Sorption Properties of Polydivinylbenzene Polymers Towards Phenolic Compounds and Pharmaceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Polymeric Microspheres
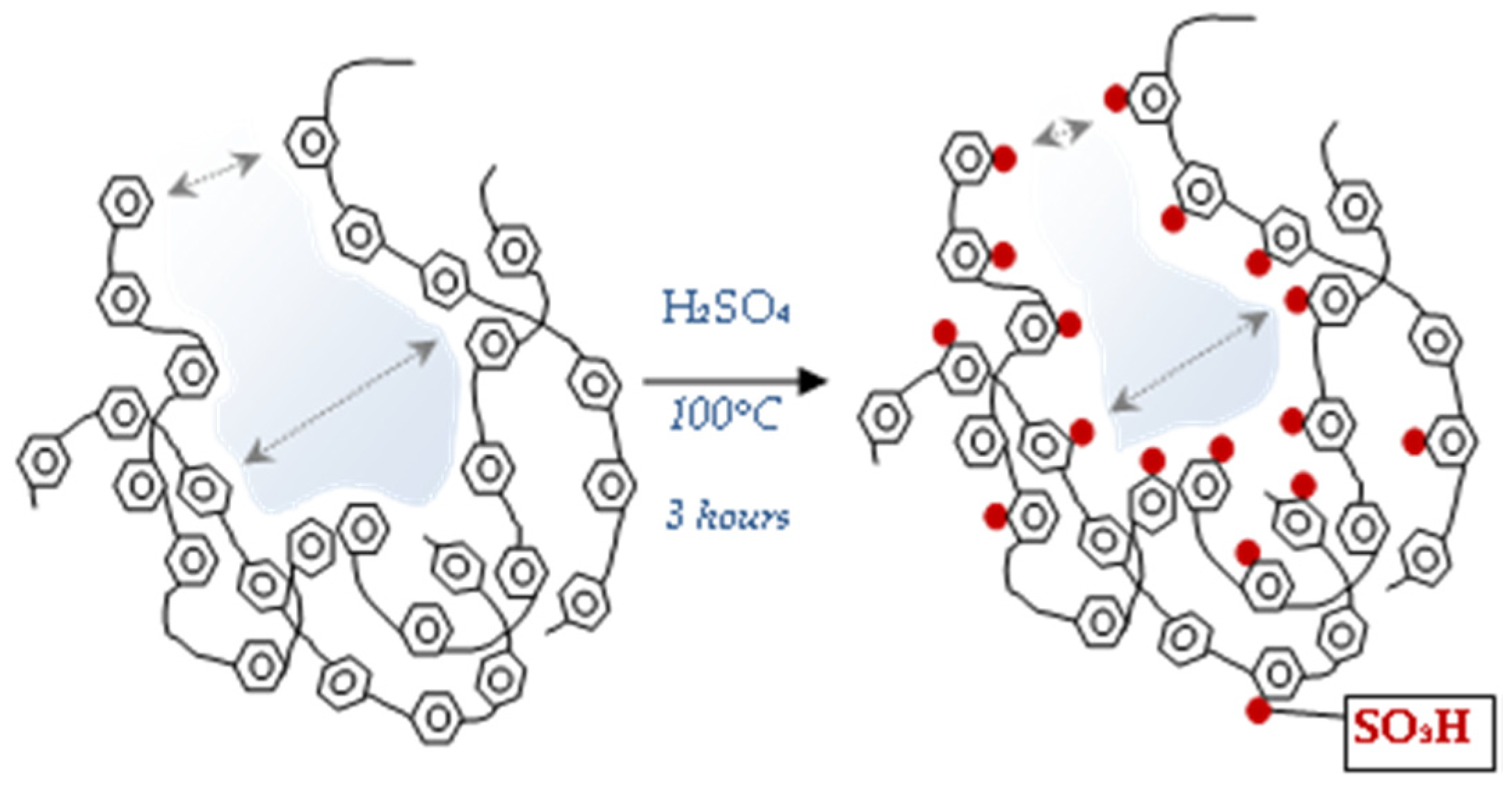
2.3. Sulphonation
2.4. Characterization
3. Results and Discussion
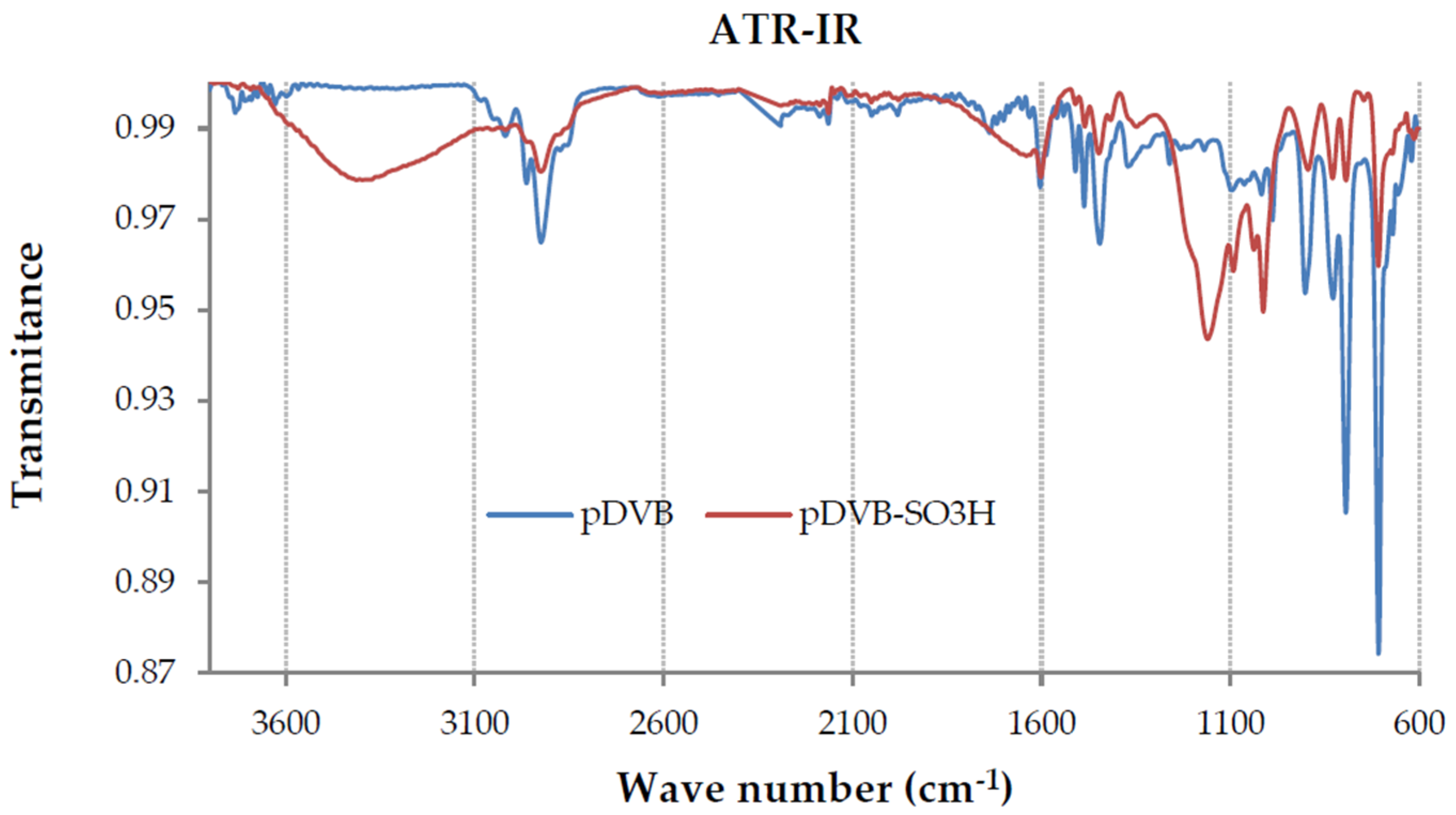
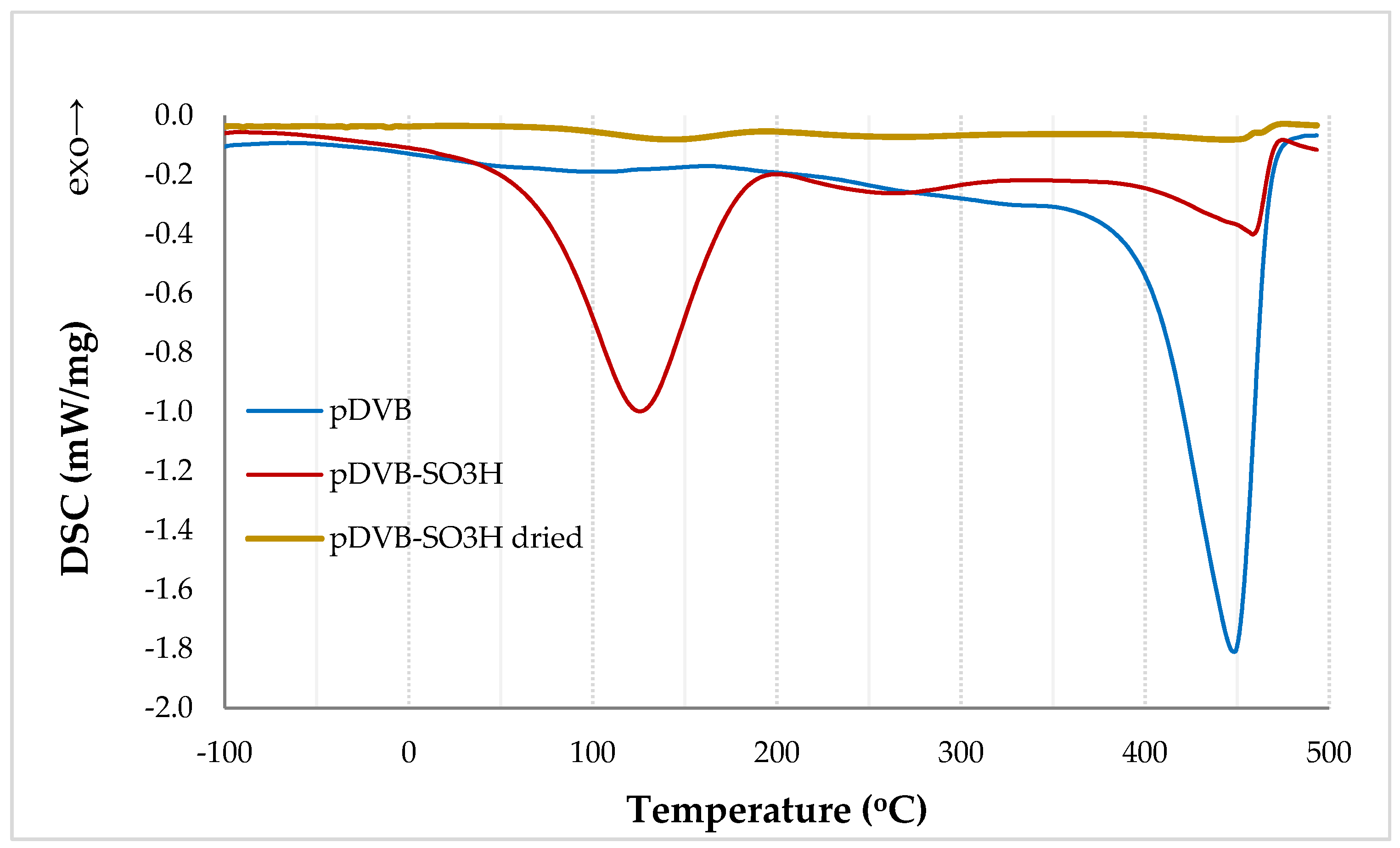
3.1. The Morphology of the Research Materials and Their Chemical Properties
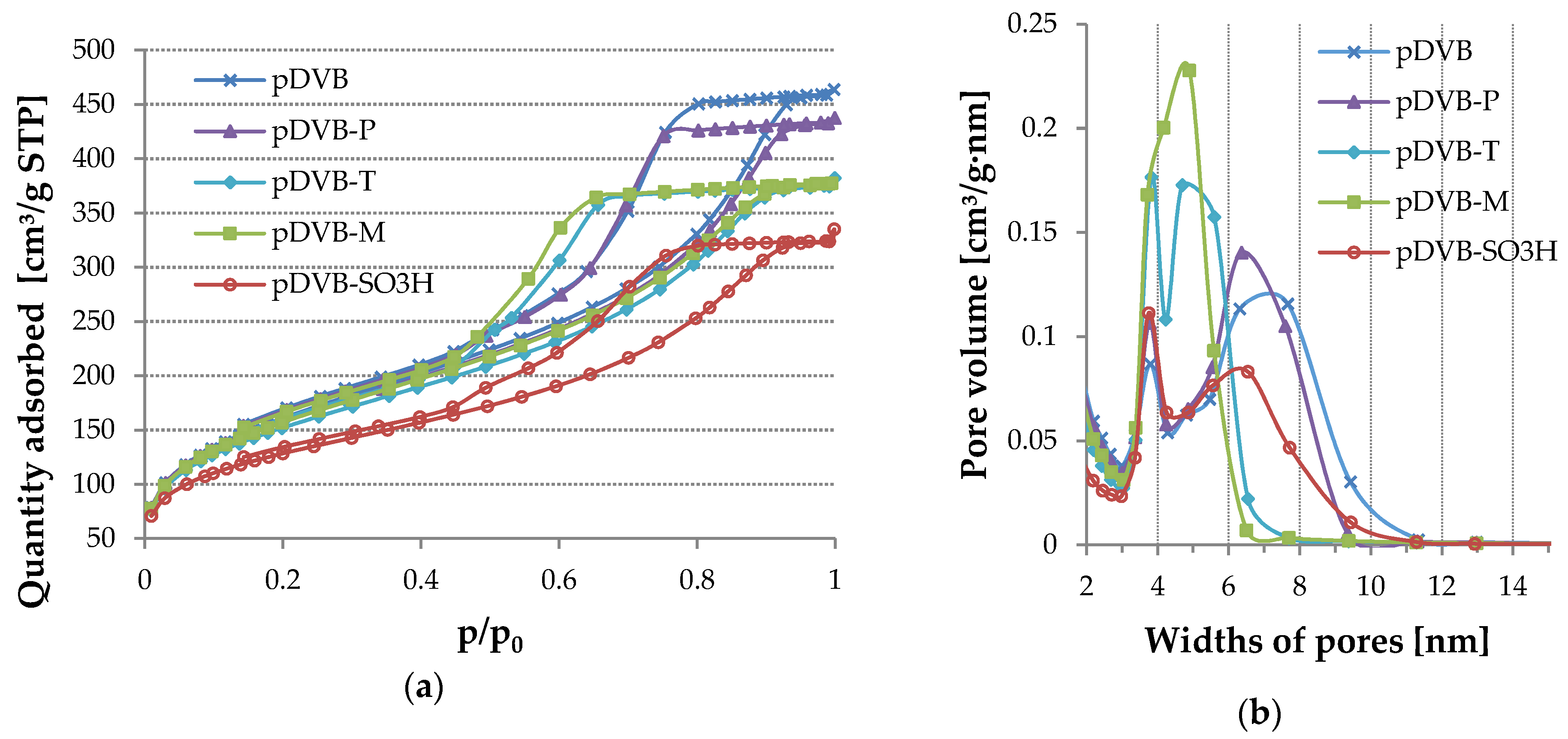
3.2. Characteristics of the Porous Structure
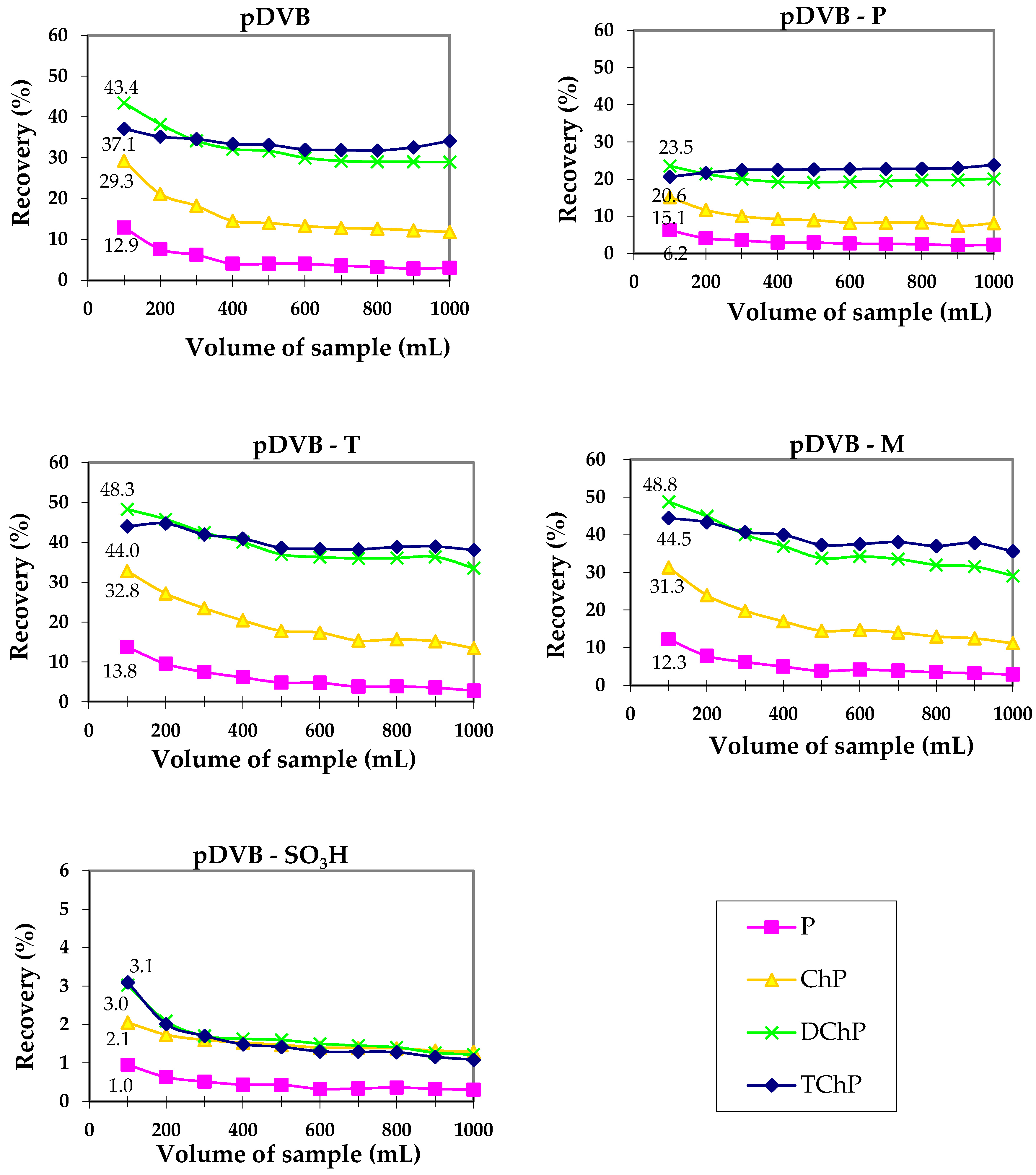
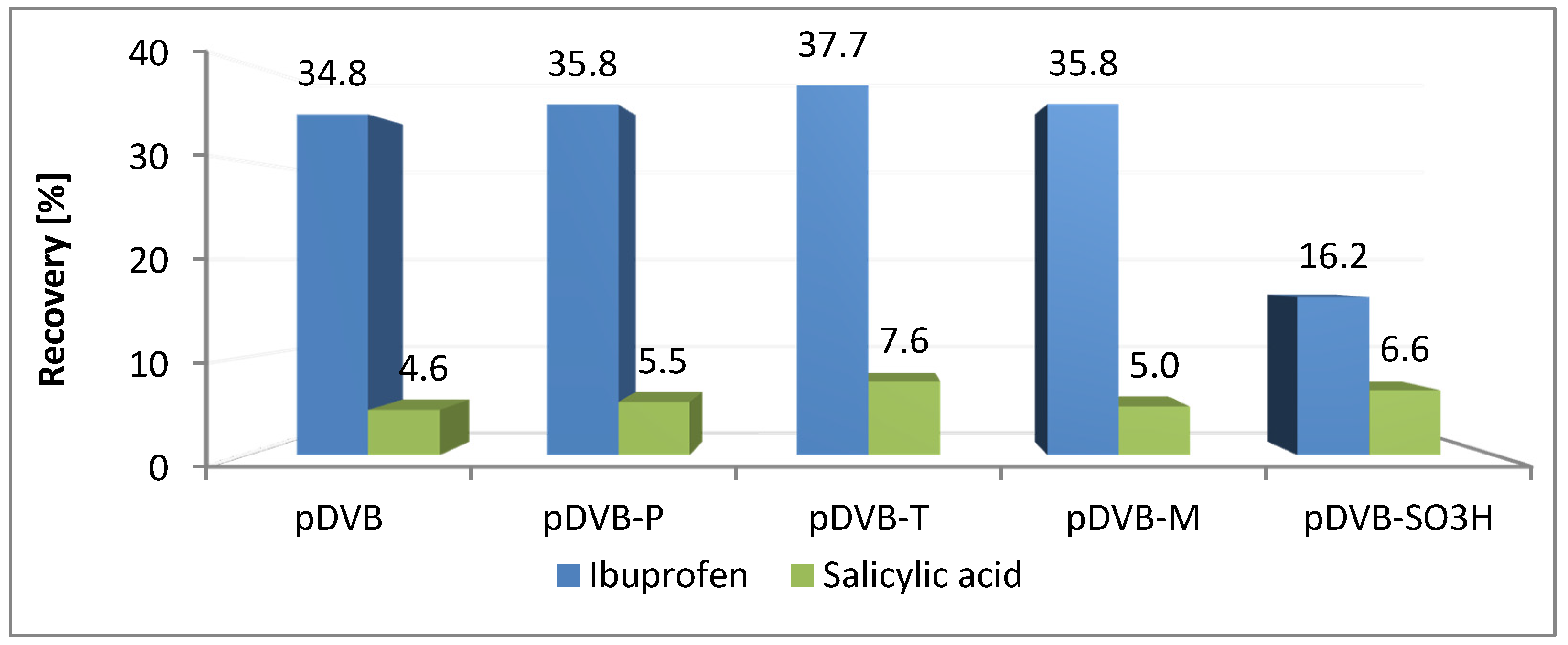
3.3. The Sorption Abilities of the Studied Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simpson, N.K.J. Solid-Phase Extraction: Principles, Techniques and Applications; Nigel, J.K.S., (Varian Associates), Eds.; Marcel Dekker Inc.: New York, NY, USA, 2000; ISBN 0-8247-09021-X. [Google Scholar]
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. (Eds.) Adsorption Processes for Water Treatment and Purification; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-58135-4. [Google Scholar]
