Cell Viability of Wharton’s Jelly-Derived Mesenchymal Stem Cells (WJ-MSCs) on 3D-Printed Resins for Temporary Dental Restorations
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Cell Isolation
2.3. Annexin V and 7-AAD Assay
2.4. Live/Dead Assay
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Presto Blue Assay
2.7. Statistical Analysis
3. Results
3.1. Annexin V and 7-AAD Assay
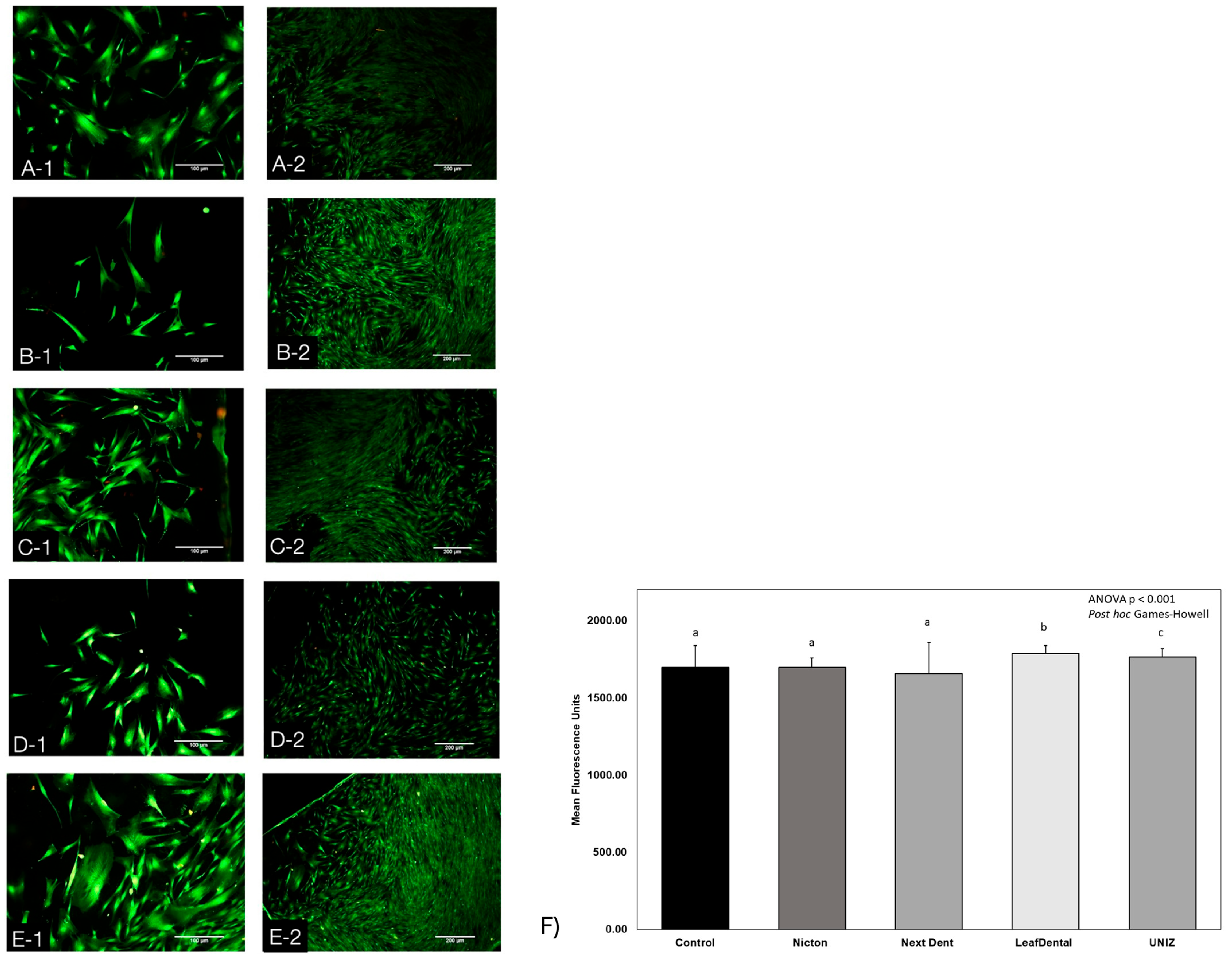
3.2. Live/Dead Assay
3.3. Scanning Electron Microscopy (SEM) Analysis
3.4. Presto Blue Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WJ-MSC | Mesenchymal stem cells derived from Wharton’s jelly |
| WBCs | Splenic white blood cells |
| CAD/CAM | Computer-aided design and manufacturing |
| DMEM-F12 | Dulbecco’s Modified Eagle Medium–Ham’s F12 |
| DS | Disc sample |
References
- Haidar, Z.S. Digital dentistry: Past, present, and future. Digit. Med. Healthc. Technol. 2023, 2, 1–16. [Google Scholar]
- Balestra, D.; Lowther, M.; Goracci, C.; Mandurino, M.; Cortili, S.; Paolone, G.; Louca, C.; Vichi, A. 3D Printed Materials for Permanent Restorations in Indirect Restorative and Prosthetic Dentistry: A Critical Review of the Literature. Materials 2024, 17, 1380. [Google Scholar] [CrossRef]
- Vitali, J.; Cheng, M.; Wagels, M. Utility and cost–effectiveness of 3D-printed materials for clinical use. J. 3D Print. Med. 2019, 3, 209–218. [Google Scholar] [CrossRef]
- de Gois Moreira, F.G.; da Silva, N.R.; Bezerra, M.G.P.G.; da Silva, S.E.G.; Butler, S.; Souza, K.B.; de Assunção E Souza, R.O. Influence of 3D printing system, postpolymerization and aging protocols on resin flexural strength and dimensional stability for printing occlusal splints, models and temporary restorations. Clin. Oral. Investig. 2024, 28, 604. [Google Scholar] [CrossRef] [PubMed]
- Alammar, A.; Att, W.; Beuer, F. The Accuracy of 3D-Printed Fixed Dental Restorations. J. Esthet. Restor. Dent 2025, 37, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Anadioti, E.; Musharbash, L.; Blatz, M.B.; Papavasiliou, G.; Kamposiora, P. 3D printed complete removable dental prostheses: A narrative review. BMC Oral. Health 2020, 20, 343. [Google Scholar] [CrossRef]
- NextDent. NextDent C&B MFH (Micro Filled Hybrid). 2025. Available online: https://nextdent.com/products/cb-mfh-micro-filled-hybrid/ (accessed on 14 November 2024).
- UNIZ. Zdental. 2025. Available online: https://uniz--en.oss-us-west-1.aliyuncs.com/pdf/zDental-IDB-TDS.pdf (accessed on 14 November 2024).
- LeafDental. LeafDental Resins for 3D Printing. 2025. Available online: https://www.uniz.com/us_en/materials/dental (accessed on 14 November 2024).
- Choudhury, M.; Nahar, N.; Yazdi, S.; Choudhury, F.; Sultana, A. Fabrication of provisional restoration on freshly prepared tooth: Indirect and direct technique. Bangladesh J. Med. Sci. 2015, 14, 59–64. [Google Scholar] [CrossRef]
