Effect of Reduced Graphene Oxide on Curing, Mechanical, and Thermal Properties of Polymethylene Tetrasulfide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of rGO
2.3. Synthesis of Na2S4, Polymer, and Nanocomposites
2.4. Curing Process
2.5. The Measurements and Characterization
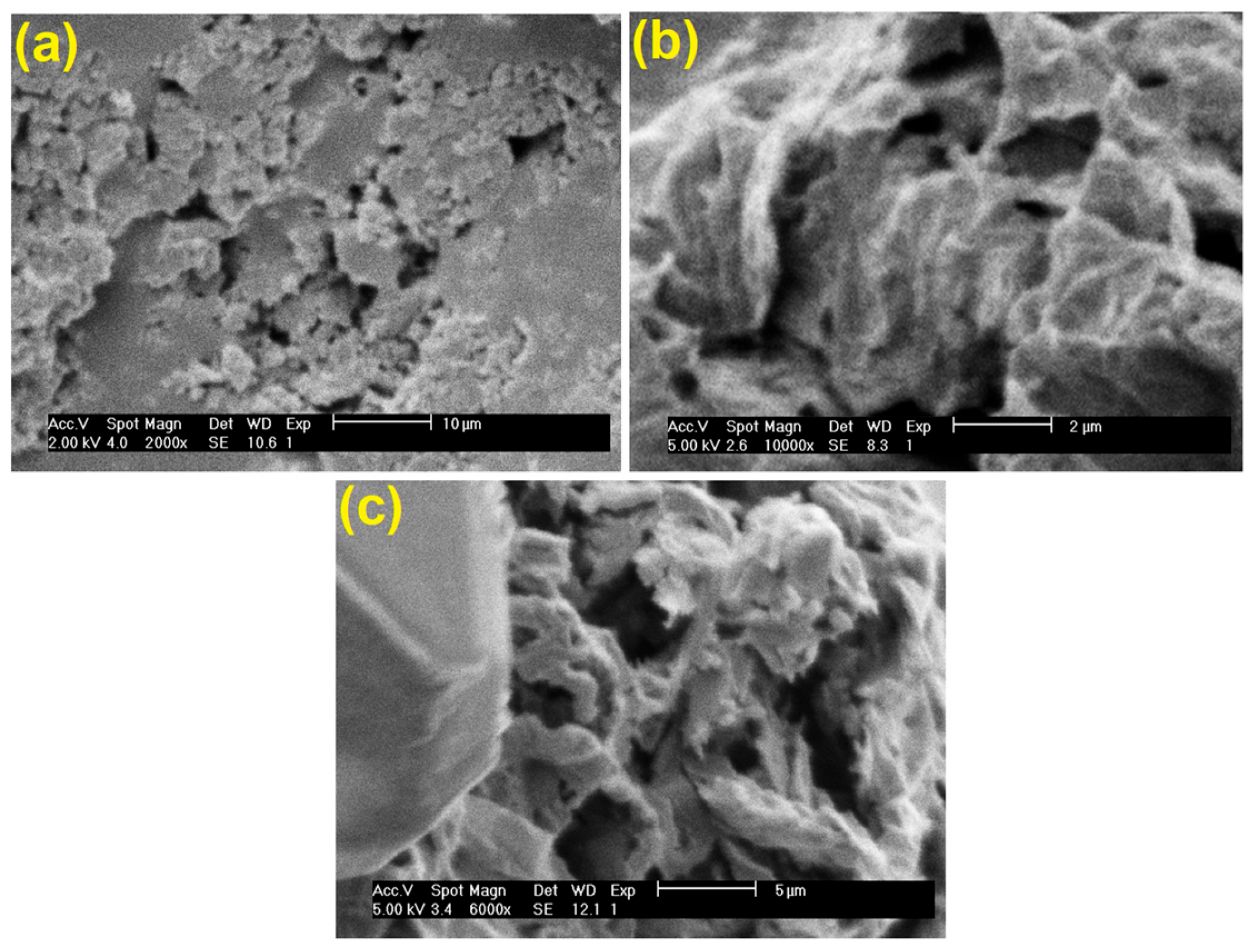
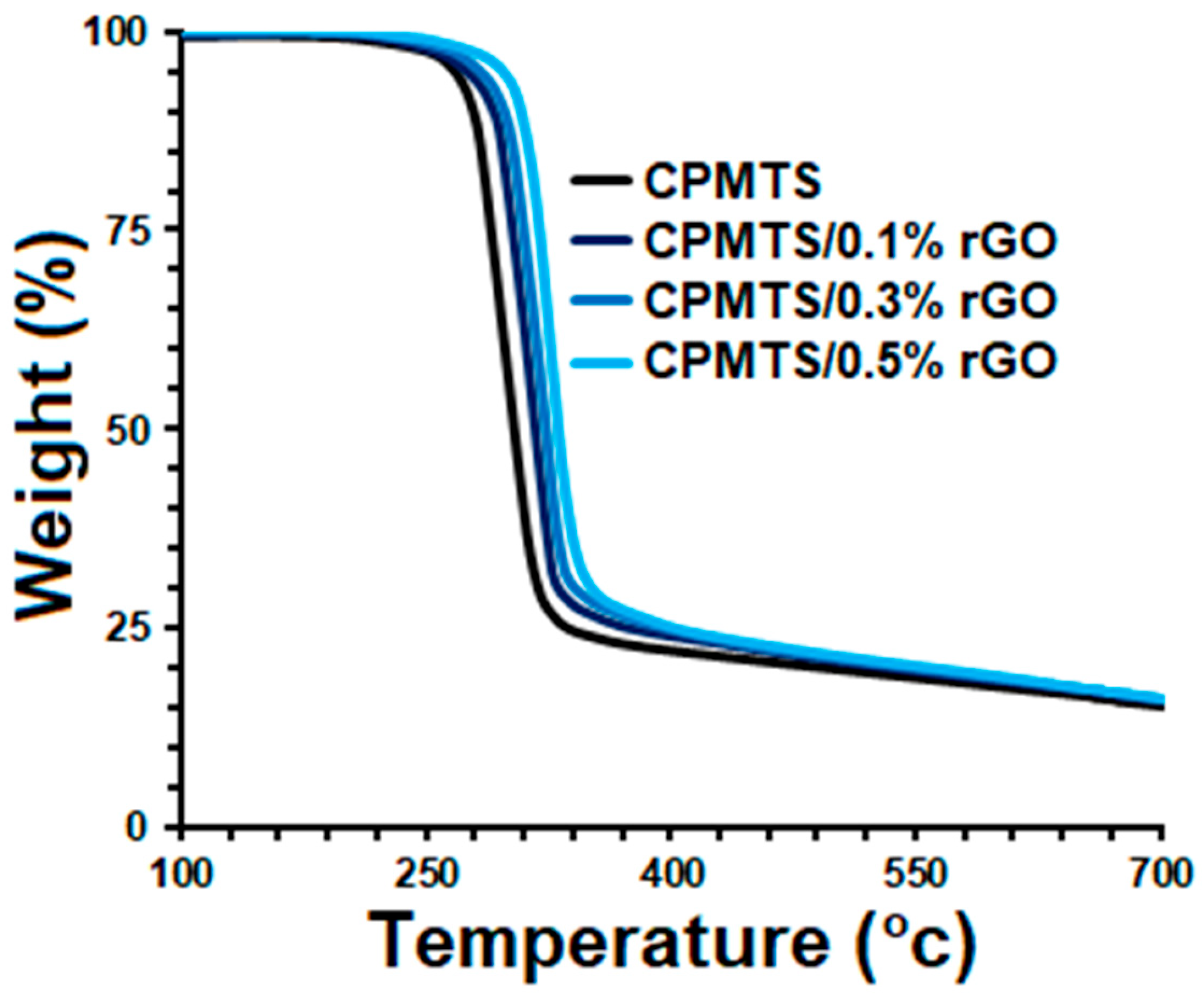
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.; Tran, P.Q.; Cowley, E.S.; Trembath-Reichert, E.; Anantharaman, K. Diversity and ecology of microbial sulfur metabolism. Nat. Rev. Microbiol. 2025, 23, 122–140. [Google Scholar] [CrossRef]
- Fakhraee, M.; Crockford, P.W.; Bauer, K.W.; Pasquier, V.; Sugiyama, I.; Katsev, S.; Raven, M.R.; Gomes, M.; Philippot, P.; Crowe, S.A.; et al. The history of Earth’s sulfur cycle. Nat. Rev. Earth Environ. 2025, 6, 106–125. [Google Scholar] [CrossRef]
- Sheydaei, M. Sodium sulfide-based polysulfide polymers: Synthesis, cure, thermal and mechanical properties. J. Sulfur Chem. 2022, 43, 643–654. [Google Scholar] [CrossRef]
- Abbasi, A.; Ghumman, A.S.M.; Nasef, M.M.; Yahya, W.Z.N.; Shamsuddin, M.R.; Moniruzzaman, M. Organosulfur Polymer Composites by Free Radical Polymerization of Sulfur with Vegetable Oils. In Advanced Composites; Springer Nature: Cham, Switzerland, 2023; pp. 601–620. [Google Scholar]
- Li, X.Y.; Zhao, M.; Song, Y.W.; Bi, C.X.; Li, Z.; Chen, Z.X.; Zhang, X.Q.; Li, B.Q.; Huang, J.Q. Polysulfide chemistry in metal–sulfur batteries. Chem. Soc. Rev. 2025, 54, 4822–4873. [Google Scholar] [CrossRef] [PubMed]
- Sheydaei, M.; Talebi, S.; Salami-Kalajahi, M. Synthesis of ethylene dichloride-based polysulfide polymers: Investigation of polymerization yield and effect of sulfur content on solubility and flexibility. J. Sulfur Chem. 2021, 42, 67–82. [Google Scholar] [CrossRef]
