Systematic Characterization of DMPC/DHPC Self-Assemblies and Their Phase Behaviors in Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Vesicle Preparation
2.3. Preparation of DMPC/DHPC Self-Assembly in an Aqueous Solution.
2.4. Turbidity Measurements of Self-Assembly Solution
2.5. Dynamic Light Scattering
2.6. Evaluation of Membrane Fluidity
2.7. Evaluation of Membrane Polarity
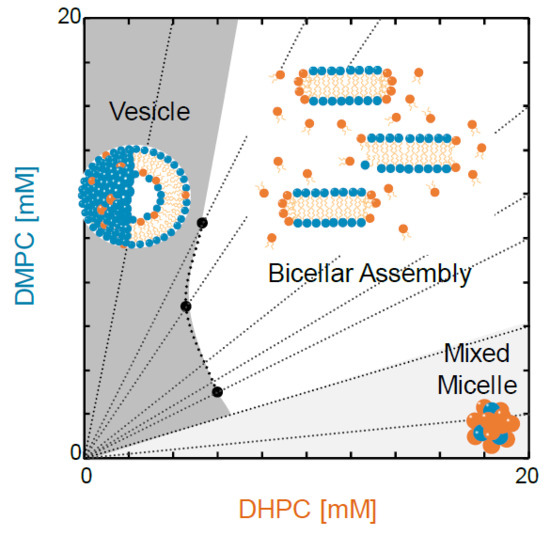
3. Results and Discussion
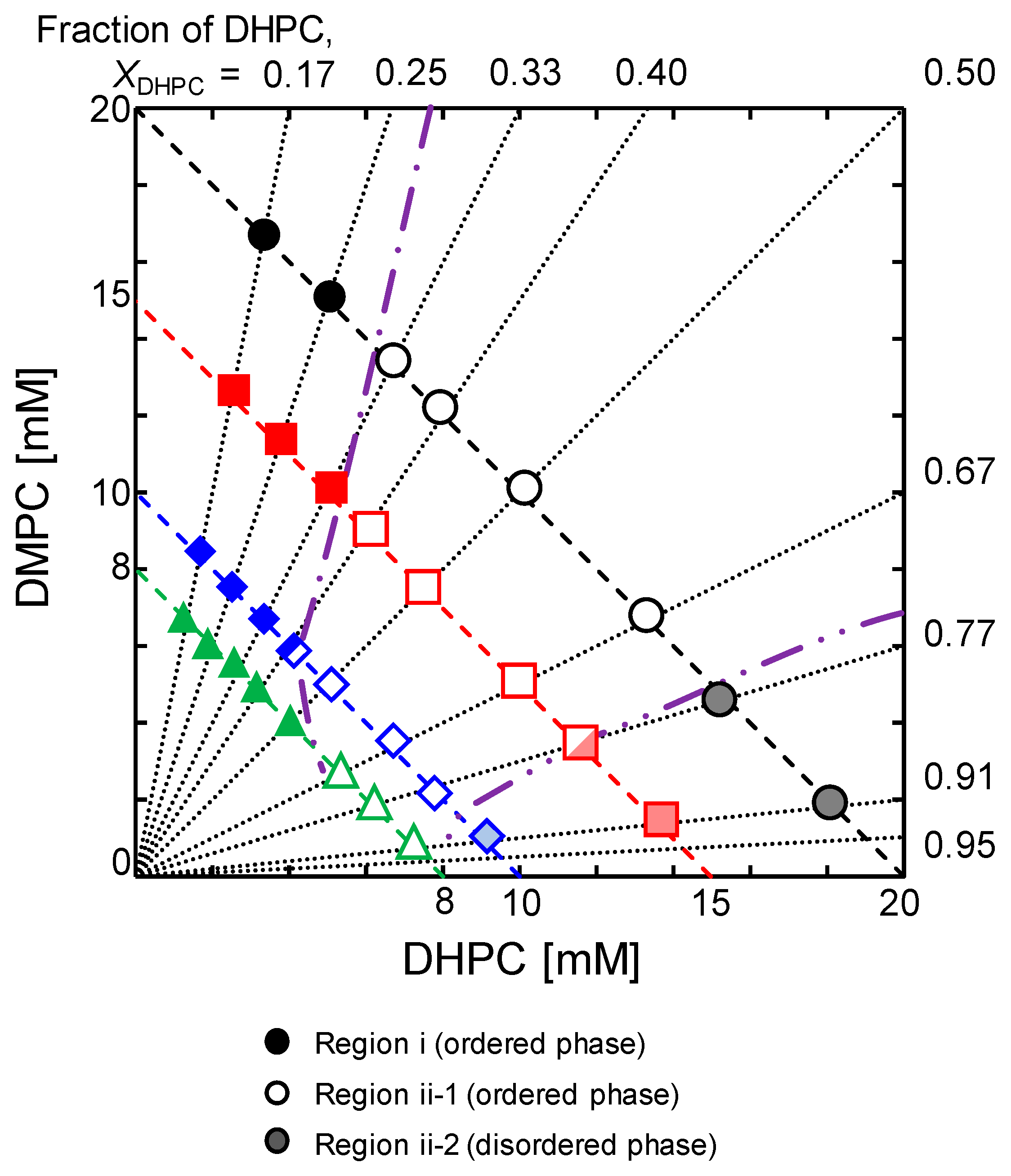
3.1. Systematic Characterization of DMPC/DHPC Assemblies Focusing on Turbidity, Size, Membrane Fluidity, and Membrane Polarity.