- Rogers, H.B.; Zhou, L.T.; Kusuhara, A.; Zaniker, E.; Shafaie, S.; Owen, B.C.; Duncan, F.E.; Woodruff, T.K. Dental resins used in 3D printing technologies release ovo-toxic leachates. Chemosphere 2021, 270, 129003. [Google Scholar] [CrossRef]
- Atria, P.J.; Bordin, D.; Marti, F.; Nayak, V.V.; Conejo, J.; Benalcázar Jalkh, E.; Witek, L.; Sampaio, C.S. 3D-printed resins for provisional dental restorations: Comparison of mechanical and biological properties. J. Esthet. Restor. Dent. 2022, 34, 804–815. [Google Scholar] [CrossRef]
- American College of Prosthodontists. Glossary of digital dental terms. 2nd edition. J. Prosthodont. 2021, 30, 172–181. [Google Scholar] [CrossRef]
- Liang, X.; Yu, B.; Dai, Y.; Wang, Y.; Hu, M.; Zhong, H.-J.; He, J. Three-Dimensional Printing Resin-Based Dental Provisional Crowns and Bridges: Recent Progress in Properties, Applications, and Perspectives. Materials 2025, 18, 2202. [Google Scholar] [CrossRef] [PubMed]
- Tigmeanu, C.V.; Ardelean, L.C.; Rusu, L.C.; Negrutiu, M.L. Additive Manufactured Polymers in Dentistry, Current State-of-the-Art and Future Perspectives-A Review. Polymers 2022, 14, 3658. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Nikzad, S. Computer-assisted implantology: Historical background and potential outcomes—A review. Int. J. Med. Robot. Comput. Assist. Surg. 2008, 4, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Shenoy, M.; Alhasmi, A.; Al Saleh, A.A.; Shivakumar, G.C.; Shivakumar, S. Biocompatibility of 3D-Printed Dental Resins: A Systematic Review. Cureus 2024, 16, e51721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alyami, M.H. The Applications of 3D-Printing Technology in Prosthodontics: A Review of the Current Literature. Cureus 2024, 16, e68501. [Google Scholar] [CrossRef]
- Kessler, A.; Reymus, M.; Hickel, R.; Kunzelmann, K.H. Three-body wear of 3D printed temporary materials. Dent. Mater. 2019, 35, 1805–1812. [Google Scholar] [CrossRef]
- Revilla-León, M.; Özcan, M. Additive Manufacturing Technologies Used for Processing Polymers: Current Status and Potential Application in Prosthetic Dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef]
- Alammar, A.; Kois, J.C.; Revilla-León, M.; Att, W. Additive Manufacturing Technologies: Current Status and Future Perspectives. J. Prosthodont. 2022, 31 (Suppl. S1), 4–12. [Google Scholar] [CrossRef]
- Hegedus, T.; Kreuter, P.; Kismarczi-Antalffy, A.A.; Demeter, T.; Banyai, D.; Vegh, A.; Geczi, Z.; Hermann, P.; Payer, M.; Zsembery, A.; et al. User Experience and Sustainability of 3D Printing in Dentistry. Int. J. Environ. Res. Public Health 2022, 19, 1921. [Google Scholar] [CrossRef]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L.V. 3d-printed vs. Heat-polymerizing and autopolymerizing denture base acrylic resins. Materials 2021, 14, 5781. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef]
