Decontamination of Uranium-Polluted Groundwater by Chemically-Enhanced, Sawdust-Activated Carbon
Abstract
:1. Introduction
2. Experiment
2.1. Chemicals and Apparatus
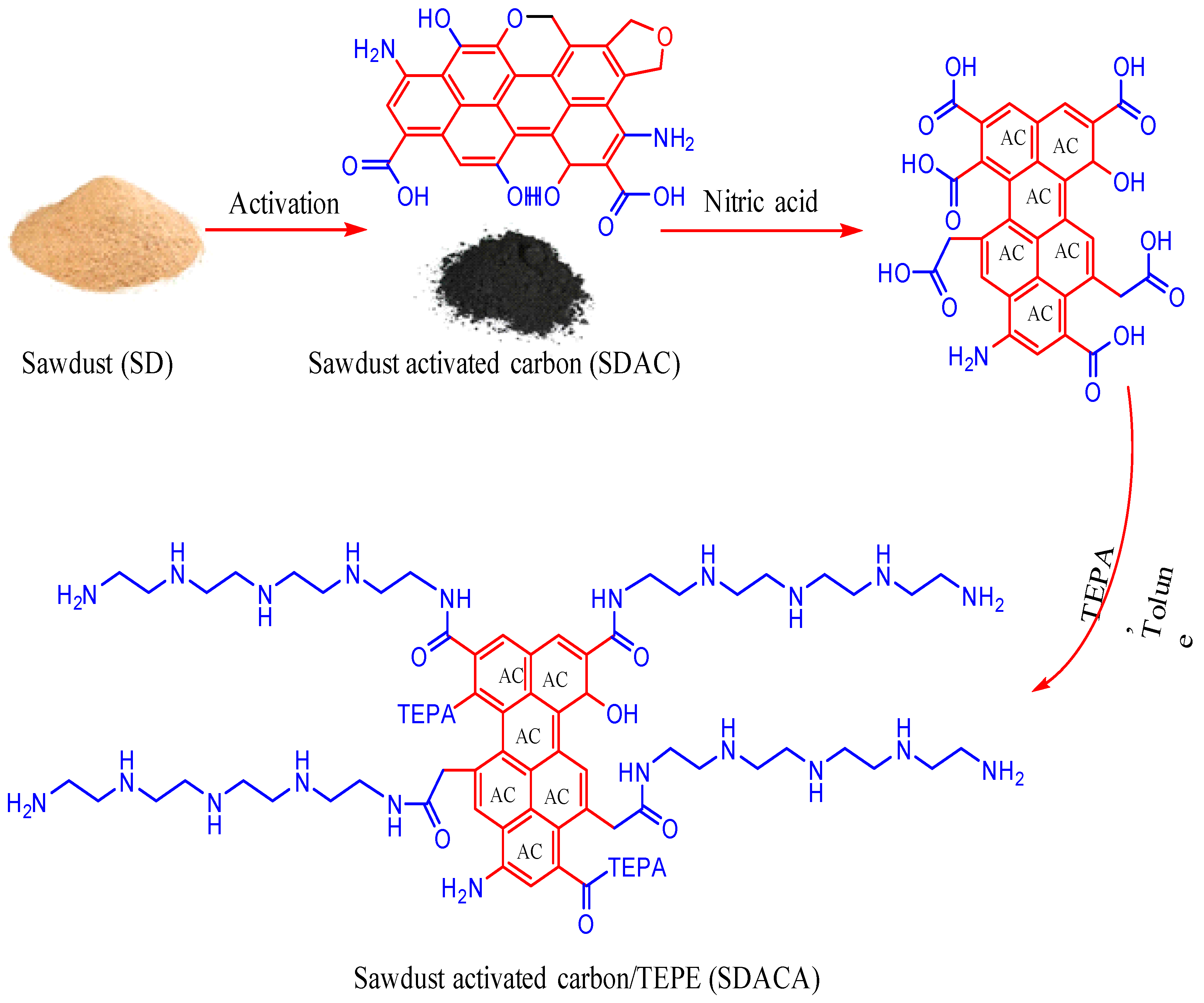
2.2. Preparation and Modification of Adsorbents
2.3. Adsorption Measurements Using the Batch Method
3. Results and Discussion
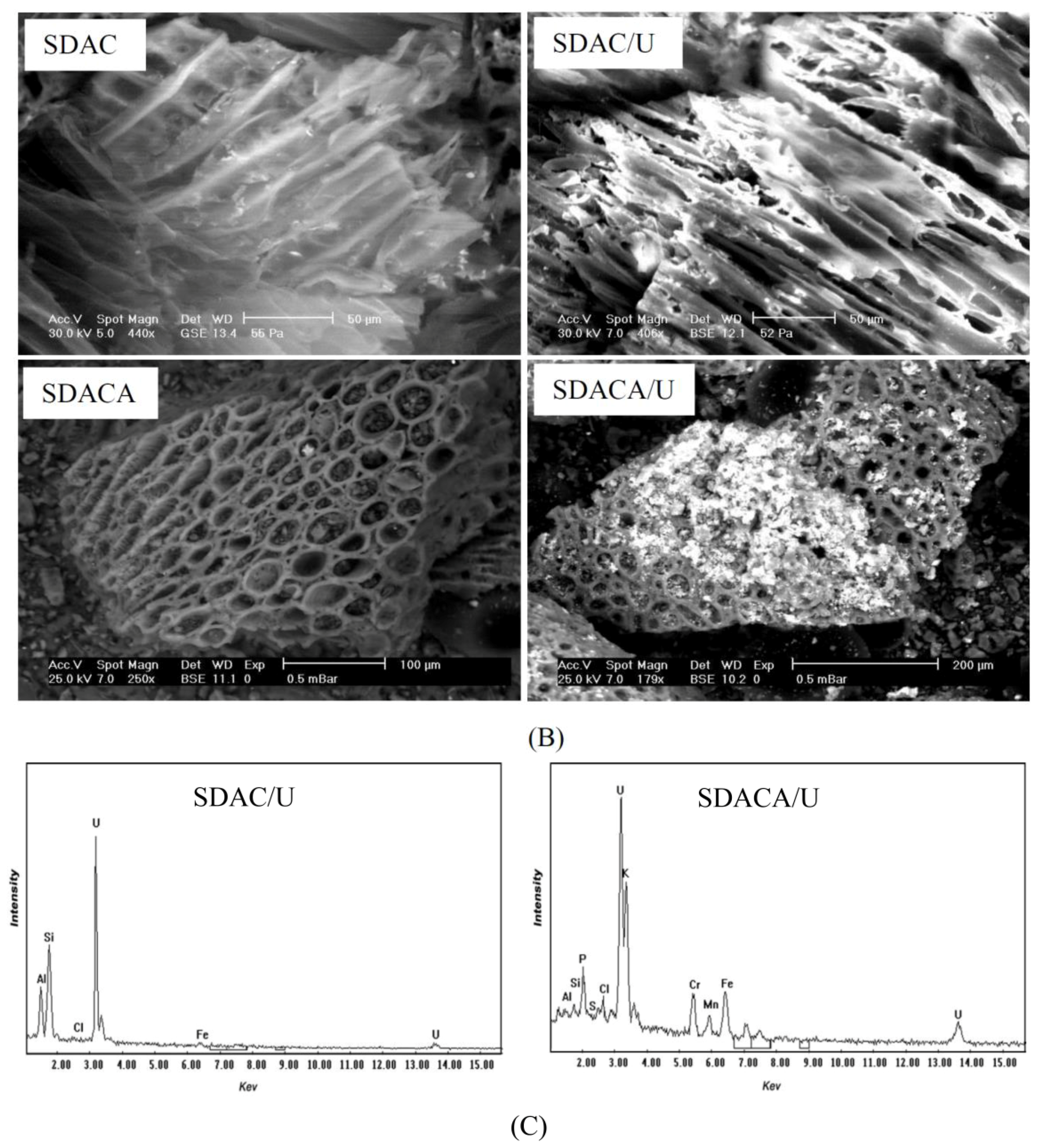
3.1. Adsorbent Structure and Characterization
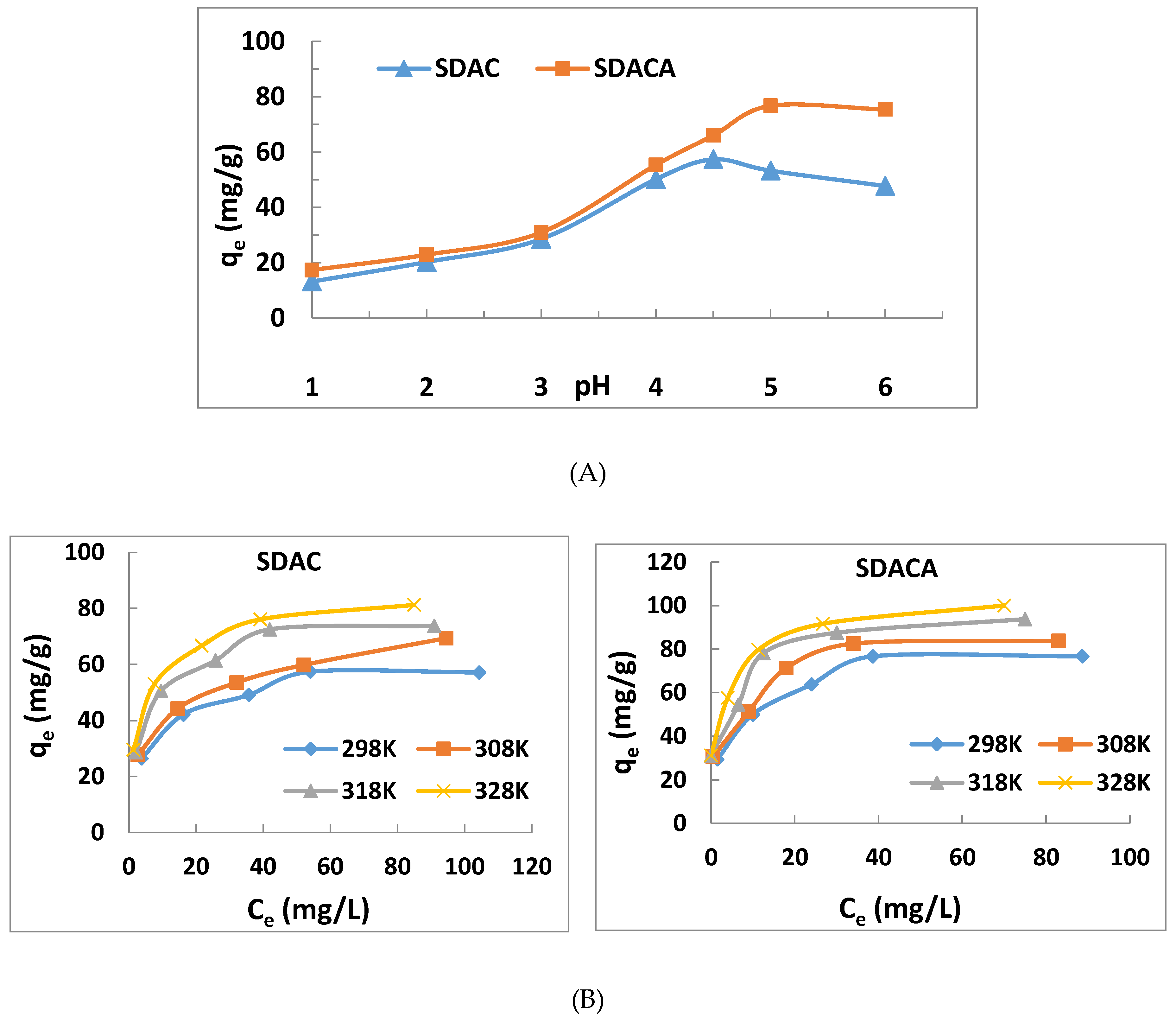
3.2. Effect of pH on U(VI) Ion Adsorption
3.3. Effect of Initial U(VI) Ion Concentration
3.4. Effect of Temperature
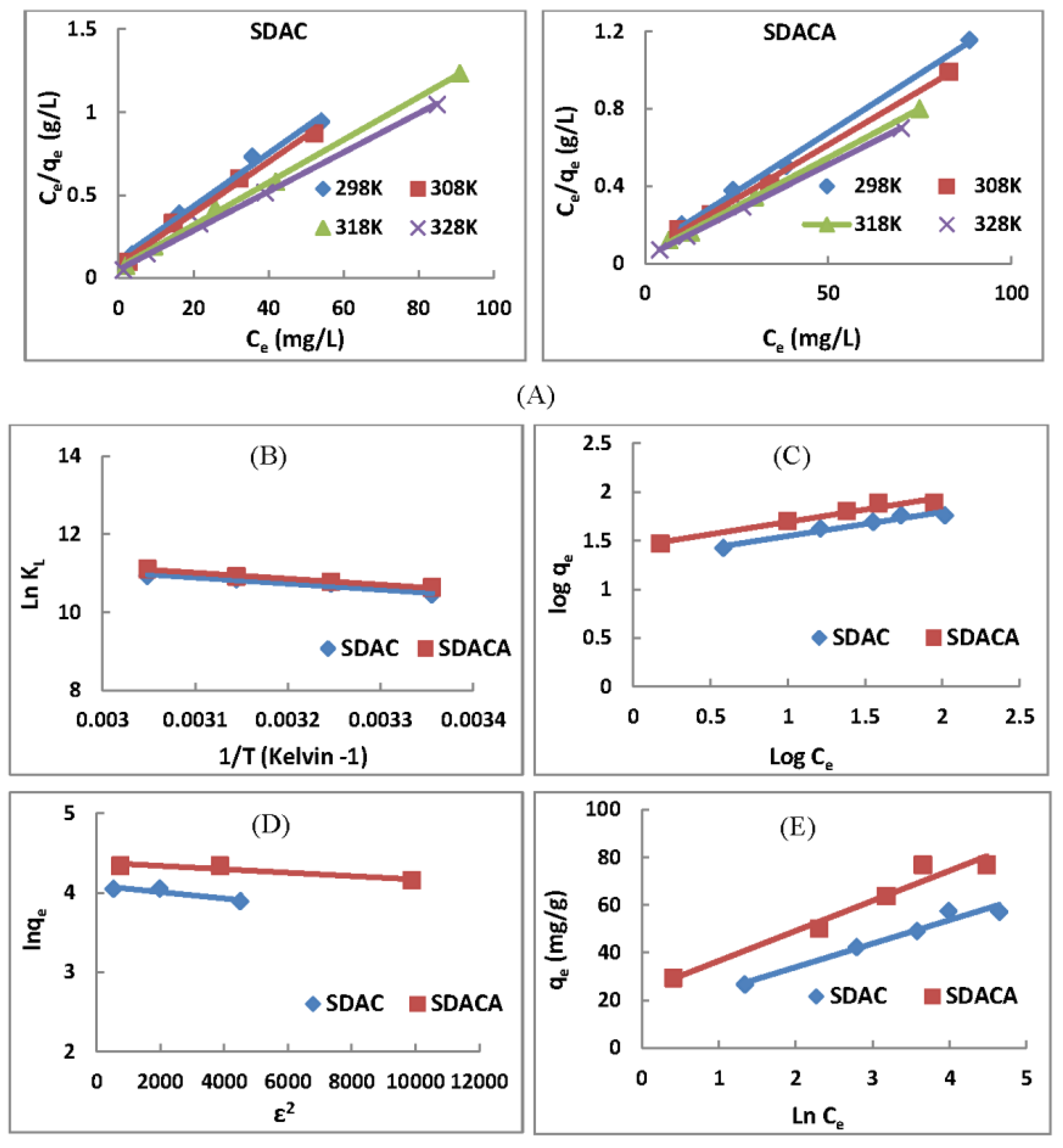
3.5. Adsorption Isotherms
3.5.1. Langmuir Isotherms
3.5.2. Freundlich Model
3.5.3. Dubinin–Radushkevich Isotherms
3.5.4. Temkin’s Model
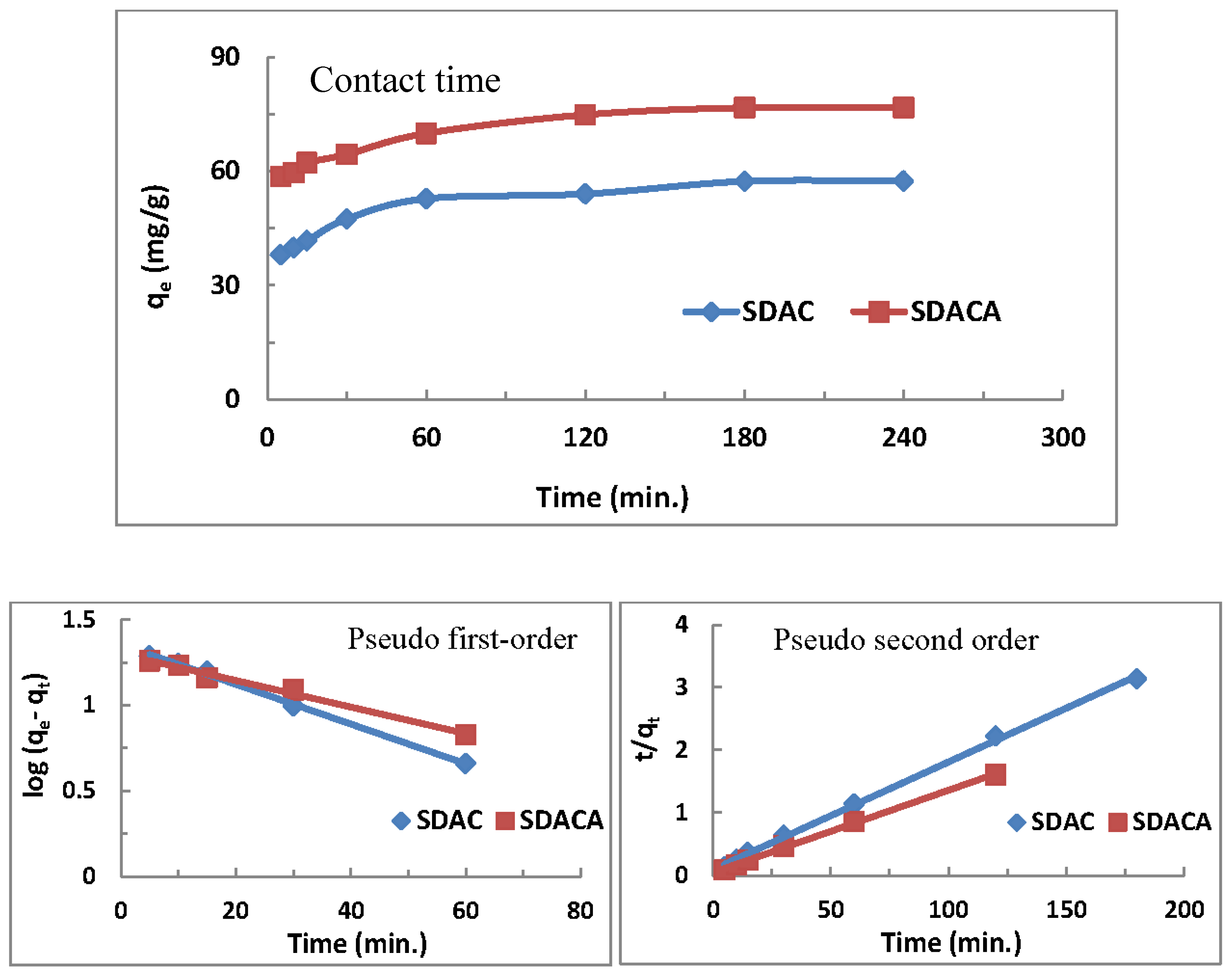
3.6. Effect of Time and Kinetic Studies
3.6.1. Pseudo-First-Order Model
3.6.2. Pseudo-Second-Order Model
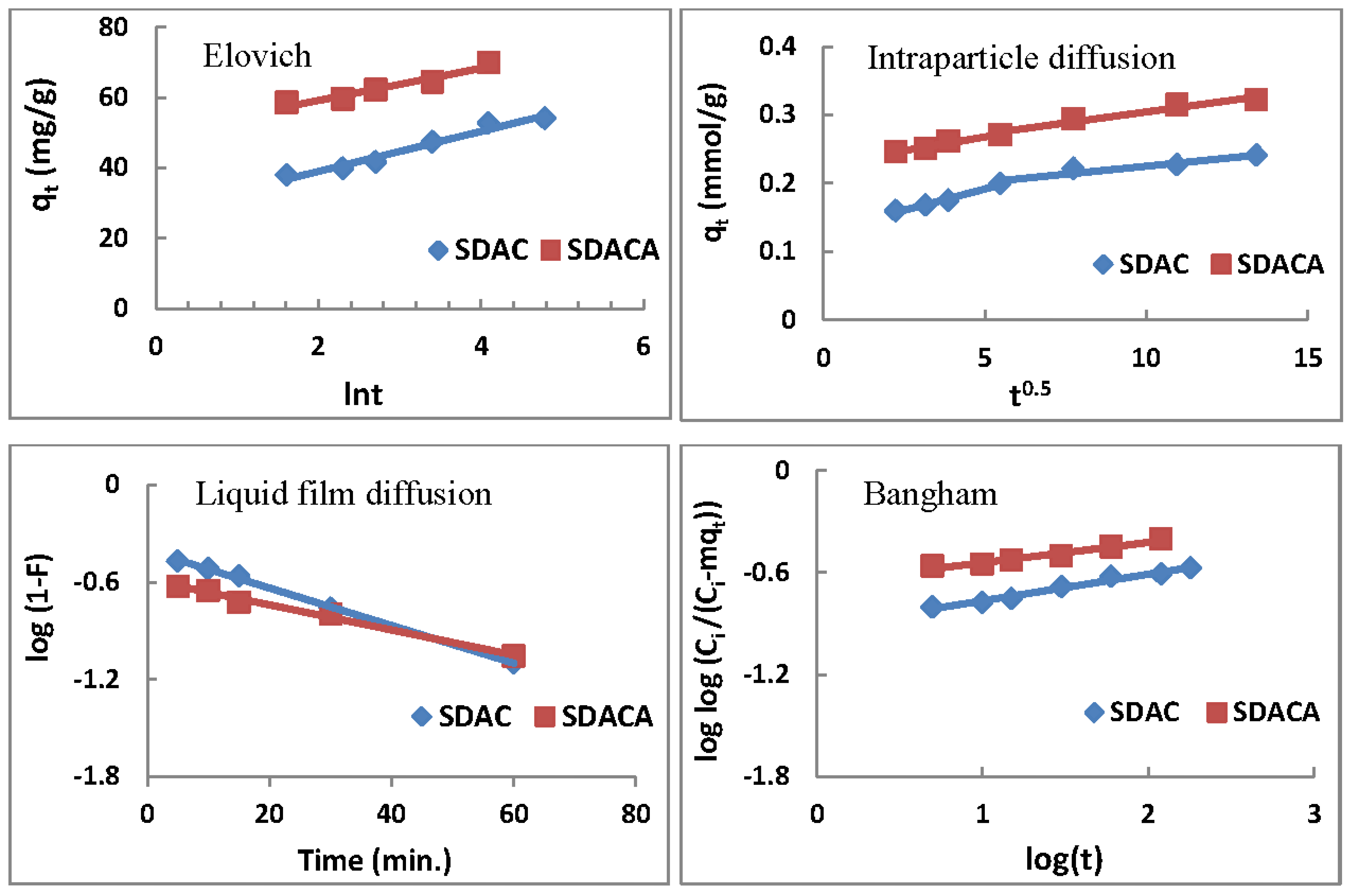
3.6.3. Elovich Equation
3.6.4. Intra-particle Diffusion Model
3.6.5. Liquid Film Diffusion Model
3.6.6. Bangham Model
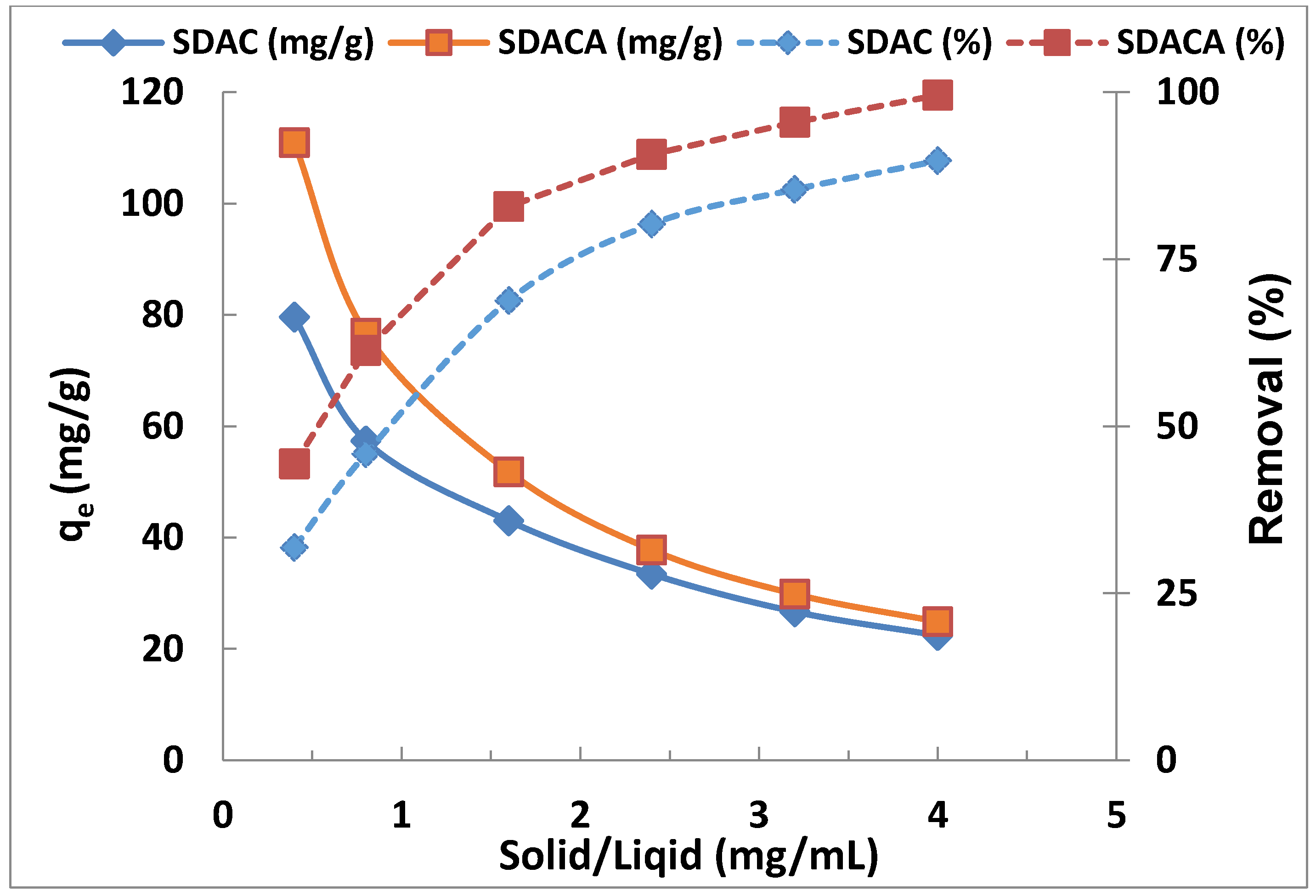
3.7. Effect of Adsorbent Dose
3.8. Adsorbent Regeneration
4. Application to Contaminated Groundwater
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Sincero, S.A.P.; Sincero, G.A. Physical-Chemical Treatment of Water and Wastewater; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Cheremisinoff, N.P. Handbook of Water and Wastewater Treatment Technologies; Butterworth-Heinemann: Oxford, UK, 2002. [Google Scholar]