3.2. Structural Properties of DMPC/DHPC Assemblies in Dilute Conditions
3.2.1. Optical Density (OD500)
3.2.2. Average Size Estimated Using DLS Analysis
3.3. Interfacial Membrane Properties of DMPC/DHPC Assemblies
3.3.1. Membrane Fluidity (1/PDPH)
3.3.2. Membrane Polarity (GP340)
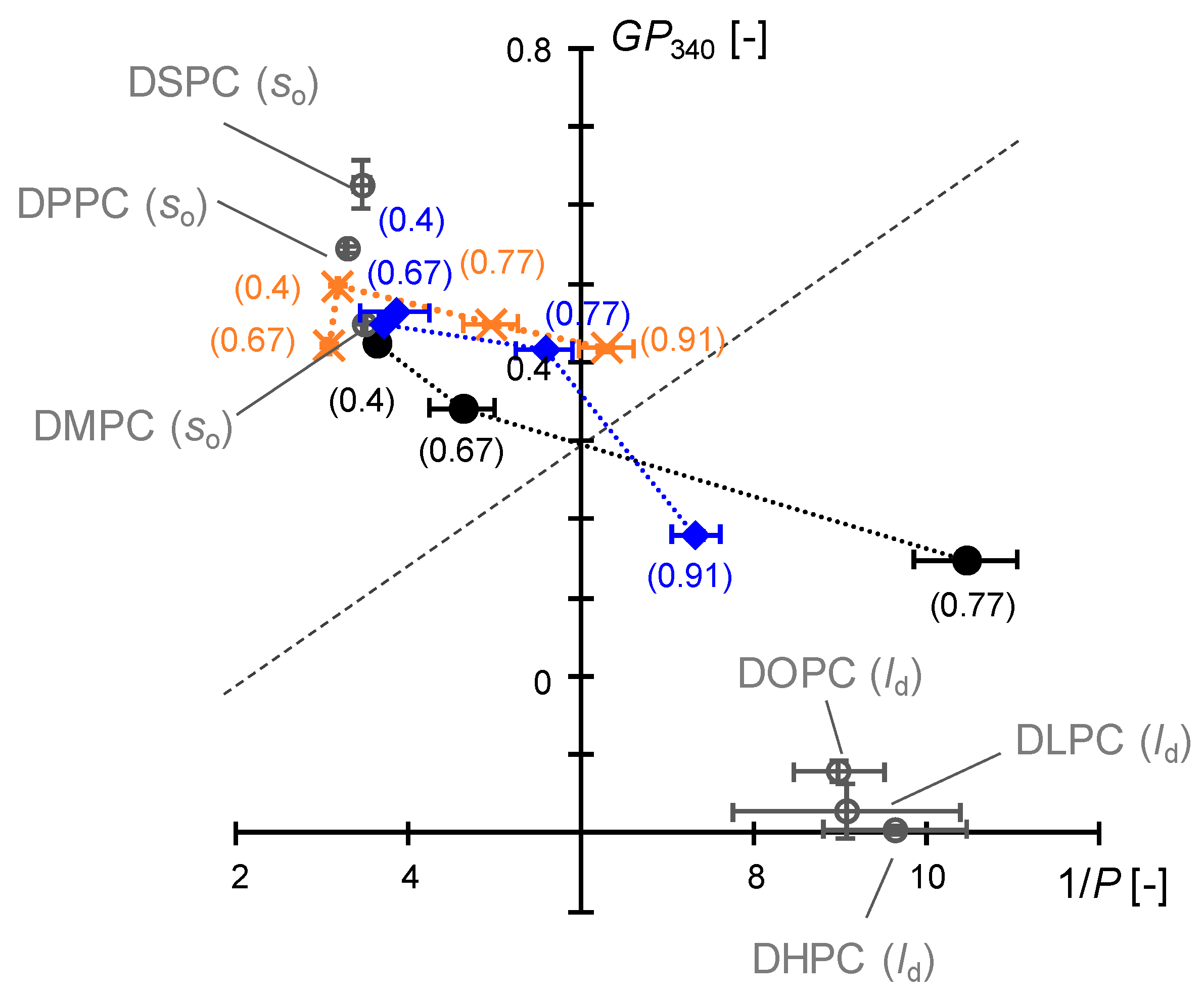
3.3.3. Comparison of Interfacial Membrane Properties in the Cartesian Diagram
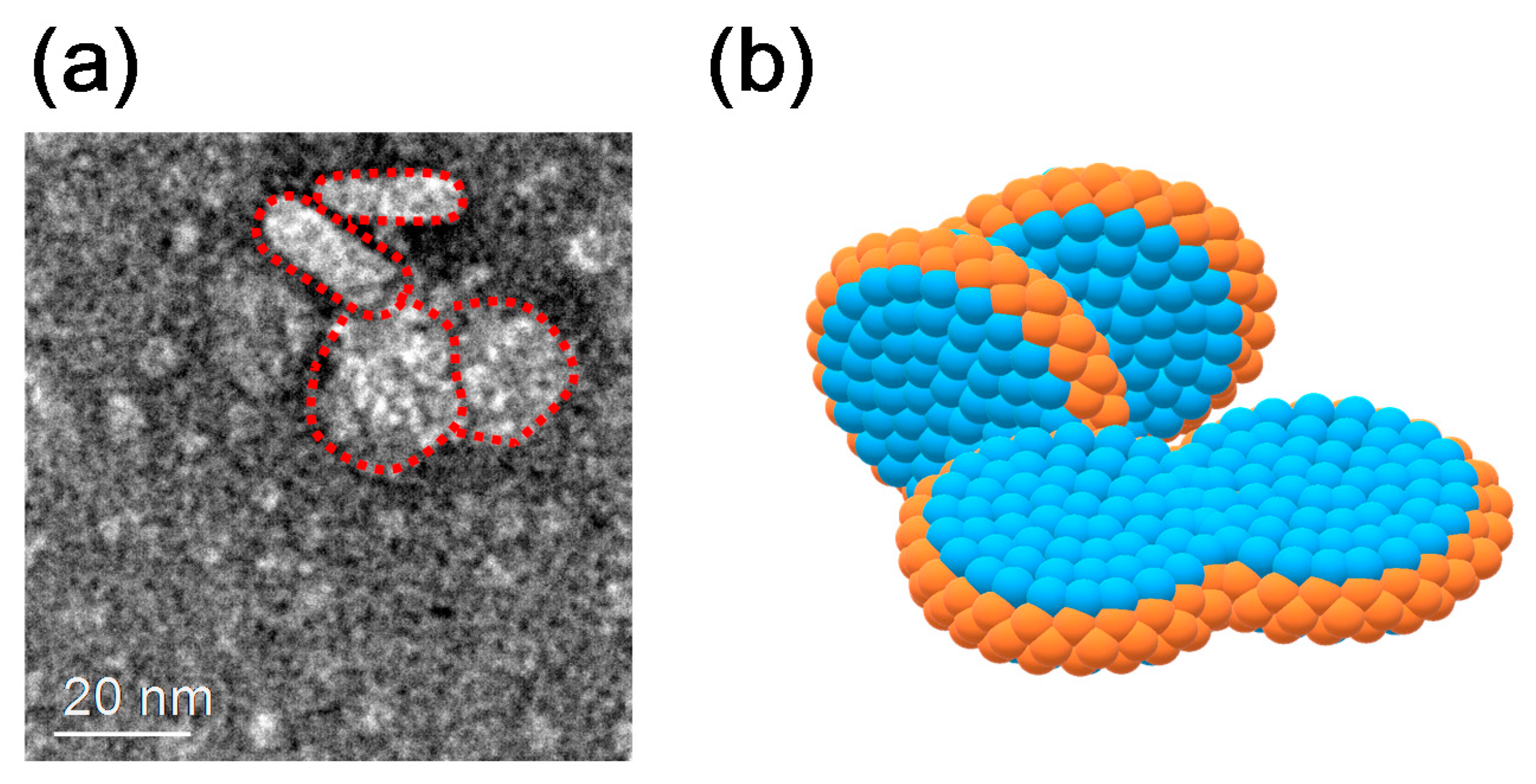
3.4. Discussion on Phase Behavior of DMPC/DHPC Assemblies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| DHPC | 1,2-dihexanoyl-sn-glycero-3-phosphocholine |
| Tm | phase transition temperature |
| CMC | critical micelle concentration |
| DLS | dynamic light scattering |
| DPH | 1,6-diphenyl-1,3,5-hexatriene |
| Laurdan | 6-dodecanoyl-N,N-dimethyl-2-naphthylamine |
| GP | general polarization |
| OD | optical density |
References
- Burns, R.A., Jr.; Roberts, M.F.; Dluhy, R.; Mendelsohn, R. Monomer-to-micelle transition of dihexanoylphosphatidylcholine: 13C and Raman studies. J. Am. Chem. Soc. 1982, 104, 430–438. [Google Scholar] [CrossRef]
- Eytan, G.D. Use of liposome for reconstitution of biological functions. Biochim. Biophys. Acta Rev. Biomembr. 1982, 692, 185–202. [Google Scholar] [CrossRef]
- Ringsdorf, H.; Schlarb, B.; Venzmer, J. Molecular Architecture and Function of Polymeric Oriented Systems: Models for the Study of Organization, Surface Recognition, and Dynamics of Biomembranes. Angew. Chem. Int. Ed. 1988, 27, 113–158. [Google Scholar] [CrossRef]
- Cyrus, S.R.; Kai, E.L. Materials chemistry: Liposomes derived from molecular vases. Nature 2012, 489, 372–374. [Google Scholar]