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, H.; Kim, J.W.; Kim, J.H. Cytocompatibility of 3D printed dental materials for temporary restorations on fibroblasts. BMC Oral Health 2020, 20, 157. [Google Scholar] [CrossRef]
- Folwaczny, M.; Ahantab, R.; Kessler, A.; Ern, C.; Frasheri, I. Cytotoxicity of 3D printed resin materials for temporary restorations on human periodontal ligament (PDL-hTERT) cells. Dent. Mater. 2023, 39, 529–537. [Google Scholar] [CrossRef]
- Guerrero-Gironés, J.; López-García, S.; Pecci-Lloret, M.R.; Pecci-Lloret, M.P.; Rodríguez Lozano, F.J.; García-Bernal, D. In vitro biocompatibility testing of 3D printing and conventional resins for occlusal devices. J. Dent. 2022, 123, 104163. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.; Kim, S.; Lim, B.-S.; Park, Y.-S.; Kim, R.J.-Y.; Chung, S.H. Dimensional Accuracy Evaluation of Temporary Dental Restorations with Different 3D Printing Systems. Materials 2021, 14, 1487. [Google Scholar] [CrossRef] [PubMed]
- Kurzmann, C.; Janjić, K.; Shokoohi-Tabrizi, H.; Edelmayer, M.; Pensch, M.; Moritz, A.; Agis, H. Evaluation of Resins for Stereolithographic 3D-Printed Surgical Guides: The Response of L929 Cells and Human Gingival Fibroblasts. Biomed. Res. Int. 2017, 2017, 4057612. [Google Scholar] [CrossRef]
- Kim, G.-T.; Go, H.-B.; Yu, J.-H.; Yang, S.-Y.; Kim, K.-M.; Choi, S.-H.; Kwon, J.-S. Cytotoxicity, Colour Stability and Dimensional Accuracy of 3D Printing Resin with Three Different Photoinitiators. Polymers 2022, 14, 979. [Google Scholar] [CrossRef]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Ríos Hernández, M.; Cepero Cañas, J. Citotoxicidad in vitro: Sistema para la evaluación de biomateriales y equipos médicos implantables en Cuba. CENIC Cienc. Biológicas 2006, 37, 173–176. [Google Scholar]
- Wulff, J.; Schweikl, H.; Rosentritt, M. Cytotoxicity of printed resin-based splint materials. J. Dent. 2022, 120, 104097. [Google Scholar] [CrossRef]
- Frasheri, I.; Aumer, K.; Keßler, A.; Miosge, N.; Folwaczny, M. Effects of resin materials dedicated for additive manufacturing of temporary dental restorations on human gingival keratinocytes. J. Esthet. Restor. Dent. 2022, 34, 1105–1112. [Google Scholar] [CrossRef]
- Oberoi, G.; Nitsch, S.; Janjić, K.; Shokoohi-Tabrizi, H.; Moritz, A.; Moscato, F.; Unger, E.; Agis, H. The impact of 3D-printed LAY-FOMM 40 and LAY-FOMM 60 on L929 cells and human oral fibroblasts. Clin. Oral. Investig. 2021, 25, 1869–1877. [Google Scholar] [CrossRef]
- Hwangbo, N.K.; Nam, N.E.; Choi, J.H.; Kim, J.E. Effects of the washing time and washing solution on the biocompatibility and mechanical properties of 3d printed dental resin materials. Polymers 2021, 13, 4410. [Google Scholar] [CrossRef]
- Sa, L.; Kaiwu, L.; Shenggui, C.; Junzhong, Y.; Yongguang, J.; Lin, W.; Li, R. 3D printing dental composite resins with sustaining antibacterial ability. J. Mater. Sci. 2019, 54, 3309–3318. [Google Scholar] [CrossRef]