- Fathy, M.; Moghny, T.A.; Awadallah, A.E.; El-Bellihi, A.-H.A.-A. Study the adsorption of sulfates by high cross-linked polystyrene divinylbenzene anion-exchange resin. Appl. Water Sci. 2017, 7, 309–313. [Google Scholar] [CrossRef]
- Ekici, S.; Güntekin, G.; Saraydın, D. The Removal of Textile Dyes with Cross-Linked Chitosan-Poly(acrylamide) Adsorbent Hydrogels. Polym. Plast. Technol. Eng. 2011, 50, 1247–1255. [Google Scholar] [CrossRef]
- Sobiesiak, M.; Podkoscielna, B. Preparation and characterization of porous DVB copolymers and their applicability for adsorption (solid-phase extraction) of phenol compounds. Appl. Surf. Sci. 2010, 257, 1222–1227. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Sobiesiak, M.; Podkościelna, B.; Podkościelny, P. New functionalised polymeric microspheres for multicomponent solid phase extraction of phenolic compounds. Adsorption 2016, 22, 653–662. [Google Scholar] [CrossRef]
- Podkościelna, B.; Sobiesiak, M.; Zhao, Y.; Gawdzik, B.; Sevastyanova, O. Preparation of lignin-containing porous microspheres through the copolymerization of lignin acrylate derivatives with styrene and divinylbenzene. Holzforschung 2015, 69, 49–56. [Google Scholar] [CrossRef]
