A Review of the Role of Machine Learning Techniques towards Brain–Computer Interface Applications
Abstract
:1. Introduction
1.1. Motivation
1.2. Paper Organization
1.3. Contributions of the Study
- To explore the role of ML in BCI applications.
- To determine the advantages of employing ML in various BCI tasks such as motor imagery classification, ERP signal classification, emotional and mental state detection, and several others.
- To study the diverse feature extraction, selection, and classification methods exploited for BCI applications.
2. Literature Survey
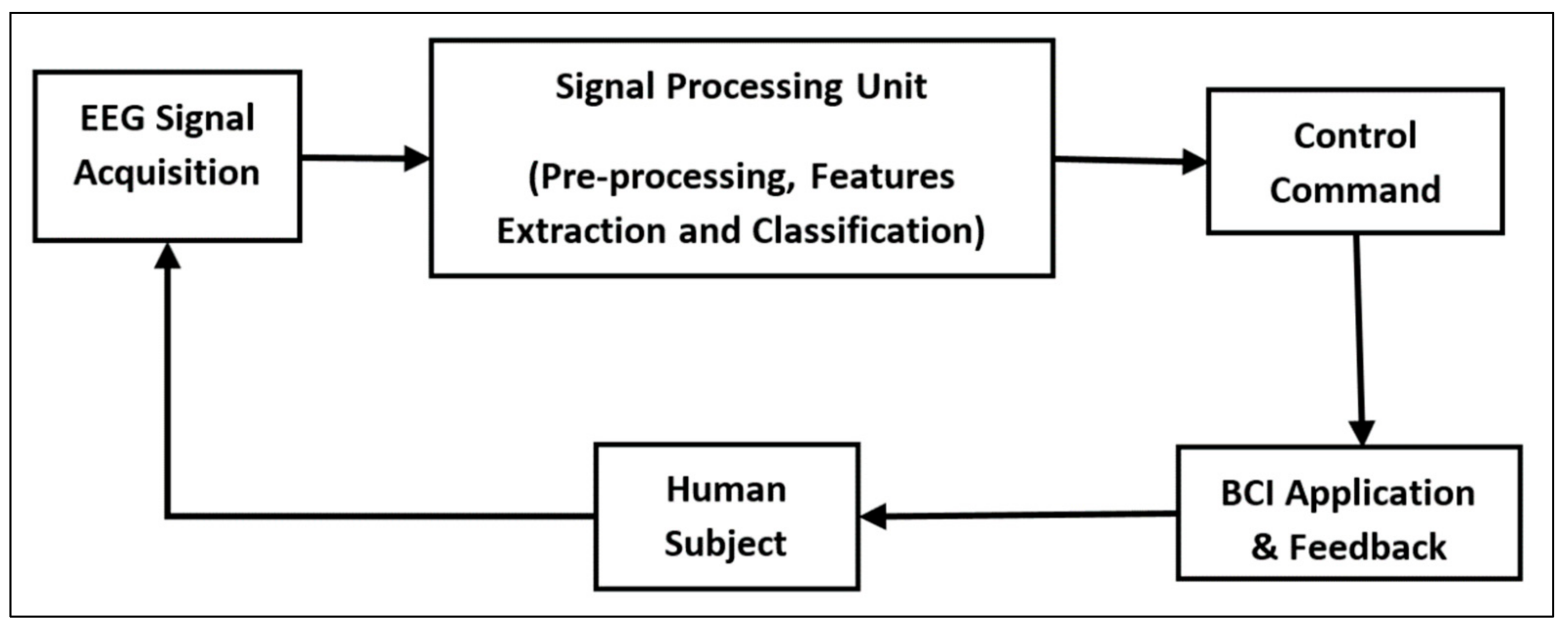
3. Role of ML in BCI
4. Methods
4.1. Feature Extraction and Selection
4.1.1. Common Spatial Pattern (CSP)
4.1.2. Principal Component Analysis (PCA)
4.1.3. Independent Component Analysis (ICA)
4.1.4. Autoregressive (AR) Method
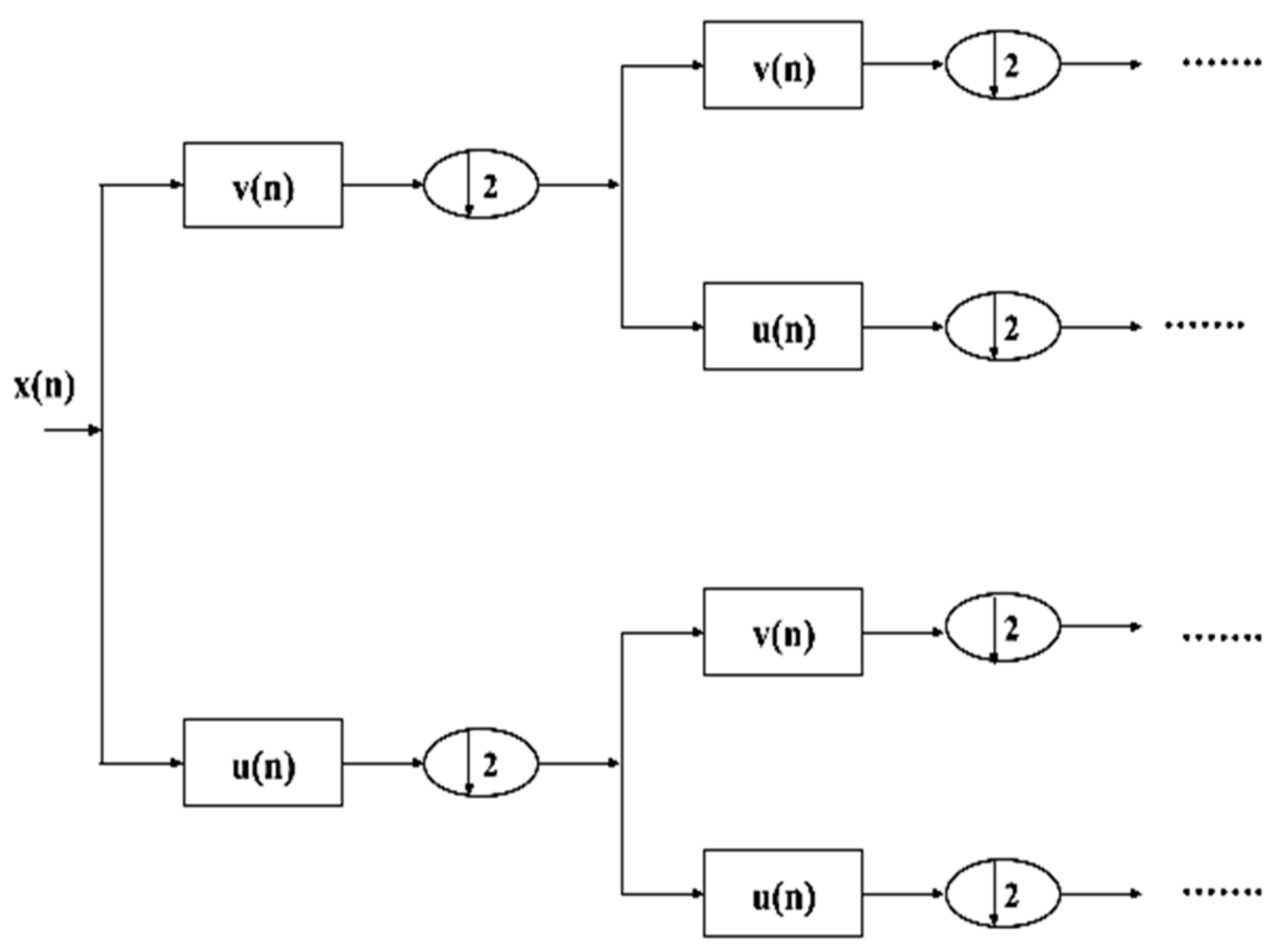
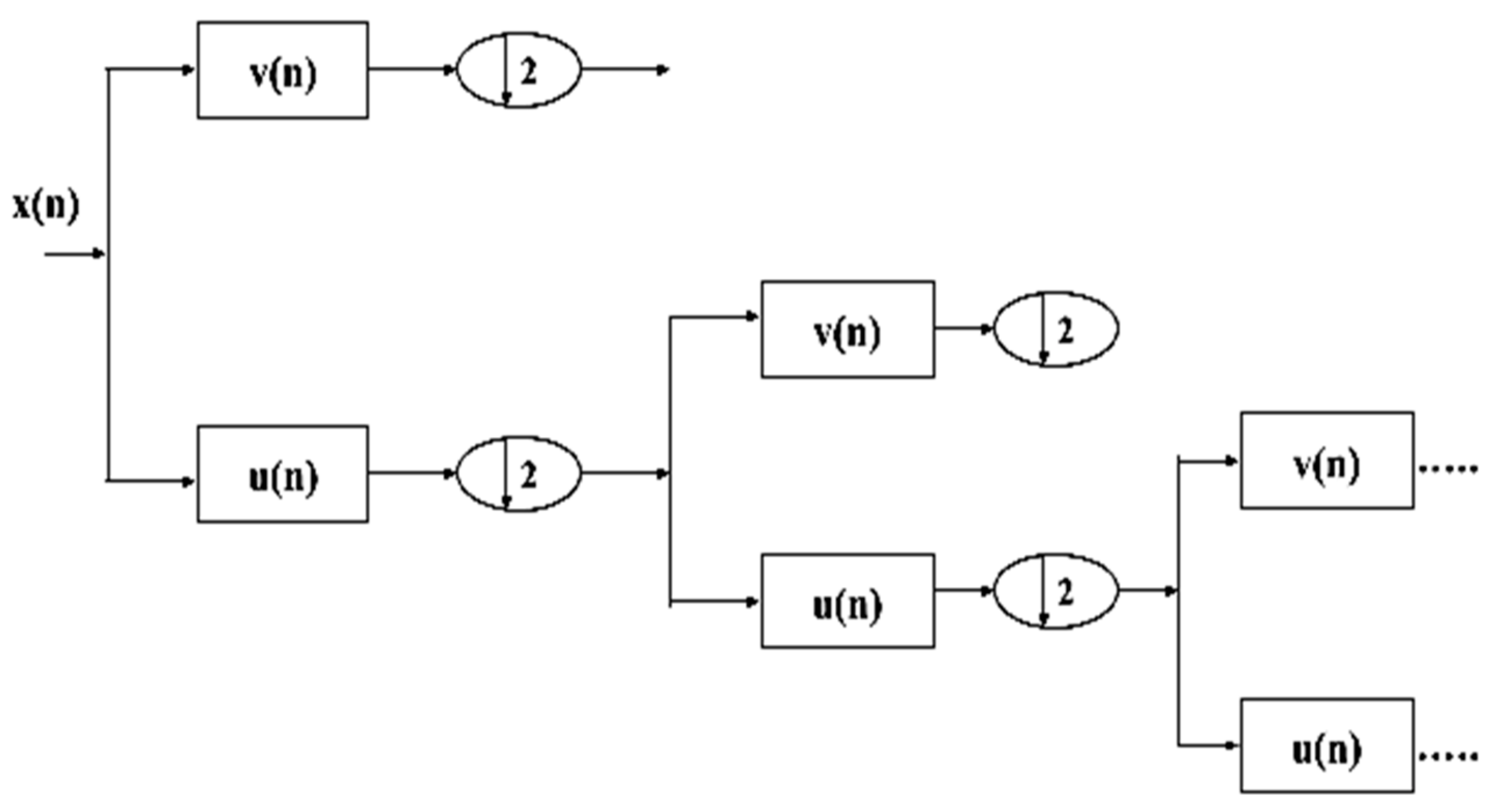
4.1.5. Wavelet Packet Decomposition (WPD)
4.1.6. Wavelet Transform (WT)
4.1.7. Fast Fourier Transform (FFT)
4.1.8. Other Techniques
4.2. Classification
4.2.1. K-Nearest Neighbor (KNN)
4.2.2. Linear Discriminant Analysis (LDA)
4.2.3. Naive Bayes
4.2.4. Extreme Learning Machine (ELM)
4.2.5. Support Vector Machine (SVM)
4.2.6. Neural Networks (NNs)
- Multilayer Perceptron (MLP)
- B.
- Artificial Neural Networks (ANN)
- C.
- Convolutional Neural Networks (CNN)
4.2.7. Other Techniques
4.3. Comparative Study
4.4. Findings of the Research
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McFarland, D.J.; Wolpaw, J.R. Brain-computer interfaces for communication and control. Commun. ACM 2011, 54, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain computer interfaces, a review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Qiao, L.; Wang, Q.; Piccialli, F. Advanced Machine-Learning Methods for Brain-Computer Interfacing. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020, 18, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Chaudhary, U.; Birbaumer, N.; Ramos-Murguialday, A. Brain–computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 2016, 12, 513–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarou, I.; Nikolopoulos, S.; Petrantonakis, P.C.; Kompatsiaris, I.; Tsolaki, M. EEG-Based Brain-Computer Interfaces for Communication and Rehabilitation of People with Motor Impairment: A Novel Approach of the 21st Century. Front. Hum. Neurosci. 2018, 12, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, H. Efficient machine learning algorithm for electroencephalogram modeling in brain–computer interfaces. Neural Comput. Appl. 2020, 1–11. Available online: https://link.springer.com/article/10.1007/s00521-020-04861-3#citeas (accessed on 10 August 2021).