- Ahuja, S. Handbook of Water Purity and Quality, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Sadeek, A.S.; Moussa, E.M.M.; El-Sayed, M.A.; Amine, M.M.; Abd El-Magied, M.O. Uranium(VI) and Thorium(IV) adsorption Studies on Chelating Resin Containing Pentaethylenehexamine as a Functional Group. J. Disper. Sci. Technol. 2014, 35, 926–933. [Google Scholar] [CrossRef]
- Merkel, B.J.; Friedrich, B.P. Groundwater Geochemistry; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1980. [Google Scholar]
- Donia, A.M.; Atia, A.A.; Moussa, E.M.M.; El-Sherif, A.M.; Abd El-Magied, M.O. Removal of Uranium(VI) from aqueous solutions using glycidyl methacrylate chelating adsorbents. Hydrometallurgy 2009, 95, 183–189. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I. Removal of uranium from contaminated drinking water: A mini review of available treatment methods. Desalin. Water Treat. 2013, 51, 2915–2925. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Althoff, H.W.; Bartel, H.; Jekel, M. The effect of groundwater composition on uranium(VI) sorption onto bacteriogenic iron oxides. Water Res. 2006, 40, 3646–3652. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Yang, P.; Huang, C.P. Preparation of graphene oxide-chitosan composite and adsorption performance for uranium. J. Radioanal. Nucl. Chem. 2017, 313, 371–378. [Google Scholar] [CrossRef]