- Maciejewska, M.; Gawdzik, J. Preparation and characterization of sorption properties of porous microspheres of 1-vinyl-2-pyrrolidone-divinylbenzene. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 950–961. [Google Scholar] [CrossRef]
- Sobiesiak, M. Bead-shaped porous polymers containing bismaleimide—their physico-chemical characteristics and sorption properties towards chlorophenols. Polish J. Appl. Chem. 2011, LV, 25–32. [Google Scholar]
- Keith, L.; Telliard, W. ES&T Special Report: Priority pollutants: I-a perspective view. Environ. Sci. Technol. 1979, 13, 416–423. [Google Scholar] [CrossRef]
- Fawell, J.K.; Hunt, S. Environmental Toxicology: Organic Pollutants; John Wiley & Sons LTD: New York, NY, USA, 1988; ISBN 0745801943. [Google Scholar]
- The European Parliament. DIRECTIVE 2006/118/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 12 December 2006. Off. J. Eur. Communities 2006, L 372, 19–31. [Google Scholar]
- The European Parliament. DECISION No 2455/2001/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 20 November 2001. Off. J. Eur. Communities 2001, L 331, 1–5. [Google Scholar]
- Lucas, D.; Castellet-Rovira, F.; Villagrasa, M.; Badia-Fabregat, M.; Barceló, D.; Vicent, T.; Caminal, G.; Sarrà, M.; Rodríguez-Mozaz, S. The role of sorption processes in the removal of pharmaceuticals by fungal treatment of wastewater. Sci. Total Environ. 2018, 610–611, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.M.; Ferrera, Z.S.; Padrón, M.E.T.; Rodríguez, J.J.S. Methodologies for the extraction of phenolic compounds from environmental samples: New approaches. Molecules 2009, 14, 298–320. [Google Scholar] [CrossRef]
- Puig, D.; Barceló, D. different sorbent materials for on-line liquid-solid extraction followed by liquid chromatographic determination of priority phenolic compounds in environmental waters. J. Chromatogr. A 1996, 733, 371–381. [Google Scholar] [CrossRef]
- Ben Hassine, S.; Hammami, B.; Touil, S.; Driss, M.R. Determination of Chlorophenols in Water Samples Using Solid-Phase Extraction Enrichment Procedure and Gas Chromatography Analysis. Bull. Environ. Contam. Toxicol. 