Effects of Short-Chain n-Alcohols on the Properties of Asphaltenes at Toluene/Air and Toluene/Water Interfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Crude Oil Fractionation
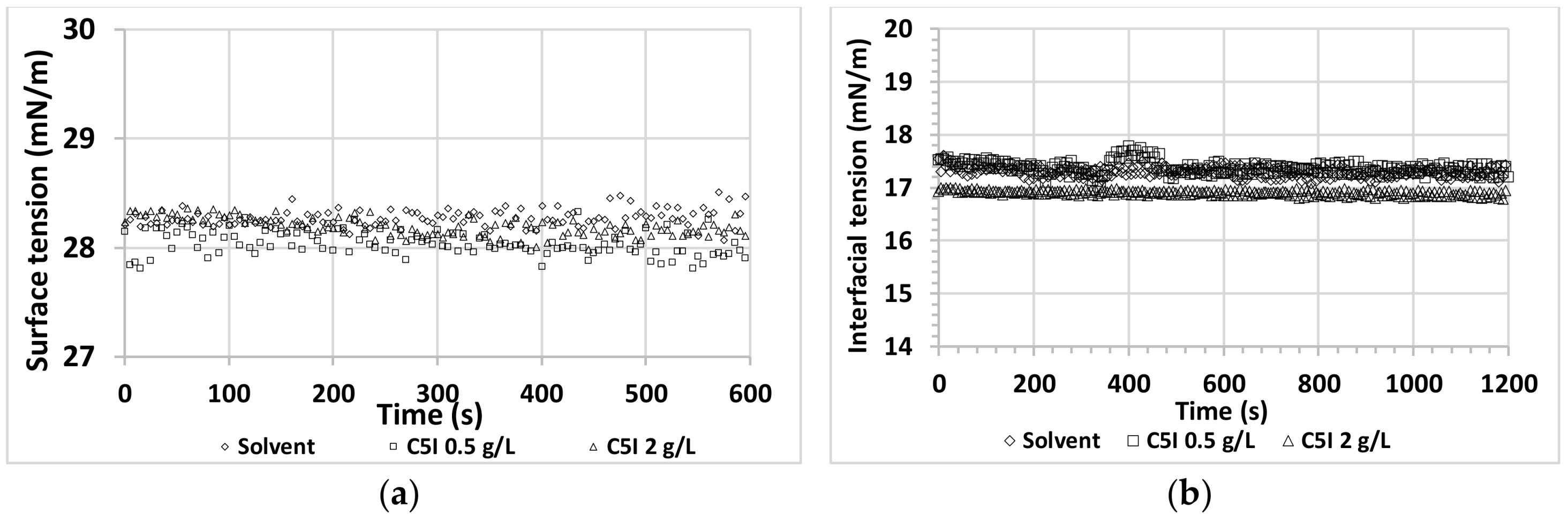
2.2.2. Surface and Interfacial Tension Measurement
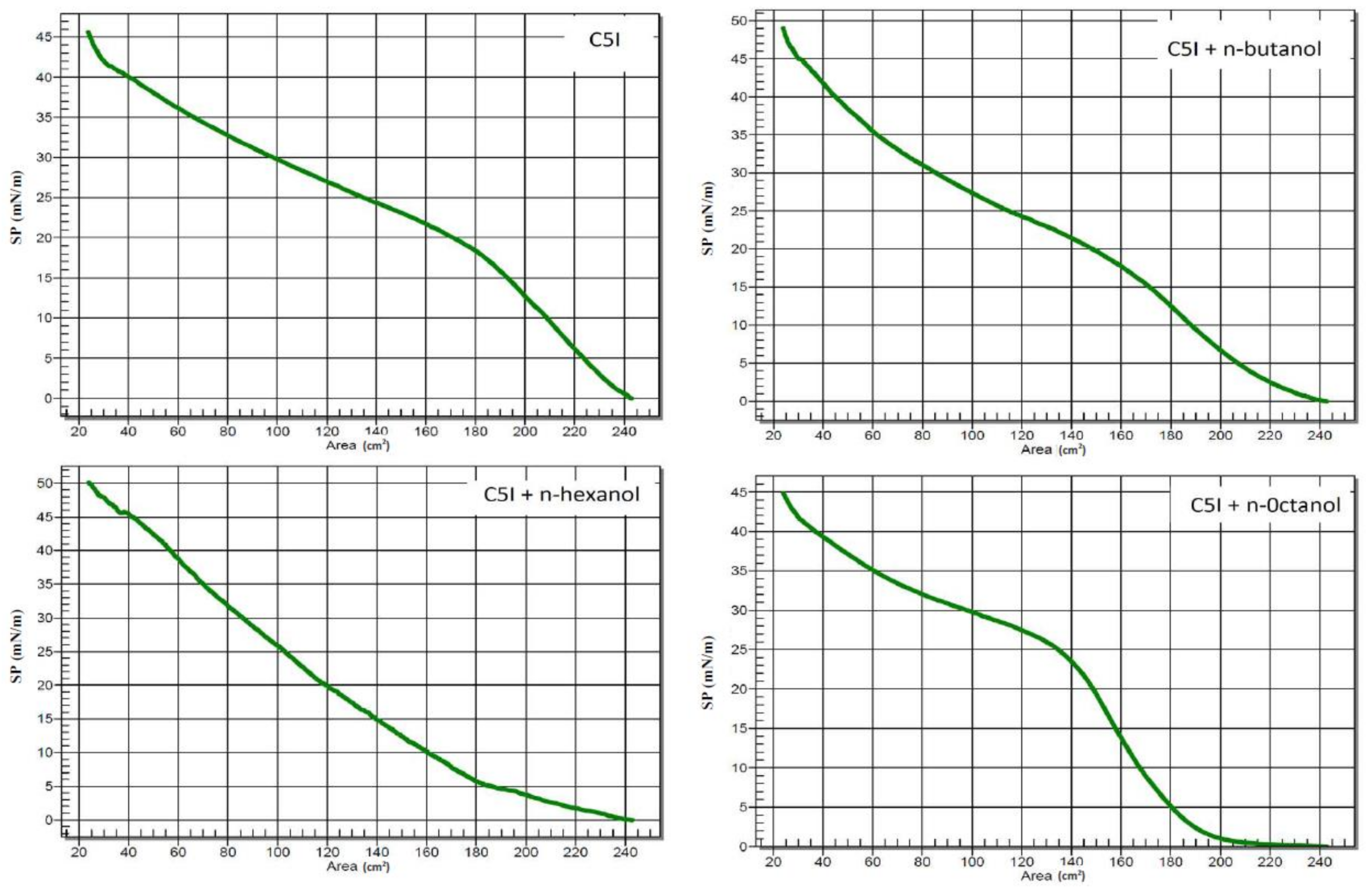
2.2.3. Surface Pressure Measurement
3. Results
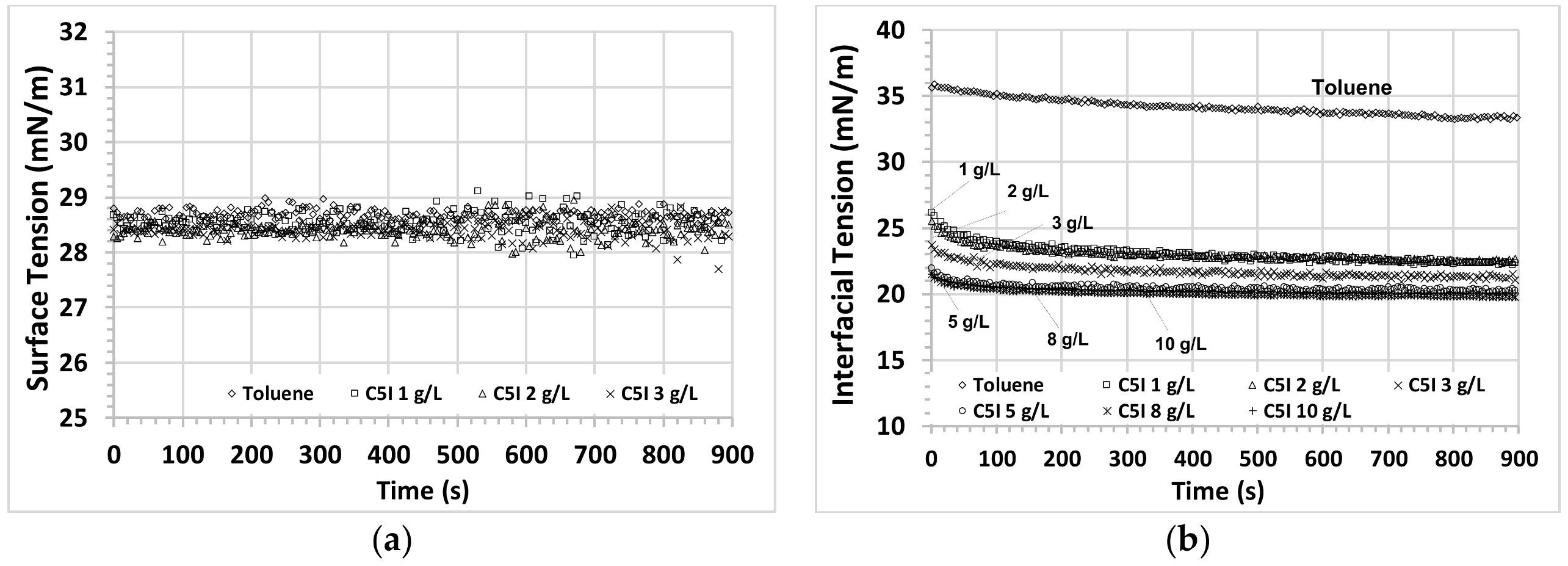
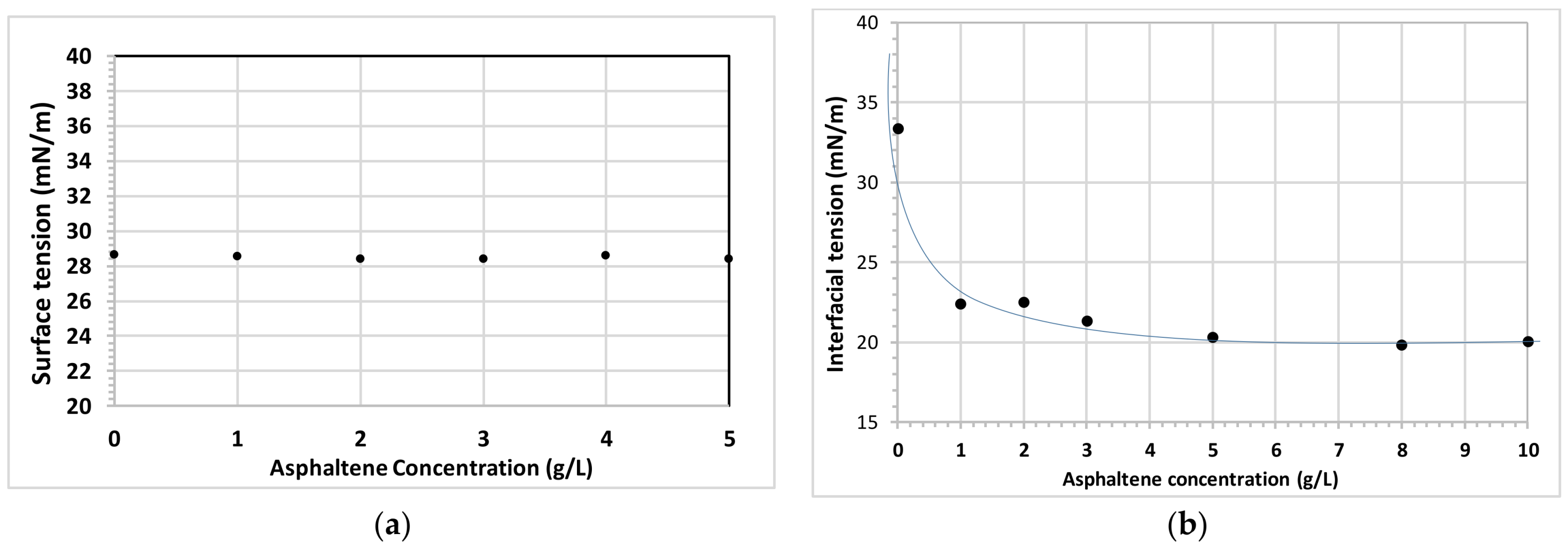
3.1. Asphaltene Interfacial Behavior
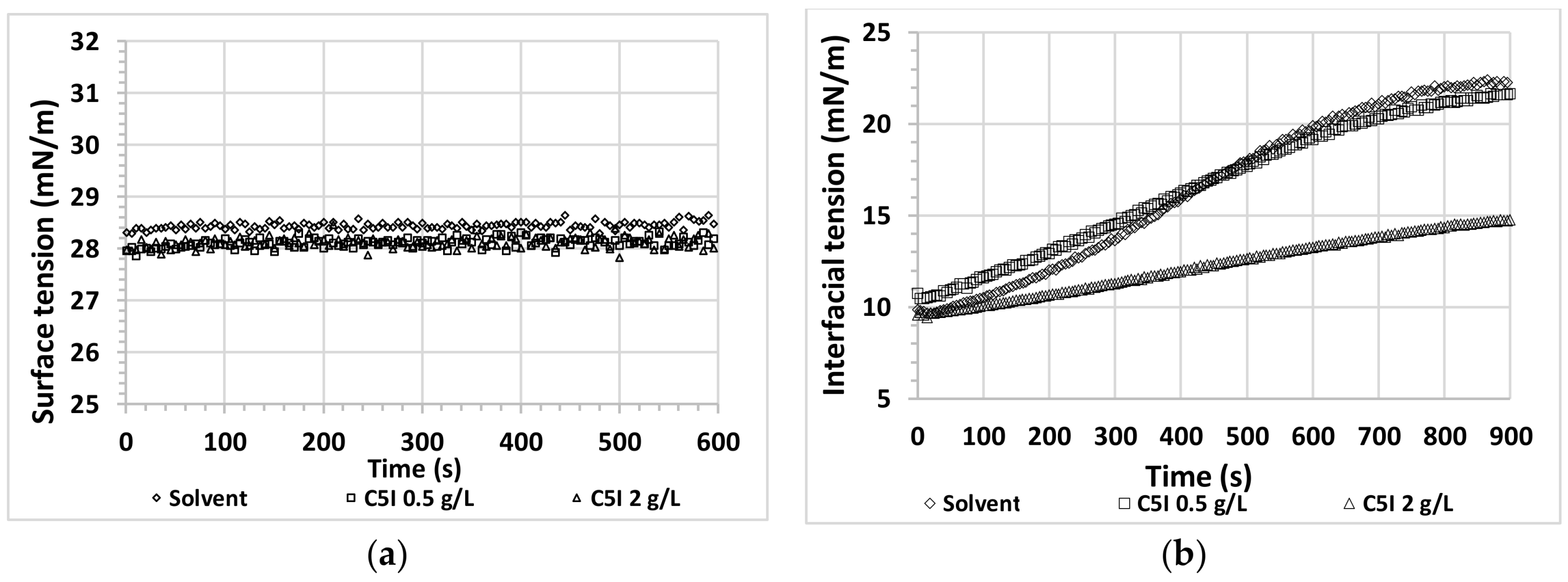
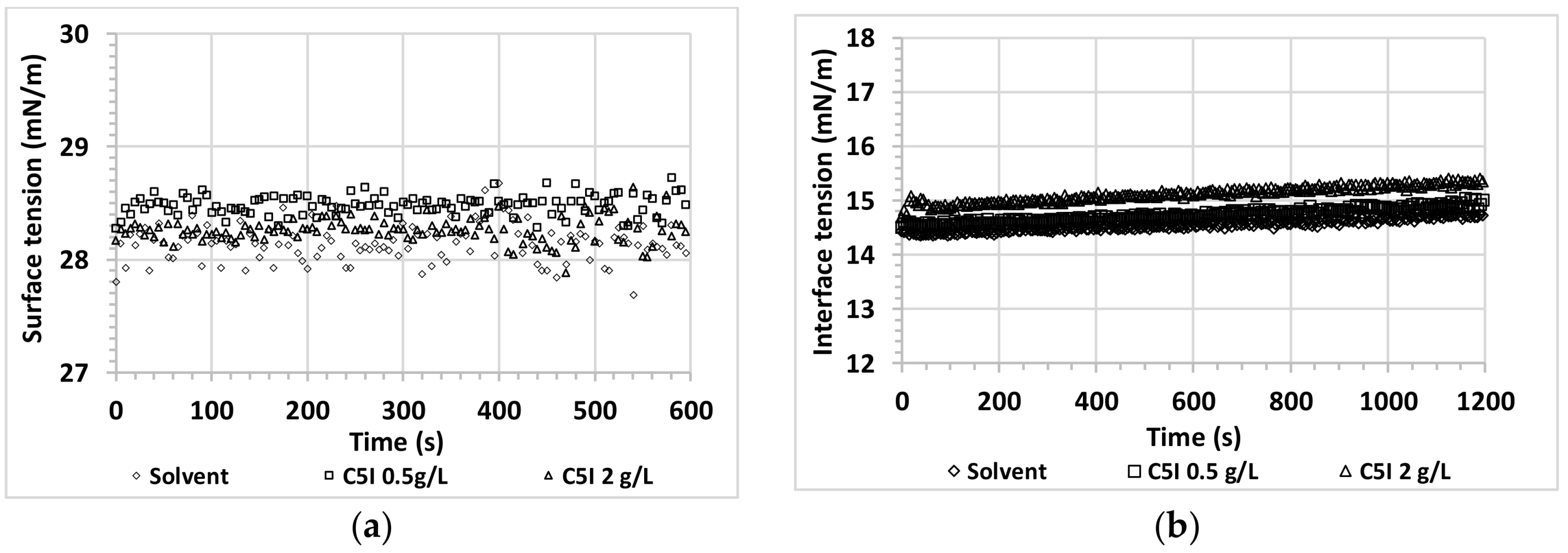
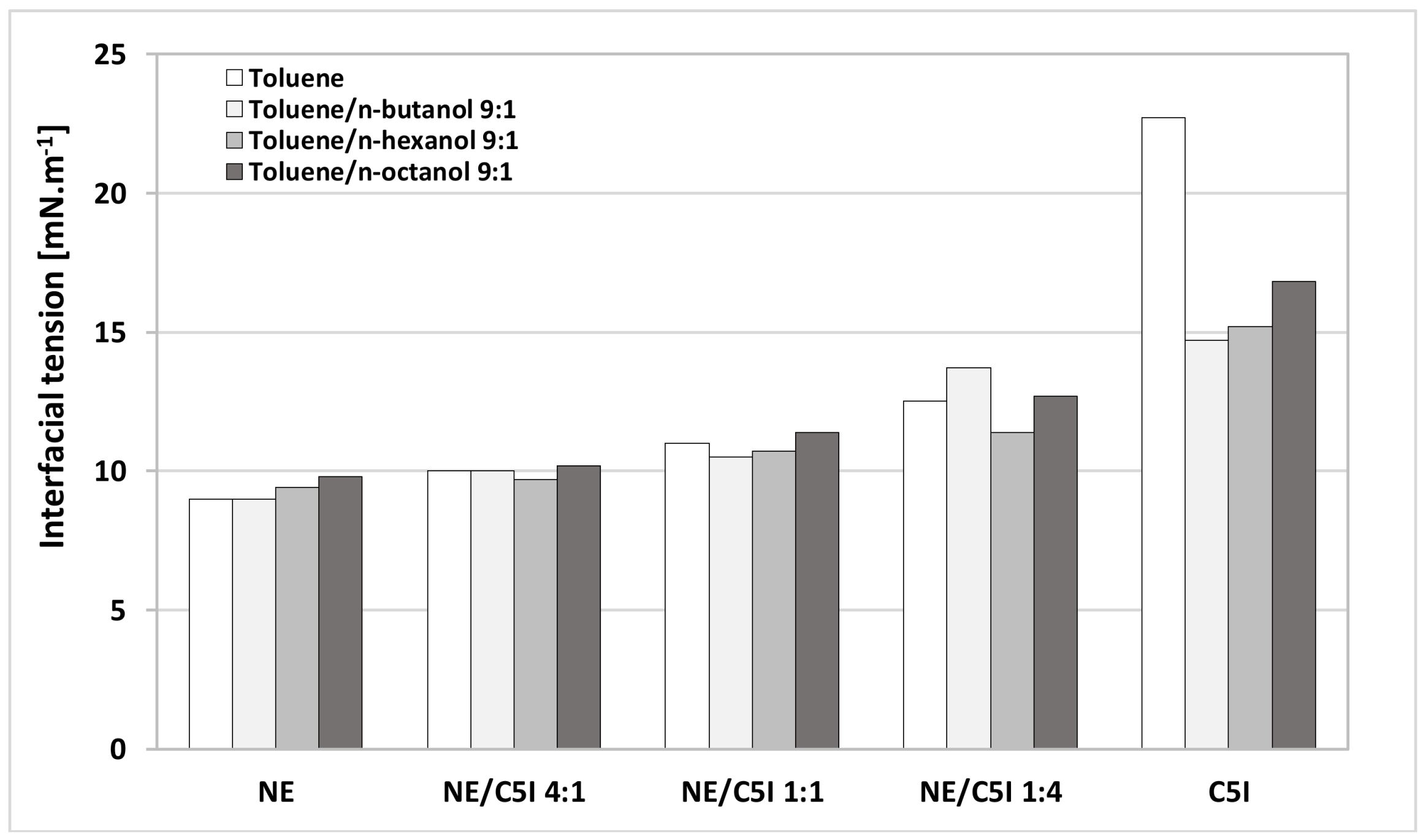
3.2. Interfacial Behavior for Asphaltene + Alcohol Systems
3.3. Langmuir Films Containing Asphaltenes and n-Alcohols
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Speight, J.G. The Chemistry and Technology of Petroleum, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Loh, W.; Mohamed, R.S.; Santos, R.G. Crude