- Mak, J.N.; Arbel, Y.; Minett, J.W.; McCane, L.M.; Yuksel, B.; Ryan, D.; Erdogmus, D. Optimizing the P300-based brain–computer interface: Current status, limitations and future directions. J. Neural Eng. 2011, 8, 025003. [Google Scholar] [CrossRef]
- Millán, J.d.R.; Rupp, R.; Müller-Putz, G.; Murray-Smith, R.; Giugliemma, C.; Tangermann, M.; Leeb, R. Combining Brain-Computer Interfaces and Assistive Technologies: State-of-the-Art and Challenges. Front. Neurosci. 2010, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-J.; Kim, S.; Choi, S.; Im, C.-H. EEG-Based Brain-Computer Interfaces: A Thorough Literature Survey. Int. J. Hum. Comput. Interact. 2013, 29, 814–826. [Google Scholar] [CrossRef]
- Gürkök, H.; Nijholt, A. Brain-Computer Interfaces for Multimodal Interaction: A Survey and Principles. Int. J. Hum. Comput. Interact. 2012, 28, 292–307. [Google Scholar] [CrossRef]
- Abdulkader, S.N.; Atia, A.; Mostafa, M.S.M. Brain computer interfacing: Applications and challenges. Egypt. Inform. J. 2015, 16, 213–230. [Google Scholar] [CrossRef] [Green Version]
- Lotte, F.; Congedo, M.; Lécuyer, A.; Lamarche, F.; Arnaldi, B. A review of classification algorithms for EEG-based brain–computer interfaces. J. Neural Eng. 2007, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Abibullaev, B.; Zollanvari, A. Learning discriminative spatiospectral features of ERPs for accurate brain–computer interfaces. IEEE J. Biomed. Health Inform. 2019, 23, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.R.; Tangermann, M.; Dornhege, G.; Krauledat, M.; Curio, G.; Blankertz, B. Machine learning for real-time single-trial EEG-analysis: From brain–computer interfacing to mental state monitoring. J. Neurosci. Methods 2008, 167, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lotte, F.; Bougrain, L.; Cichocki, A.; Clerc, M.; Congedo, M.; Rakotomamonjy, A.; Yger, F. A review of classification algorithms for EEG-based brain–computer interfaces: A 10 year update. J. Neural Eng. 2018, 15, 031005. [Google Scholar] [CrossRef] [Green Version]
- Fouad, M.M.; Amin, K.M.; El-Bendary, N.; Hassanien, A.E. Brain computer interface: A review. Brain-Comput. Interfaces 2015, 74, 3–30. [Google Scholar]
- Ramadan, R.A.; Refat, S.; Elshahed, M.A.; Ali, R.A. Basics of brain computer interface. In Brain-Computer Interfaces; Springer: Cham, Germany, 2015; pp. 31–50. [Google Scholar]
- Combaz, A.; Chumerin, N.; Manyakov, N.V.; Robben, A.; Suykens, J.A.; Van Hulle, M.M. Towards the detection of error-related potentials and its integration in the context of a P300 speller brain–computer interface. Neurocomputing 2012, 80, 73–82. [Google Scholar] [CrossRef]
- Padfield, N.; Zabalza, J.; Zhao, H.; Masero, V.; Ren, J. EEG-Based Brain-Computer Interfaces Using Motor-Imagery: Techniques and Challenges. Sensors 2019, 19, 1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blankertz, B.; Tangermann, M.; Vidaurre, C.; Dickhaus, T.; Sannelli, C.; Popescu, F.; Müller, K.R. Detecting mental states by machine learning techniques: The Berlin brain–computer interface. In Brain-Computer Interfaces; Springer: Berlin/Heidelberg, Germany, 2009; pp. 113–135. [Google Scholar]
- Torres, E.P.; Torres, E.A.; Hernández-Álvarez, M.; Yoo, S.G. EEG-Based BCI Emotion Recognition: A Survey. Sensors 2020, 20, 5083. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Gu, X.; Xu, K.; Ren, H.; Chen, W. Independent decision path fusion for bimodal asynchronous brain–computer interface to discriminate multiclass mental states. IEEE Access 2019, 7, 165303–165317. [Google Scholar] [CrossRef]
- Mohanty, R.; Sinha, A.M.; Remsik, A.B.; Dodd, K.C.; Young, B.M.; Jacobson, T.; Prabhakaran, V. Machine learning classification to identify the stage of brain-computer interface therapy for stroke rehabilitation using functional connectivity. Front. Neurosci. 2018, 12, 353. [Google Scholar] [CrossRef]
- Bablani, A.; Edla, D.R.; Tripathi, D.; Cheruku, R. Survey on Brain-Computer Interface: An Emerging Computational Intelligence Paradigm. ACM Comput. Surv. 2019, 52, 1–32. [Google Scholar] [CrossRef]
- Chakladar, D.D.; Chakraborty, S. Feature extraction and classification in brain-computer interfacing: Future research issues and challenges. In Natural Computing for Unsupervised Learning; Springer: Cham, Germany, 2019; pp. 101–131. [Google Scholar]
- Salazar-Varas, R.; Gutiérrez, D. An optimized feature selection and classification method for using electroencephalographic coherence in brain–computer interfaces. Biomed. Signal Process. Control. 2015, 18, 11–18. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, C. Adaptive feature extraction for EEG signal classification. Med. Biol. Eng. Comput. 2006, 44, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Garrett, D.; Peterson, D.A.; Anderson, C.W.; Thaut, M.H. Comparison of linear, nonlinear, and feature selection methods for EEG signal classification. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Ahangi, A.; Karamnejad, M.; Mohammadi, N.; Ebrahimpour, R.; Bagheri, N. Multiple classifier system for EEG signal classification with application to brain–computer interfaces. Neural Comput. Appl. 2013, 23, 1319–1327. [Google Scholar] [CrossRef]
- Mirzaei, S.; Ghasemi, P. EEG motor imagery classification using dynamic connectivity patterns and convolutional autoencoder. Biomed. Signal Process. Control. 2021, 68, 102584. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Z.; Gu, Z.; Li, Y. Deep Temporal-Spatial Feature Learning for Motor Imagery-Based Brain–Computer Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2356–2366. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, X.; Zhang, H.; Bai, R.; Li, T. EEG Motor Imagery Classification with Sparse Spectrotemporal Decomposition and Deep Learning. IEEE Trans. Autom. Sci. Eng. 2020, 18, 541–551. [Google Scholar] [CrossRef]
- Burke, D.P.; Kelly, S.P.; De Chazal, P.; Reilly, R.B.; Finucane, C. A parametric feature extraction and classification strategy for brain-computer interfacing. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majidov, I.; Whangbo, T. Efficient Classification of Motor Imagery Electroencephalography Signals Using Deep Learning Methods. Sensors 2019, 19, 1736. [Google Scholar] [CrossRef] [Green Version]
- Alomari, M.H.; Samaha, A.; AlKamha, K. Automated Classification of L/R Hand Movement EEG Signals using Advanced Feature Extraction and Machine Learning. Int. J. Adv. Comput. Sci. Appl. 2013, 4, 207–212. [Google Scholar]
- Atmaji, C.; Putra, A.E.; Tontowi, I.A. Three-Class Classification of EEG Signals Using Support Vector Machine Methods. In Proceedings of the 2018 4th International Conference on Science and Technology (ICST), Yogyakarta, Indonesia, 7–8 August 2018; pp. 1–4. [Google Scholar]
- Chaudhary, S.; Taran, S.; Bajaj, V.; Sengur, A. Convolutional Neural Network Based Approach Towards Motor Imagery Tasks EEG Signals Classification. IEEE Sens. J. 2019, 19, 4494–4500. [Google Scholar] [CrossRef]
- Jafarifarmand, A.; Badamchizadeh, M.A.; Khanmohammadi, S.; Nazari, M.A.; Tazehkand, B.M. A New Self-Regulated Neuro-Fuzzy Framework for Classification of EEG Signals in Motor Imagery BCI. IEEE Trans. Fuzzy Syst. 2018, 26, 1485–1497. [Google Scholar] [CrossRef]