- Sanders, C.R.; Schwonek, J.P. Characterization of Magnetically Orientable Bilayers in Mixtures of Dihexanoylphosphatidylcholine and Dimyristoylphosphatidylcholine by Solid-State NMR. Biochemistry 1992, 31, 8898–8905. [Google Scholar] [CrossRef] [PubMed]
- Ottiger, M.; Bax, A. Characterization of magnetically oriented phospholipid micelles for measurement of dipolar couplings in macromolecules. J. Biomol. NMR 1998, 12, 361–372. [Google Scholar] [CrossRef]
- McKibbin, C.; Farmer, N.A.; Jeans, C.; Reeves, P.J.; Khorana, H.G.; Wallace, B.A.; Edwards, P.C.; Villa, C.; Booth, P.J. Opsin Stability and Folding: Modulation by Phospholipid Bicelles. J. Mol. Biol. 2007, 374, 1319–1332. [Google Scholar] [CrossRef]
- Li, Y.; To, J.; Verdià-Baguena, C.; Surya, S.; Huang, M.; Paulmichl, M.; Liu, D.X.; Aguilella, V.M.; Torres, J. Inhibition of the Human Respiratory Syncytial Virus Small Hydrophobic Protein and Structural Variations in a Bicelle Environment. J. Virology 2014, 88, 11899–11914. [Google Scholar] [CrossRef]
- Sanders, C.R.; Landis, G.C. Reconstitution of Membrane Proteins into Lipid-Rich Bilayered Mixed Micelles for NMR Studies. Biochemistry 1995, 34, 4030–4040. [Google Scholar] [CrossRef]
- van Dam, L.; Karlsson, G.; Edwards, K. Direct observation and characterization of DMPC/DHPC aggregates under conditions relevant for biological solution NMR. Biochim. Biophys. Acta Biomembr. 2004, 1664, 241–256. [Google Scholar] [CrossRef]