- Chmielewska, E.; Tylus, W. Adsorption of Al(III), Sb(III), chromate and halides onto some natural versus commercial materials. J. Radioanal. Nucl. Chem. 2016, 308, 887–893. [Google Scholar] [CrossRef]
- Petrov, V.G.; Kuchinskaya, T.S.; Perfiliev, Y.D.; Dedushenko, S.K.; Kalmykov, S.N. Radionuclide removal from aqueous solutions using potassium ferrate(VI). J. Radioanal. Nucl. Chem. 2016, 310, 347–352. [Google Scholar] [CrossRef]
- Yi, Z.; Yuan, Z.; Yao, J.; Liu, X.; Zhu, M.J.; Chen, H.L.; Wang, F. Batch study of uranium biosorption by Elodea canadensis biomass. J. Radioanal. Nucl. Chem. 2016, 310, 505–513. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Ma, J.; Cui, Y.; Sun, G. Extraction and separation of uranium(VI) and Fe(III) from lanthanide chlorides with N,N,N,N-tetra substituted alkyl diglycolamide extractants. J. Radioanal. Nucl. Chem. 2017, 313, 327–332. [Google Scholar] [CrossRef]
- Yakout, S.M.; Metwally, S.S.; El-Zakla, T. Uranium sorption onto activated carbon prepared from rice straw: Competition with humic acids. Appl. Surface Sci. 2013, 280, 745–750. [Google Scholar] [CrossRef]
- Taskaev, E.; Apostolov, D. On uranium(VI) adsorption on activated carbon. J. Radio Anal. Chem. 1978, 45, 65–71. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Sharaby, C.M.; El Gammal, E.M. Uranium extraction from sulfuric acid medium using trioctylamine impregnated activated carbon. Hydrometallurgy 2013, 134–135, 150–157. [Google Scholar] [CrossRef]