- Atkinson, J.; Campos, D. Improving BCI-based emotion recognition by combining EEG feature selection and kernel classifiers. Expert Syst. Appl. 2016, 47, 35–41. [Google Scholar] [CrossRef]
- Hsu, W.Y. Continuous EEG signal analysis for asynchronous BCI application. Int. J. Neural Syst. 2011, 21, 335–350. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Devipriya, A.; Maniraj, J.; Sivaram, M.; Ambikapathy, A.; Iraj, S.A. A novel method of motor imagery classification using eeg signal. Artif. Intell. Med. 2020, 103, 101787. [Google Scholar]
- Sreeja, S.R.; Rabha, J.; Nagarjuna, K.Y.; Samanta, D.; Mitra, P.; Sarma, M. Motor imagery EEG signal processing and classification using machine learning approach. In Proceedings of the 2017 International Conference on New Trends in Computing Sciences (ICTCS), Amman, Jordan, 11–13 October 2017; pp. 61–66. [Google Scholar]
- El Bahy, M.M.; Hosny, M.; Mohamed, W.A.; Ibrahim, S. EEG signal classification using neural network and support vector machine in brain computer interface. In International Conference on Advanced Intelligent Systems and Informatics; Springer: Cham, Germany, 2016; pp. 246–256. [Google Scholar]
- Gao, L.; Cheng, W.; Zhang, J.; Wang, J. EEG classification for motor imagery and resting state in BCI applications using multi-class Adaboost extreme learning machine. Rev. Sci. Instrum. 2016, 87, 085110. [Google Scholar] [PubMed]
- Rakshit, A.; Khasnobish, A.; Tibarewala, D.N. A Naïve Bayesian approach to lower limb classification from EEG signals. In Proceedings of the 2016 2nd International Conference on Control, Instrumentation, Energy & Communication (CIEC), Kolkata, India, 28–30 January 2016; pp. 140–144. [Google Scholar]
- Bird, J.J.; Manso, L.J.; Ribeiro, E.P.; Ekart, A.; Faria, D.R. A study on mental state classification using eeg-based brain-machine interface. In Proceedings of the 2018 International Conference on Intelligent Systems (IS), Funchal, Portugal, 25–27 September 2018; pp. 795–800. [Google Scholar]
- Liang, N.Y.; Saratchandran, P.; Huang, G.B.; Sundararajan, N. Classification of mental tasks from EEG signals using extreme learning machine. Int. J. Neural Syst. 2006, 16, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrakhteh, S.; Mosavi, M.R.; Khishe, M.; Ayatollahi, A. Accurate classification of EEG signals using neural networks trained by hybrid population-physic-based algorithm. Int. J. Autom. Comput. 2020, 17, 108–122. [Google Scholar] [CrossRef]
- Yasoda, K.; Ponmagal, R.S.; Bhuvaneshwari, K.S.; Venkatachalam, K. Automatic detection and classification of EEG artifacts using fuzzy kernel SVM and wavelet ICA (WICA). Soft Comput. 2020, 24, 16011–16019. [Google Scholar] [CrossRef]
- El-Kafrawy, N.M.; Hegazy, D.; Tolba, M.F. Features extraction and classification of eeg signals using empirical mode decomposition and support vector machine. In International Conference on Advanced Machine Learning Technologies and Applications; Springer: Cham, Germany, 2014; pp. 189–198. [Google Scholar]
- Chaudhary, S.; Taran, S.; Bajaj, V.; Siuly, S. A flexible analytic wavelet transform based approach for motor-imagery tasks classification in BCI applications. Comput. Methods Programs Biomed. 2020, 187, 105325. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Han, M. Classification of EEG signals using hybrid feature extraction and ensemble extreme learning machine. Neural Process. Lett. 2019, 50, 1281–1301. [Google Scholar] [CrossRef]
- Ieracitano, C.; Mammone, N.; Hussain, A.; Morabito, F.C. A novel multi-modal machine learning based approach for automatic classification of EEG recordings in dementia. Neural Netw. 2020, 123, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhou, H.; Wang, Y.; Huang, J. Fuzzy support vector machine for classification of EEG signals using wavelet-based features. Med. Eng. Phys. 2009, 31, 858–865. [Google Scholar] [CrossRef]
- Anh, N.T.H.; Hoang, T.H.; Thang, V.T.; Bui, T.Q. An artificial neural network approach for electroencephalographic signal classification towards brain-computer interface implementation. In Proceedings of the 2016 IEEE RIVF International Conference on Computing & Communication Technologies, Research, Innovation, and Vision for the Future (RIVF), Hanoi, Vietnam, 7–9 November 2016; pp. 205–210. [Google Scholar]
- Ilyas, M.Z.; Saad, P.; Ahmad, M.I.; Ghani, A.R.I. Classification of EEG signals for brain-computer interface applications: Performance comparison. In Proceedings of the 2016 International Conference on Robotics, Automation and Sciences (ICORAS), Melaka, Malaysia, 5–6 November 2016; pp. 1–4. [Google Scholar]
- Aydemir, O.; Kayikcioglu, T. Decision tree structure based classification of EEG signals recorded during two dimensional cursor movement imagery. J. Neurosci. Methods 2014, 229, 68–75. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Hashemi, S.M.R.; Houshmand, N. Classification of EEG-based emotion for BCI applications. In Proceedings of the 2017 Artificial Intelligence and Robotics (IRANOPEN), Qazvin, Iran, 9 April 2017; pp. 127–131. [Google Scholar]
- Wang, X.W.; Nie, D.; Lu, B.L. Emotional state classification from EEG data using machine learning approach. Neurocomputing 2014, 129, 94–106. [Google Scholar] [CrossRef]
- Rashid, M.; Sulaiman, N.; Mustafa, M.; Khatun, S.; Bari, B.S. The classification of EEG signal using different machine learning techniques for BCI application. In International Conference on Robot Intelligence Technology and Applications; Springer: Singapore, 2018; pp. 207–221. [Google Scholar]
- Rodríguez-Bermúdez, G.; García-Laencina, P.J. Automatic and adaptive classification of electroencephalographic signals for brain computer interfaces. J. Med. Syst. 2012, 36, 51–63. [Google Scholar]
- Suk, H.I.; Lee, S.W. A novel Bayesian framework for discriminative feature extraction in brain-computer interfaces. IEEE Trans. Pattern Anal. Mach. Intell. 2012, 35, 286–299. [Google Scholar] [CrossRef] [Green Version]
- Shahlaei, F.; Bagh, N.; Zambare, M.S.; Machireddy, R.; Shaligram, A.D. Detection of Event Related Patterns using Hilbert Transform in Brain Computer Interface. In Proceedings of the 2019 IEEE Canadian Conference of Electrical and Computer Engineering (CCECE), Edmonton, AB, Canada, 5–8 May 2019; pp. 1–4. [Google Scholar]
- Miao, M.; Zeng, H.; Wang, A.; Zhao, C.; Liu, F. Discriminative spatial-frequency-temporal feature extraction and classification of motor imagery EEG: An sparse regression and Weighted Naïve Bayesian Classifier-based approach. J. Neurosci. Methods 2017, 278, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kuncheva, L.I.; Rodríguez, J.J. Interval feature extraction for classification of event-related potentials (ERP) in EEG data analysis. Progress Artif. Intell. 2013, 2, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Y.; Zhou, G.; Jin, J.; Wang, B.; Wang, X.; Cichocki, A. Multi-kernel extreme learning machine for EEG classification in brain-computer interfaces. Expert Syst. Appl. 2018, 96, 302–310. [Google Scholar] [CrossRef]
- Appriou, A.; Cichocki, A.; Lotte, F. Modern Machine-Learning Algorithms: For Classifying Cognitive and Affective States from Electroencephalography Signals. IEEE Syst. Man Cybern. Mag. 2020, 6, 29–38. [Google Scholar] [CrossRef]