- Wu, H.; Su, K.; Guan, X.; Sublette, M.E.; Stark, R.E. Assessing the Size, Stability, and Utility of Isotropically Tumbling Bicelle Systems for Structural Biology. Biochim. Biophys. Acta Biomembr. 2010, 1798, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Beaugrand, M.; Arnold, A.A.; Hénin, J.; Warshawski, D.E.; Williamson, P.T.F.; Marcotte, I. Lipid Concentration and Molar Ratio Boundaries for the Use of Isotropic Bicelles. Langmuir 2014, 30, 6162–6170. [Google Scholar] [CrossRef] [PubMed]
- Mineev, K.S.; Nadezhdin, K.D.; Goncharuk, S.A.; Areseniev, A.S. Characterization of Small Isotropic Bicelles with Various Compositions. Langmuir 2016, 32, 6624–6637. [Google Scholar] [CrossRef] [PubMed]
- Triba, M.N.; Warschawski, D.E.; Devaux, P.F. Reinvestigation by phosphorus NMR of lipid distribution in bicelles. Biophys. J. 2005, 88, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Lind, J.; Eriksson, J.; Mäler, L. Characterization of the Morphology of Fast-Tumbling Bicelles with Varying Composition. Langmuir 2014, 30, 5488–5496. [Google Scholar] [CrossRef] [PubMed]
- Struppe, J.; Vold, R.R. Dilute Bicellar Solutions for Structural NMR Work. J. Magn. Reson. 1998, 135, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Imaz, A.; Callejas-Fernández, J.; Barbosa-Barros, L.; Estelrich, J.; Quesada-Pérez, M.; Forcada, J. Soft Nanoparticles (Thermos-responsive Nanogels and Bicelles) with Biotechnological Applications: From Synthesis to Simulation Through Colloidal Characterization. Soft Matter 2010, 7, 5067–5082. [Google Scholar] [CrossRef]
- Matsui, R.; Ohtani, M.; Yamada, K.; Hikima, T.; Takata, M.; Nakamura, T.; Koshino, H.; Ishida, Y.; Aida, T. Chemically Locked Bicelles with High Thermal and Kinetic Stability. Angew. Chem. Int. Ed. 2015, 54, 13284–13288. [Google Scholar] [CrossRef]
