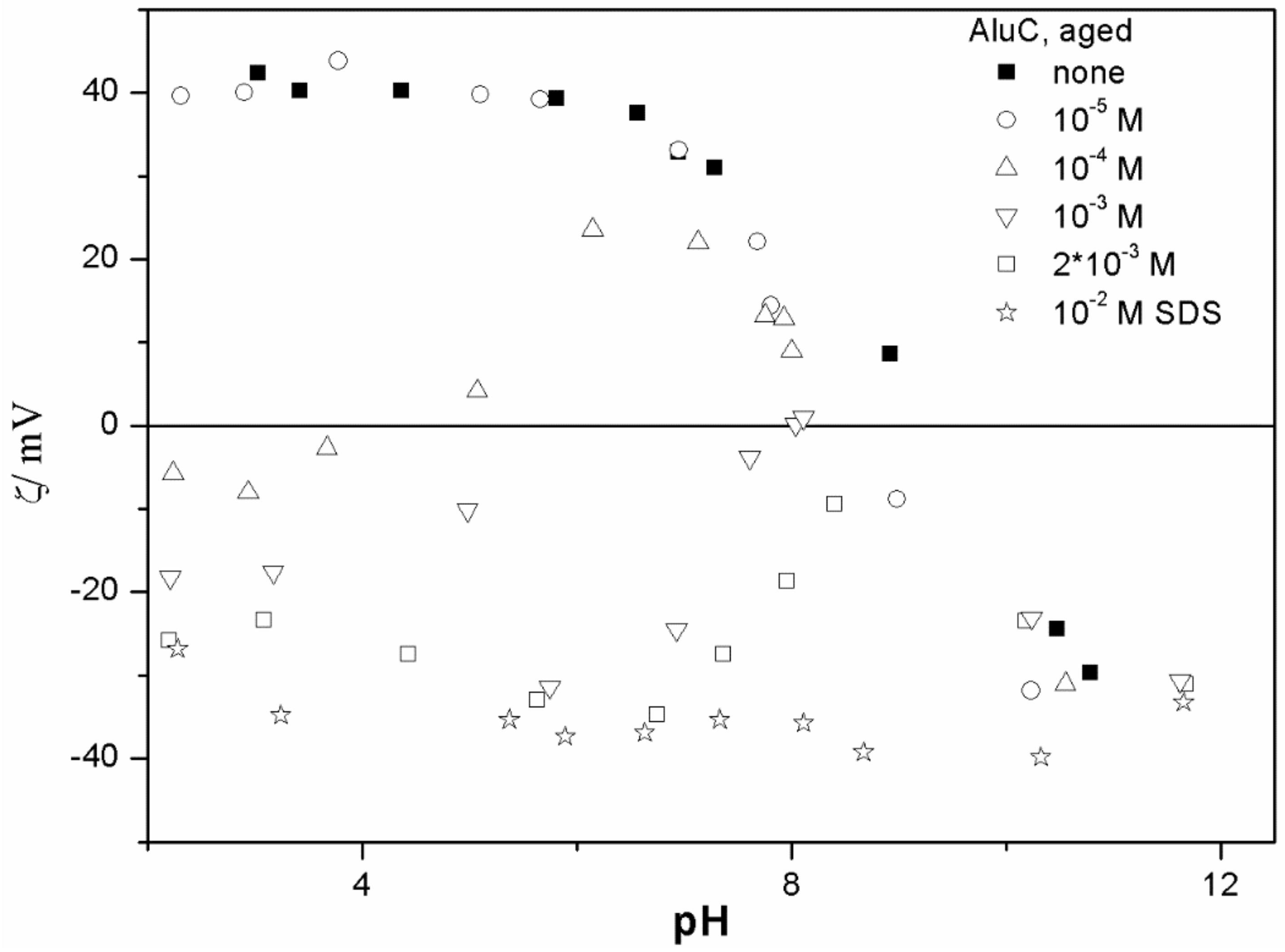
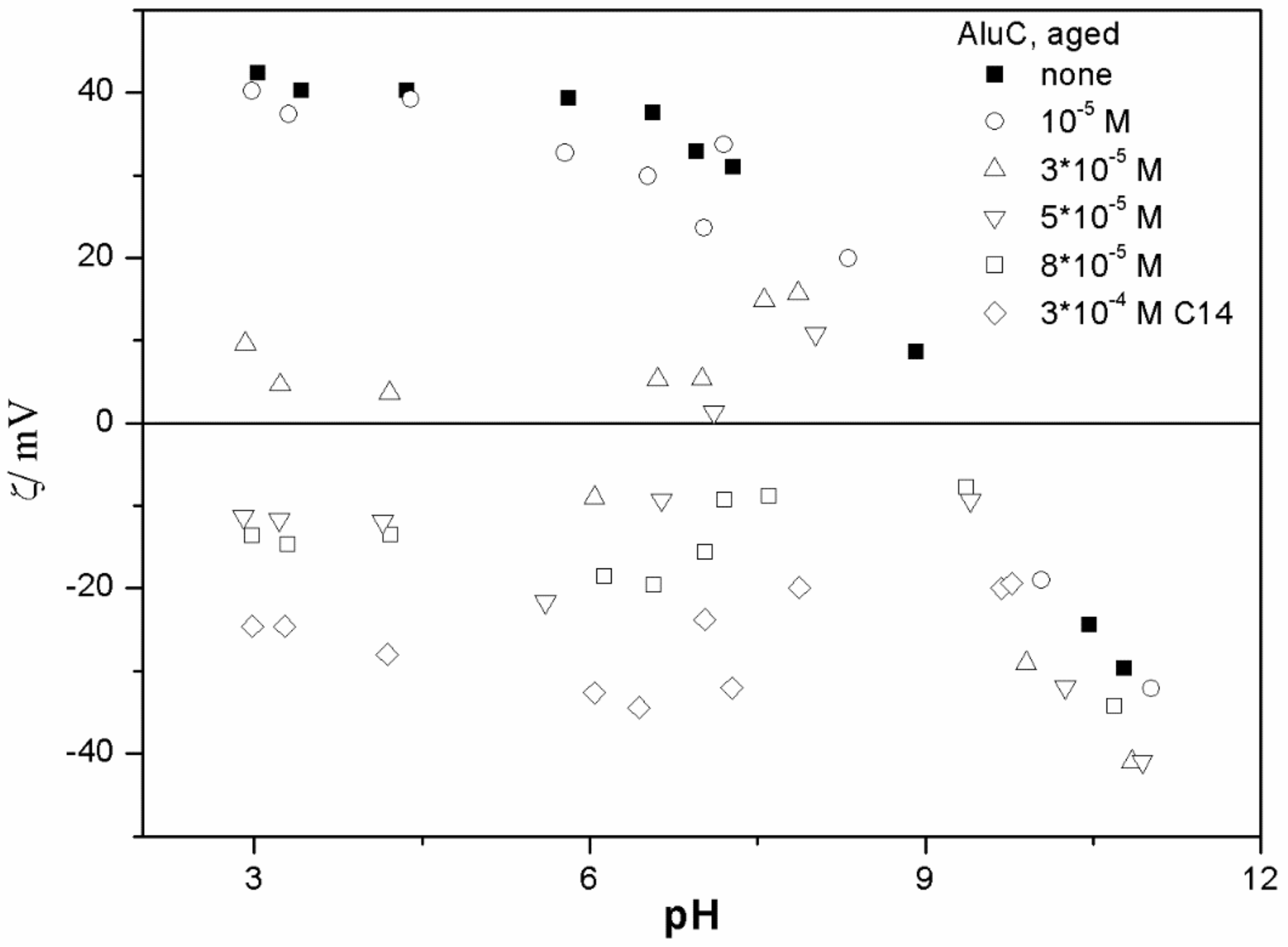
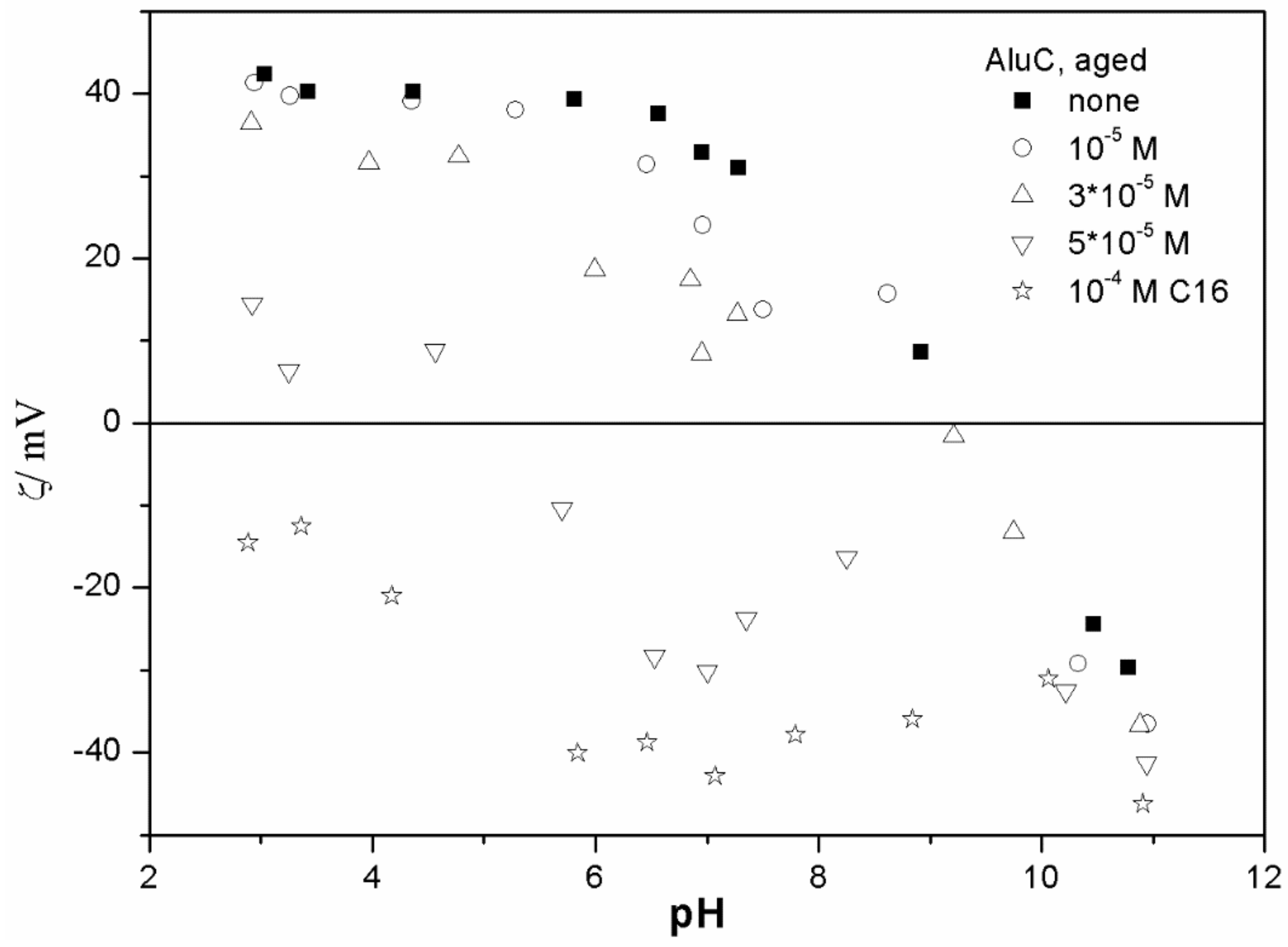
The ζ potentials shown in
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8 are very similar to the results reported for fresh dispersions [
20]; that is, aging has rather insignificant effect on the ζ potentials of metal oxides in the presence of alkylsulfates. Two types of electrokinetic behavior have been observed. In dispersions containing SDS (
Figure 3 and
Figure 6), and in titania- tetradecylsulfate (
Figure 4) and alumina- tetradecylsulfate (
Figure 7) systems, the specific adsorption of anionic surfactants resulted in a reversal of the sign of the ζ potential of metal oxide from positive to negative in the acidic range, but a rather insignificant effect in pH-neutral range. Thus, the electrokinetic curves showed a clear maximum. In contrast, in dispersions containing sodium hexadecylsulfate, the presence of the surfactant resulted in a shift in the IEP to low pH (no clear maximum in the electrokinetic curves), which is a typical behavior observed in most anions other than SDS, and discussed in the introduction. The unusual electrokinetic behavior observed in the presence of SDS was interpreted in terms of surfactant adsorption in aggregated form (admicelles, hemimicelles) [
20]. It should be emphasized that the ζ potentials shown in
Figure 1–8 are only valid for certain solid-to-liquid ratios (here 1:10,000). With higher solid-to-liquid ratio, higher concentrations of surfactants are required to induce the same effect on the ζ potential. The sets of electrokinetic curves obtained at various solid-to-liquid ratios can be normalized in terms of concentration of surfactant per unit of surface area [
2].