- Poorna, S.S.; Baba, P.S.; Ramya, G.L.; Poreddy, P.; Aashritha, L.S.; Nair, G.J.; Renjith, S. Classification of EEG based control using ANN and KNN—A comparison. In Proceedings of the 2016 IEEE International Conference on Computational Intelligence and Computing Research (ICCIC), Chennai, India, 15–17 December 2016; pp. 1–6. [Google Scholar]
- Thulasidas, M.; Guan, C.; Wu, J. Robust classification of EEG signal for brain-computer interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, C.; Zhang, D. An experimental evaluation of ensemble methods for EEG signal classification. Pattern Recognit. Lett. 2007, 28, 2157–2163. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, W.; You, Y.; Jiang, Y.; Li, M.; Zhang, T. Ensemble deep learning for automated visual classification using EEG signals. Pattern Recognit. 2020, 102, 107147. [Google Scholar] [CrossRef]
- Aayesha, Q.M.B.; Afzaal, M.; Qureshi, M.S.; Fayaz, M. Machine learning-based EEG signals classification model for epileptic seizure detection. Multimed. Tools Appl. 2021, 80, 17849–17877. [Google Scholar] [CrossRef]
- Aliakbaryhosseinabadi, S.; Kamavuako, E.N.; Jiang, N.; Farina, D.; Mrachacz-Kersting, N. Classification of EEG signals to identify variations in attention during motor task execution. J. Neurosci. Methods 2017, 284, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayram, K.S.; Kızrak, M.A.; Bolat, B. Classification of EEG signals by using support vector machines. In Proceedings of the 2013 IEEE INISTA, Albena, Bulgaria, 19–21 June 2013; pp. 1–3. [Google Scholar]
- Vazquez, R.A.; Salazar-Varas, R. Classification of EEG signals using fractal dimension features and artificial neural networks. In Proceedings of the 2017 IEEE Symposium Series on Computational Intelligence (SSCI), Honolulu, HI, USA, 27 November–1 December 2017 2017; pp. 1–6. [Google Scholar]
- She, Q.; Hu, B.; Gan, H.; Fan, Y.; Nguyen, T.; Potter, T.; Zhang, Y. Safe semi-supervised extreme learning machine for EEG signal classification. IEEE Access 2018, 6, 49399–49407. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Yan, Y.; Wei, W.; Wang, Z.J. Classification of EEG signals using a multiple kernel learning support vector machine. Sensor 2014, 14, 12784–12802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuvaneswari, P.; Kumar, J.S. Support vector machine technique for EEG signals. Int. J. Comput. Appl. 2013, 63, 1–5. [Google Scholar] [CrossRef]
- Tan, P.; Sa, W.; Yu, L. Applying extreme learning machine to classification of EEG BCI. In Proceedings of the 2016 IEEE International Conference on Cyber Technology in Automation, Control, and Intelligent Systems (CYBER), Chengdu, China, 19–22 June 2016; pp. 228–232. [Google Scholar]
- Zhang, Y.; Zhou, G.; Jin, J.; Zhao, Q.; Wang, X.; Cichocki, A. Sparse Bayesian classification of EEG for brain–computer interface. IEEE Trans. Neural Netw. Learn. Syst. 2015, 27, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhou, G.; Gao, D.; Zhang, Y. EEG classification using sparse Bayesian extreme learning machine for brain–computer interface. Neural Comput. Appl. 2020, 32, 6601–6609. [Google Scholar] [CrossRef]
- Tabar, Y.R.; Halici, U. A novel deep learning approach for classification of EEG motor imagery signals. J. Neural Eng. 2017, 14, 16003. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.; Yu, Z.; Chen, T.; Wang, F.; Hou, Y. A simplified CNN classification method for MI-EEG via the electrode pairs signals. Front. Hum. Neurosci. 2020, 14, 338. [Google Scholar] [CrossRef]
- Alwasiti, H.; Yusoff, M.Z.; Raza, K. Motor imagery classification for brain computer interface using deep metric learning. IEEE Access 2020, 8, 109949–109963. [Google Scholar] [CrossRef]
- Dose, H.; Møller, J.S.; Iversen, H.K.; Puthusserypady, S. An end-to-end deep learning approach to MI-EEG signal classification for BCIs. Expert Syst. Appl. 2018, 114, 532–542. [Google Scholar] [CrossRef]
- He, L.; Hu, D.; Wan, M.; Wen, Y.; Von Deneen, K.M.; Zhou, M. Common Bayesian network for classification of EEG-based multiclass motor imagery BCI. IEEE Trans. Syst. Man Cybern. Syst. 2015, 46, 843–854. [Google Scholar] [CrossRef]
- Cecotti, H.; Graser, A. Convolutional neural networks for P300 detection with application to brain-computer interfaces. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 33, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Blankertz, B.; Lemm, S.; Treder, M.; Haufe, S.; Müller, K.R. Single-trial analysis and classification of ERP components—a tutorial. NeuroImage 2011, 56, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, G.; Jin, J.; Zhao, Q.; Wang, X.; Cichocki, A. Aggregation of sparse linear discriminant analyses for event-related potential classification in brain-computer interface. Int. J. Neural Syst. 2014, 24, 1450003. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Zhao, Q.; Jin, J.; Wang, X.; Cichocki, A. Spatial-temporal discriminant analysis for ERP-based brain-computer interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Hortal, E.; Iáñez, E.; Úbeda, A.; Planelles, D.; Costa, Á.; Azorín, J.M. Selection of the best mental tasks for a svm-based bci system. In Proceedings of the 2014 IEEE International Conference on Systems, Man, and Cybernetics (SMC), San Diego, CA, USA, 5–8 October 2014; pp. 1483–1488. [Google Scholar]
- Ieracitano, C.; Mammone, N.; Hussain, A.; Morabito, F.C. A novel explainable machine learning approach for EEG-based brain-computer interface systems. Neural Comput. Appl. 2021, 1–14. [Google Scholar] [CrossRef]
- Hong, K.S.; Khan, M.J.; Hong, M.J. Feature extraction and classification methods for hybrid fNIRS-EEG brain-computer interfaces. Front. Hum. Neurosci. 2018, 12, 246. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Guan, C. Bayesian learning for spatial filtering in an EEG-based brain–computer interface. IEEE Trans. Neural Netw. Learn. Syst. 2013, 24, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre, C.; Kawanabe, M.; von Bünau, P.; Blankertz, B.; Müller, K.R. Toward unsupervised adaptation of LDA for brain–computer interfaces. IEEE Trans. Biomed. Eng. 2010, 58, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, Y.; Liu, J.; Sun, J.; Cichocki, A.; Gao, F. Regularized group sparse discriminant analysis for P300-based brain–computer interface. Int. J. Neural Syst. 2019, 29, 1950002. [Google Scholar] [CrossRef]
- Kundu, S.; Ari, S. P300 detection with brain–computer interface application using PCA and ensemble of weighted SVMs. IETE J. Res. 2018, 64, 406–414. [Google Scholar] [CrossRef]
- Bhatnagar, V.; Yede, N.; Keram, R.S.; Chaurasiya, R.K. A modified approach to ensemble of SVM for P300 based brain computer interface. In Proceedings of the 2016 International Conference on Advances in Human Machine Interaction (HMI), Kodigehalli, India, 3–5 March 2016; pp. 1–5. [Google Scholar]
- Manyakov, N.V.; Chumerin, N.; Combaz, A.; Van Hulle, M.M. Comparison of classification methods for P300 brain-computer interface on disabled subjects. Comput. Intell. Neurosci. 2011, 519868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramoser, H.; Muller-Gerking, J.; Pfurtscheller, G. Optimal spatial filtering of EEG during imagined hand movement. IEEE Trans. Rehabil. Engi. 2000, 8, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