- King-Poole, C.; Thérien-Aubin, H. Sulfur-Rich Polymers Coatings. Adv. Funct. Mater. 2024, 34, 2405608. [Google Scholar] [CrossRef]
- Pirayesh, A.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Najafi, F. Polysulfide polymers: Synthesis, blending, nanocomposites, and applications. Polym. Rev. 2019, 59, 124–148. [Google Scholar] [CrossRef]
- Moqadam, S.; Salami Kalajahi, M.; Mahdavian, M. Synthesis and characterization of sunflower oil-based polysulfide polymer/cloisite 30B nanocomposites. Iran. J. Chem. Chem. Eng. 2018, 37, 185–192. [Google Scholar]
- Amangah, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Nanoconfinement effect of graphene on thermophysical properties and crystallinity of matrix-grafted graphene/crosslinked polysulfide polymer nanocomposites. Diam. Relat. Mater. 2018, 83, 177–183. [Google Scholar] [CrossRef]
- Hadavand, B.S.; Javid, K.M.; Gharagozlou, M. Mechanical properties of multi-walled carbon nanotube/epoxy polysulfide nanocomposite. Mater. Des. 2013, 50, 62–67. [Google Scholar] [CrossRef]
- Sheydaei, M.; Edraki, M.; Mousazadeh Moghaddampour, I.; Alinia-Ahandani, E. Poly (butylene trisulfide)/SiO2 nanocomposites: Cure and effect of SiO2 content on mechanical and thermophysical properties. J. Sulfur Chem. 2022, 43, 413–425. [Google Scholar] [CrossRef]
- Pirayesh, A.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Dehghani, E. Amine-modified graphene oxide as co-curing agent of epoxidized polysulfide prepolymer: Thermophysical and mechanical properties of nanocomposites. Diam. Relat. Mater. 2018, 86, 109–116. [Google Scholar] [CrossRef]
