1. Introduction
Despite numerous studies carried out on the effect of biomaterial surface on the cellular response, it has not been possible yet to specify which factors influence on interactions between the cell and the material to the largest extent. The cell is surrounded by a biological membrane which is a lipid–protein structure exhibiting some fluidity and polarity. Visually, this is a double lipid layer (containing mainly phospholipids, glycolipids, and steroids) in which also integral proteins are found. The most popular phospholipid of Eukaryotes used by scientists as a biological membrane model is 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC). For better understanding the interactions between the cell membrane and the material surface, research on the effects of topography, wettability, chemical composition, and surface energy can be most helpful. Precise specification of above mentioned properties of the biomaterial surface would allow to achieve the expected response from the biological environment. Moreover, knowledge of these parameters makes the appropriate control of the surface layer properties and its use for the directional cells behavior on the biomaterial surface possible.
Wettability of the surface determines interactions of the biomaterials with the biological environment. In the human body, the first contact is between the biomaterial surface and the water molecules, then ions and proteins are adsorbed, and finally different kinds of surrounding cells approach the material. At the same time, bacteria can compete with the cells for surface colonization. That is why eventual relationships between the physicochemical surface characteristics of the different materials and their biological response (blood wettability, protein adsorption, cell adhesion, bacterial adhesion, and biofilm formation) should be considered separately for particular cases.
The goal of our studies was to determine the wettability of the DPPC monolayers deposited on the glass by means of LB technique from the subphase containing chitosan (Ch), hyaluronic acid (HA) and/or titanium dioxide (TiO
2). These compounds were chosen due to their specific features useful for medical applications. Chitosan is a copolymer containing two units: 2-acetamido-2-deoxy-β-
d-glucopyranose and 2-amino-2-deoxy-β-
d-pyranose, connected by 1,4-β glycosidic linkages. In the chitosan structure, the letter mentioned part is more important, therefore in relation to chitin it is much more soluble in numerous aqueous solutions of organic or inorganic acids [
1]. The aminoglucose moieties present in the chitosan molecule arise from the deacetylation of chitin. As a result, complete or partial conversion of acetamide to amine unit may occur. The parameter which determines the molar content of deacetylated mers in the chitosan structure is a degree of deacetylation (DD). In the literature DD is defined as the ratio of the number of glucosamine groups to the overall number of N-acetylglucosamine (GlcNAc) and glucosamine (GtcN) units [
2]. Their presence in the macromolecule chain is the most important feature of chitosan, decisive in its widespread use. They are protonated in the acidic environment, so on this basis it can be deduced that the main parameter is the number of free groups, enabling the start of various processes with the chitosan contribution, e.g., it can adhere or aggregate with negatively charged molecules or biological membrane [
2]. In addition, it has primary and secondary hydroxyl groups in its structure that are responsible for the adsorption capacity of the compound. The above-mentioned properties as well as the antibacterial properties [
1,
3,
4] are very important for the creation of hybrid systems with controlled release of active substances [
2,
5].
The other selected component—titanium dioxide—is found in all areas of life. It has the form of a white, odorless powder, owing to which it is widely applied as a natural pigment. It is also used as a synthetic mineral dye in food industry. Its pH, close to that of skin and hydrophilic nature, allows it to be a factor that improves stability in the drug delivery systems [
6,
7,
8]. An additional advantage is its non-toxicity and anti-bacterial activity [
9,
10,
11,
12].
To improve the biocompatibility of the systems, hyaluronic acid (HA) can be added. It is a hydrophilic polysaccharide found in the extracellular matrix of living organisms [
13]. This biopolymer is often used for medical applications due to its non-toxicity, film-forming capability, bioactivity, biodegradability, and anticoagulant properties [
13,
14,
15]. HA existing as a polyanion under physiological pH strongly interacts with Ch (cationic polysaccharide) via electrostatic forces to form the polymeric network. Ch and HA based materials had the high tendency to the structural disintegration and their physicochemical properties can thus exhibit different behavior while the TiO
2 particles can increase the system stability and mechanical properties by intermolecular connection.
To the best of our knowledge the studies with using the Langmuir–Blodgett technique and model biological membrane have been not conducted yet. Variable wettability can be a convenient parameter to inform about the properties of artificial skin. Getting more profound insight into the interactions between model membrane and components of imitation skin with liquids of different character (apolar and polar) can make the subsequent biological effects more predictable. It is expected that innovative composite materials based on natural ingredients will be biocompatible with the human body and will not interfere with processes occurring in it. Therefore, the characteristics of the surface wettability of materials with potential applications in medicine, pharmacy, and cosmetics, both for internal and external use, is very important, mainly due to the fact that many biochemical processes occurring in the human body require an appropriate environment.
The research presented in this paper was aimed at seeking the most suitable system which can be used for skin substitution. At this stage of our investigations, we concluded that degree of surface hydrophilicity can be the most essential parameter for more profound characteristics.