The anticorrelation between the absolute value of the ζ potential and the particle size has been widely discussed in the literature, and the maximum in the particle size as the function of pH has been considered as a method of determination of the IEP. Indeed, many studies indicate a correlation between the pristine IEP of metal oxides and the pH of the maximum in the particle size [
1]. The literature is full of reports on the aggregation of the materials studied in this paper (especially P25 and AluC) in dilute 1-1 electrolytes in the absence of ionic surfactants, including several publications by the present authors. While the correlation between the maximum in the particle size and IEP is commonplace, in most studies, primary particles were not observed, but a certain degree of aggregation was detected even far from IEP, and with absolute values of ζ potentials higher than 50 mV.
The fresh and aged dispersions were turbid, but they were not homogeneous. The particles were deposited on the walls of plastic vials, and sediment was visible on their bottoms.
The relationship between the absolute value of the ζ potential and the particle size in fresh and aged dispersions of metal oxides containing anionic surfactants is illustrated in
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13,
Figure 14,
Figure 15 and
Figure 16. The same results in pH-size coordinates are presented in
Table S2. We only present a few selected results (a few concentrations of the surfactants), and the other (unpublished) results follow the trends presented and discussed below. The average particle size produced by Malvern Zetasizer is calculated according to certain model, and it does not reflect the actual size of the aggregates, especially when their shape is far from spherical. Nevertheless, the high values of average particle size reflect a high degree of aggregation of primary particles, and they are on the same order of magnitude as the size of the actual aggregates. The Malvern Zetasizer also produces the degree of polydispersity, which is not reported here, and which was high in most specimens (typically about 0.5 and >0.2 in most specimens), and best-fit uni-, bi-, or trimodal distributions of particle size. Many authors report such graphs in their publications, but these distributions reflect the best-fit model parameters rather than the actual distributions, and their relevance to the actual dispersions is limited.
In many papers, including our publications [
17,
22], the results of electrophoretic measurements are presented as electrophoretic mobility vs. pH rather than as ζ vs. pH curves. Both approaches have their pros and cons. With irregularly-shaped and flexible aggregates and 1
