Synthesis and Evaluation of a Photocatalytic TiO2-Ag Coating on Polymer Composite Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Synthesis of the Coatings
2.1.2. Substrate Preparation
2.2. Coating Methodology and Characterization
2.2.1. Coating Thickness Analysis
2.2.2. Analysis of Photocatalytic Activity
2.2.3. Analysis of Photocatalytic Performance
2.2.4. Optical Analysis
2.2.5. Analysis of Crystalline Properties
2.2.6. Microscopic Analysis
2.2.7. Analysis of Antimicrobial Properties
2.2.8. Analysis of Self-Cleaning Performance
3. Results
3.1. Characterization of Photoactive Coatings
3.2. Characterization of Photoactive Substrates
3.2.1. Crystalline and Optical Properties
3.2.2. Photocatalytic Activity Analysis
3.2.3. Photocatalytic Performance Analysis
3.2.4. Humectability
3.2.5. Self-Cleaning Performance
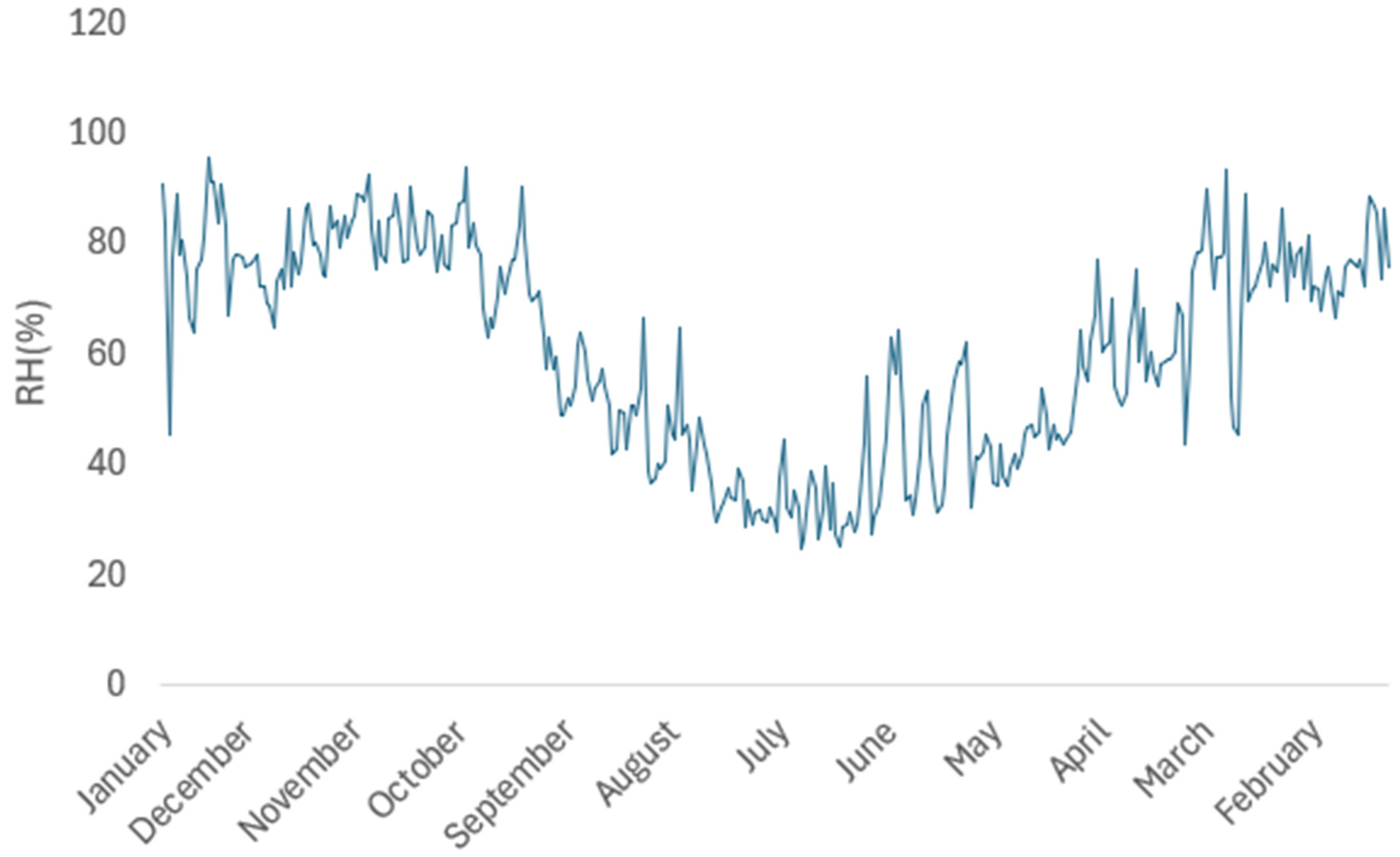