3. Results and Discussion
The wettability of surfaces by probe liquids depends primarily on the equilibrium of the cohesive forces in the liquid and the adhesion forces at the solid-liquid interface. It is obvious that the contact angle is not quantitative parameter but only a general determinant of the hydrophobicity/hydrophilicity of the layers. It allows determining the character/nature of the surface depending of the test liquid used. To determine the type of interactions, polar liquids such as water and formamide are used which have the ability to interact with hydrogen bonds as well as dispersion forces and diiodomethane which is a non-polar liquid that interacts mainly with dispersion forces.
3.1. Contact Angle and Its Hysteresis on the Active Glass Plate
In order to determine the wetting properties of the analyzed systems, the advancing and receding contact angles were measured on the surfaces obtained by transferring the DPPC monolayer from the subphase (AA, AA/Ch, AA/HA, AA/TiO
2, AA/Ch/TiO
2, AA/Ch/HA, AA/HA/TiO
2, AA/Ch/HA/TiO
2) to the glass support. For comparative purposes, the wettability of clean plates before and after air plasma modification (1 min) was also evaluated. The results are included in the table below (
Table 1).
As a result of modification of the glass plates by plasma the contact angles values for all test liquids were reduced, wherein the largest changes were noted for the polar liquids, i.e., formamide and water. There was a decrease in the contact angle values from 37.2° to 4.3° for formamide and from 40.5° to 10.9° for water. This indicates an increase in polarity of the activated plates surface. Treatment of the material surface by air plasma provides the additional functional groups containing oxygen and nitrogen. The contact of polar liquids with such activated surface results in strong interactions and thus an increase in polarity. The test liquids give much lower values of receding contact angles (diiodomethane, water) or are completely spread out on the activated glass (formamide). The lower values of hysteresis for the polar liquids result from the lower contact angles and also confirm the polar character of surface.
Roughly analyzing the diiodomethane contact angles on the
plasma untreated and treated glass plates and the combined error bars, the
values seem to be similar. Usually it is recognized that the surface tension of
non-polar diiodomethane is only due to the intermolecular interactions of
Lifshitz–van der Waals . Mainly these are London dispersion
interactions. For this reason, we can assume that in both cases (before and
after plasma treatment) this type of interactions is similar. However, after
air plasma modification the effect is particularly visible for the receding
contact angles and hysteresis. This is caused by the fact that after plasma
action the surface structure changes in time as an effect of newly created
groups.
3.2. Analysis of the Surface Wettability with or without the DPPC Monolayer
After transfer of the phospholipid DPPC monolayer from the subphase to the glass support, a significant increase in the contact angles of the measuring liquids was noted in all the studied systems. In this case, the largest changes were observed for formamide. The higher contact angles values were probably due to the specific orientation of the DPPC molecules with the hydrocarbon chains towards the air. Analysis of wettability after application of the DPPC film indicated a decrease in polarity for all tested systems.
The polarity of most chemical compounds depends on the distribution of the electric charge in the molecule, or the presence of functional groups that may have a dipole moment. However, there are substances that can be both hydrophilic or lipophilic depending on the environment. These compounds have both polar and non-polar groups in their structure and are called amphiphilic. This property is also possessed by phospholipids.