<κa<100, exact calculation of ζ from electrophoretic mobility is not possible, and the ζ potential can only be estimated [
1], while the electrophoretic mobility is an exact experimental result. In this present paper, we show ζ rather than mobility in the figures, although we do realize that our ζ is only an approximation. Our approach has the following advantages:
Our ζ can be directly compared with experimental results from other publications, including the results obtained by electroosmosis and electroacoustic methods.
Our ζ can be directly compared with the results of model calculations.
Our ζ can be directly used in calculations, e.g., of interaction curves in the DLVO (Derjaguin-Landau-Verwey-Overbeek) theory.
The electrophoretic mobilities corresponding to the data points from
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13,
Figure 14,
Figure 15 and
Figure 16 are presented in
Table S3. They can also be calculated from the data presented in the figures by the division of ζ (expressed in mV) by 12.8, and the resulting mobility is expressed in 10
−8 m
2/V/s.
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13,
Figure 14,
Figure 15 and
Figure 16 indicate a substantial degree of aggregation of the particles even at relatively high absolute values of the ζ potential. In dispersions of hematite, the lowest observed average radii were about 70 nm, while the radii from TEM (transmission electron microscopy) were about 25 nm. In dispersions of titania, the lowest observed average radii were about 100 nm, while the radii from TEM were about 15 nm. In dispersions of alumina, the lowest observed average radii were about 110 nm, while the radii from TEM were about 10 nm.
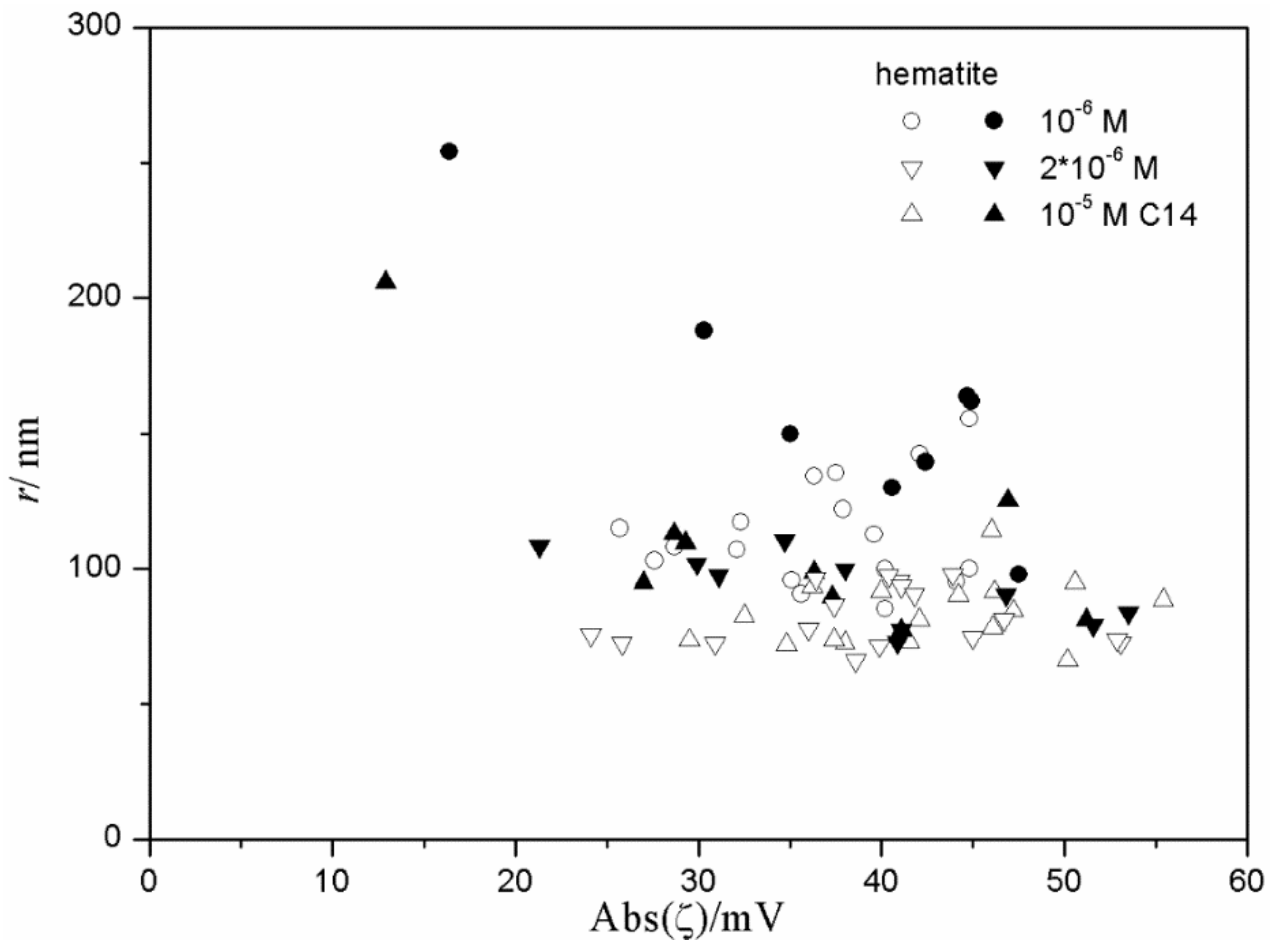
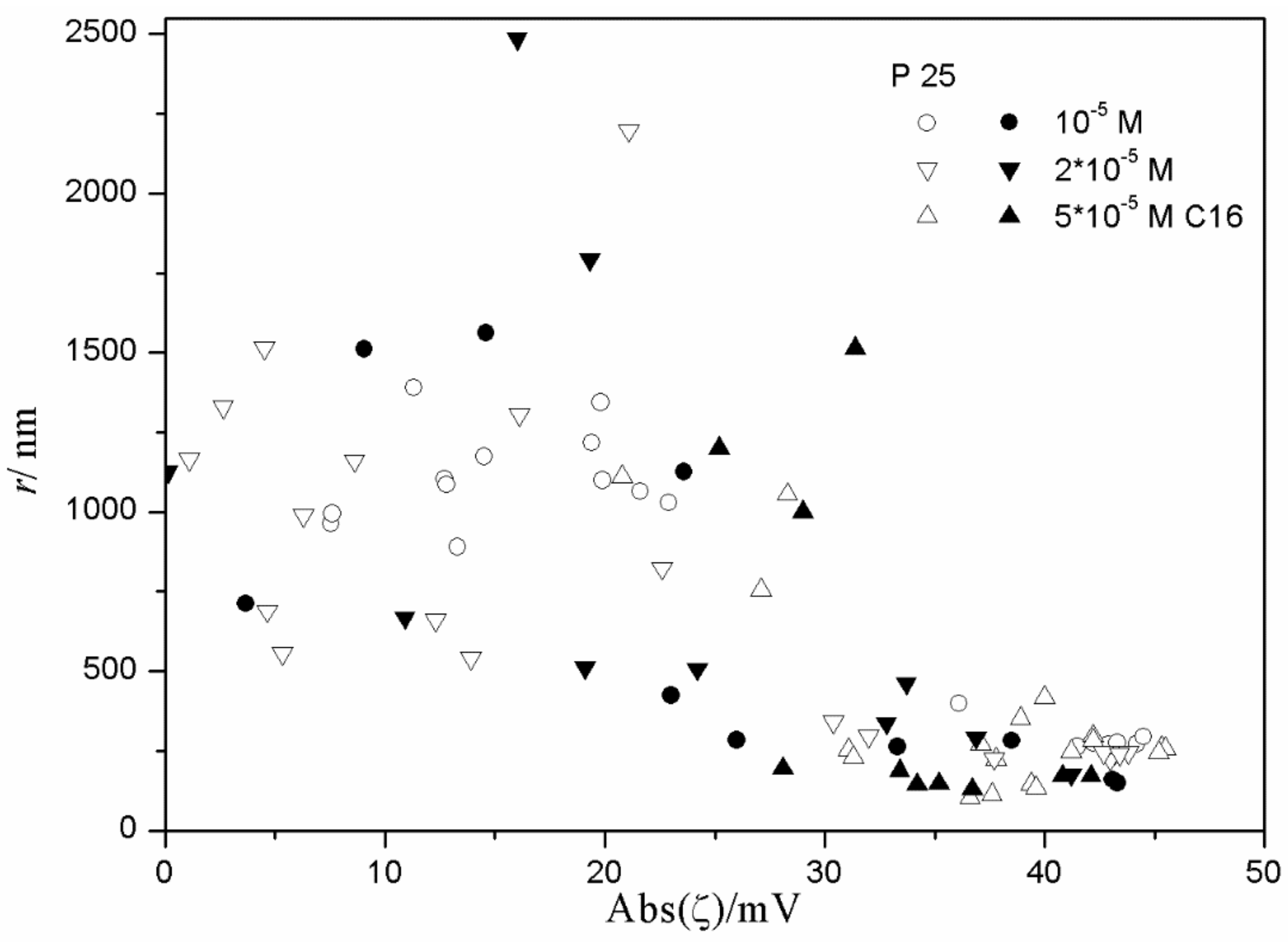
Figure 9 and
Figure 10 show very weak correlation between the absolute value of ζ potential and the particle size of hematite. Interestingly enough, the highest degree of aggregation (particle radii of 200–500 nm) was only observed in the presence of 10
−5 M hexadecylsulfate, in fresh and aged dispersions, while at lower concentrations of hexadecylsulfate the particles were smaller. This may suggest that a high concentration of hexadecylsulfate induces particle aggregation. However, several dispersions containing 10
-5 M hexadecylsulfate showed low degree of aggregation (particle radii <100 nm). Almost all data points shown in
Figure 9 and
Figure 10 refer to a relatively high absolute value of ζ potential (>25 mV), and two data points corresponding to low absolute value of ζ potential show a substantial degree of aggregation.