- Saleh Alghamdi, S.; John, S.; Roy Choudhury, N.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.J.; Garcia-Godoy, F. 13—Stem cells and dental tissue reconstruction. In Material-Tissue Interfacial Phenomena; Spencer, P., Misra, A., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 325–353. [Google Scholar]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Cullen, D.K.; Le, A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018, 8, 6634. [Google Scholar] [CrossRef] [PubMed]
- Simancas-Escorcia, V.; Díaz-Caballero, A.; Vergara-Hernández, C. Aspectos morfológicos in vivo e in vitro de fibroblastos gingivales en pacientes con agrandamiento gingival. Entramado 2020, 16, 276–284. [Google Scholar] [CrossRef]
- Baust, J.M.; Buehring, G.C.; Campbell, L.; Elmore, E.; Harbell, J.W.; Nims, R.W.; Price, P.; Reid, Y.A.; Simione, F. Best practices in cell culture: An overview. Vitr. Cell Dev. Biol. Anim. 2017, 53, 669–672. [Google Scholar] [CrossRef]
- Coecke, S.; Balls, M.; Bowe, G.; Davis, J.; Gstraunthaler, G.; Hartung, T.; Hay, R.; Merten, O.-W.; Price, A.; Schechtman, L.; et al. Guidance on Good Cell Culture Practice. Altern. Lab. Anim. 2005, 33, 261–287. [Google Scholar] [CrossRef]
- Oyeleye, O.O.; Ogundeji, S.T.; Ola, S.I.; Omitogun, O.G. Basics of animal cell culture: Foundation for modern science. Biotechnol. Mol. Biol. Rev. 2016, 11, 6–16. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem Cells: Their Source, Potency and Use in Regenerative Therapies with Focus on Adipose-Derived Stem Cells—A Review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Shi, L.; Lu, Y.; Wang, B.; Tang, T.; Fu, W.; He, W.; Li, G.; Zhang, J. Linc-ROR Promotes Osteogenic Differentiation of Mesenchymal Stem Cells by Functioning as a Competing Endogenous RNA for miR-138 and miR-145. Mol. Ther.–Nucleic Acids 2018, 11, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Bassir, S.H.; Wisitrasameewong, W.; Raanan, J.; Ghaffarigarakani, S.; Chung, J.; Freire, M.; Andrada, L.C.; Intini, G. Potential for Stem Cell-Based Periodontal Therapy. J. Cell. Physiol. 2016, 231, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Inchingolo, A.D.; Nardelli, P.; Latini, G.; Trilli, I.; Ferrante, L.; Malcangi, G.; Palermo, A.; Inchingolo, F.; Dipalma, G. Stem Cells: Present Understanding and Prospects for Regenerative Dentistry. J. Funct. Biomater. 2024, 15, 308. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, Y.; Ji, Y.; Li, X.; Xing, Y.; Wen, Y.; Huang, H.; Xu, X. Comparative Analysis of lncRNA and mRNA Expression Profiles Between Periodontal Ligament Stem Cells and Gingival Mesenchymal Stem Cells. Gene 2019, 699, 155–164. [Google Scholar] [CrossRef]