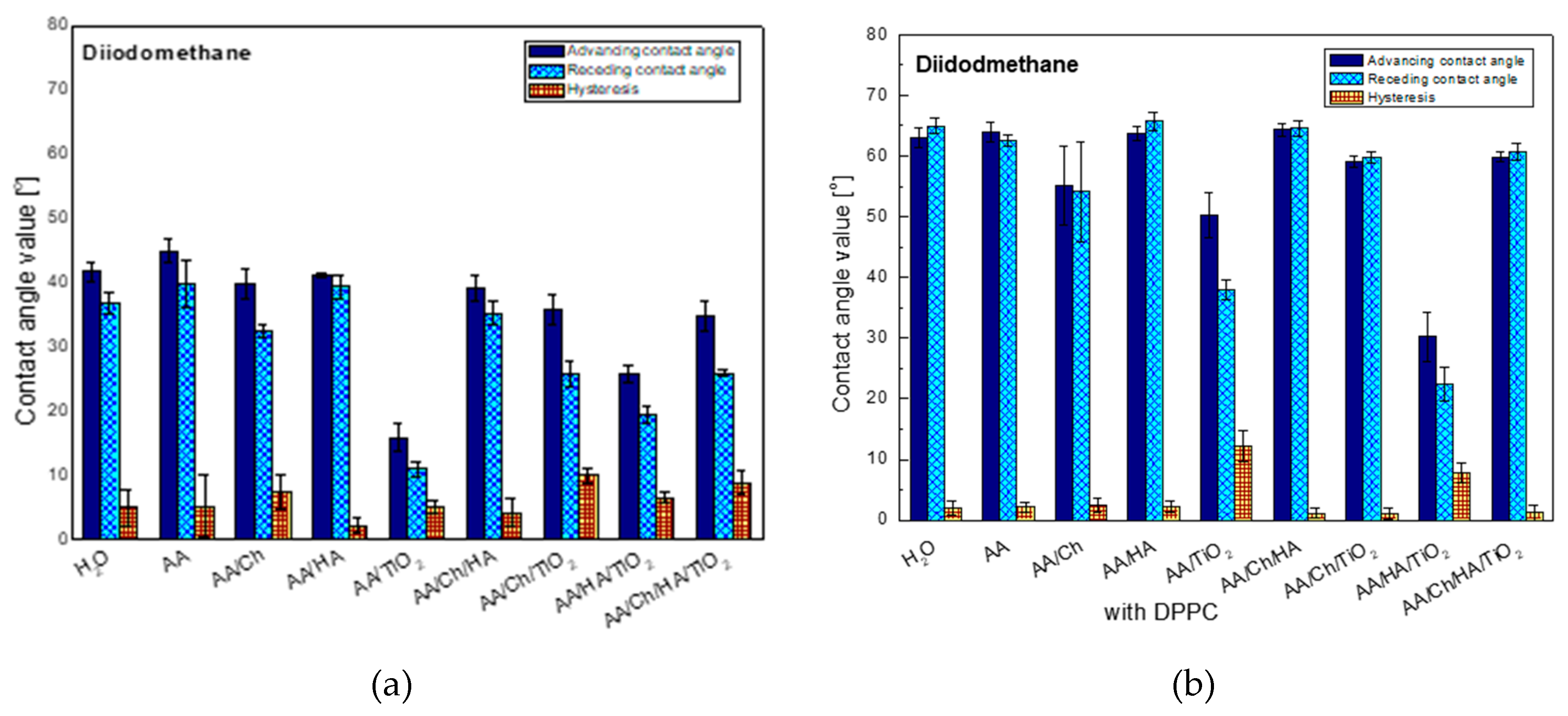
3.2.1. Diiodomethane Contact Angle and Its Hysteresis
The following graph (
Figure 1) shows the averaged contact angle values (advancing, receding and hysteresis) of non-polar liquid-diiodomethane (DM), obtained on the glass plate surface after contacting with different subphases. It can be noticed that the contact angle of this liquid on the glass plate after its contact with the water subphase is close to that obtained on the inactivated clean plate. This suggests that the glass plate surface is modified by water whose molecules are likely to bind by physical forces and/or chemical bonds. Such water modified (Glass/W) surface interacts with the DM droplets. The highest contact angle values were obtained on the plate with the embedded acetic acid layer (glass/AA). In this case this indicates that the least apolar surface was obtained compared to the other studied systems. The biggest changes of advancing contact angles, compared to those on Glass/W, were noted for the systems containing titanium dioxide where there was a detectable reduction of contact angles: by about 26° for the glass/AA/TiO
2 system, about 6°—for glass/AA/Ch/TiO
2, 16°—for the glass/AA/HA/TiO
2 and 7°—for glass/AA/Ch/HA/TiO
2. This indicates that TiO
2 interacts via dispersion forces. In addition, the oxide additive reduced the values of contact angles compared to the systems that do not contain it. Therefore, the largest difference was noted for glass/AA/HA/TiO
2 which had a contact angle of 25.8° that is 15.3° less than for glass/AA/HA. Moreover, these systems were characterized by one of the highest contact angle hysteresis values which were probably caused by surface irregularities resulting from the presence of insoluble, nanometric TiO
2 particles and/or strong dispersive interactions of TiO
2-DM.
Significant hysteresis also occurred in the case of the glass/AA/Ch system. This can indicate an increase of the interactions between the liquid and the biopolymer film and/or the penetration of the liquid into its structure during the measurement of the receding contact angle. However, the lowest hysteresis value was obtained for glass/AA/HA, suggesting a close packing of the HA film deposited on the surface of the plate and/or weak interactions by the London dispersion forces.
When analyzing only the effect of chitosan on the obtained results, some analogy was noticed. Namely, the addition of biopolymer caused an increase in the hysteresis value for all tested systems. Moreover, the addition of Ch to the other systems (except these containing TiO
2) resulted in a reduction of contact angle. All ingredients AA, HA, and Ch have groups capable of forming hydrogen bonds. However, the construction of TiO
2 allows it to interact with the environment mainly through London forces. The increase in the values of contact angles after the addition of Ch to the systems containing TiO
2 indicates oxide binding to the polymer confirmed in our earlier investigations [
20] which may result in weaker interactions with DM.
The general trend of changes in contact angle values for diiodomethane was analogous to that obtained for this liquid but on the plates without a DPPC monolayer (
Figure 1) the biggest change was in the case of the glass/AA/TiO
2 system.
After the transfer of the DPPC monolayer, the averaged contact angle was 50.3° which was higher by 35.5° than in the case of the system without a phospholipid film. However, the smallest changes were observed for glass/AA/HA/TiO2 where only a 4.4° increase in the contact angle was observed (glass/AA/HA/TiO2—25.8°, glass/AA/HA/TiO2/DPPC—30.2°).