Oil Asphaltenes: Colloidal Aspects. In Encyclopedia of Surface and Colloid Science; Somasundaram, P., Ed.; Taylor & Francis: New York, NY, USA, 2007; Volume 1, pp. 1–18. [Google Scholar]
- Santos, R.G.; Loh, W.; Bannwart, A.; Trevisan, O. An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef]
- Ovalles, C.; Garcia, M.D.; Lujano, E.; Aular, W.; Bermudez, R.; Cotte, E. Structure-interfacial activity relationships and thermal stability studies of Cerro Negro crude oil and its acid, basic and neutral fractons. Fuel 1998, 77, 121–126. [Google Scholar] [CrossRef]
- Nenningsland, A.; Simon, S.; Sjoblom, J. Surface properties of basic components extracted from petroleum crude oil. Energy Fuels 2010, 24, 6501–6505. [Google Scholar] [CrossRef]
- Ramos, A.C.S.; Haraguchi, L.H.; Notrispe, F.R.; Loh, W.; Mohamed, R.S. Interfacial and colloidal behavior of asphaltenes obtained from Brazilian crude oils. J. Pet. Sci. Eng. 2001, 32, 201–216. [Google Scholar] [CrossRef]
- Sheu, E.Y. Petroleum asphaltenes—Properties, characterization, and issues. Energy Fuels 2002, 16, 74–82. [Google Scholar] [CrossRef]
- Stephenson, K. Producing asphaltenic crude oils: Problems and solutions. Pet. Eng. Int. 1990, 8, 24–31. [Google Scholar]
- De Boer, R.B.; Leerlooyer, K.; Eigner, M.R.P.; van Berger, A.R.D. Screening of crude oils for asphalt precipitation: Theory, practice and the selection of inhibitors. Soc. Pet. Eng. 1992, 24987, 259–270. [Google Scholar] [CrossRef]
- Mohamed, R.S.; Loh, W.; Ramos, A.C.S.; Delgado, C.C.; Almaeida, V.R. Reversibility and inhibition of asphaltene precipitation in Brazilian crude oils. Pet. Sci. Technol. 1999, 17, 877–896. [Google Scholar] [CrossRef]
- Kabbach, C.B.; Santos, R.G. Effects of pH and temperature on the phase behavior and properties of asphaltene liquid films. Energy Fuels 2017. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Santos, I.C.V.M.; Vieira, H.V.P.; Fraga, A.K.; Mansur, C.R.E. Interfacial rheology of asphaltene emulsions in the presence of nanoemulsions based on a polyoxide surfactant and asphaltene dispersant. Fuel 2017, 193, 220–229. [Google Scholar] [CrossRef]
- Langevin, D.; Poteau, S.; Hénaut, I.; Argillier, J.F. Crude oil emulsion properties and their application to heavy oil transportation. Oil Gas Sci. Technol. 2004, 59, 511–521. [Google Scholar] [CrossRef]
- Santos, R.G.; Bannwart, A.C.; Loh, W. Phase segregation, shear thinning and rheological behavior of crude oil-in-water emulsions. Chem. Eng. Res. Des. 2014, 92, 1629–1636. [Google Scholar] [CrossRef]
- Salager, J.L.; Briceño, M.I.; Brancho, C.L. Heavy hydrocarbon emulsions. In Encyclopedic Handbook of Emulsion Technology; Sjöblom, J., Ed.; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Santos, R.G.; Briceño, M.I.; Bannwart, A.C.; Loh, W. Physico-chemical properties of heavy crude oil-in-water emulsions stabilized by mixtures of ionic and non-ionic ethoxylated nonylphenol surfactants and medium chain alcohols. Chem. Eng. Res. Des. 2011, 89, 957–967. [Google Scholar] [CrossRef]
- Graciaa, A.; Lachaise, J.; Cucuphat, C.; Bourrel, M.; Salager, J.L. Improving solubilization in microemulsions with additives. 2. Long chain alcohols as lipophilic linkers. Langmuir 1993, 9, 3371–3374. [Google Scholar]
- Bourrel, M.; Chambu, C. The rules for achieving high solubilization of brine and oil by amphiphilic molecules. Soc. Pet. Eng. J. 1983, 23, 327–338. [Google Scholar] [CrossRef]
- Ross, S. The Change of Surface Tension with Time. I. Theories of Diffusion to the Surface. J. Am. Chem. Soc. 1945, 67, 990–994. [Google Scholar]