2015, 95, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Insa, S.; Salvadó, V.; Anticó, E. Development of solid-phase extraction and solid-phase microextraction methods for the determination of chlorophenols in cork macerate and wine samples. J. Chromatogr. A 2004, 1047, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.-Z.; Zhao, L.-X.; Yan, W.; Lin, J.-M.; Zheng, Z.-X. Molecularly imprinted solid-phase extraction combined with high performance liquid chromatography for analysis of phenolic compounds from environmental water samples. J. Hazard. Mater. 2009, 167, 282–288. [Google Scholar] [CrossRef]
- An, F.; Du, R.; Wang, X.; Wan, M.; Dai, X.; Gao, J. Adsorption of phenolic compounds from aqueous solution using salicylic acid type adsorbent. J. Hazard. Mater. 2012, 201–202, 74–81. [Google Scholar] [CrossRef]
- Cai, Y.; Cai, Y.; Mou, S.; Lu, Y. Multi-walled carbon nanotubes as a solid-phase extraction adsorbent for the determination of chlorophenols in environmental water samples. J. Chromatogr. A 2005, 1081, 245–247. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M. Green adsorbents for wastewaters: A critical review. Materials (Basel) 2014, 7, 333–364. [Google Scholar] [CrossRef]
- Grochowicz, M. Investigation of the thermal behavior of 4-vinylpyridine-trimethylolpropane trimethacrylate copolymeric microspheres. J. Therm. Anal. Calorim. 2014, 118, 1603–1611. [Google Scholar] [CrossRef]
- Maciejewska, M. Thermal properties of TRIM–GMA copolymers with pendant amine groups. J. Therm. Anal. Calorim. 2016, 126, 1777–1785. [Google Scholar] [CrossRef]
- Beyler, C.L.; Hirschler, M.M. Section One—Fundamentals, Chapter 7: Thermal Decomposition of Polymers; 2002; ISBN 0877653542 9780877653547. [Google Scholar]
- Matsuda, M.; Funabashi, K.; Yusa, H.; Kikuchi, M. Influence of functional sulfonic acid group on pyrolysis characteristics for cation exchange resin. J. Nucl. Sci. Technol. 1987, 24, 124–128. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sobiesiak, M. Chemical Structure of Phenols and Its Consequence for Sorption Processes. In Phenolic Compounds—Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., García-Mateos, R., Eds.; InTech: London, UK, 2017; pp. 3–26. ISBN 978-953-51-2958-5. [Google Scholar]