- Rodrıguez, R.V.; Cabrera, M.E.M.; Ponce, H.E.E.; Peraza, E.F.H.; Casarrubias, M.L.B. Uranium removal from water using cellulose triacetate membranes added with activated carbon. Appl. Radiat. Isot. 2012, 70, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Afsari, M.; Safdari, J.; Towfighi, J.; Mallah, M.H. The adsorption characteristics of uranium hexafluoride onto activated carbon in vacuum conditions. Ann. Nucl. Energy 2012, 46, 144–151. [Google Scholar] [CrossRef]
- Saleha, T.A.; Naeemullahb; Tuzenb, M.; Sarı, A. Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chem. Eng. Res. Des. 2017, 117, 218–227. [Google Scholar] [CrossRef]
- Gad, H.M.H.; Mohammaden, T.F.; Mahmoud, M.A. Solid phase extractive pre-concentration of uranium(VI) from liquid waste onto peach stone steam pyrolysis activated carbon. Asian J. Chem. 2013, 28, 751–760. [Google Scholar] [CrossRef]
- Zhu, M.; Yi, Z.; Liu, R.; Chai, H.; Yao, J.; Chen, Y. Hazelnut shell activated carbon: A potential adsorbent material for the decontamination of uranium(VI) from aqueous solutions. J. Radioanal. Nucl. Chem. 2016, 310, 1147–1154. [Google Scholar] [CrossRef]
- Zheng, Z.; Cao, X.; Zhang, Z.; Wang, Y.; Dai, Y.; Liu, Y.; Zhao, W.; Le, Z.; Xiong, G.; Yu, S. Adsorptive removal of uranyl ions in aqueous solution using hydrothermal carbon spheres functionalized with 4-aminoacetophenone oxime group. J. Radioanal. Nucl. Chem. 2017, 312, 187–198. [Google Scholar] [CrossRef]