- Raveau, S.; Jordana, F. Tissue Engineering and Three-Dimensional Printing in Periodontal Regeneration: A Literature Review. J. Clin. Med. 2020, 9, 4008. [Google Scholar] [CrossRef]
- Abdal-Wahab, M.; Abdel Ghaffar, K.A.; Ezzatt, O.M.; Hassan, A.A.A.; El Ansary, M.M.S.; Gamal, A.Y. Regenerative potential of cultured gingival fibroblasts in treatment of periodontal intrabony defects (randomized clinical and biochemical trial). J. Periodontal Res. 2020, 55, 441–452. [Google Scholar] [CrossRef]
- Guo, B.; Tang, C.; Wang, M.; Zhao, Z.; Shokoohi-Tabrizi, H.A.; Shi, B.; Andrukhov, O.; Rausch-Fan, X. In vitro biocompatibility of biohybrid polymers membrane evaluated in human gingival fibroblasts. J. Biomed. Mater. Res. Part. B 2020, 108, 2590–2598. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral. Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef]
- Dave, J.R.; Chandekar, S.S.; Behera, S.; Desai, K.U.; Salve, P.M.; Sapkal, N.B.; Mhaske, S.T.; Dewle, A.M.; Pokare, P.S.; Page, M.; et al. Human gingival mesenchymal stem cells retain their growth and immunomodulatory characteristics independent of donor age. Sci. Adv. 2022, 8, eabm6504. [Google Scholar] [CrossRef]
- Grawish, M.E.; Saeed, M.A.; Sultan, N.; Scheven, B.A. Therapeutic Applications of Dental Pulp Stem Cells in Regenerating Dental, Periodontal and Oral-Related Structures. World J. Meta-Anal. 2021, 9, 176–192. [Google Scholar] [CrossRef]
- Ma, L.; Liu, A.Q.; Guo, H.; Xuan, K. Dental pulp stem cells in tooth regeneration: Advancement and emerging directions. Chin. J. Stomatol. 2024, 59, 496–501. [Google Scholar]
- Cai, H.; Xu, X.; Lu, X.; Zhao, M.; Jia, Q.; Jiang, H.-B.; Kwon, J.-S. Dental Materials Applied to 3D and 4D Printing Technologies: A Review. Polymers 2023, 15, 2405. [Google Scholar] [CrossRef]
- Zeng, W.Y.; Ning, Y.; Huang, X. Advanced technologies in periodontal tissue regeneration based on stem cells: Current status and future perspectives. J. Dent. Sci. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Hassanpour, M.; Narongdej, P.; Alterman, N.; Moghtadernejad, S.; Barjasteh, E. Effects of Post-Processing Parameters on 3D-Printed Dental Appliances: A Review. Polymers 2024, 16, 2795. [Google Scholar] [CrossRef]
- Alharbi, S.; Alshabib, A.; Algamaiah, H.; Aldosari, M.; Alayad, A. Influence of Post-Printing Polymerization Time on Flexural Strength and Microhardness of 3D Printed Resin Composite. Coatings 2025, 15, 230. [Google Scholar] [CrossRef]
- Bayarsaikhan, E.; Gu, H.; Hwangbo, N.-K.; Lim, J.-H.; Shim, J.-S.; Lee, K.-W.; Kim, J.-E. Influence of different postcuring parameters on mechanical properties and biocompatibility of 3D printed crown and bridge resin for temporary restorations. J. Mech. Behav. Biomed. Mater. 2022, 128, 105127. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ. U.S. National Institute of Health, Bethesda, MD, USA, 1997–2018. Available online: http://imagej.net/ij/ (accessed on 14 November 2024).