3.2.6. Analysis in Real Environmental Conditions
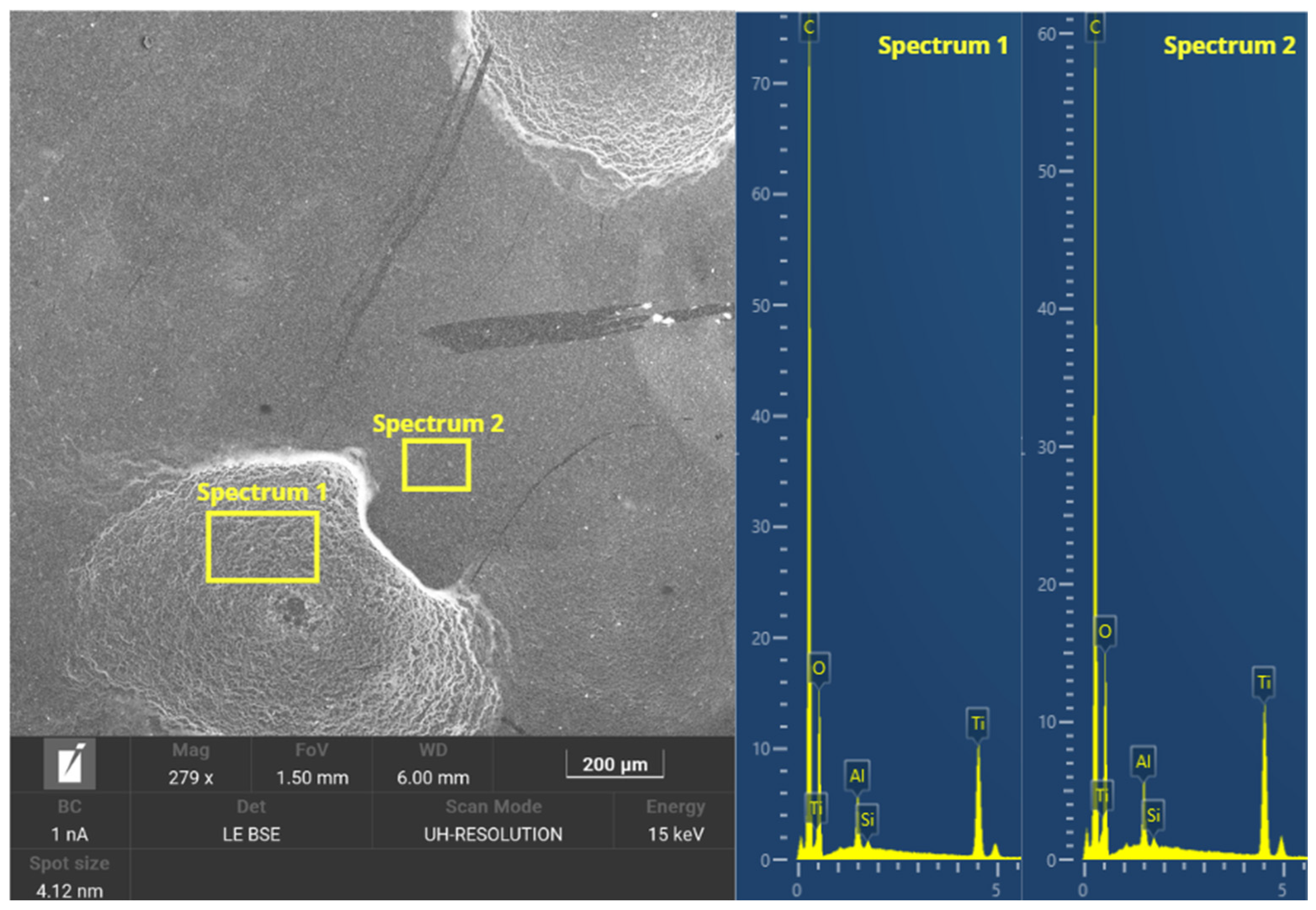
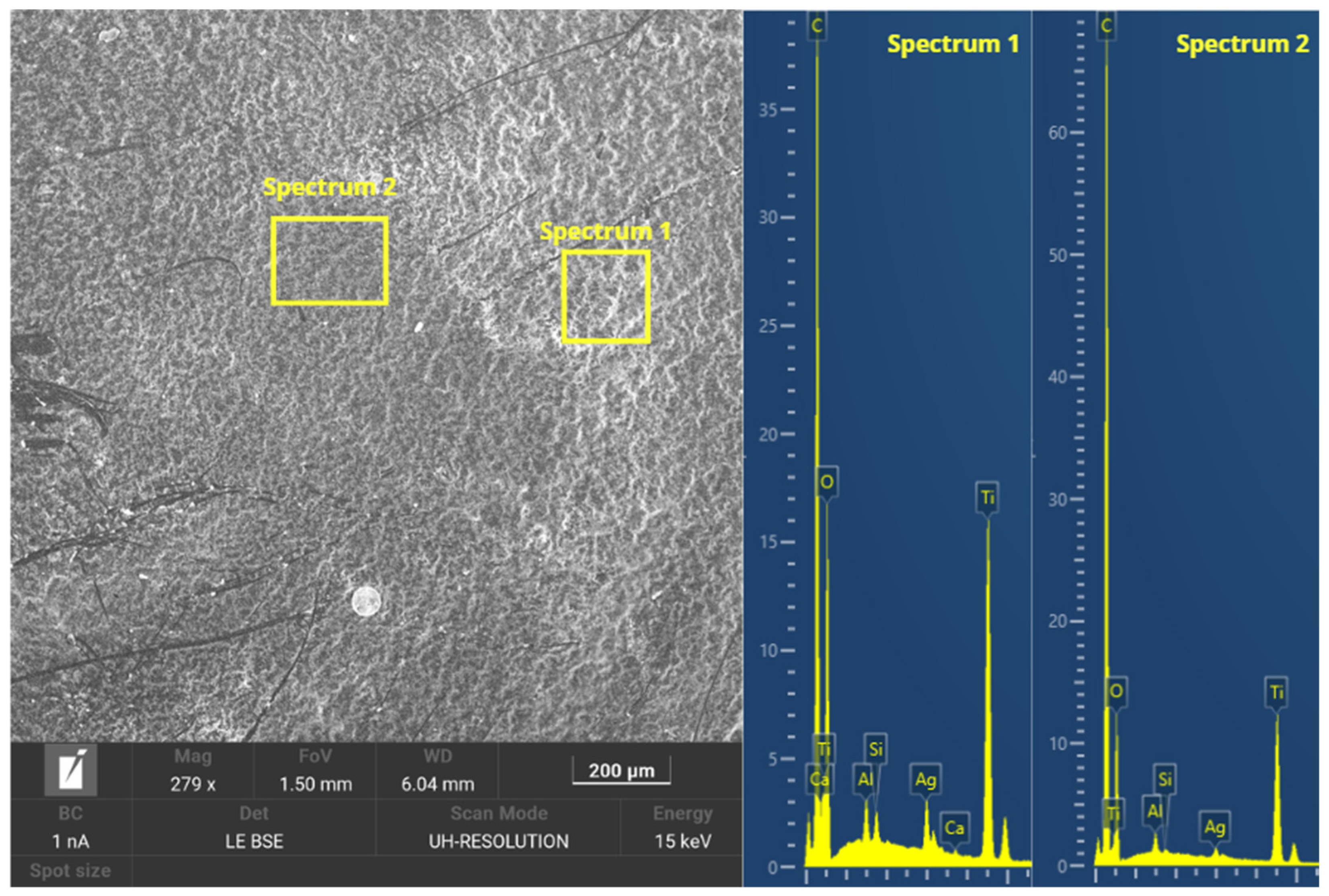
3.2.7. Morphological Analysis
3.2.8. Antimicrobial Properties
4. Conclusions
- The TiO2-Ag coatings presented a contact angle close to 10°, indicating an outstanding improvement in hydrophilic properties, a behavior that translates into higher interaction with water and higher efficiency in photocatalytic applications.