- Kariminejad, B.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Noparvar-Qarebagh, A. Effect of surface chemistry of graphene and its content on the properties of ethylene dichloride-and disodium tetrasulfide-based polysulfide polymer nanocomposites. Polym. Compos. 2017, 38, E515–E524. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Liu, X.; Li, L.; Yu, J.; Li, S.; Guo, G.; Gao, D.; Dai, Y. Preparation of epoxy resin with disulfide-containing curing agent and its application in self-healing coating. Materials 2023, 16, 4440. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, C.Y.; Zhang, C.; Yang, H.C.; Xu, Z.K. Thermodynamic and kinetic understanding for managing the controllability of interfacial polymerization. Prog. Polym. Sci. 2024, 152, 101815. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Luo, Z.; Wang, H.; Liu, C.; Ma, X.; Li, Y.; Bai, J.; Yuan, C. Alkaline stability of polyester nanofil-tration membranes prepared by interfacial polymerization. Desalination 2024, 589, 117961. [Google Scholar] [CrossRef]
- Wu, X.; Chen, T.; Dong, G.; Tian, M.; Wang, J.; Zhang, R.; Zhang, G.; Zhu, J.; Zhang, Y. A critical review on polyamide and polyesteramide nanofiltration membranes: Emerging monomeric structures and interfacial polymerization strategies. Desalination 2024, 577, 117379. [Google Scholar] [CrossRef]
- Jeon, Y.; Ahn, C.S.; Char, K.; Lim, J. Size Control and Antioxidant Properties of Sulfur-Rich Polymer Colloids from Interfacial Polymerization. Macromol. Rapid Commun. 2024, 45, 2300747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Wang, L.; Wang, J.; Wang, X.; Hao, J. Probing the construction mechanism of polyamide membranes regulated by interfacial polymerization from the novel micro-and macro-perspectives: A review. Desalination 2024, 578, 117422. [Google Scholar] [CrossRef]
- ASTM D412; Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension. ASTM International: West Conschohocken, PA, USA, 2002.