- Menacer, S.; Lounis, A.; Guedioura, B.; Bayou, N. Uranium removal from aqueous solutions by adsorption on Aleppo pine sawdust, modified by NaOH and neutron irradiation. Desalin. Water Treat. 2015, 57, 1–12. [Google Scholar] [CrossRef]
- Sadeek, A.S.; El-Sayed, M.A.; Amine, M.M.; Abd, E.L.; Magied, M.O. A chelating resin containing trihydroxybenzoic acid as the functional group; synthesis and adsorption behavior for Th(IV) and U(VI) ions. J. Radioanal. Nucl. Chem. 2014, 299, 1299–1306. [Google Scholar] [CrossRef]
- Abd El-Magied, M.O.; Tolba, A.A.; El-Gendy, H.S.; Zaki, S.A.; Atia, A.A. Studies on the recovery of Th(IV) ions from nitric acid solutions using amino-magnetic glycidyl methacrylate adsorbents and application to granite leach liquors. Hydrometallurgy 2017, 169, 89–98. [Google Scholar] [CrossRef]
- Abd El-Magied, M.O. Sorption of uranium ions from their aqueous solution by adsorbents containing nanomagnetite particles. J. Eng. 2016, 2016, 7214348. [Google Scholar] [CrossRef]
- Abd El-Magied, M.O.; Elshehy, E.A.; Manaa, E.A.; Tolba, A.A.; Atia, A.A. Kinetics and thermodynamics studies on the recovery of thorium ions using amino adsorbents with magnetic properties. Ind. Eng. Chem. Res. 2016, 55, 11338–11345. [Google Scholar] [CrossRef]
- El Nagdy, M.S.; Ali, B.H.; El Wakil, A.F.; Gaber, K.A. Effect of physical parameters in measuring some poisonous and radionuclides in groundwater using ICP-OES: A case study in Southwestern Sinai, Egypt. Arab J. Nucl. Sci. Appl. 2016, 94, 74–84. [Google Scholar]