- Morphological and microstructural analysis showed that the dispersion of the TiO2-Ag coating was not homogeneous on the GFRE substrate, presenting agglomeration in certain areas, suggesting a worse adhesion to the material.
- Aging tests conducted under outdoor conditions showed that the TiO2-Ag coating on GFRP exhibits enhanced durability compared to other coatings, such as those based on GFRE, which showed signs of incompatibility between the resin and the photocatalytic layer after 351 days of exposure. This aging resistance gives TiO2-Ag coatings on GFRP substrates a considerable advantage in outdoor applications where durability is crucial. As a future line, the photocatalytic and antimicrobial properties will be reevaluated.
- The antiviral activity of the TiO2-Ag coating achieved 80% vaccinia virus conversion under UV-A irradiation, evidencing its ability to efficiently inactivate pathogens. This performance positions TiO2-Ag as a powerful option for applications in environments requiring microbial inactivation.
- As future lines, it is proposed to extend the application to biopolymers and to assess the environmental impact by means of life cycle analysis (LCA), promoting more sustainable solutions in construction and transport.
- Although the TiO2-Ag coating showed good performance, toxicity testing, leaching, and life cycle or cost analyses, key aspects for indoor application were not performed. These evaluations are considered future work.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GFRP | Glass-fiber-reinforced polyester |
| GFRE | Glass-fiber-reinforced epoxy |
References
- Wu, S.; Li, L.; Jiang, T.; Gui, Y.; Song, K.; Li, J.; Ma, D.; Zhang, Y.; Liang, L.; Zhang, Z.; et al. Excellent self-cleaning surface achieved through the synergistic effect of superhydrophilicity and photocatalysis of PVDF-TiO2-HAP coating. Surf. Interfaces 2024, 51, 104676. [Google Scholar] [CrossRef]
- Esteban-Cantillo, O.J.; Menendez, B.; Quesada, B. Climate change and air pollution impacts on cultural heritage building materials in Europe and Mexico. Sci. Total Environ. 2024, 921, 170945. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dakshina Murthy, N.R.; Sumanth, C. Effect of air pollution on building materials. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Meda, U.S.; Vora, K.; Athreya, Y.; Mandi, U.A. Titanium dioxide based heterogeneous and heterojunction photocatalysts for pollution control applications in the construction industry. Process Saf. Environ. Prot. 2022, 161, 771–787. [Google Scholar] [CrossRef]
- Byeon, H.; Sunil, J. Exploring the novel environmentally friendly highly hydrophobic TiO2 coating for enhanced anti-corrosion performance of steel in potential industrial applications. Results Chem. 2025, 14, 102087. [Google Scholar] [CrossRef]
- Yu, D.L.; Jiang, B.; Qi, X.; Wang, C.; Song, R.G. Study on the microstructure and comprehensive properties of black MAO/TiO2 coatings prepared on 6063 aluminum alloy. Mater. Today Commun. 2025, 42, 111317. [Google Scholar] [CrossRef]
- Liao, G.; Yao, W.; She, A. Enhanced self-cleaning capacity of RBP@TiO2 based building coating: Synergetic effect of photocatalysis and photo-induced superhydrophilicity. Constr. Build. Mater. 2023, 388, 131699. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. Multi-functional TiO2-based nanocomposite coating with durable superhydrophobicity and enhanced photocatalytic and antimicrobial properties for the sustainable maintenance of building stones. Constr. Build. Mater. 2023, 404, 133139. [Google Scholar] [CrossRef]