Another important difference between the systems with and without the DPPC film is the fact that for the first one (glass/subphase/DPPC) much lower hysteresis values were obtained (0.3° for glass/AA/Ch/HA/DPPC to 2.1° for glass/AA/HA/DPPC). This may testify to: (I) tight order/packing of the phospholipid monolayer [
21], (II) predominance of the cohesion forces over those in DM (which can be confirmed also by the higher values of the receding contact angle than the advancing one obtained in some cases), (III) high repeatability of results as the effect of surface homogeneity. Only for the systems exhibiting the lowest contact angles values (glass/AA/TiO
2/DPPC, glass/AA/HA/TiO
2/DPPC) hysteresis of the order of 12.3° and 7.8°, respectively was recorded.
In general, receding contact angles give information about liquid sorption/retention and/or liquid penetration into the deposited layer whereas the contact angle hysteresis results from the molecule size of the test liquid [
22,
23,
24], i.e., larger molecules have a lower tendency to penetrate the film layer and have a low hysteresis of the contact angle. When phospholipid molecules are in contact with diiodomethane having an average molecular surface (compared to water) [
25], their reorganization is almost impossible. As a consequence, low hysteresis is observed. Higher hysteresis values would suggest liquid penetration into the film structure due to lower condensation and/or ordering of molecules in the lipid film.
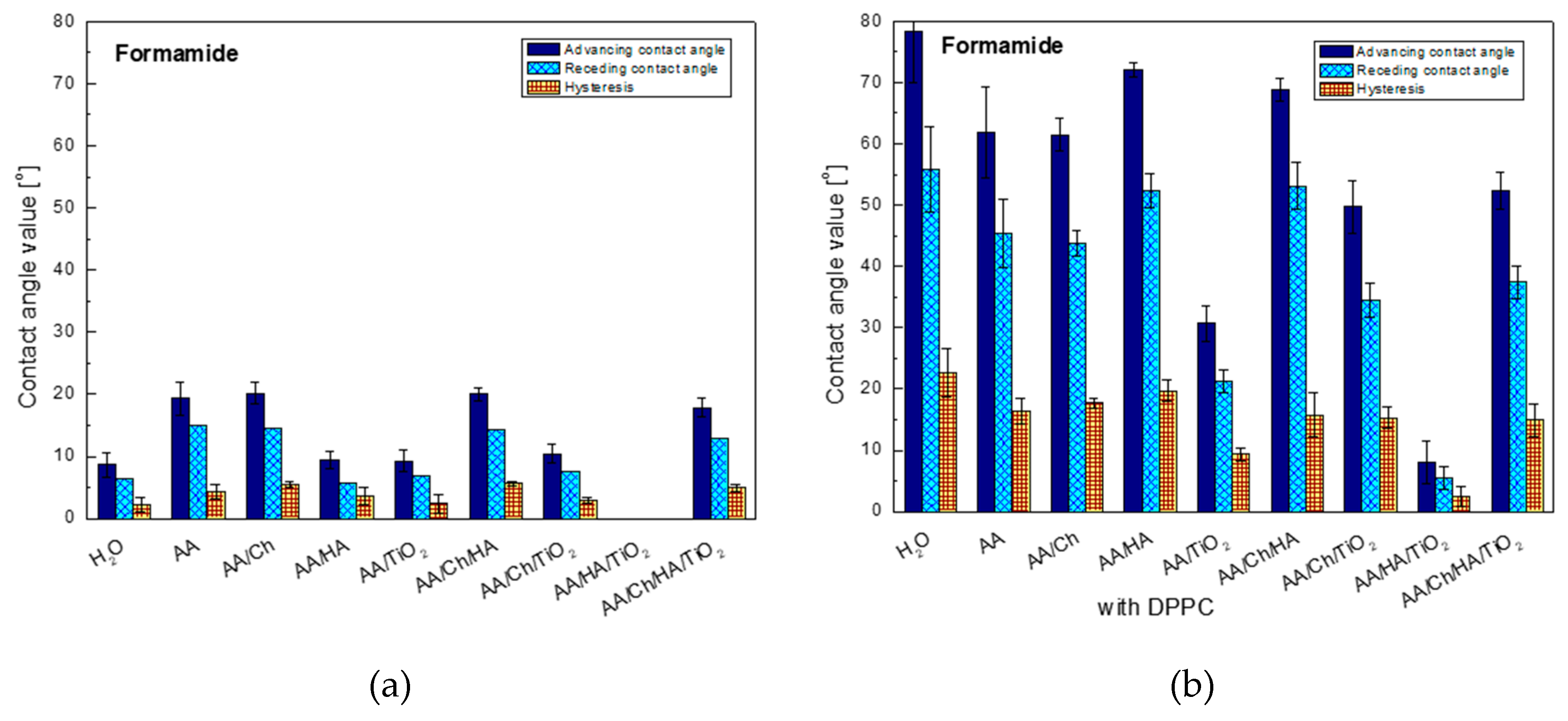
3.2.2. Formamide Contact Angle and Its Hysteresis
The values of contact angles of formamide and water give us information about the polarity of the examined surfaces, their hydrophilic-hydrophobic nature (in the case of water) as well as existence or not of strong interactions by hydrogen bonds type.