- Hamza, M.F.; El Aassy, I.E. Solid phase extraction of uranium removal from underground water, Wadi Naseib, Southwestern Sinai, Egypt. Desalin. Water Treat. 2014, 52, 331–338. [Google Scholar] [CrossRef]



| Isotherms Model | Parameters | (SDAC) | (SDACA) | Temperature |
|---|---|---|---|---|
| Langmuir isotherm | qmax, (mg/g) | 62.50 | 82.645 | 298 K |
| 64.516 | 89.286 | 308 K | ||
| 77.519 | 99.0099 | 318 K | ||
| 84.746 | 105.263 | 328 K | ||
| KL, (L/mg) | 0.144 | 0.175 | 298 K | |
| 0.191 | 0.20 | 308 K | ||
| 0.214 | 0.231 | 318 K | ||
| 0.225 | 0.281 | 328 K | ||
| R2 | 0.989 | 0.9969 | 298 K | |
| Freundlich isotherm | KF, (mg/g) | 20.091 | 27.568 | 298 K |
| N | 4.085 | 3.957 | ||
| R2 | 0.9503 | 0.9612 | ||
| D-R isotherm | qmax, (mg/g) | 59.806 | 80.0698 | 298 K |
| Kad, (mol2/kJ2) | 4.00 × 10−8 | 2.00 × 10−8 | ||
| E, (kJ/mol) | 3.536 | 5.00 | ||
| R2 | 0.8513 | 0.8868 | ||
| Temkin isotherm | β (kJ/mol) | 9.877 | 12.60 | 298 K |
| AT (L/g) | 4.177 | 6.690 | ||
| B | 250.838 | 196.586 | ||
| R2 | 0.9628 | 0.9577 |
| Adsorbents | Temp. (Kelvin) | Thermodynamic Parameters | |||
|---|---|---|---|---|---|
| ∆H° (kJ/mol) | ∆S° (KJ/(mol·K)) | T∆S° (kJ/mol) | ∆G° (kJ/mol) | ||
| SDAC | 298 | 12.93 | 0.131 | 38.93 | −5.99 |
| 308 | 40.23 | −27.30 | |||
| 318 | 41.54 | −28.61 | |||
| 328 | 42.84 | −29.91 | |||
| SDACA | 298 | 12.73 | 0.131 | 304 | −26.31 |
| 308 | 40.35 | −27.62 | |||
| 318 | 41.66 | −28.93 | |||