- Cedillo-González, E.I.; Hernández-López, J.M.; Ruiz-Valdés, J.J.; Barbieri, V.; Siligardi, C. Self-cleaning TiO2 coatings for building materials: The influence of morphology and humidity in the stain removal performance. Constr. Build. Mater. 2020, 237, 117692. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Shen, H.; Shuai, D.; Xiong, X.; Wang, Y.; Huang, H.; Li, Y. Superior self-cleaning surfaces via the synergy of superhydrophobicity and photocatalytic activity: Principles, synthesis, properties, and applications. J. Clean. Prod. 2023, 428, 139430. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, X.; Chen, D.; Ding, H. Fabrication of sericite-TiO2/HDTMS superhydrophobic self-cleaning coatings by hydrothermal method. J. Alloys Compd. 2025, 1014, 178677. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Yang, D.Q. A durable superhydrophilic self-cleaning coating based on TiO2–SiO2-PAA nanocomposite for photovoltaic applications: Long-term outdoor study. Sol. Energy Mater. Sol. Cells 2024, 268, 112731. [Google Scholar] [CrossRef]
- Bento, D.H.; Matias, M.L.; Magalhães, M.; Quitério, C.; Pimentel, A.; Sousa, D.; Amaral, P.; Galhano, C.; Fortunato, E.; Martins, R.; et al. Self-cleaning stone Façades using TiO2 Microwave-Synthesised Coatings. Clean. Mater. 2025, 15, 100294. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Xu, X.; Yang, L.; Wang, F. Study on photocatalytic performance of carbonated coating supported TiO2@SiO2. Constr. Build. Mater. 2024, 411, 134574. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Shen, Y.; Xu, O.; Wan, S.; Zhu, X. Construction of stable Ti3+-TiO2 photocatalytic membrane for enhanced photoactivity and emulsion separation. J. Membr. Sci. 2021, 618, 118748. [Google Scholar] [CrossRef]
- Nunes, D.; Fortunato, E.; Martins, R. Flexible nanostructured TiO2-based gas and UV sensors: A review. Discov. Mater. 2022, 2, 2. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, J.; Ma, R.; Lv, H.; Jiang, X.; Zhang, J.; Kong, L.; Shen, Y. The ultra efficient magnetic recyclable photocatalyst CoFe2O4/TiO2 based on kaolinite for mineral processing wastewater treatment. Environ. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Vajda, K.; Saszet, K.; Kedves, E.Z.; Kása, Z.; Danciu, V.; Baia, L.; Magyari, K.; Hernádi, K.; Kovács, G.; Pap, Z. Shape-controlled agglomeration of TiO2 nanoparticles. New insights on polycrystallinity vs. single crystals in photocatalysis. Ceram. Int. 2016, 42, 3077–3087. [Google Scholar] [CrossRef]
- Khalaghi, M.; Atapour, M.; Momeni, M.M.; Karampoor, M.R. Visible light photocatalytic efficiency and corrosion resistance of Zn, Ni, and Cu-doped TiO2 coatings. Results Chem. 2025, 13, 102032. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, D.; Jiang, Z.; Zheng, B.; Yu, Z. Effect of Cu content on the wear resistant behavior of arc-sprayed laminated-structured Cu–TiN/TiO2 composite coating. Ceram. Int. 2024, 50, 11966–11979. [Google Scholar] [CrossRef]
- Adebanjo, A.U.; Abbas, Y.M.; Shafiq, N.; Khan, M.I.; Farhan, S.A.; Masmoudi, R. Optimizing nano-TiO2 and ZnO integration in silica-based high-performance concrete: Mechanical, durability, and photocatalysis insights for sustainable self-cleaning systems. Constr. Build. Mater. 2024, 446, 138038. [Google Scholar] [CrossRef]