The first conclusion, that can be drawn based on
Figure 2, is that high polarity of the AA/HA/TiO
2 system surface on which the formamide spread out completely. However, the highest contact angles were recorded on the glass/AA/Ch (20.1°), glass/AA/Ch/HA (20.0°), glass/AA (19.3°), and glass/AA/Ch/HA/TiO
2 (17.8°) surfaces where the similar hysteresis values 5.5°, 5.6°, 4.4°, and 5.0°, respectively were also observed. Interestingly, the addition of TiO
2 to the other systems resulted in the reduction of angles by about 10°, only in the case of the most complicated system containing all components (glass/AA/Ch/HA/TiO
2), the change was in the order of only 2° compared to the glass/AA/Ch/HA system. This suggests that it is actually mainly responsible for the decrease in surface polarity. A similar trend of changes was observed in the contact angles noted for the other polar test liquid, water (
Figure 3).
Analysis of wettability of the glass plate surface (with the deposited DPPC layer, transferred from different subphases) using formamide shows a significant increase in the contact angles for all tested systems, with the highest increase (9-fold) for the DPPC monolayer transferred from H2O and almost 8-fold for glass/AA/HA/DPPC, while the smallest one for the glass/AA/HA/TiO2/DPPC system was obtained. In other cases, the increase was 3-fold (only for glass/AA/Ch/TiO2/DPPC—5-fold). The high values of contact angles of polar formamide indicate the specific orientation of phospholipid molecules with the non-polar hydrocarbon chains towards air, and also suggest the existence of significant interactions/forces between the polar phospholipid heads and the biopolymers groups contained in the solution. The least polar surfaces were: glass/H2O/DPPC, glass/AA/HA/DPPC, and glass/AA/Ch/HA/DPPC, on which the contact angles were 78.4°, 72.1°, and 68.8°, respectively. In these cases also the highest values of the contact angle hysteresis were obtained, i.e., 22.7°, 19.7°, 15.7°, respectively. The reason for this may be: (I) existence of significant cohesion forces; (II) penetration of formamide droplets between the hydrocarbon chains of DPPC molecules during the receding contact angle measurement; (III) looser monolayer packing or reorganization as an effect of contact with the liquid being measured.
On the other hand, the addition of TiO
2 to the systems caused a decrease in the contact angle for all cases. The biggest change was recorded for glass/AA/HA/DPPC→glass/AA/HA/TiO
2/DPPC where the difference among the studied systems was 64.1°. This can suggest the penetration of nanoparticle TiO
2 between the DPPC molecules in the monolayer which may further be accessible to the formamide droplets of, causing them to spread over the ‘plate’ surface. The fact of oxide particles migration into the lipid film was also observed during the analysis of surface pressure isotherms as a function of the area per molecule (π-A) which was described in detail in our last paper [
26].
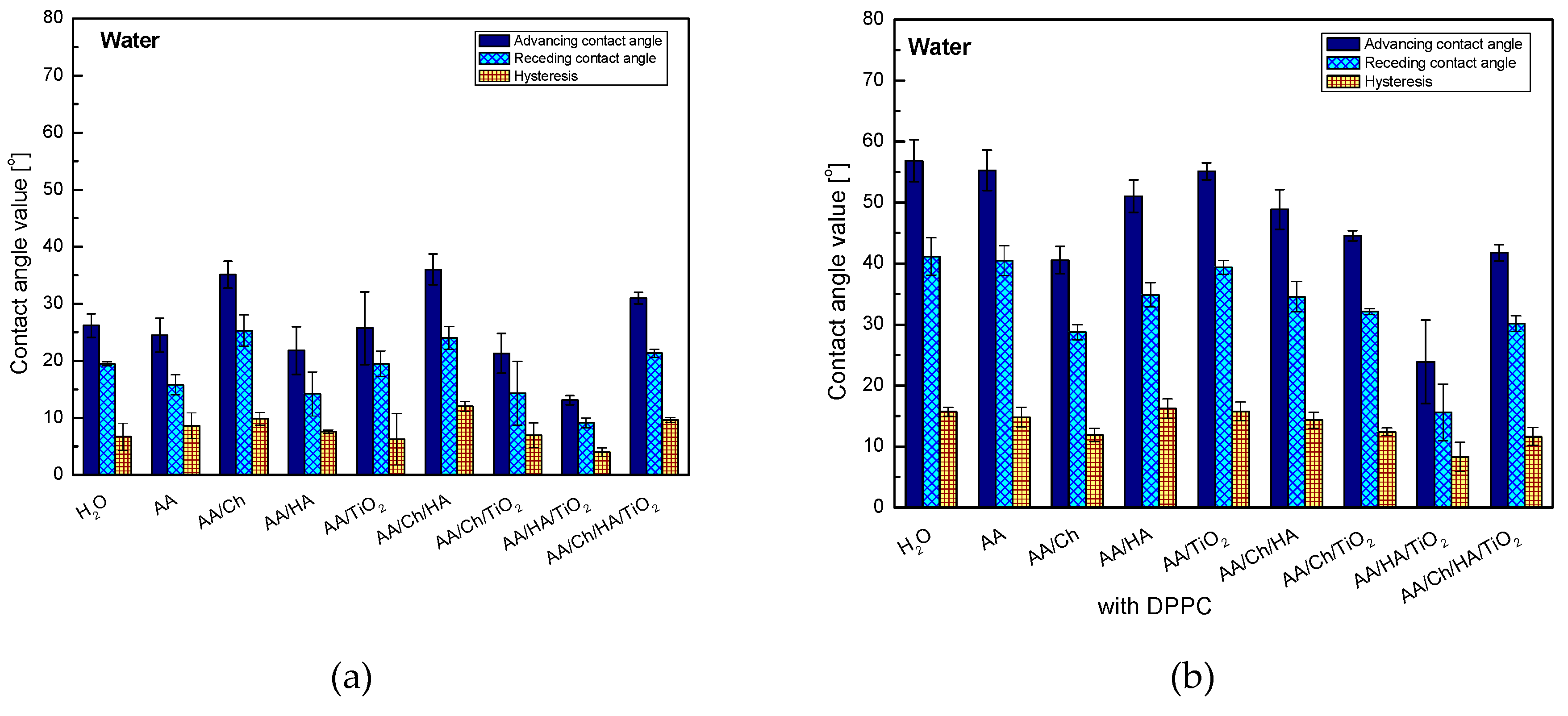
3.2.3. Water Contact Angle and its Hysteresis