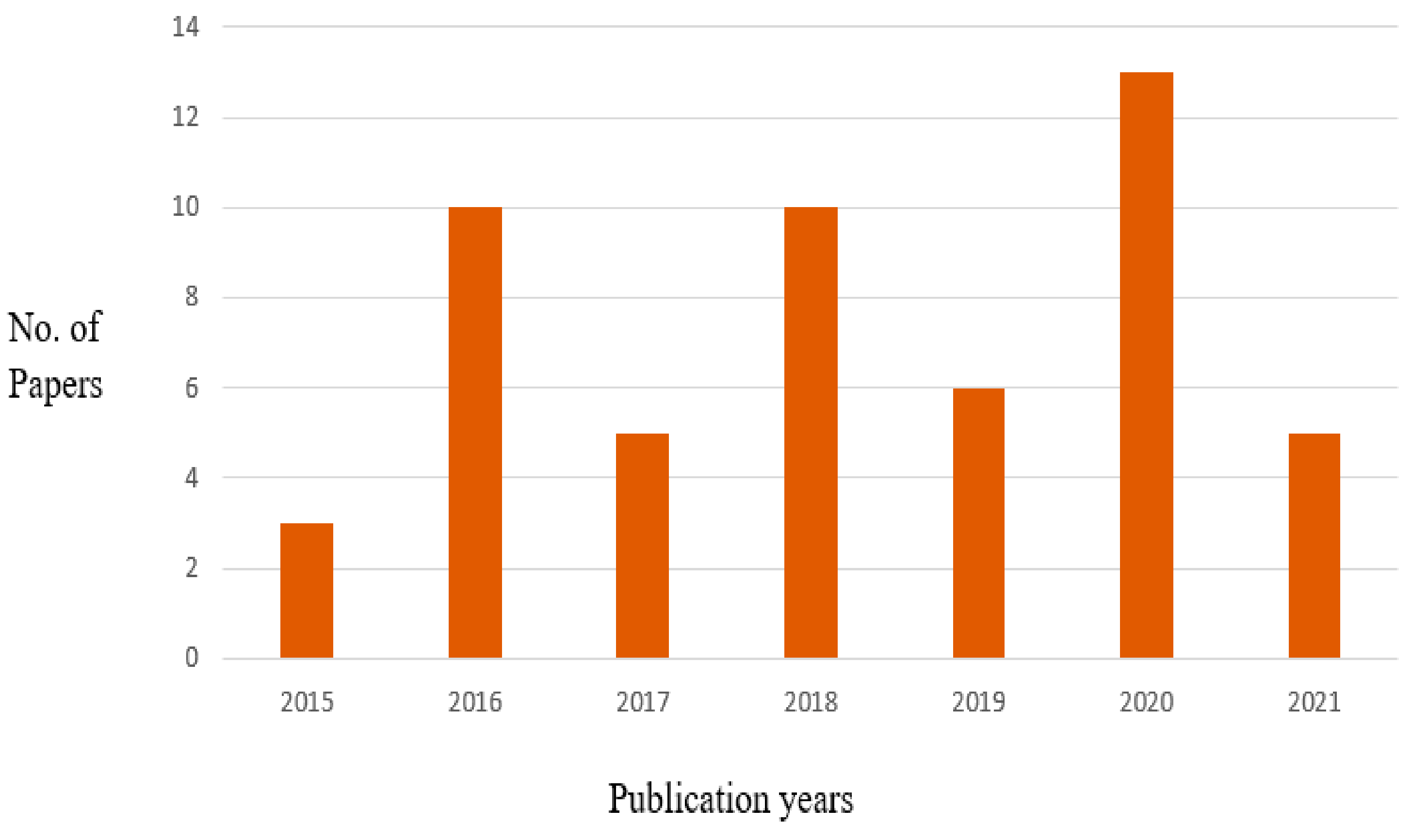
| Reference | Publication Year | Feature Extraction/Selection Method | Classification Method | Performance Measure | Accuracy Level | BCI Task | Merits | Demerits or Future Directions |
|---|---|---|---|---|---|---|---|---|
| [7] | 2020 | - | Linear regression | - | 95% | EEG signal categorization | Showed high accuracy. | Involved numerous mathematical computations. |
| [15] | 2008 | CSP | LDA | - | - | EEG signal analysis | Analyzed EEG signals effectively. | Distinct physiological features should be combined for multi-category classification. |
| [19] | 2012 | - | SVM | Classification accuracy | 90.55% | ERP signal categorization | Effectively categorized ERP signals. | More participants should be considered in the work. |
| [23] | 2019 | CSP | Independent decision path integration | Classification accuracy, specificity, sensitivity | 70.32% | Mental state categorization | Categorized multicategory mental states. | Accuracy should be improved. |
| [24] | 2018 | PCA | SVM | Classification accuracy, specificity, sensitivity | 92.5% | BCI therapy stage classification | Assisted in stroke rehabilitation. | More participants should be included in the study. |
| [27] | 2015 | EEG coherence-based method | Fisher’s linear discriminant | Classification accuracy | 73.6% | EEG coherence selection | Effectively discriminated motor and cognitive tasks. | The accuracy level requires improvement. |
| [28] | 2006 | ACSP | SVM | Classification accuracy | 65.12% | Classification of EEG signals | Extracted most discriminative attributes pertinent to brain states. | Failed to show improvements in EEG signal categorization. |
| [29] | 2003 | GA | SVM, LDA, and NNs | Classification accuracy | 76% | Classification of EEG signals | Improved feature selection. | Parameter variations and additional databases must be considered. |
| [30] | 2013 | WT, GA | KNN, SVM, LDA, Bayesian, MLP and weighted majority voting | Classification accuracy | - | EEG signal categorization | The proposed multiclassifier outperformed the individual classifiers. | - |
| [31] | 2021 | PCA | Convolutional autoencoder | Classification accuracy | - | MI classification | Showed promising MI categorization performance compared to contemporary methods. | Performance evaluation using additional and more discriminative subjects is required. |
| [32] | 2020 | Spatial filtering | Temporal-spatial CNN | Classification accuracy | 65.7% | MI classification | Displayed significant performance enhancement compared with classical strategies. | Required separate training of classification layers and feature extraction layers. |
| [33] | 2020 | SSD | CNN | Kappa value, classification accuracy | 79.3% | MI classification | Displayed high robustness and classification quality. | DL network structure and layer selection require optimization. |
| [34] | 2005 | ARX | LDA | Accuracy | 79.1% | EEG feature extraction | ARX method outperformed the AR technique in EEG feature extraction. | Classification performance needs further improvement. |
| [35] | 2019 | Riemannian geometry, CSP and PSO | CNN | Classification accuracy | 80.44% | EEG signal categorization | Improved EEG signal categorization accuracy for multifarious subjects. | Required data augmentation. |
| [36] | 2013 | ICA | NNs and SVM | Classification accuracy | 89.8%, 97.1% | EEG signal categorization | Displayed promising feature extraction outputs for classifying EEG signals. | Performance evaluation with additional databases is required. |
| [37] | 2018 | WT | SVM | Sensitivity, classification accuracy | >90% | EEG signal categorization | Effectively performed multiclass classification with three distinct subjects. | - |
| [38] | 2019 | STFT, Continuous WT | CNN | Classification accuracy, specificity, error percent, kappa value, sensitivity, f1-score, | 99.35% | MI classification | Achieved greater accuracy scores in MI categorization than existing strategies. | MI categorization using ResNet, GoogleNet, and VGGNet frameworks must be explored. |
| [39] | 2018 | CSP | Neuro-fuzzy scheme | Classification accuracy, the kappa value | 91.43% | EEG signal categorization | Outperformed related existing techniques by 4.5%. | Intelligent optimization schemes are required for adjusting classification model parameters. |
| [40] | 2016 | mRMR | SVM | Classification accuracy | 62.33% | BCI-oriented emotion recognition | Recognized multiple emotion types without employing additional classifiers. | Highly exhaustive setup assessments are required for assessing model parameters affecting the efficacy of the approach. |
| [41] | 2011 | WT | SVM | Classification accuracy | 88.6% | EEG signal categorization | Exhibited excellent potential and encouraging outcomes towards asynchronous BCI applications. | EEG signals linked with MI should be analyzed. |
| [42] | 2020 | PCA, FLD | K-ELM | Classification accuracy | 96.54% | MI classification | Achieved higher accuracy score than other approaches. | Experienced certain data loss due to the energy function of compressed input information. |
| [43] | 2017 | WT, Lasso regularization, mRMR | GNB, LDA, SVM | Confusion matrix, k-score, classification accuracy | 95.47% (GNB), 91.10% (LDA), 92.26% (SVM) | MI classification | Improved two-class MI categorization using only a few feature vectors. | Multiclass MI categorization should be conducted. |
| [44] | 2016 | WT, PCA, FFT | SVM, ANN | Classification accuracy | 84% | EEG signal categorization | Effectively categorized EEG signals linked with five distinct mental tasks. | EEG signals linked with MI and emotions need to be categorized. |
| [45] | 2016 | Kolmogorov complexity | Adaboost ELM | Classification accuracy | 79.5% | EEG signal categorization | Improved EEG signal categorization for multi-category samples. | Performance of devised approach in distinct mental task categorization for BCI development should be inspected. |
| [46] | 2016 | WT | Probabilistic NB | Classification accuracy | 78.33% | Limb movement categorization | Considered both spatial and frequency domain attributes of EEG without immolating the accuracy. | The accuracy level should be upgraded. |
| [47] | 2018 | Short-term windowing, symmetrical uncertainty, information gain, oneR, correlation, and evolutionary technique | Bayesian networks, random forest, SVM | Classification accuracy | 87% | Mental state categorization | Effectively categorized diverse mental states. | DL models should be exploited for further incrementing the accuracy. |
| [48] | 2006 | AR | ELM, SVM, BPNN | Classification accuracy | 9.11% (SVM) | Mental task categorization | ELM utilized a shorter training time than BPNN and SVM. | The accuracy score should be intensified. |
| [49] | 2020 | CSP | MLP | Mean square error, classification accuracy | 97.8214% | EEG signal categorization | Displayed acceptable performance than the rest. | Additional metrics must be employed for assessing the EEG signal categorization performance. |