- ASTM D5289; Standard Test Method for Rubber Property—Vulcanization Using Rotorless Cure Meters; Annual Book of Standards, Vol. 09.01. ASTM International: West Conshohocken, PA, USA, 2021.
- Allahbakhsh, A.; Yari, S.; Safari, M.; Dubal, D.P. Poly (ethylene disulfide)/graphene oxide nanocomposites: Dynamic-mechanical and electrochemical properties. Eur. Polym. J. 2020, 130, 109694. [Google Scholar] [CrossRef]
- Samadaei, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Banaei, M. A structural study on ethylenediamine-and poly (amidoamine)-functionalized graphene oxide: Simultaneous reduction, functionalization, and formation of 3D structure. RSC adv. 2015, 5, 71835–71843. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, H.; Zhang, Y.; Qu, X. C–S bonds in sulfur-embedded graphene, carbon nanotubes, and flake graphite cathodes for lithium–sulfur batteries. ACS Omega 2019, 4, 16352–16359. [Google Scholar] [CrossRef] [PubMed]
- Perez Beltran, S.; Balbuena, P.B. Formation of multilayer graphene domains with strong sulfur–carbon interaction and enhanced sulfur reduction zones for lithium–sulfur battery cathodes. ChemSusChem 2018, 11, 1970–1980. [Google Scholar] [CrossRef]
- Nazari, N.; Bahramian, A.R.; Allahbakhsh, A. Thermal storage achievement of paraffin wax phase change material systems with regard to novolac aerogel/carbon monofilament/zinc borate form stabilization. J. Energy Storage 2022, 50, 104741. [Google Scholar] [CrossRef]