- Saleem, Q.; Zhang, Z.; Petretic, A.; Gradinaru, C.C.; Macdonald P., M. Single Lipid Bilayer Deposition on Polymer Surfaces Using Bicelles. Biomacromol. 2015, 16, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzan, K.; Jackman, J.A.; Yoon, B.K.; Kim, M.C.; Johal, M.S.; Cho, N.-J. Optimizing the Formation of Supported Lipid Bilayers from Bicellar Mixtures. Langmuir 2017, 33, 5052–5064. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimanouchi, T.; Kato, K.; Miyazaki, T.; Nakamura, A.; Umakoshi, H. Span80 Vesicles have a More Fluid, Flexible and “Wet” Surface Than Phospholipid Liposomes. Colloid Surf. B 2011, 87, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Suga, K.; Umakoshi, H. Roles of Sterol Derivatives in Regulating the Properties of Phospholipid Bilayer Systems. Langmuir 2016, 32, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Taguchi, S.; Suga, K.; Hayashi, K.; Jung, H.-S.; Umakoshi, H. Characterization of the physicochemical properties of phospholipid vesicles prepared in CO2/water systems at high pressure. Biointerphases 2015, 10, 031005. [Google Scholar] [CrossRef]
- Suga, K.; Umakoshi, H. Detection of Nanosized Ordered Domains in DOPC/DPPC and DOPC/Ch Binary Lipid Mixture Systems of Large Unilamellar Vesicles Using a TEMPO Quenching Method. Langmuir 2013, 29, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. Lipid-protein interactions in biological membranes: A structural perspective. Biochim. Biophys. Acta Biomembr. 2003, 1612, 1–40. [Google Scholar] [CrossRef]