- Raes, A.; Minja, A.C.; AG, K.R.; Verbruggen, S.W. Recent advances in metal-doped defective TiO2 for photocatalytic CO2 conversion. Curr. Opin. Chem. Eng. 2024, 44, 101013. [Google Scholar] [CrossRef]
- Hassanin, E.A.E.M.; Ibrahim, H.; Moussa, I.; El-Aziz, E.A. Imparting self-cleaning and antimicrobial properties to cellulosic fabrics using Ti (3+) self-doped rutile TiO2 nanopowder. Int. J. Biol. Macromol. 2025, 304, 140783. [Google Scholar] [CrossRef] [PubMed]
- Irshad, A.; Jawad, R.; Ishtiaq, U.; Joly, N.; Bejaoui, B.; M’Hamdi, N.; Martin, P.; Mubashar, F. Unveiling the power of TiO2 doped ZnO nanomaterial as an effective antimicrobial solution in the leather industry. Heliyon 2024, 10, e38414. [Google Scholar] [CrossRef] [PubMed]
- Abualnaja, K.M.; ElAassar, M.R.; Ghareeb, R.Y.; Ibrahim, A.A.; Abdelsalam, N.R. Development of photo-induced Ag0/TiO2 nanocomposite coating for photocatalysis, self-cleaning and antimicrobial polyester fabric. J. Mater. Res. Technol. 2021, 15, 1513–1523. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wang, Y.; Gu, Z.; Chen, L. Bi-functional water-purification materials derived from natural wood modified TiO2 by photothermal effect and photocatalysis. RSC Adv. 2022, 12, 26245–26250. [Google Scholar] [CrossRef] [PubMed]
- Vindhya, P.S.; Suresh, S.; Kavitha, V.T. Evaluation of antimicrobial, cytotoxic, antioxidant and photocatalytic properties of Ni-doped TiO2 nanoparticles produced in green method. Ceram. Int. 2025, 51, 12901–12917. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Wang, H.; Lu, X.; Wang, Y.; Xu, M.; Tan, X.; Ju, J.; Shao, Y.; Liu, H.; et al. Preparation and antibacterial activity of Ag-doped TiO2 coating on the porous surface of aluminum. J. Mater. Res. Technol. 2024, 32, 1801–1808. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Z.; Herout, R.; Liu, C.; Yu, K.; Lange, D.; Godin, R.; Kizhakkedathu, J.N.; Troczynski, T.; Wang, R. Axial suspension plasma sprayed Ag-TiO2 coating for enhanced photocatalytic and antimicrobial properties. Surf. Interfaces 2024, 45, 103856. [Google Scholar] [CrossRef]
- Chobba, M.B.; Weththimuni, M.L.; Messaoud, M.; Urzi, C.; Bouaziz, J.; de Leo, F.; Licchelli, M. Ag-TiO2/PDMS nanocomposite protective coatings: Synthesis, characterization, and use as a self-cleaning and antimicrobial agent. Prog. Org. Coat. 2021, 158, 106342. [Google Scholar] [CrossRef]
- Yan, L.; Xu, H. Lightweight composite materials in automotive engineering: State-of-the-art and future trends. Alex. Eng. J. 2025, 118, 1–10. [Google Scholar] [CrossRef]
- Nayak, S.; Rout, P.K.; Jena, P.K. Investigation of mechanical, thermal and morphological properties of rattan fiber-reinforced polymer composites to produce lightweight material for automotive industries. Therm. Adv. 2025, 2, 100014. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Ishak, M.R.; Jawaid, M.; Sapuan, S.M.; Leman, Z. Low velocity impact properties of natural fiber-reinforced composite materials for aeronautical applications. Sustain. Compos. Aerosp. Appl. 2018, 293–313. [Google Scholar] [CrossRef]
- Kotcharat, P.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Development of bacterial cellulose and polycaprolactone (PCL) based composite for medical material. Sustain. Chem. Pharm. 2021, 20, 100404. [Google Scholar] [CrossRef]