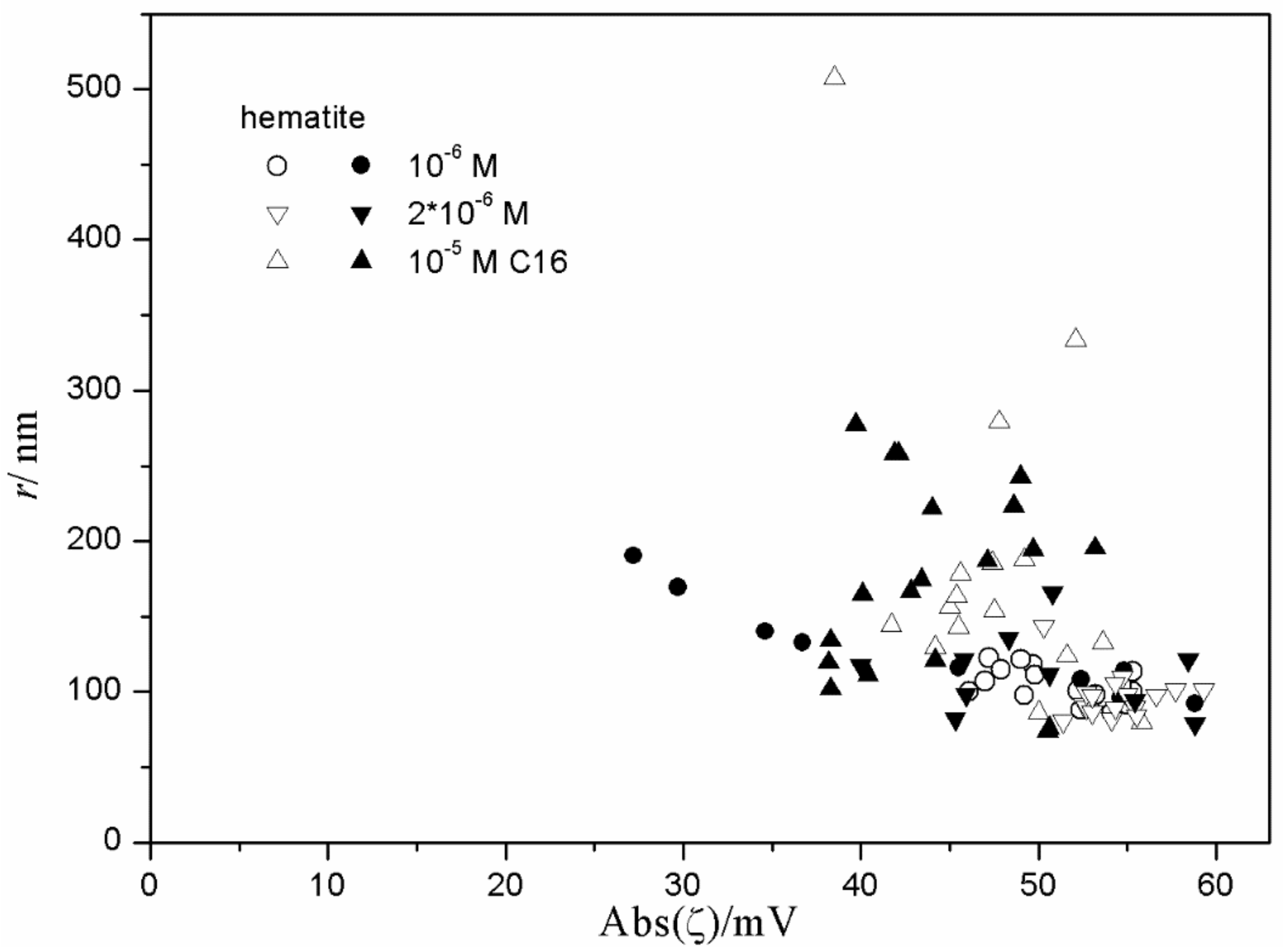
Figure 11,
Figure 12 and
Figure 13 show anticorrelation between the absolute value of ζ potential and the particle size of titania, especially in dispersions stabilized with tetradecyl- and hexadecylsulfate. Relatively low degrees of aggregation (particle radii <250 nm) are only observed at absolute values of ζ potential >30 mV, while for absolute values of ζ potential <30 mV the particle radii are >500 nm, with a few exceptions. There is no systematic effect on aging on the particle size. The results obtained in the presence of 10
−3 and 10
−2 M SDS (
Figure 11) are exceptional; in spite of high absolute value of ζ potential (>40 mV), most dispersions show substantial aggregation (particle radii >500 nm). Such an aggregation was not observed in dispersions stabilized with tetradecyl- or hexadecylsulfate or in 10
−4 M SDS. This may suggest that a high concentration of SDS induces particle aggregation.
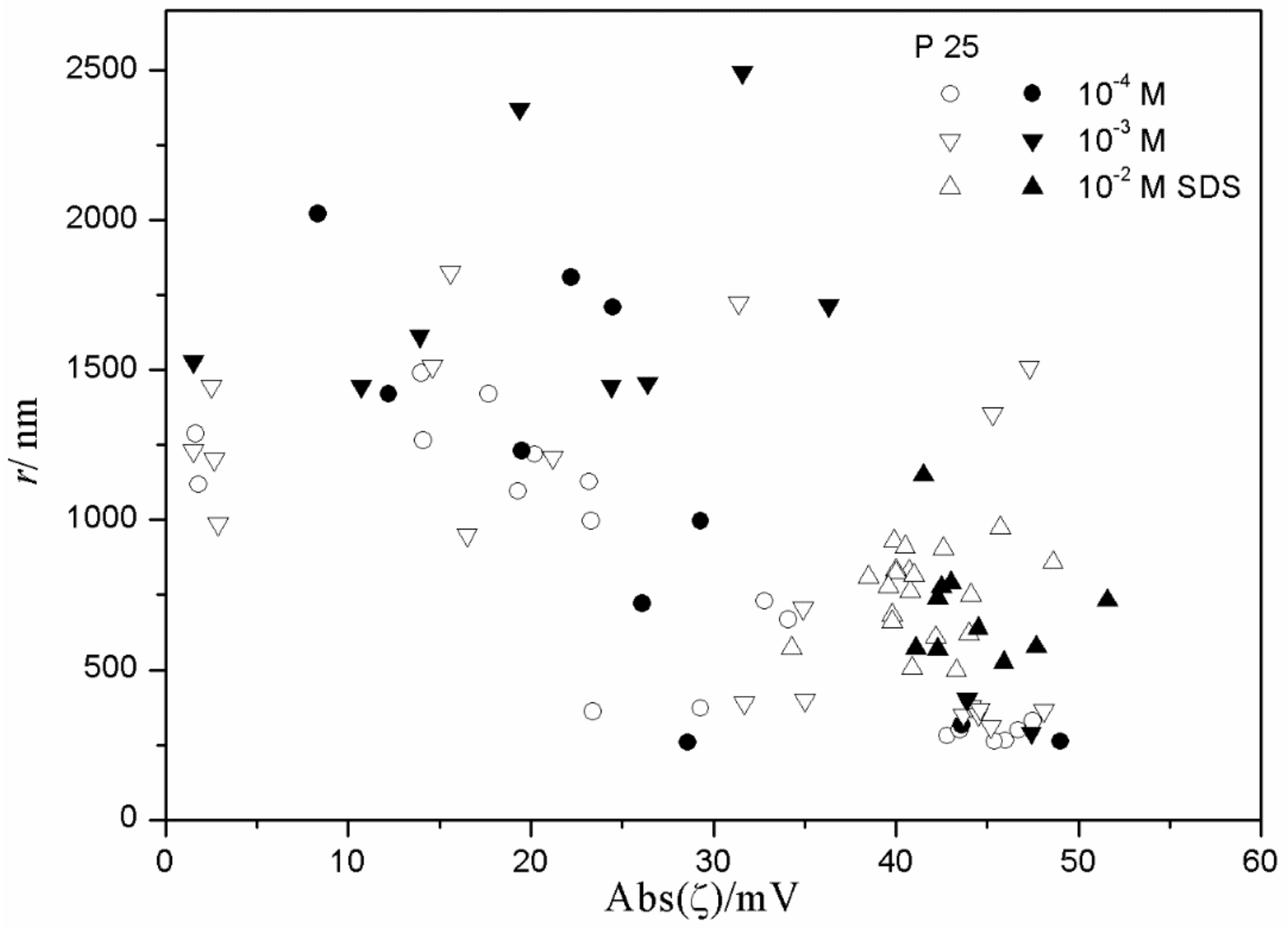
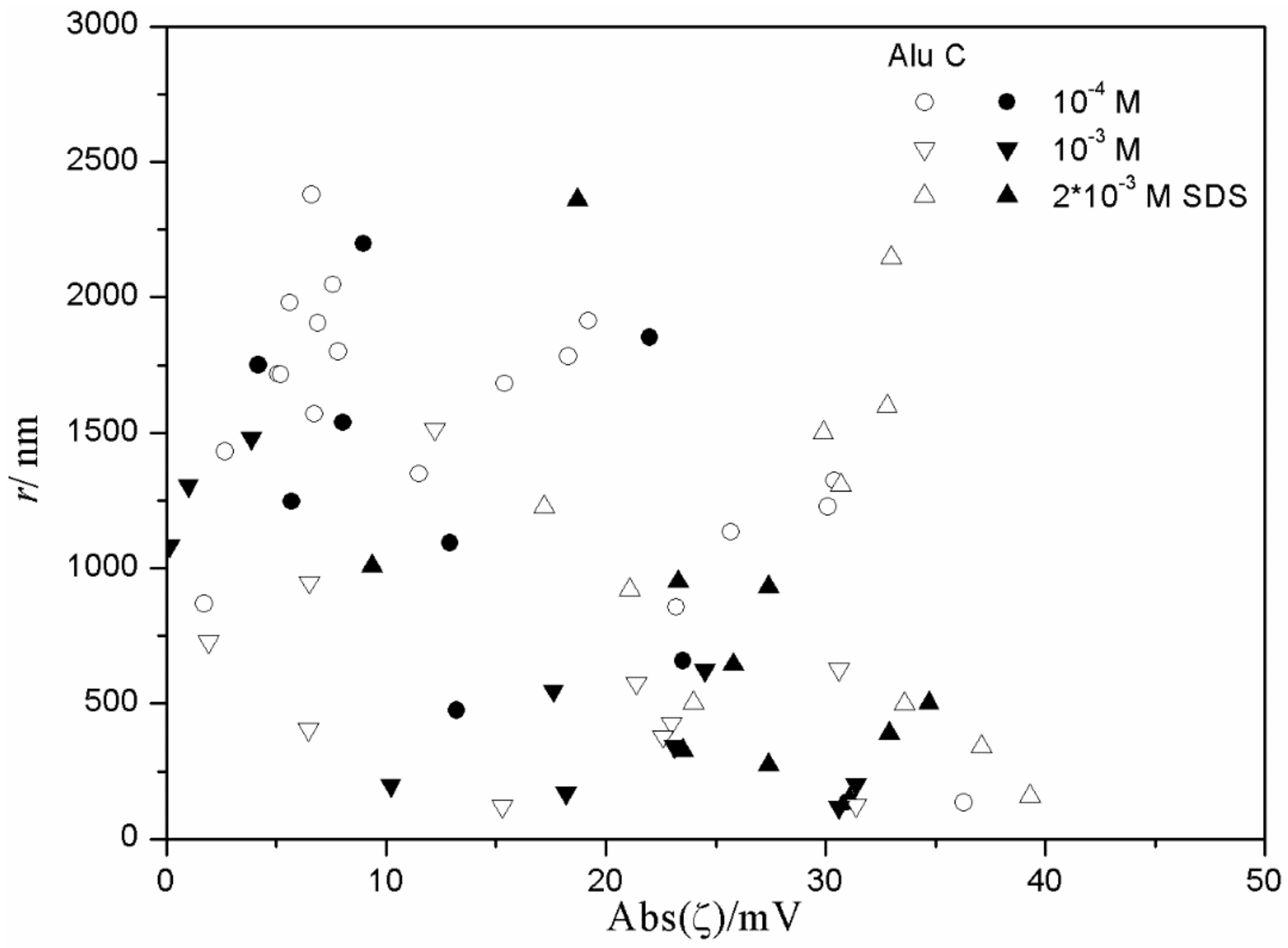
The results shown in
Figure 14 for the alumina-SDS system indicate a random scatter rather than any correlation between the absolute value of ζ potential and the particle size. The only correlation is that in dispersions with very low absolute value of ζ potential (<10 mV) the degree of aggregation is always high (particle radii >400 nm). In alumina dispersions stabilized with tetradecyl- and hexadecylsulfate (