| 328 | 42.97 | −30.24 | |||
| Kinetic Model | Model Parameters | (SDAC) | (SDACA) |
|---|---|---|---|
| Pseudo-first-order kinetics | q1st, (mg/g) | 22.6308 | 19.9388 |
| k1, (min−1) | 0.0267 | 0.0177 | |
| R2 | 0.9984 | 0.9909 | |
| Pseudo-second-order kinetics | q2nd, (mg/g) | 58.1395 | 76.3358 |
| K2, (g/(mg·min)) | 0.0031 | 0.0038 | |
| R2 | 0.9987 | 0.9986 | |
| Elovich kinetic model | β (g/mg) | 0.1758 | 0.2191 |
| α (mg/(g·min)) | 744.2934 | 266,639.15 | |
| R2 | 0.9644 | 0.9387 | |
| Intraparticle diffusion model | Kip, (mg/(g·min0.5)) | 0.0047 | 0.0065 |
| I | 0.1778 | 0.2397 | |
| R2 | 0.9109 | 0.9563 | |
| Liquid film diffusion model | Kfd | 0.0267 | 0.0177 |
| R2 | 0.9984 | 0.9909 | |
| Bangham kinetic model | Kb (mL/g/L) | 55.1345 | 100.5596 |
| Α | 0.1556 | 0.119 | |
| R2 | 0.9767 | 0.9595 |
| Constituents | Concentration, mg/L | Constituents | Concentration, mg/L |
|---|---|---|---|
| pH | 7.2 | U | 1.8 |
| TDS | 2810 | Cu | 0.018 |
| Ca2+ | 343 | Co | 0.022 |
| Mg2+ | 277 | Cr | 0.004 |
| Na+ | 314 | As | 0.057 |
| K+ | 35 | Zr | 0.036 |
| SO42− | 452 | Cd | 0.008 |
| CO32− | 510 | Pb | 0.091 |
| Cl− | 370 | Fe | 2.067 |
| Adsorbents | Elements | Permissible Levels (mg/L) | Well-1 | Well-2 |
|---|---|---|---|---|
| SDAC | U | 0.03–0.015 | 0.01 | 0.006 |
| Ni | 0.1 | 0.09 | - | |
| Cu | 1.0 | 0.005 | - | |
| Co | 0.002 | 0.003 | - | |
| Cr | 0.1 | - | - | |
| As | 0.01 | 0.011 | 0.004 | |
| Cd | 0.005 | - | - | |