- Ding, X.; Yu, Q.; Xue, H.; Zhang, W.; Ren, H.; Geng, J. Photochemical behavior of extracellular polymeric substances in intimately coupled TiO2 photocatalysis and biodegradation system. Bioresour. Technol. 2025, 416, 131752. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, R.; Fanaie, N.; Mousavi-Bafrouyi, S.M.S.; Lee, J. Experimental investigation on flexural behavior and energy absorption of lightweight sandwich panels with aluminum honeycomb core embedded by thin-ply carbon-glass fibers/epoxy face sheets. Structures 2024, 60, 105961. [Google Scholar] [CrossRef]
- Frazão, C.; Barros, J.; Toledo Filho, R.; Ferreira, S.; Gonçalves, D. Development of sandwich panels combining Sisal Fiber-Cement Composites and Fiber-Reinforced Lightweight Concrete. Cem. Concr. Compos. 2018, 86, 206–223. [Google Scholar] [CrossRef]
- Yudha, N.K.; Nugroho, A.D.; Erlangga, W.; Jamasri Fiedler, B.; Muflikhun, M.A. Sustainable High-Performance Materials: The Role of Bamboo and Glass Fibers in Hybrid Composites. Hybrid Adv. 2025, 9, 100416. [Google Scholar] [CrossRef]
- Hasan, M.M.; Islam, M.A.; Hassan, T. Analysis of jute-glass fiber reinforced epoxy hybrid composite. Heliyon 2024, 10, e40924. [Google Scholar] [CrossRef] [PubMed]
- Devendrappa, S.K.; Puttegowda, M.; Ballupete Nagaraju, S. Enhancing wear resistance, mechanical properties of composite materials through sisal and glass fiber reinforcement with epoxy resin and graphite filler. J. Indian Chem. Soc. 2024, 101, 101349. [Google Scholar] [CrossRef]
- Gurmu, D.N.; Gebrelibanos, H.M.; Tefera, C.A.; Sirahbizu, B. Experimental investigation the effect of bamboo micro filler on performance of bamboo-sisal-E-glass fiber-reinforced epoxy matrix hybrid composites. Heliyon 2024, 10, e40176. [Google Scholar] [CrossRef] [PubMed]
- Salino, R.E.; Catai, R.E. A study of polyurethane waste composite (PUR) and recycled plasterboard sheet cores with polyurethane foam for acoustic absorption. Constr. Build. Mater. 2023, 387, 131201. [Google Scholar] [CrossRef]
- Liang, F.; Chen, Q.; Shi, Z.; Dan, R.; Liang, H.; Feng, X.; Tang, C.; Yu, X.; Shang, X.; Zhang, D.; et al. Multifunctional composites made from waste polyester cotton fabric and waste rigid polyurethane foam for potential building wall material applications. Energy Build. 2025, 329, 115274. [Google Scholar] [CrossRef]
- Rathi, J.S.L.; Ruban, Y.J.V.; Ginil Mon, S. Waste iron swarf reinforced epoxy/unsaturated polyester particulate composite films fabrication, characterization and peculiarities. J. Indian Chem. Soc. 2025, 102, 101619. [Google Scholar] [CrossRef]
- Gervásio, H.; Simões da Silva, L.; Eizaguirre-Iribar, A.; Olano-Azkune, X.; Lange, J.; Pradhan, E.M.; Renaux, T.; Gelders, T.; Huet, V.; Izabel, D.; et al. Experimental characterization of the mechanical and functional performance of innovative ultra-low carbon sandwich panels and envelope systems for buildings. Thin-Walled Struct. 2025, 210, 112999. [Google Scholar] [CrossRef]
- Castillo-Lara, J.F.; Flores-Johnson, E.A.; Valadez-Gonzalez, A.; Herrera-Franco, P.J.; Carrillo, J.G.; Gonzalez-Chi, P.I.; Agaliotis, E.; Li, Q.M. Mechanical behaviour of composite sandwich panels with foamed concrete core reinforced with natural fibre in four-point bending. Thin-Walled Struct. 2021, 169, 108457. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Z.W.; Xu, B.; Zhou, C.; Zhang, Z. Investigating the static flexural properties of prefabricated sandwich panels utilizing bamboo fiber recycled aggregate concrete. Eng. Struct. 2025, 326, 119558. [Google Scholar] [CrossRef]