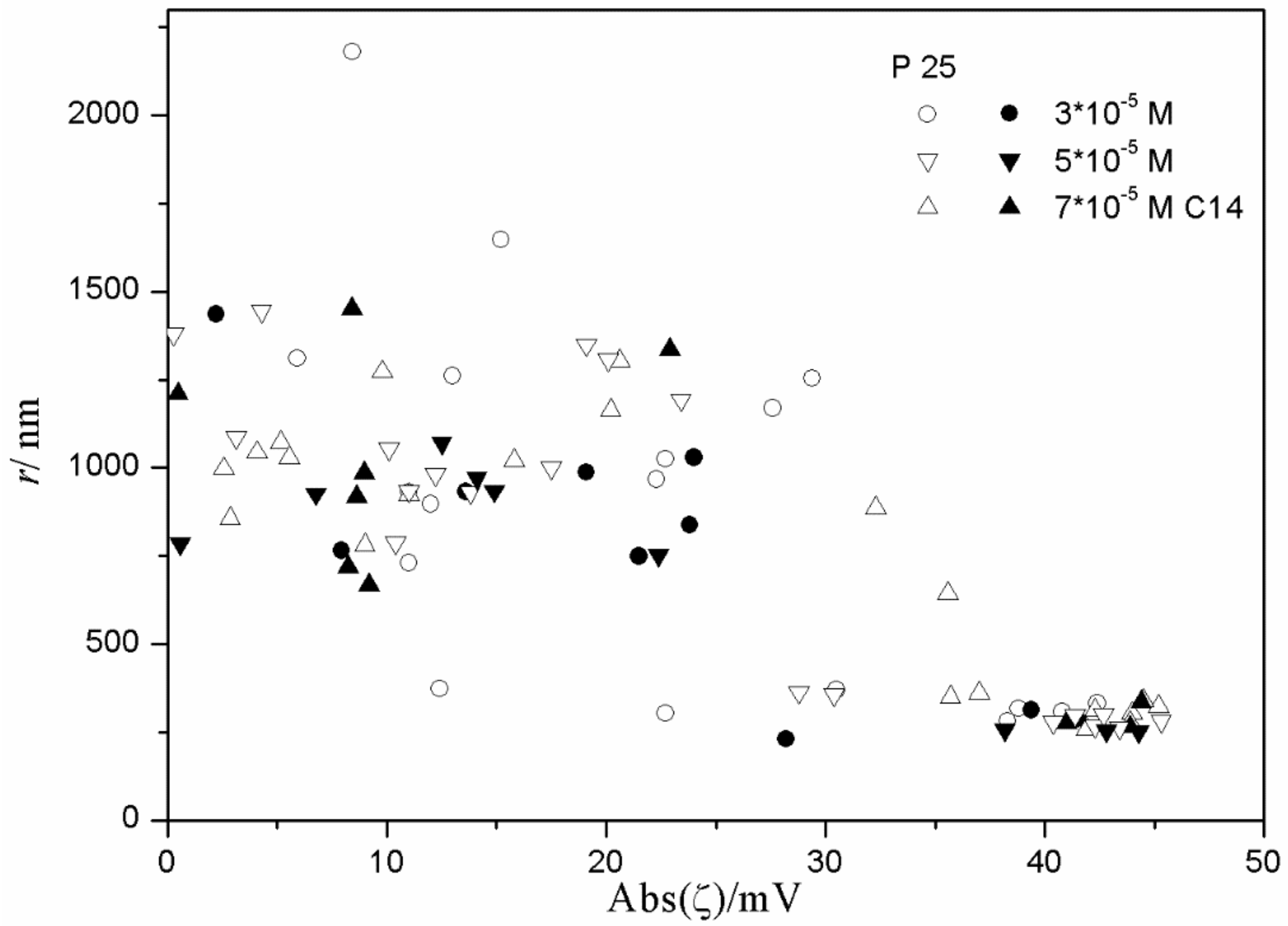
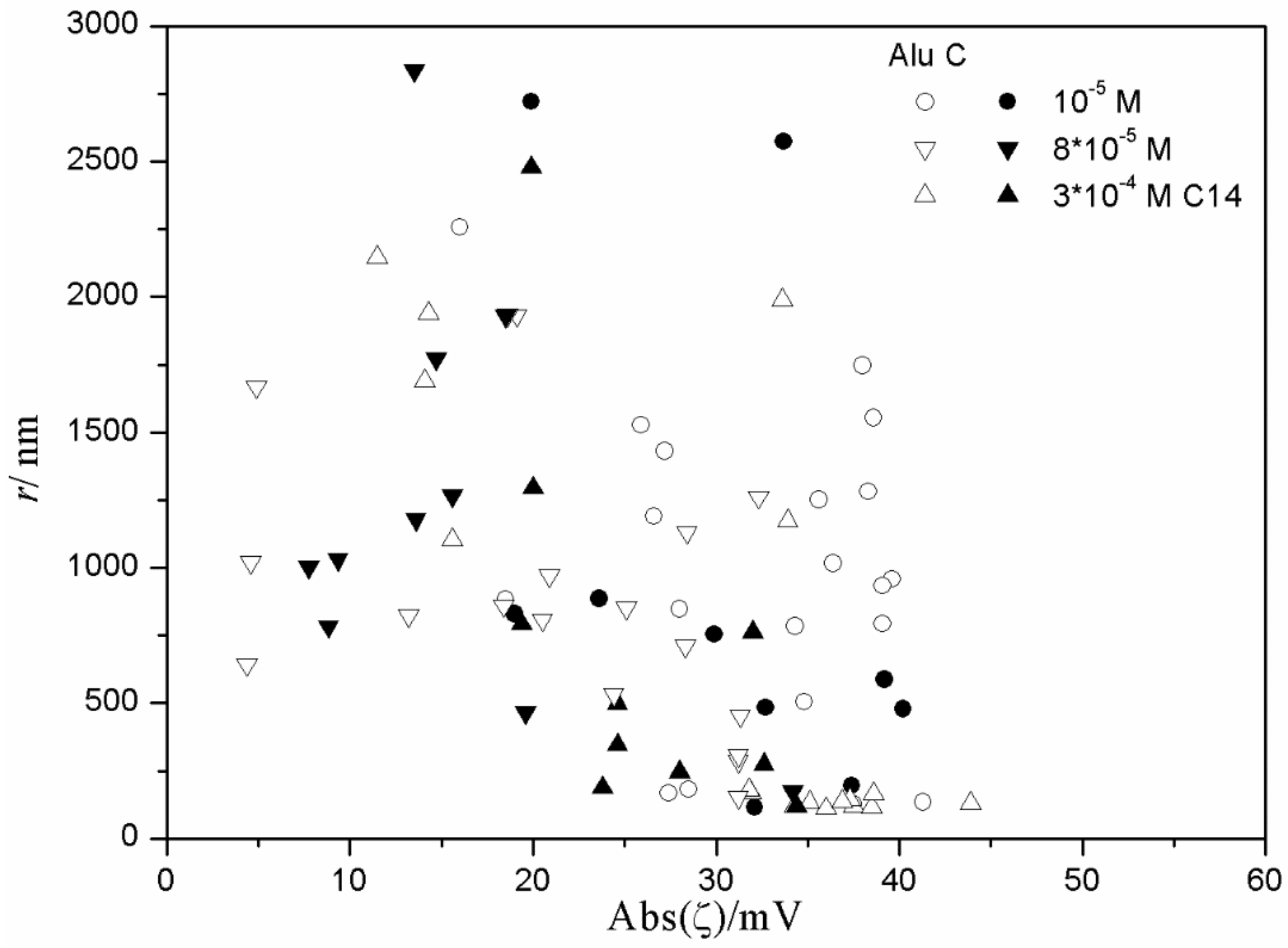
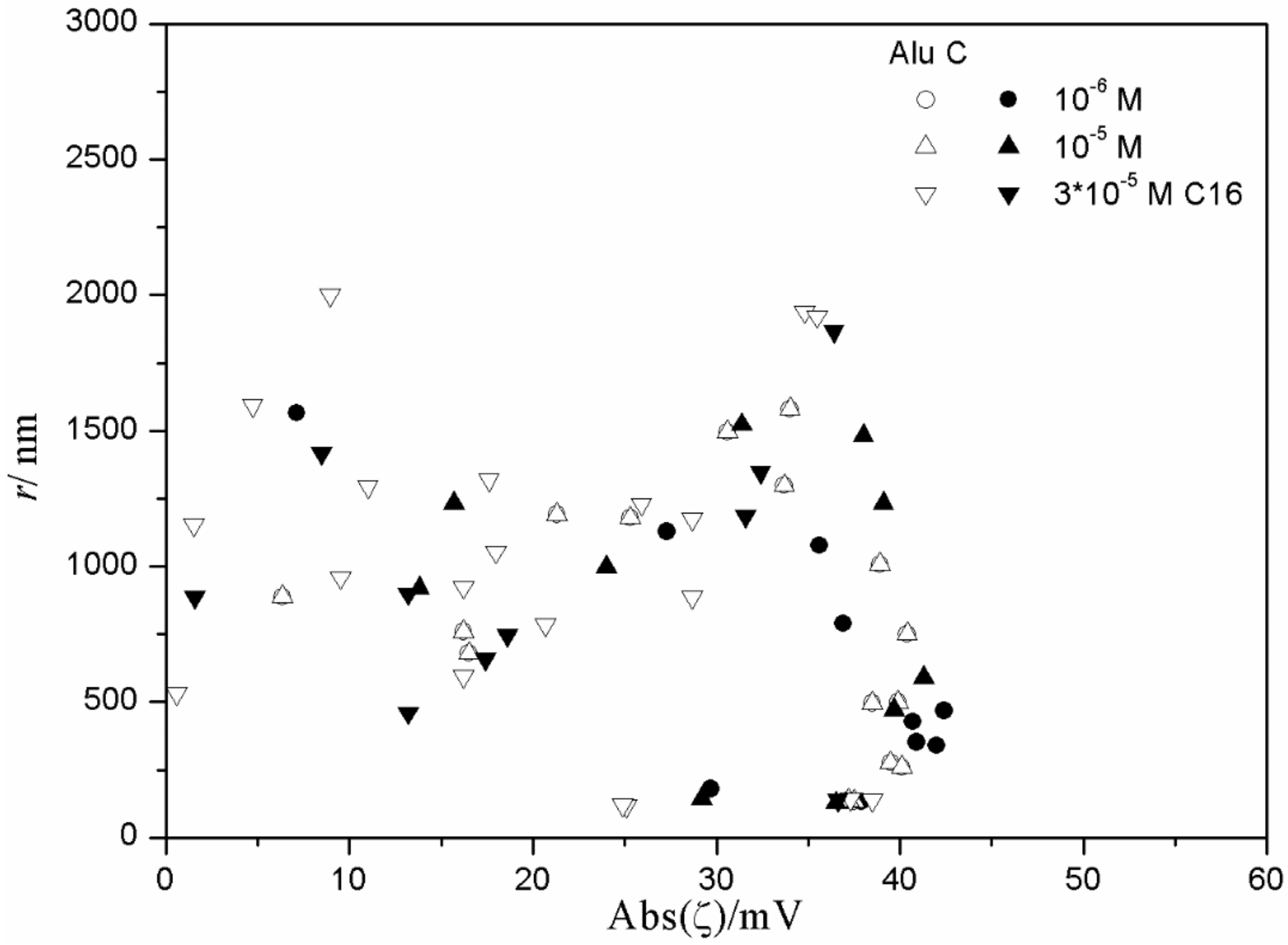
Figure 15 and
Figure 16), low degrees of aggregation (particle radii <200 nm) are only observed at high absolute values of ζ potential (>25 mV), but in many systems with high absolute values of ζ potential (>25 mV), the degree of aggregation was very high.
In spite of the similarity in surface-charging behaviors of different metal oxides, which is often emphasized in the literature [
1], the electrokinetic and aggregation behaviors of hematite, titania, and alumina found in this study showed substantial differences. The apparent ζ potentials of hematite showed the highest absolute values (up to 65 mV), the ζ potentials of titania showed absolute values up to 52 mV, and the ζ potentials of alumina showed absolute values up to 45 mV. These absolute values are probably underrated, because they were calculated from the Smoluchowski equation, that is
κa>100 was assumed. Unfortunately, there is no method to accurately calculate the ζ potentials at 1<