The presence of oxide in individual systems caused a decrease in the contact angle values by 13.8° for glass/AA/Ch/TiO2, about 8.7° for glass/AA/HA/TiO2 and 5.0° for glass/AA/Ch/HA/TiO2. However, only (glass/AA/TiO2) showed an intermediate value between that obtained for the H2O and AA systems, namely 25.7°. This can demonstrate the existence of other strong interactions beyond the dispersion forces. In addition, a significant increase in the hydrophilic character may be due to the hydrophilic properties of the oxide. The water droplets interact strongly with the TiO2 particles, and as a result, they spread out more freely (conversely they are more strongly attracted by the surface). Furthermore, the smallest hysteresis values (except for glass/AA/Ch/HA/TiO2) were found in these systems showing high stability and evident organization of the deposited film. This may suggest that the polymers combine with the titanium dioxide particles in a manner allowing them for the contact of groups capable of hydrogen bonds formation with the activated plate surface, and also interact with the polar water droplets. In the case of other systems, the highest values of contact angles were obtained of all the tested ones and very high hysteresis values were noted from 7.6° for glass/AA/HA to 12.0° for glass/AA/Ch/HA, suggesting that the liquid penetrates into the subphase layers.
The great ability of HA to bind water molecules may be a reason for obtaining a smaller contact angle value for the glass/AA/HA system (21.8°) compared to that of glass/W (26.1°). This property was ‘strengthened’ after the addition of TiO2, and ‘weakened’ in combination with Ch—resulting in the highest contact angle value (36.7°), of all tested systems, and also, as mentioned before, its highest hysteresis.
The values of contact angles of water on the DPPC monolayer have highlighted the analogous trend of changes (except for the glass/AA/Ch/DPPC system) to those obtained on the subphase layers but without the lipid film, wherein the values of the glass/subphase/DPPC systems are higher from about 10.8° for glass/AA/HA/TiO
2/DPPC and glass/AA/Ch/HA/TiO
2/DPPC to 30.8° for glass/AA/DPPC. On the other hand, glass/AA/Ch/DPPC showed only a 5.5° higher water contact angle value compared to the system without DPPC (glass/AA/Ch—35.1°, glass/AA/Ch/DPPC—40.6°). However, TiO
2 in the DPPC film systems did not cause such a significant reduction in contact angle values for all systems in comparison with the non-lipid systems. Only in the case of glass/AA/HA/DPPC → glass/AA/HA/TiO
2/DPPC there was a decrease of this parameter (27.3°) after the addition of TiO
2. This may be related to the slight loosening of the monolayer structure at the transfer pressure after the inorganic substance addition to the subphase [
26] which makes it more permeable than glass/AA/HA/DPPC. As a result, water molecules may have access to and interact with subphase components. A similar relationship was also observed for the glass/AA/HA/Ch/DPPC → glass/AA/HA/Ch/TiO
2/DPPC systems.
The most hydrophobic DPPC monolayer was obtained by transferring it from the AA (55.3°) and AA/TiO
2 subphase (55.1°), and the least one from AA/HA/TiO
2 (23.9°)—in this case, the lowest value of the contact angle hysteresis was also noted, namely 8.3°, suggesting the existence of weak interactions with water. In the first case (glass/AA/DPPC), the monolayer was highly packed [
26]. It is well-known that the more condensed monolayer is, the less permeable to water molecules and other liquids. In other cases, very high values of contact angle hysteresis appeared which indicates the existence of strong interactions between the DPPC and the test liquid, preventing the water droplets ‘detaching’ from the monolayer surface.