- Firooz, A.; Chen, P. Surface tension and adsorption kinetics of amphiphiles in aqueous solutions: The role of carbon chain length and temperature. J. Colloid Interface Sci. 2012, 370, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Nordli, K.G.; Sjoblom, J.; Kizling, J.; Stenius, P. Water-in-crude oil-emulsions from the Norwegian continental-shelf. 4. Monolayer properties of the interfacially active crude-oil fraction. Colloids Surf. 1991, 57, 83–98. [Google Scholar] [CrossRef]
- Poteau, S.; Argillier, J.; Langevin, D.; Pincet, F.; Perez, E. Influence of pH on Stability and dynamic properties of asphaltenes and other amphiphilic molecules at the oil-water interface. Energy Fuels 2005, 19, 1337–1341. [Google Scholar] [CrossRef]
- Vieira, V.C.C.; Severino, D.; Oliveira, O.N.; Pavinatto, F.J.; Zaniquelli, M.E.D.; Ramos, A.P.; Baptista, M.S. Langmuir films of petroleum at the air-water interface. Langmuir 2009, 25, 12585–12590. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.G.; Martins, L.S.; Santos, R.G. Effects of Short-Chain n-Alcohols on the Properties of Asphaltenes at Toluene/Air and Toluene/Water Interfaces. Colloids Interfaces 2018, 2, 13. https://doi.org/10.3390/colloids2020013
Martins RG, Martins LS, Santos RG. Effects of Short-Chain n-Alcohols on the Properties of Asphaltenes at Toluene/Air and Toluene/Water Interfaces. Colloids and Interfaces. 2018; 2(2):13. https://doi.org/10.3390/colloids2020013
Chicago/Turabian StyleMartins, Raphael G., Lilian S. Martins, and Ronaldo G. Santos. 2018. "Effects of Short-Chain n-Alcohols on the Properties of Asphaltenes at Toluene/Air and Toluene/Water Interfaces" Colloids and Interfaces 2, no. 2: 13. https://doi.org/10.3390/colloids2020013
APA StyleMartins, R. G., Martins, L. S., & Santos, R. G. (2018). Effects of Short-Chain n-Alcohols on the Properties of Asphaltenes at Toluene/Air and Toluene/Water Interfaces. Colloids and Interfaces, 2(2), 13. https://doi.org/10.3390/colloids2020013