- Gerebtzoff, G.; Li-Blatter, X.; Fischer, H.; Frentzel, A.; Seelig, A. Halogenation of drugs enhances membrane binding and permeation. ChemBioChem. 2004, 5, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, T.; Suga, K.; Umakoshi, H. Chiral Recognition of L-Amino Acids on Liposomes Prepared with L-Phospholipid. ACS Appl. Mater. Interfaces 2015, 7, 21065–21072. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, F.; Luginbühl, S.; Suga, K.; Walde, P.; Umakoshi, H. Fluorescent Probe Study of AOT Vesicle Membranes and Their Alteration upon Addition of Aniline or the Aniline Dimer p-Aminodiphenylamine (PADPA). Langmuir 2017, 33, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, K.; Mattila, J.-P.; Kinnunen, P.K.J. Interfacial behavior of cholesterol, ergosterol, and lanosterol in mixtures with DPPC and DMPC. Biophys. J. 2008, 95, 2340–2355. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Vinson, P.K.; Kaplun, A.; Talmon, Y. Intermediate Structures in the Cholate-Phosphatidylcholine Vesicle-micelle Transition. Biophys. J. 1991, 60, 1315–1325. [Google Scholar] [CrossRef]
- Guo, Z.; Hauser, N.; Moreno, A.; Ishikawa, T.; Walde, P. AOT Vesicles as Templates for the Horseradish Peroxidase-triggered Polymerization of Aniline. Soft Matter 2011, 7, 180–193. [Google Scholar] [CrossRef]
- Craig, A.F.; Clark, E.E.; Sahu, I.D.; Zhang, R.; Frantz, N.D.; Al-Abdul-Wahid, M.S.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. Tuning the Size of Styrene-maleic Acid Copolymer-lipid Nanoparticles (SMALPs) using RAFT Polymerization for Biophysical Studies. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Shintzky, M.; Barenholz, Y. Fluidity parameters of lipid regions determined by flurescence polarization. Biochim. Biophys. Acta Rev. Biomembr. 1978, 515, 367–394. [Google Scholar] [CrossRef]