The addition of chitosan to all systems caused the decrease of advancing contact angle probably due to interactions by hydrogen bonds. Both acetylated and deacetylated chitosan units have donor/acceptor groups which can be involved in hydrogen bonding [
27]. Chitosan below its pKa value (6.1–6.5) is positively charged [
22,
28] but its -NH
3+ groups are close to deprotonation. In contrast, the phosphate groups -OPO
3− in bipolar DPPC have a negative charge, and the ammonium group -N
+(CH
3)
3 is positive, keeping the uncharged monolayer at neutral pH [
23]. During the contact of both substances it is very likely that the phospholipid molecules combine with chitosan by hydrogen bonding (amino/ammonium and/or hydroxyl groups of chitosan with phospholipid polar heads).
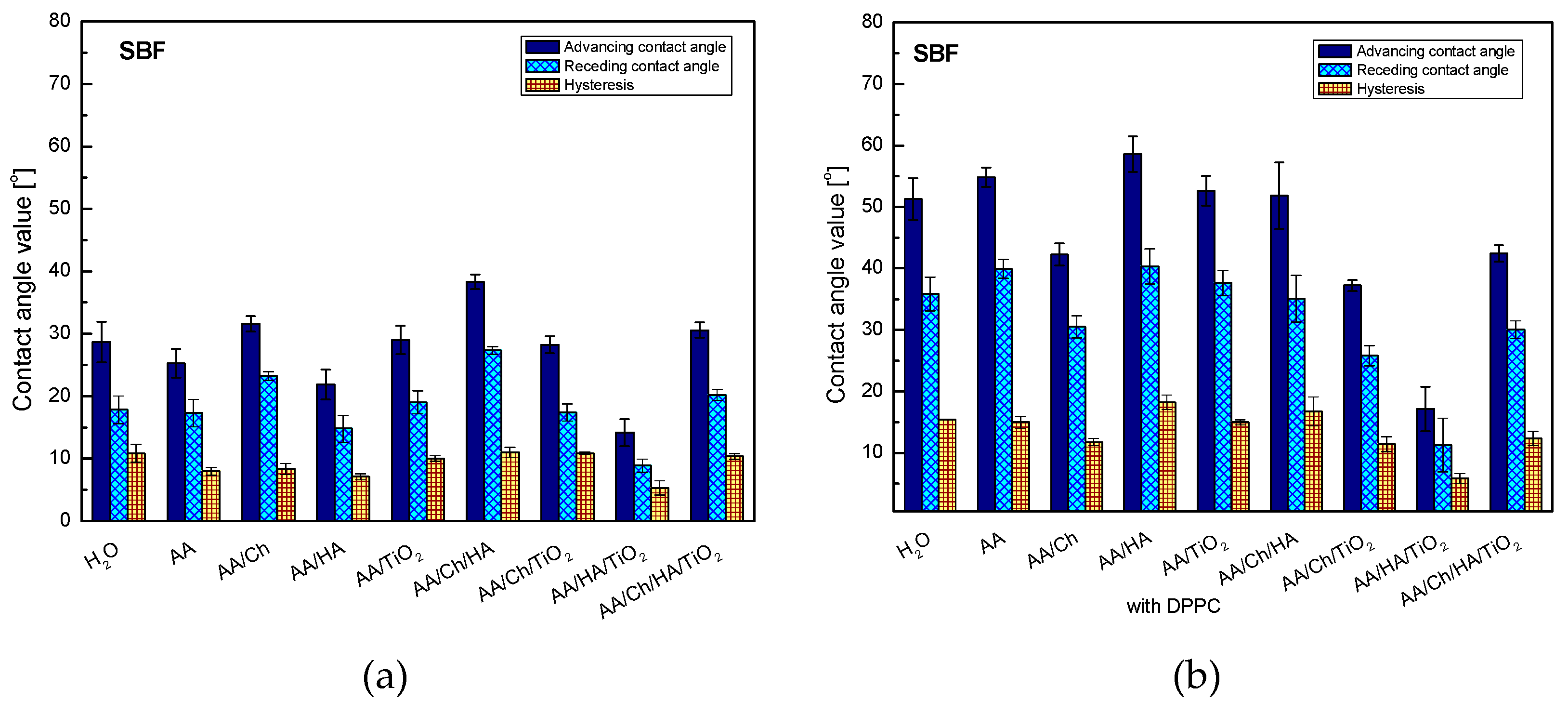
3.2.4. SBF Contact Angle and Its Hysteresis
In order to analyze the biocompatibility of the tested systems, they were subjected to a wettability test by using the SBF solution (
Figure 4). SBF is a mixture with the ions concentration similar to that of human blood plasma. The exact composition of the SBF solution is presented in [
16,
17]. As 79% of blood includes water, it is expected that the contact angles for this liquid will be close to those obtained for water.