| Pb | 0.015 | - | - | |
| Fe | 0.3 | 0.30 | 0.05 | |
| SDACA | U | 0.03–0.015 | - | - |
| Ni | 0.1 | - | - | |
| Cu | 1.0 | - | - | |
| Co | 0.002 | - | - | |
| Cr | 0.1 | - | - | |
| As | 0.01 | - | - | |
| Cd | 0.005 | - | - | |
| Pb | 0.015 | - | - | |
| Fe | 0.3 | 0.04 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Magied, M.O.A.; Mohammaden, T.F.; El-Aassy, I.K.; Gad, H.M.H.; Hassan, A.M.; Mahmoud, M.A. Decontamination of Uranium-Polluted Groundwater by Chemically-Enhanced, Sawdust-Activated Carbon. Colloids Interfaces 2017, 1, 2. https://doi.org/10.3390/colloids1010002
El-Magied MOA, Mohammaden TF, El-Aassy IK, Gad HMH, Hassan AM, Mahmoud MA. Decontamination of Uranium-Polluted Groundwater by Chemically-Enhanced, Sawdust-Activated Carbon. Colloids and Interfaces. 2017; 1(1):2. https://doi.org/10.3390/colloids1010002
Chicago/Turabian StyleEl-Magied, Mahmoud O. Abd, Tarek F. Mohammaden, Ibrahim K. El-Aassy, Hamdi M. H. Gad, Ali M. Hassan, and Mohamed A. Mahmoud. 2017. "Decontamination of Uranium-Polluted Groundwater by Chemically-Enhanced, Sawdust-Activated Carbon" Colloids and Interfaces 1, no. 1: 2. https://doi.org/10.3390/colloids1010002
APA StyleEl-Magied, M. O. A., Mohammaden, T. F., El-Aassy, I. K., Gad, H. M. H., Hassan, A. M., & Mahmoud, M. A. (2017). Decontamination of Uranium-Polluted Groundwater by Chemically-Enhanced, Sawdust-Activated Carbon. Colloids and Interfaces, 1(1), 2. https://doi.org/10.3390/colloids1010002