- Podkościelna, B.; Sobiesiak, M. Synthesis and characterization of organic–inorganic hybrid microspheres. Adsorption 2016, 22, 631–638. [Google Scholar] [CrossRef]
| Polymer | Monomer (g) | Pore Forming Agents | Initiator (g) | ||
|---|---|---|---|---|---|
| DVB | Toluene (mL) | Phenol (P) (g) | 2,4,6-Tricholrophenol (T) (g) | AIBN (g) | |
| pDVB | 14 | 21 | 0.186 | ||
| pDVB-P | 14 | 20 | 1.07 | 0.186 | |
| pDVB-T | 14 | 20 | 1.68 | 0.186 | |
| pDVB-M | 14 | 20 | 0.54 | 0.84 | 0.186 |
| Polymer | C (%) | H (%) | N (%) | Other (%) | AN (mg/g) |
|---|---|---|---|---|---|
| pDVB | 89.2 | 8.17 | 0.48 | 2.15 | - |
| pDVB-P | 89.01 | 8.25 | 0.31 | 2.43 | - |
| pDVB-T | 89.53 | 8.2 | 0.4 | 1.87 | - |
| pDVB-M | 84.08 | 7.82 | 0.41 | 7.69 | - |
| pDVB-SO3H | 61.43 | 6.91 | 0.25 | 31.41 | 18.68 |
| Polymer | SBET (m2/g) | Vtot (cm3/g) | Smi (m2/g) | Vmi (cm3/g) | W (nm) |
|---|---|---|---|---|---|
| pDVB | 560 | 0.717 | 1 | 0 | 3.75 and 7.15 |
| pDVB -P | 553 | 0.677 | 5 | 0 | 3.75 and 6.35 |
| pDVB - T | 529 | 0.591 | 9 | 0 | 3.75 and 4.75 |