- Vincent, M.; de Foresta, B.; Gallay, J. Nanosecond Dynamics of a Mimicked Membrane-Water Interface Observed by Time Resolved Stokes Shift of LAURDAN. Biophys. J. 2005, 88, 4337–4350. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; De Stasio, G.; d’Ubaldo, A.; Gratton, E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 1990, 57, 1179–1186. [Google Scholar] [CrossRef]
- Knight, C.; Rahmani, A.; Morrow, M.R. Effect of an Anionic Lipid on the Barotropic Behavior of a Ternary Bicellar Mixture. Langmuir 2016, 32, 10259–10267. [Google Scholar] [CrossRef] [PubMed]
- Mély-Goubert, B.; Freedman, M.H. Lipid fluidity and membrane protein monitoring using 1,6-diphenyl-1,3,5-hexatriene. Biochim. Biophys. Acta Biomembr. 1980, 601, 315–327. [Google Scholar]
- Takajo, Y.; Matsuki, H.; Matsubara, H.; Tsuchiya, K.; Aratono, M.; Yamanaka, M. Structural and morphological transition of long-chain phospholipid vesicles induced by mixing with short-chain phospholipid. Colloid Surf. B 2010, 76, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Nieh, M.-P.; Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Hilty, C.; Wider, G.; Wüthrich, K. Lipid-protein Interactions in DHPC Micelles Containing the Integral Membrane Protein OmpX Investigated by NMR Spectroscopy. Proc. Natl. Acad. Sci. USA 2002, 99, 13533–13537. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Murase, O.; Sugishita, K.; Yoneyama, S.; Akada, K.; Ueha, M.; Nakamura, A.; Kobayashi, K. Optical characterization of liposomes by right angle light scattering and turbidity measurement. Biochim. Biophys. Acta Biomembr. 2000, 1467, 219–226. [Google Scholar] [CrossRef]