- Kalaee, M.R.; Famili, M.H.N.; Mahdavi, H.; Naderi, A. Synthesis, characterization and properties of poly (methylenetetrasulfide) using interfacial catalysis. Polym. Sci. Ser. B 2010, 52, 286–291. [Google Scholar] [CrossRef]
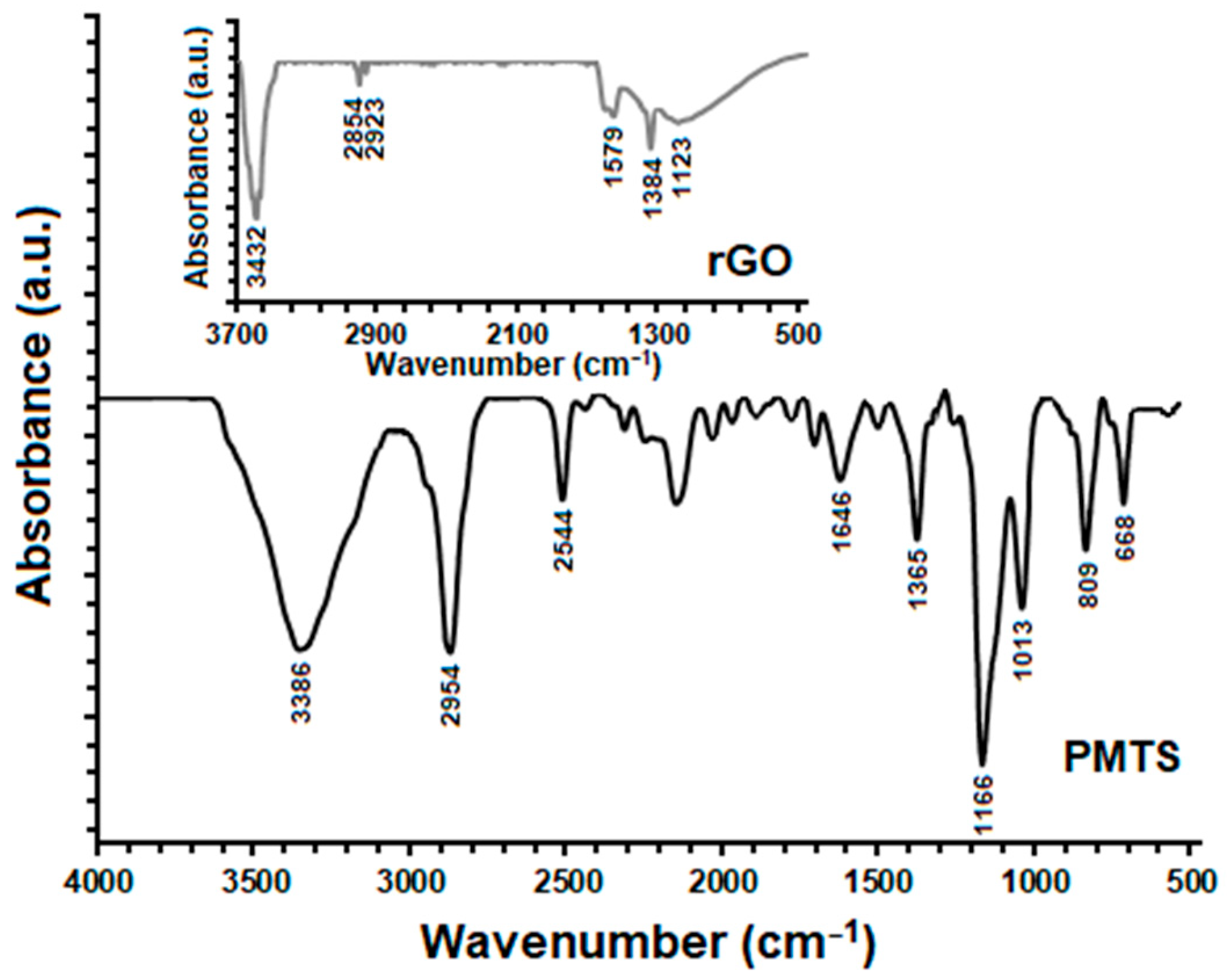

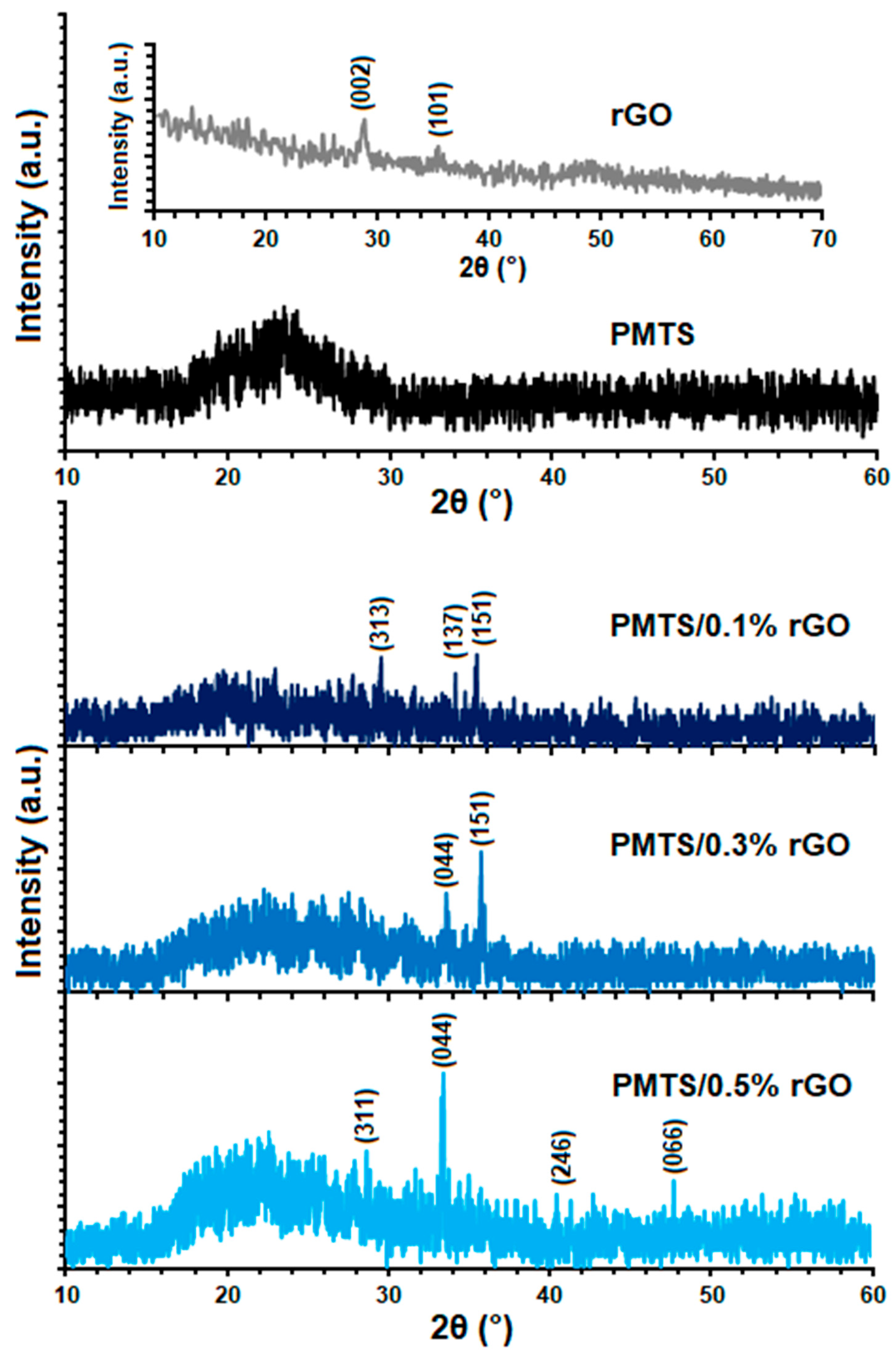

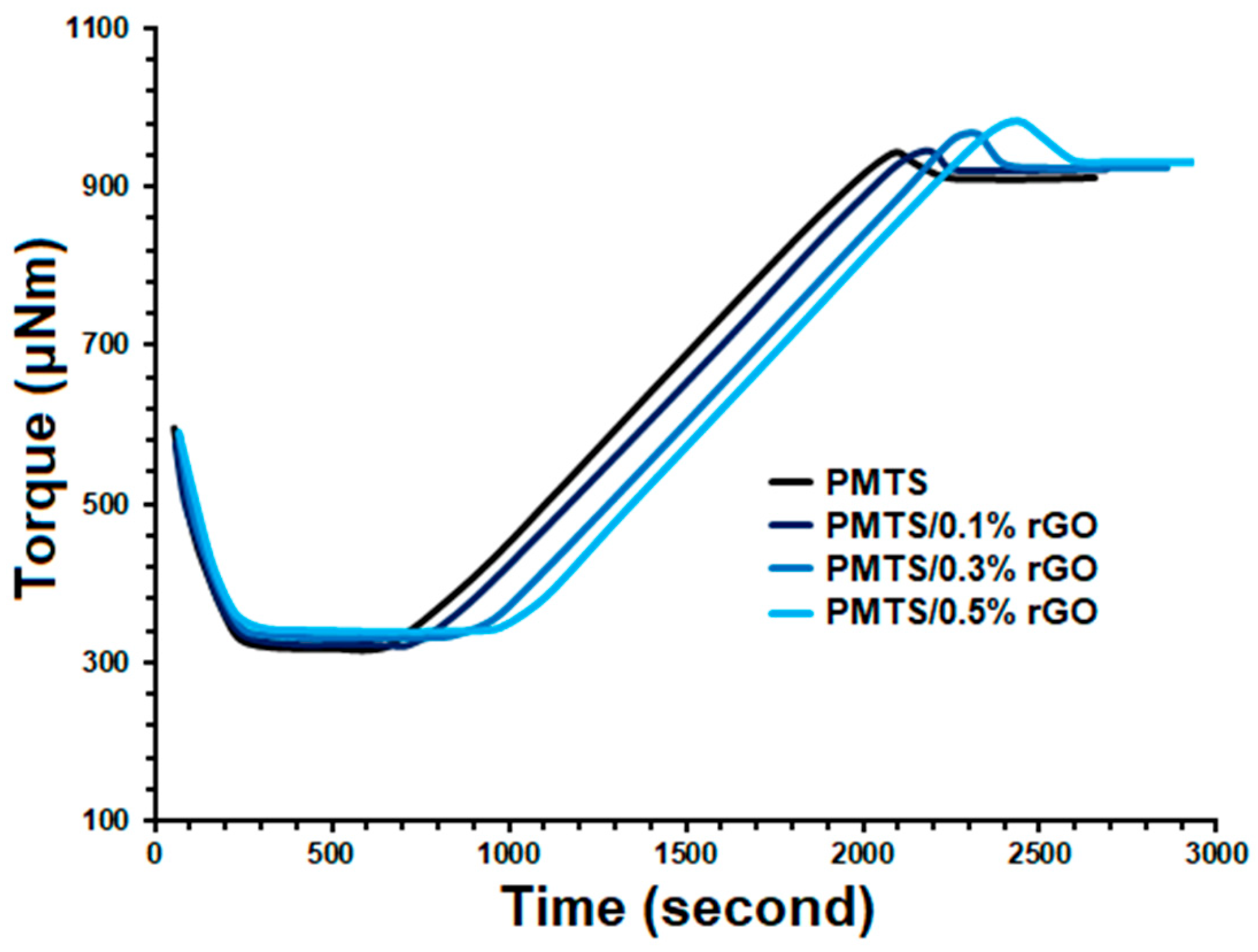

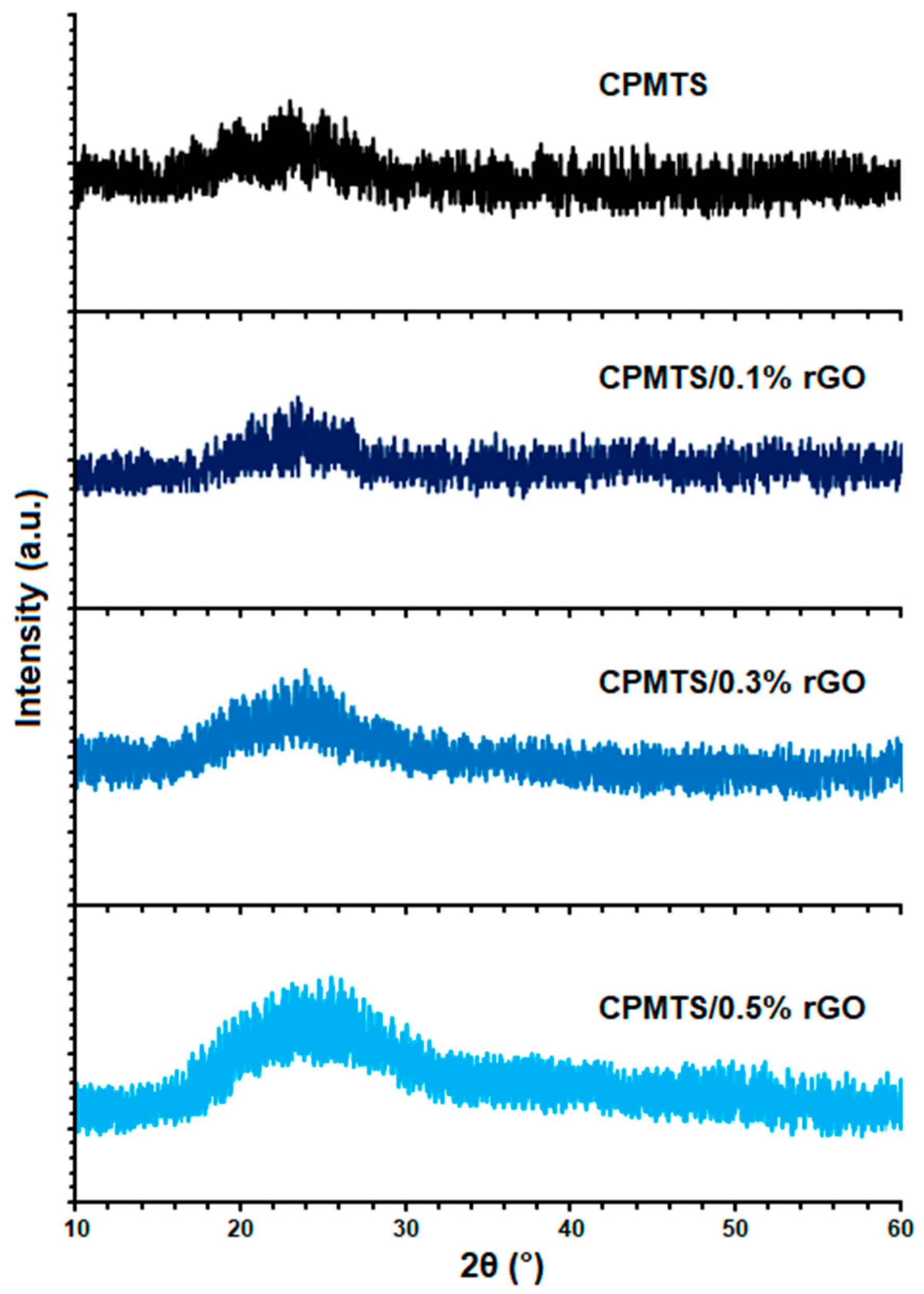
| Compounds | Cure Temperature (°C) | ML (μNm) | MH (μNm) | tscorch (Min) | t10 (Min) | t50 (Min) | t90 (Min) | tcure (Min) |
|---|---|---|---|---|---|---|---|---|
| PMTS | 140 | 319 | 929 | 10.1 | 12.6 | 22.4 | 32.2 | 34.6 |