κa<100 in dispersions of irregularly-shaped particles or aggregates, and we cannot use the radii of the primary particles (which are nearly spherical) in the calculations because of the substantial degree of aggregation indicated in
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13,
Figure 14,
Figure 15 and
Figure 16. High degrees of aggregation in dispersions of alumina and titania (apparent particle radii in excess of 1000 nm are commonplace) and lower degrees of aggregation in dispersions of hematite (typical apparent particle radii of 100 nm) are in line with high absolute values of ζ potentials of hematite and lower absolute values of ζ potentials of alumina and titania. The electrokinetic curves shown in
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8 are rather smooth, and the data points showing the apparent particle radii are very scattered. This is because the relationship between electrophoretic mobility and ζ potential is relatively insensitive to the shape and orientation of the particles, especially in the range of the ζ potentials low in absolute value. In contrast, the calculated particle size is very sensitive to the model assumptions. The scatter in the apparent particle radii indicates that the model used in the calculations by the Malvern software is not suitable for the studied systems, especially when large and irregularly shaped aggregates are present. Yet the order of magnitude of the particle sizes shown in
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13,
Figure 14,
Figure 15 and
Figure 16 is relevant to the order of magnitude of the actual aggregates, as discussed in the experimental section.
The idea that adsorbed surfactant molecules can act as bridges between primary particles and induce their aggregation was coined in early papers by Colic and co-workers [
4,
11], and this argument was repeated in many other publications [
17,
18,
19,
20]. Moreover, the effective dielectric constant within the aggregate is lower than in bulk water (due to the presence of hydrocarbon chains), thus, the effective Hamaker constant of particles interacting through such a medium is higher than of the same particles interaction through water. Moreover, the electrokinetic measurements produce the ζ potential of aggregates actually present in dispersion rather than of primary particles. The alkylsulfate anions are not uniformly distributed within the aggregate, and the ζ potential of aggregates is not representative for primary particles. High concentration of surfactant in the external corona of the aggregate may result in high ζ potential of the aggregate and substantial electrostatic repulsion between the aggregates, but not between particular primary particles within the same aggregate.