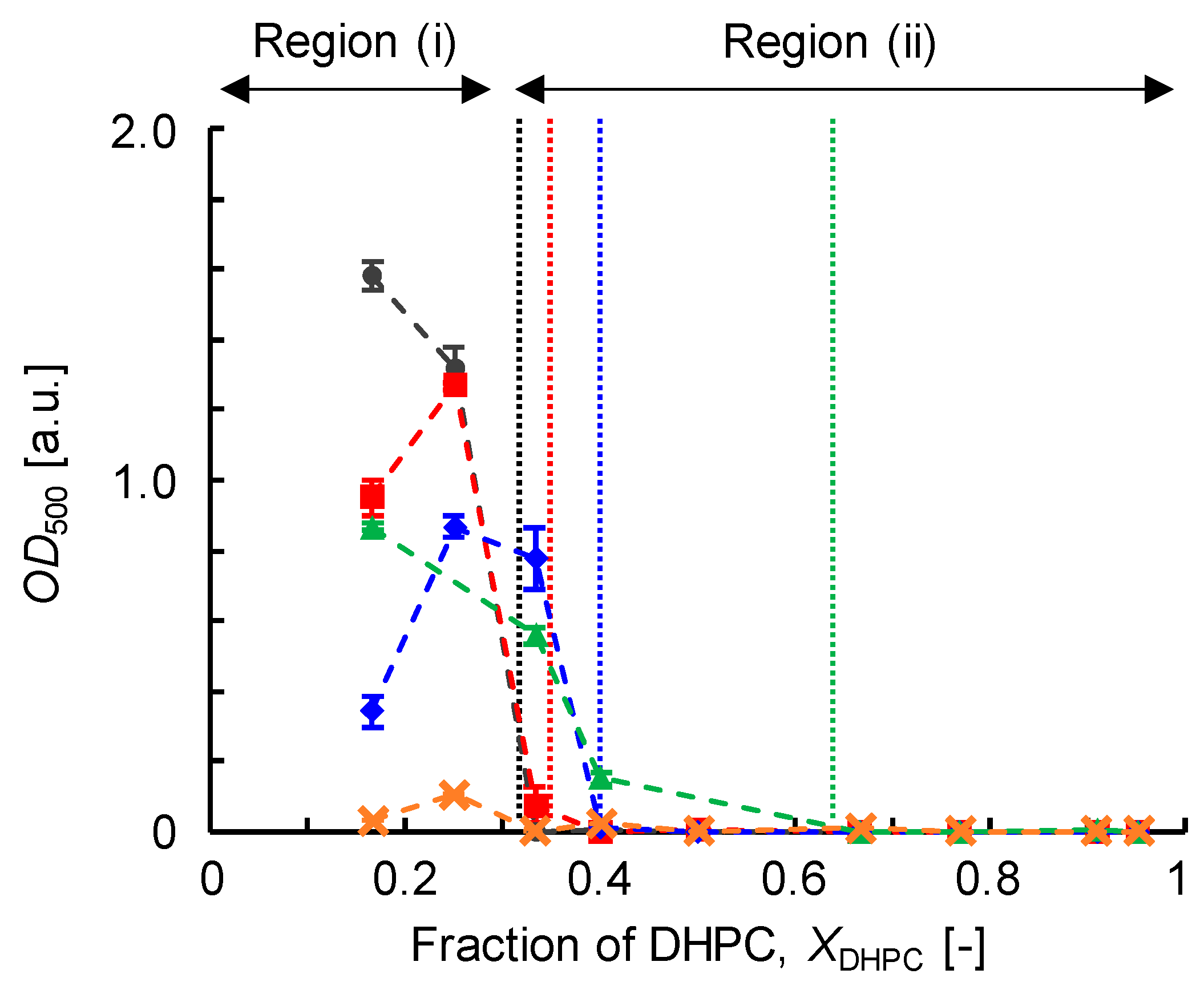
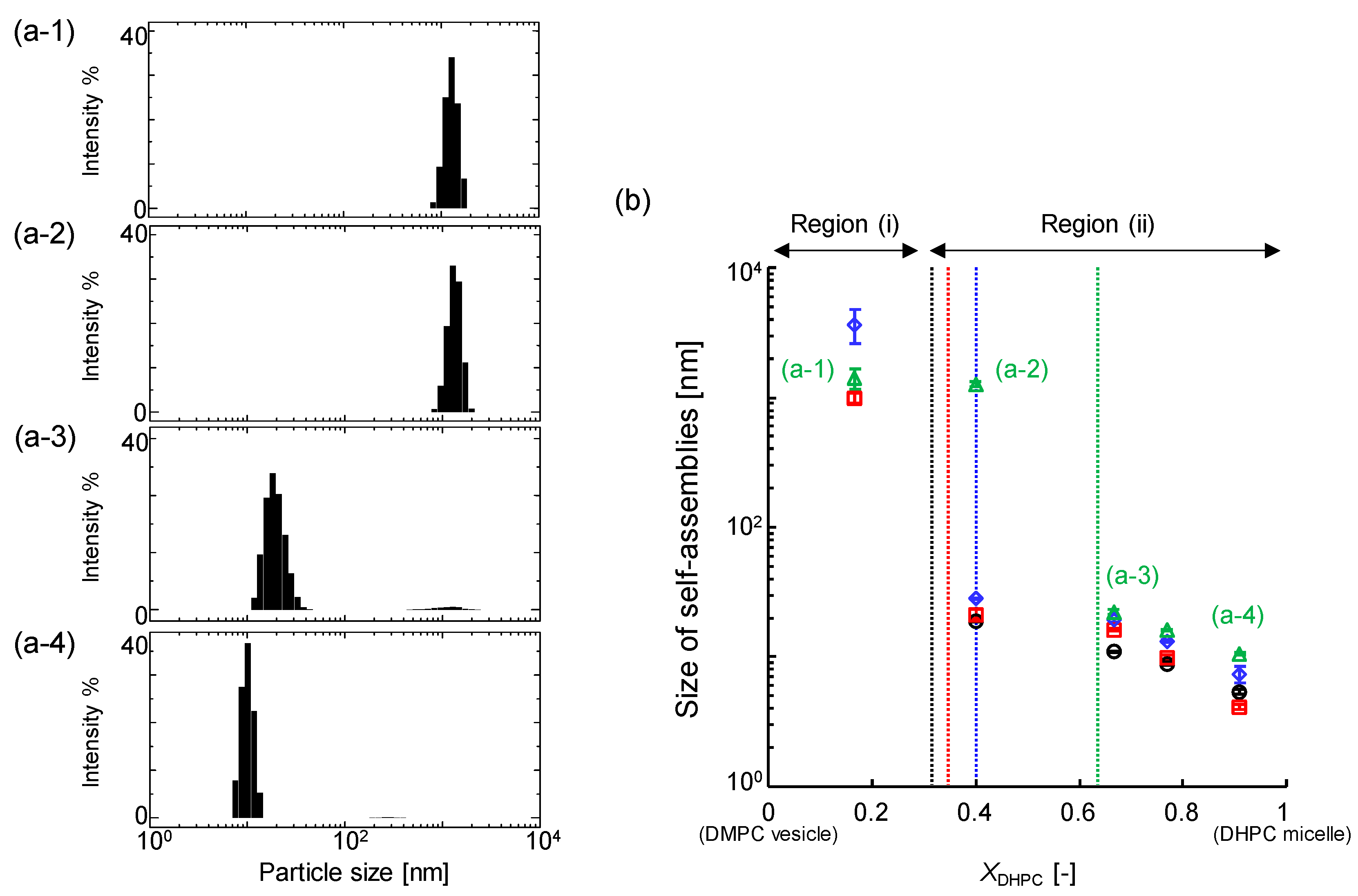
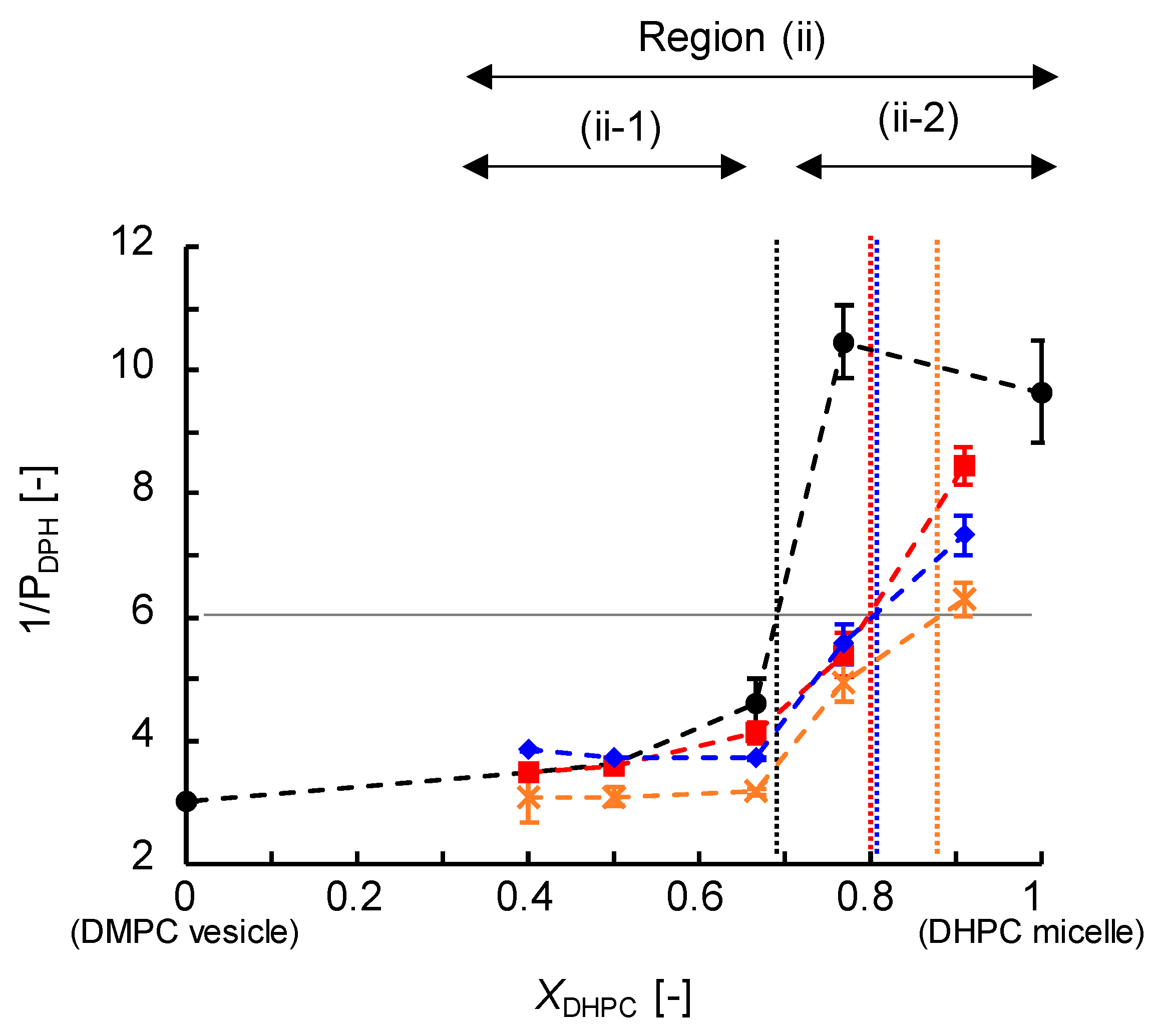

| Size (DLS) | Turbidity, OD500 | Membrane Fluidity, 1/PDPH | Membrane Polarity, GP340 | |
|---|---|---|---|---|
| Region (i) | >30 nm | ≥0.1 | <6 | >0.3 |
| Region (ii-1) | ≤30 nm | <0.1 | <6 | >0.3 |
| Region (ii-2) | ≤9 nm | <0.1 | >6 | <0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taguchi, S.; Suga, K.; Hayashi, K.; Okamoto, Y.; Jung, H.-S.; Nakamura, H.; Umakoshi, H. Systematic Characterization of DMPC/DHPC Self-Assemblies and Their Phase Behaviors in Aqueous Solution. Colloids Interfaces 2018, 2, 73. https://doi.org/10.3390/colloids2040073
Taguchi S, Suga K, Hayashi K, Okamoto Y, Jung H-S, Nakamura H, Umakoshi H. Systematic Characterization of DMPC/DHPC Self-Assemblies and Their Phase Behaviors in Aqueous Solution. Colloids and Interfaces. 2018; 2(4):73. https://doi.org/10.3390/colloids2040073
Chicago/Turabian StyleTaguchi, Shogo, Keishi Suga, Keita Hayashi, Yukihiro Okamoto, Ho-Sup Jung, Hidemi Nakamura, and Hiroshi Umakoshi. 2018. "Systematic Characterization of DMPC/DHPC Self-Assemblies and Their Phase Behaviors in Aqueous Solution" Colloids and Interfaces 2, no. 4: 73. https://doi.org/10.3390/colloids2040073
APA StyleTaguchi, S., Suga, K., Hayashi, K., Okamoto, Y., Jung, H.-S., Nakamura, H., & Umakoshi, H. (2018). Systematic Characterization of DMPC/DHPC Self-Assemblies and Their Phase Behaviors in Aqueous Solution. Colloids and Interfaces, 2(4), 73. https://doi.org/10.3390/colloids2040073