- Alami, S.Y.; Hampton, J.W.; Race, G.J.; Speer, R.J. Fibrin stabilizing factor (factor XIII). Am. J. Med. 1968, 44, 1. [Google Scholar] [CrossRef]
- Anilkumar, T.V.; Muhamed, J.; Jose, A.; Jyothi, A.; Mohanan, P.V.; Krishnan, L.K. Advantages of hyaluronic acid as a component of fibrin sheet for care of acute wound. Biologicals 2011, 39, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Mellor, S.; Hao, L.; Zhang, D. Additive manufacturing: A framework for implementation. Int. J. Prod. Econ. 2014, 149, 194–201. [Google Scholar] [CrossRef]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J.N. Additive technology: Update on current materials and applications in dentistry. J. Prosthodont. 2017, 26, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Meyers, M.J.; Zandinejad, A.; Özcan, M. A review on chemical composition, mechanical properties, and manufacturing work flow of additively manufactured current polymers for interim dental restorations. J. Esthet. Restor. Dent. 2019, 31, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Kalberer, N.; Kamnoedboon, P.; Mekki, M.; Durual, S.; Özcan, M.; Müller, F. CAD-CAM complete denture resins: An evaluation of biocompatibility, mechanical properties, and surface characteristics. J. Dent. 2021, 114, 103785. [Google Scholar] [CrossRef] [PubMed]
- Konidena, A. 3D printing: Future of dentistry? J. Indian. Acad. Oral. Med. Radiol. 2016, 28, 109. [Google Scholar] [CrossRef]
- Alshamrani, A.; Alhotan, A.; Kelly, E.; Ellakwa, A. Mechanical and biocompatibility properties of 3D-printed dental resin reinforced with glass silica and zirconia nanoparticles: In vitro study. Polymers 2023, 15, 2523. [Google Scholar] [CrossRef]
- Piedra-Cascón, W.; Krishnamurthy, V.R.; Att, W.; Revilla-León, M. 3D printing parameters, supporting structures, slicing, and post-processing procedures of vat-polymerization additive manufacturing technologies: A narrative review. J. Dent. 2021, 109, 103630. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lin, C.-H.; Lin, Y.-M.; Lai, Y.-L.; Lee, S.-Y. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2020, 123, 349–354. [Google Scholar] [CrossRef]
- Almeida, S.M.; Meereis, C.T.; Leal, F.B.; Carvalho, R.V.; Boeira, P.O.; Chisini, L.A.; Cuevas-Suárez, C.E.; Lima, G.S.; Piva, E. Evaluation of alternative photoinitiator systems in two-step self-etch adhesive systems. Dent. Mater. 2020, 36, e29–e37. [Google Scholar] [CrossRef]
- Zeng, B.; Cai, Z.; Lalevée, J.; Yang, Q.; Lai, H.; Xiao, P.; Liu, J.; Xing, F. Cytotoxic and cytocompatible comparison among seven photoinitiators-triggered polymers in different tissue cells. Toxicol. Vitr. 2021, 72, 105103. [Google Scholar] [CrossRef]
- Kurt, A.; Altintas, S.H.; Kiziltas, M.V.; Tekkeli, S.E.; Guler, E.M.; Kocyigit, A.; Usumez, A. Evaluation of residual monomer release and toxicity of self-adhesive resin cements. Dent. Mater. J. 2018, 37, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, S.; Mascarenhas, P.; Luís, A.R.; Catrau, M.; Bekman, E.; Ribeiro, A.C.; Félix, S.; Caldeira, J.; Barahona, I. In vitro and in silico evaluations of resin-based dental restorative material toxicity. Clin. Oral. Investig. 2020, 24, 2691–2700. [Google Scholar] [CrossRef] [PubMed]