| PMTS/0.1% rGO | 140 | 322 | 940 | 10.8 | 13.3 | 23.2 | 33.1 | 35.5 |
| PMTS/0.3% rGO | 140 | 331 | 965 | 12.9 | 15.5 | 25.8 | 36.2 | 38.7 |
| PMTS/0.5% rGO | 140 | 339 | 980 | 15.3 | 17.8 | 27.8 | 37.8 | 40.3 |
| Samples | Elongation at Break (%) | Strength (MPa) | Elastic Modulus (MPa) |
|---|---|---|---|
| CPMTS | 284.4 ± 1 | 2.1 ± 0.1 | 40.2 ± 1 |
| CPMTS/0.1% rGO | 280.3 ± 1 | 2.3 ± 0.1 | 43.4 ± 1 |
| CPMTS/0.3% rGO | 274.5 ± 1 | 2.7 ± 0.1 | 47.1 ± 1 |
| CPMTS/0.5% rGO | 264.4 ± 1 | 3.3 ± 0.1 | 52.4 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheydaei, M. Effect of Reduced Graphene Oxide on Curing, Mechanical, and Thermal Properties of Polymethylene Tetrasulfide. J. Compos. Sci. 2025, 9, 431. https://doi.org/10.3390/jcs9080431
Sheydaei M. Effect of Reduced Graphene Oxide on Curing, Mechanical, and Thermal Properties of Polymethylene Tetrasulfide. Journal of Composites Science. 2025; 9(8):431. https://doi.org/10.3390/jcs9080431
Chicago/Turabian StyleSheydaei, Milad. 2025. "Effect of Reduced Graphene Oxide on Curing, Mechanical, and Thermal Properties of Polymethylene Tetrasulfide" Journal of Composites Science 9, no. 8: 431. https://doi.org/10.3390/jcs9080431
APA StyleSheydaei, M. (2025). Effect of Reduced Graphene Oxide on Curing, Mechanical, and Thermal Properties of Polymethylene Tetrasulfide. Journal of Composites Science, 9(8), 431. https://doi.org/10.3390/jcs9080431