| [50] | 2020 | Wavelet ICA | Fuzzy kernel-SVM | Specificity, accuracy, sensitivity | 86.1% | EEG signal categorization | Automatically removed EEG signal artifacts from the raw database. | Further increment in accuracy score is necessary. |
| [51] | 2014 | EMD | RBF kernel with SVM | Classification accuracy | 100% | EEG signal categorization | Displayed superior EEG signal categorization accuracy. | Additional samples must be incorporated for assessing EEG categorization performance. |
| [52] | 2020 | FAWT | Subspace KNN, LDA, SVM, Decision Trees (DT), standard KNN | Accuracy, specificity, kappa value, sensitivity, f1-score | 99.33% (Subspace KNN), 81.1% (LDA), 95.72% (SVM), 91.79% (DT), 92.8% (standard KNN) | MI classification | Outperformed the previously available methods in MI categorization with the same dataset. | Parameters employed should be optimized for incrementing the accuracy. |
| [53] | 2019 | AR, WT, WPD, class separability feature selection | Ensemble ELM with LDA | Classification accuracy | 99.43% | EEG signal categorization | Achieved higher class separability compared to previously existing schemes. | Applicability of ensemble ELM with LDA approach to other BCI-pertinent biomedical signals must be examined. |
| [54] | 2020 | Continuous WT | Autoencoder, SVM, logistic regression and MLP | Accuracy, recall, precision, f-score | 0.5% (MLP) | EEG signal categorization | Outperformed the cutting-edge learning frameworks and yielded greater accuracy rates. | Utility of approach in diverse BCI-pertinent tasks must be assayed. |
| [55] | 2009 | WT | Fuzzy SVM | Classification accuracy, classification time, minimal misclassification rate, | 80.71% | EEG signal categorization | Provided a contemporary means for online EEG signal categorization. | Required huge computational effort and training time. |
| [56] | 2016 | PCA | ANN, naive bayes, KNN, LDA, SVM | Classification accuracy | 71.80% (ANN), 55.52% (naive bayes), 65.51% (KNN), 62.94% (LDA), 68.56% (SVM) | EEG signal categorization | Efficiently classified EEG signals linked with mental states. | Further increment in accuracy score is needed. |
| [57] | 2016 | FFT | MLP-ANN, KNN, SVM, logistic regression | Classification accuracy | 66.42% (MLP-ANN), 56.71% (KNN), 68.97% (SVM), 73.03% (logistic regression) | EEG signal categorization | Classified EEG signals linked with MI within a limited period. | Further increment in accuracy levels is required. |
| [58] | 2014 | AR, Continuous WT | LDA, KNN, SVM | Classification accuracy | 55.92% (LDA), 57.90% (KNN), 82.24% (SVM) | EEG signal categorization | Showed a 12.25% improvement in EEG signal categorization. | Utilized more time for decision formulation. |
| [59] | 2017 | WT, PCA | Naive Bayes, KNN, ANN, SVM | Classification accuracy | 50.7% (naive bayes), 49.82% (KNN), 55.58% (ANN), 51.82% (SVM) | EEG-dependent emotion classification | Classified EEG-dependent emotions accurately. | Lesser electrodes should be employed for experimentation. |
| [60] | 2014 | WT, FFT, PCA, LDA, correlation-dependent feature selection | SVM | Classification accuracy | 91.77% | EEG-dependent emotion classification | Provided a promising procedure for visualizing the subject’s emotional condition/state. | More subjects and experiments are required for improving the presented model’s efficiency. |
| [61] | 2018 | FFT | LDA, KNN, SVM | Classification accuracy | 95% (LDA), 100% (KNN), 100% (SVM) | EEG signal categorization | Provided a better method for examining EEG signals from diverse human cognitive conditions. | Investigated EEG attributes considering only two mental exercises. |
| [62] | 2012 | AR, PSD, Hjorth parameter, LOO, LAR | LDA, SVM | Classification accuracy | 74.3% (LDA), 70.5% (SVM) | EEG signal categorization | Did not require tunable parameter for classification and attribute selection phases. | Conducted EEG signal categorization only using an offline approach. |
| [63] | 2012 | Bayesian approach with SSO | SVM | Classification accuracy | >95% | MI classification | Outperformed the cutting-edge techniques in MI categorization. | Other features such as cortical potential, readiness potential, or Bereitschafts potential should be incorporated apart from ERS/ERD features. |
| [64] | 2019 | Hilbert transform | SVM, Naive bayes, LDA | Classification accuracy, kappa coefficient | 82.22% (SVM), 71.64% (naive bayes), 75.52% (LDA) | MI categorization | EEG attributes extracted via Hilbert transform showed the finest performance than previously exploited methods. | Applicability of approach towards diverse BCI-pertinent tasks must be inspected. |
| [65] | 2017 | CSP with sparse regression | Weighted naive Bayes | Classification accuracy | 85.24% | MI categorization | Improved MI classification performance. | Required large computation time. |
| [66] | 2013 | ICA, WT, PCA, AR, interval feature extracting method, fast correlation-dependent filter | Perceptron SVM, linear SVM, random forest, KNN | Classification accuracy | 97% (Perceptron SVM), 86% (Linear SVM), 97% (Random Forest), 97% (KNN) | ERP signal categorization | Classified ERP signals accurately. | Electrode information should be coupled with extracted valuable features for further incrementing the accuracy. |
| [67] | 2018 | CSP | Multi-kernel ELM | Classification accuracy | 10.5% | EEG signal categorization | Provided an improved solution for devising an MI-oriented BCI. | An increment in accuracy score for undersized samples is necessary. |
| [68] | 2020 | CSP | CNNs | Classification accuracy | 72.7296% (mental state categorization), 48.0469% (subject independent emotion categorization) | Emotional and mental state categorization | Performed both emotional and mental state categorization using EEG signals. | Emotional and mental state categorization using other bio-medical signals must be investigated. |
| [69] | 2016 | - | ANN, KNN | Classification accuracy, sensitivity | 98.58% (ANN), 96.06% (KNN) | EEG signal categorization | Displayed fine performance in EEG signal categorization. | Performance must be incremented by combining EEG with diverse biomedical signals. |
| [70] | 2006 | PCA | SVM | Classification accuracy | >95% | EEG signal categorization | Lowered training time and substantially incremented speed and accuracy. | - |
| [71] | 2007 | - | SVM, KNN and DT | Classification accuracy | 64.92% (SVM), 64.63% (KNN), 56.74% (DT) | EEG signal categorization | Ensemble schemes displayed better EEG signal categorization over an individual base classifier. | Issues regarding online evaluation and parameter tuning should be probed. |
| [72] | 2020 | - | LSTM | Classification accuracy, recall, f1-score, precision | 97.13% | EEG signal categorization | Offered a reliable platform for intelligent visual classification. | Sophisticated techniques are required for distinguishing EEG signals for additional image categories. |
| [73] | 2021 | - | KNN | - | - | EEG signal categorization | Displayed highest accuracy scores. | - |
| [74] | 2017 | PCA | LDA | Classification accuracy | 10.9% | EEG signal categorization | Extracted both time-frequency and temporal attributes effectively from three distinct brain lobes. | The accuracy level must be incremented. |