| pDVB - M | 548 | 0.584 | 1 | 0 | 4.89 |
| pDVB-SO3H | 444 | 0.518 | 60 | 0.025 | 3.75 and 6.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobiesiak, M.; Grochowicz, M.; Osypiuk Tomasik, J. Sorption Properties of Polydivinylbenzene Polymers Towards Phenolic Compounds and Pharmaceuticals. Colloids Interfaces 2019, 3, 19. https://doi.org/10.3390/colloids3010019
Sobiesiak M, Grochowicz M, Osypiuk Tomasik J. Sorption Properties of Polydivinylbenzene Polymers Towards Phenolic Compounds and Pharmaceuticals. Colloids and Interfaces. 2019; 3(1):19. https://doi.org/10.3390/colloids3010019
Chicago/Turabian StyleSobiesiak, Magdalena, Marta Grochowicz, and Joanna Osypiuk Tomasik. 2019. "Sorption Properties of Polydivinylbenzene Polymers Towards Phenolic Compounds and Pharmaceuticals" Colloids and Interfaces 3, no. 1: 19. https://doi.org/10.3390/colloids3010019
APA StyleSobiesiak, M., Grochowicz, M., & Osypiuk Tomasik, J. (2019). Sorption Properties of Polydivinylbenzene Polymers Towards Phenolic Compounds and Pharmaceuticals. Colloids and Interfaces, 3(1), 19. https://doi.org/10.3390/colloids3010019