Analysis of SBF wettability confirmed the primary presumption. The trend of changes in the contact angle values for individual systems was analogous to that obtained for the water droplets (
Figure 3), showing only small differences from 0.1° for glass/AA/HA to 6.9° for glass/AA/Ch/TiO
2. The contact angles values of SBF were slightly higher than those obtained for water, except for glass/AA/Ch and glass/AA/Ch/HA/TiO
2 where the lower values of 3.5° and 0.4° were recorded, respectively.
As mentioned before, apart from determining the wettability and hydrophilic–hydrophobic character of the studied systems, the aim of the paper was also to determine their biocompatibility, which can be checked by the analysis of the contact angles of the SBF solution. The SBF contact angle values for individual systems with the DPPC monolayer were higher than those for the analogous systems without a phospholipid film, and also the general trend of changes was completely different. The highest increase in value was found in the Glass/AA/HA system (Glass/AA/HA—21.9°, Glass/AA/HA/DPPC—58.6°) and the smallest for Glass/AA/HA/TiO2 (Glass/AA/HA/TiO2—14.2°, Glass/AA/HA/TiO2/DPPC—17.2°). When comparing the values of water and SBF contact angles on the surfaces of the studied systems containing the phospholipid monolayer, significant differences were noted in the range from 0.4° for Glass/AA to 7.6° for Glass/AA/HA. This may indicate their good biocompatibility with the components of blood plasma and probably with the entire human body (in the case of the former, the water content is 79% while in the human body it is up to 75%).
Moreover, the obtained higher values of the contact angle hysteresis indicate the existence of strong interactions between the DPPC molecules. Biological membranes cannot be treated as isolated systems because they remain in constant contact with various substances. Therefore, the obtained results give better insight into their complicated characteristics.
3.3. Summary
The analysis of the wettability of the DPPC monolayer surface allowed to determine: (I) specific orientation of phospholipid molecules polar part towards the glass plate with the subphase film while the nonpolar one towards the air, which more or less interacted with the test liquids such as water, formamide, and diiodomethane; (II) strong interactions between the DPPC heads and the subphase components; (III) specific penetration of individual components of the subphase, i.e., Ch, TiO
2, and HA between the DPPC molecules in the monolayer; and (IV) biocompatibility of the tested systems with the blood plasma components. By playing with our components content it will be possible to obtain the biomaterial characterized by adequate wettability. However, to choose which surfaces, hydrophobic or hydrophilic, are more appropriate for the extensive biological studies involving cell differentiation in the human skin
in vitro studies are necessary. Based on our present and previous results [
17,
19,
26,
29] we think that the moderate hydrophilic surfaces will be the most useful for skin substitution. Also as reported by Rivero et al. [
30] the moderate hydrophilic surfaces are favorable for cell adhesion and proliferation.
Our studies are preliminary but the use of these surfaces for
in vitro studies at laboratory level would help define which of the medical treatments will be more suitable to apply
in vivo. For example, fibronectin has the ability to change the spatial structure depending on the environment and the body’s needs. It shows a marked reduction in its cell-adhesive activity if adsorbed onto hydrophobic substrates, while its cell-stimulating functionality is maintained if adsorbed on hydrophilic ones [
31]. In the aspect of skin replacement it takes part along with fibrin in the process of rebuilding damaged tissues. We suppose that behavior of HA-based systems strictly depending on HA amount will be similar to that of fibronectin, but such a hypothesis needs to be verified.
4. Conclusions
The aim of the study was to determine the wettability and hydrophobic-hydrophilic character of the systems containing common biopolymers—chitosan and hyaluronic acid—as well as biocompatible titanium dioxide as having the potential of medical, pharmaceutical, or cosmetic applications.
The wettability of DPPC monolayers deposited on the glass plates using the Langmuir–Blodgett (LB) technique was determined based on the contact angle measurements of the three test liquids: water, formamide and diiodomethane. In addition, the biocompatibility of the proposed systems has been analyzed based on the measurements of the contact angles of a simulated body fluid solution (SBF) in which the concentration of ions is close to that of human plasma. After the deposition of the DPPC monolayer on the plates, a significant increase in the contact angles of all the measurement liquids was found in comparison to the plates immersed in the subphase without DPPC. The presence of phospholipid increases the hydrophobic nature of the surface due to the orientation of the molecules with the hydrocarbon chains towards the air. The subphase type determines the properties of the DPPC film. Differences are visible in the changes of contact angles values. Variations of these parameters are dependent on the film permeability to the used test liquid which is related to the characteristic orientation of the particles and/or molecules and their packing.
Analysis of the obtained data allowed general estimation of the type of forces by means of which individual components in the system can interact with the environment. The obtained results provide information on the functionality of biological membranes as well as on precise cellular processes such as the transport of water molecules, the kind of ions and substances through the membrane. However, contact angle values are only a general determinant of interactions proceeding in the solution. More precise information, such as the type of interactions and their magnitude/contribution, can be provided from the values of surface free energy and its components, which can be determined using commonly available theoretical models. Analysis of these data will be presented in our next paper [
29].