| [75] | 2013 | - | SVM | Classification accuracy | 71.43% | EEG signal categorization | Exhibited good EEG signal categorization performance. | The accuracy score must be upgraded. |
| [76] | 2017 | - | ANN, LDA, Bayesian classifier | Classification accuracy | 78.84% (ANN), 70.05% (LDA), 65.08% (Bayesian) | EEG signal categorization | Performed better with diminutive training sets and time-variant brain signals. | Further increment in accuracy rate is needed. |
| [77] | 2018 | - | Semi-supervised ELM | Classification accuracy | 1.38% | EEG signal categorization | Provided an efficient and safe approach for categorizing EEG signals. | Additional evaluation measures for examining the risk level of unlabeled information instances should be incorporated. |
| [78] | 2014 | - | Multiple kernel-SVM | Classification accuracy | 99.20% (2-class), 81.25% (3-class), 76.76% (4-class), 75.25% (5-class) | EEG signal categorization | Effectively executed multi-class categorization of EEG signals linked with mental tasks. | The categorization of EEG signals for BCI-pertinent tasks should be scrutinized. |
| [79] | 2013 | - | SVM | - | - | EEG signal analysis | Discussed the role of SVM in EEG signal analysis. | Experiment validation of SVM and its variants in EEG signal categorization should be conducted. |
| [80] | 2016 | Band-power scheme | ELM, LDA, SVM | Mutual information, classification accuracy | 82.02% (ELM), 77.1% (LDA), 78.18% (SVM) | EEG signal categorization | Displayed greater accuracy level and mutual information. | - |
| [81] | 2015 | - | SBLaplace method | Classification accuracy | - | EEG signal categorization | Automatically estimated model parameters without requiring cross-validation. | The performance of EEG signal categorization should be further intensified. |
| [82] | 2020 | - | Sparse Bayesian ELM | Classification accuracy | 14.3% | EEG signal categorization | Exhibited good EEG signal categorization accuracy. | More distinctive and high-level attributes should be learned for augmenting the categorization performance. |
| [83] | 2017 | STFT | CNN-SA | Classification accuracy, kappa value | 90.0% | MI classification | Performed swift classification with few samples and yielded greater performance. | More pooling layers should be incorporated for boosting MI categorization performance. |
| [84] | 2020 | - | CNN | Classification accuracy | 97.28% | MI classification | Classified raw EEG signals linked with MI without any synthetic feature extraction and preprocessing operations. | A system for real-time EEG wave/signal acquisition should be constructed. |
| [85] | 2020 | Stockwell transform | DML | Classification accuracy, recall, precision | 64.7% | MI classification | Offered a promising procedure for classifying MI signals with just fewer training samples. | The accuracy level must be further augmented. |
| [86] | 2018 | - | CNN | Classification accuracy | 86.13% | MI classification | Showed 6–9% of mean improvement in MI categorization accuracy. | Categorization of EEG signals linked with other BCI functions is needed. |
| [87] | 2015 | - | Bayesian network | Kappa coefficient | - | MI classification | Displayed excellent multiclass MI categorization performance. | Alternatives for minimizing the time involved for training information collection should be probed. |
| [88] | 2010 | - | CNN | Classification accuracy, noise, precision, error, f1 measure, silence, recall | 95.5% | ERP signal classification | Provided a contemporary approach for examining brain activities. | The impact of P300 waves on character identification problems should be studied. |
| [89] | 2011 | - | LDA | Classification accuracy | - | ERP signal classification | Provided an effective procedure for ERP signal classification. | The accuracy level must be augmented. |
| [90] | 2014 | - | Aggregated sparse LDA | Classification accuracy | 19.2% | ERP signal classification | Demonstrated better performance even with inadequate training samples. | Applicability of aggregated sparse LDA for diverse BCI-related functions must be reconnoitered. |
| [91] | 2013 | - | STDA | Classification accuracy | 6.32% | ERP signal classification | Displayed superior ERP signal categorization performance with scarce training samples. | Categorization performance must be further augmented. |
| [92] | 2014 | - | SVM | Classification accuracy | 59.75%, 72.89% and 91.42% for four, three and two mental tasks | Mental task categorization | Effectively executed multiclass mental task categorization. | Involved in an additional procedure for determining the best electrodes and tasks. |
| [93] | 2021 | - | CNN | - | - | EEG signal categorization | Displayed good EEG signal categorization performance. | Cortical regions involved with hand unlock/close gestures must be studied. |
| [94] | 2018 | CSP | LDA, ELM, SVM | - | - | EEG signal analysis | Described brain activities leading to a substantial rise in hemodynamic response. | Suggested recognition of brain areas and categorization of hemodynamic reactions. |
| [95] | 2013 | Spatial filtering | Bayesian network | Classification accuracy | - | EEG signal analysis | Effectively analyzed EEG signals. | The accuracy score should be further enhanced. |
| [96] | 2010 | - | Unsupervised LDA | Mean, PMean | - | EEG signal analysis | Outperformed the cutting-edge approaches. | Analysis pertinent to asynchronous BCI tasks is required. |
| [97] | 2019 | - | Sparse discriminant analysis | Classification accuracy, time complexity | >60% | P300 detection and categorization | Exhibited good P300 categorization accuracy. | The accuracy score must be further incremented. |
| [98] | 2018 | PCA | Weighted SVM | - | - | P300 detection | Performed better in P300 signal recognition. | Training time must be lowered. |
| [99] | 2016 | - | SVM | Accuracy | 92.5% | P300-based BCI operation | Improved P300 detection performance. | Augmentation of the test set for accuracy up-gradation is required. |
| [100] | 2011 | - | Linear SVM, stepwise-LDA, fisher’s LDA, Bayesian-LDA, ANN, non-linear SVM | Intensification sequences | - | P300-based BCI operation | Bayesian LDA exhibited remarkable performance. | The impact of the P300 speller on stroke patients should be scrutinized. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, S. A Review of the Role of Machine Learning Techniques towards Brain–Computer Interface Applications. Mach. Learn. Knowl. Extr. 2021, 3, 835-862. https://doi.org/10.3390/make3040042
Rasheed S. A Review of the Role of Machine Learning Techniques towards Brain–Computer Interface Applications. Machine Learning and Knowledge Extraction. 2021; 3(4):835-862. https://doi.org/10.3390/make3040042
Chicago/Turabian StyleRasheed, Saim. 2021. "A Review of the Role of Machine Learning Techniques towards Brain–Computer Interface Applications" Machine Learning and Knowledge Extraction 3, no. 4: 835-862. https://doi.org/10.3390/make3040042
APA StyleRasheed, S. (2021). A Review of the Role of Machine Learning Techniques towards Brain–Computer Interface Applications. Machine Learning and Knowledge Extraction, 3(4), 835-862. https://doi.org/10.3390/make3040042