| Brand Name | Manufacturer | Type | * Matrix | Processing Method |
|---|---|---|---|---|
| NicTone | MDC Dental | PMMA-based | Liquid: methyl methacrylate; powder: methacrylate copolymers, initiators, and pigments. | Conventional: auto polymerization |
| C&B MFH | NextDent | Methacrylate-based | Methacrylates 7,7,9(or 7,9,9)-trimethyl-4,13-dioxo-3,14-dioxa-5,12-diazahexadecane-1,16-diyl bismethacrylate; ethylene dimethacrylate; HEMA; TPO; E-BPA; titanium dioxide; mequinol; 4-methoxyphenol; hydroquinone monomethyl ether. | 3D printing: photopolymerization |
| Seed C&B | Leaf Dental | Methacrylate-based | Methyl methacrylate, ethyl methacrylate, urethane dimethacrylate (UDMA), Bisphenol A glycidyl methacrylate (BisGMA); photo initiators: camphor quinone and benzophenone; additives: diphenyl phthalate, aluminum hydroxide, and titanium dioxide. | 3D printing: photopolymerization |
| zDental C&B | UNIZ | Methacrylate-based | Only data available: Acrylate monomer, acrylate oligomers, and photo initiators. | 3D printing: photopolymerization |
| 1d | 4d | 10d | ANOVA p | |
|---|---|---|---|---|
| Nic Tone | 84.76 ± 7.67 a,£ | 75.67 ± 5.25 a,£ | 78.17 ± 12.81 a,£ | 0.494 |
| NextDent | 90.23 ± 7.60 a,£ | 74.35 ± 6.14 a,£,¥ | 68.48 ± 9.15 a,¥ | 0.032 * |
| Leaf Dental | 90.08 ± 9.24 a,£ | 73.63 ± 14.34 a,£ | 69.41 ± 3.23 a,£ | 0.095 |
| Uniz | 91.42 ± 1.86 a,£ | 78.41 ± 5.34 a,¥ | 75.98 ± 5.99 a,¥ | 0.015 * |
| ANOVA p | 0.517 | 0.908 | 0.466 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonio-Flores, M.; Castell-Rodríguez, A.E.; Piñón-Zárate, G.; Hernández-Téllez, B.; Flores-Ledesma, A.; Pérez-Martínez, E.; Sámano-Valencia, C.; Quiroz-Petersen, G.; Jarquín-Yáñez, K. Cell Viability of Wharton’s Jelly-Derived Mesenchymal Stem Cells (WJ-MSCs) on 3D-Printed Resins for Temporary Dental Restorations. J. Compos. Sci. 2025, 9, 404. https://doi.org/10.3390/jcs9080404
Antonio-Flores M, Castell-Rodríguez AE, Piñón-Zárate G, Hernández-Téllez B, Flores-Ledesma A, Pérez-Martínez E, Sámano-Valencia C, Quiroz-Petersen G, Jarquín-Yáñez K. Cell Viability of Wharton’s Jelly-Derived Mesenchymal Stem Cells (WJ-MSCs) on 3D-Printed Resins for Temporary Dental Restorations. Journal of Composites Science. 2025; 9(8):404. https://doi.org/10.3390/jcs9080404
Chicago/Turabian StyleAntonio-Flores, Mónica, Andrés Eliú Castell-Rodríguez, Gabriela Piñón-Zárate, Beatriz Hernández-Téllez, Abigailt Flores-Ledesma, Enrique Pérez-Martínez, Carolina Sámano-Valencia, Gerardo Quiroz-Petersen, and Katia Jarquín-Yáñez. 2025. "Cell Viability of Wharton’s Jelly-Derived Mesenchymal Stem Cells (WJ-MSCs) on 3D-Printed Resins for Temporary Dental Restorations" Journal of Composites Science 9, no. 8: 404. https://doi.org/10.3390/jcs9080404
APA StyleAntonio-Flores, M., Castell-Rodríguez, A. E., Piñón-Zárate, G., Hernández-Téllez, B., Flores-Ledesma, A., Pérez-Martínez, E., Sámano-Valencia, C., Quiroz-Petersen, G., & Jarquín-Yáñez, K. (2025). Cell Viability of Wharton’s Jelly-Derived Mesenchymal Stem Cells (WJ-MSCs) on 3D-Printed Resins for Temporary Dental Restorations. Journal of Composites Science, 9(8), 404. https://doi.org/10.3390/jcs9080404