- Liang, Y.C.; Kuo, C.C. Enhanced dual function of Ag nanoparticles decorated one-dimensional polymorphic TiO2 composites for sustainable environmental applications. J. Sci. Adv. Mater. Devices 2024, 9, 100696. [Google Scholar] [CrossRef]
- Stojadinović, S.; Radić, N.; Vasilić, R.; Petković, M.; Stefanov, P.; Zeković, L.; Grbić, B. Photocatalytic properties of TiO2/WO3 coatings formed by plasma electrolytic oxidation of titanium in 12-tungstosilicic acid. Appl. Catal. B Environ. 2012, 126, 334–341. [Google Scholar] [CrossRef]
- Du, Q.; Lin, Y.; Cheng, S.; Wei, D.; Wang, Y.; Zhou, Y. In situ synthesis of three-dimensional flower-like TiO2/WO3 heterojunction: Enhanced visible photocatalytic properties and theoretical calculations. Ceram. Int. 2024, 50, 30605–30616. [Google Scholar] [CrossRef]
- Szołdra, P.; Frąc, M.; Pichór, W. Photocatalytic degradation of nitrogen oxides (NOx) in a continuous-flow photoreactor with aluminosilicate microspheres coated with TiO2 thin films. Chem. Eng. Process.-Process Intensif. 2024, 202, 109856. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, K.; He, W.; Luo, Y.; Wang, X.; Le, H. Enhanced tribological and antimicrobial performance by sputtered heterojunction of ZnO-TiO2 multilayer thin film. Surf. Interfaces 2025, 58, 105835. [Google Scholar] [CrossRef]
- Paniagua-Méndez, J.; Ramírez-Sandoval, S.L.; Reyes-Uribe, E.; Contreras-García, M.E. Photocatalytic activity and biocide effect of nanostructured SnO2/ZnO/TiO2 thin film heterostructure obtained by sol-gel spin coating technique. Ceram. Int. 2024, 50, 34421–34430. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, L.; Xin, Y.; Lu, Y.; Guo, M.; Liu, P.; Xu, H.; Duan, T. Effect of coating layers on microstructure and electrochemical properties of Ti/RuO2-IrO2-TiO2 anode prepared by polymeric sol-gel method. Int. J. Electrochem. Sci. 2025, 20, 100957. [Google Scholar] [CrossRef]
- Valenzuela Expósito, J.J.; Picazo Camilo, E.; Corpas Iglesias, F.A. Development of a Modular Sandwich Panel with a Composite Core of Recycled Material for Application in Sustainable Building. Polymers 2024, 16, 3604. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M. The effect of pH on the physical and structural properties of TiO2 nanoparticles. J. Cryst. Growth 2022, 585, 126603. [Google Scholar] [CrossRef]
- Muhmood, T.; Cai, Z.; Lin, S.; Xiao, J.; Hu, X. Dimensions controllable synthesis of silver Nano-morphologies via moderate one step methodology. Adv. Powder Technol. 2021, 32, 3388–3394. [Google Scholar] [CrossRef]
- Muhmood, T.; Ahmad, F.; Hu, X.; Yang, X. Silver nanowires: A focused review of their synthesis, properties, and major factors limiting their commercialization. Nano Futures 2022, 6, 032006. [Google Scholar] [CrossRef]
- Ling, L.; Feng, Y.; Li, H.; Chen, Y.; Wen, J.; Zhu, J.; Bian, Z. Microwave induced surface enhanced pollutant adsorption and photocatalytic degradation on Ag/TiO2. Appl. Surf. Sci. 2019, 483, 772–778. [Google Scholar] [CrossRef]
- Hariharan, D.; Thangamuniyandi, P.; Jegatha Christy, A.; Vasantharaja, R.; Selvakumar, P.; Sagadevan, S.; Pugazhendhi, A.; Nehru, L.C. Enhanced photocatalysis and anticancer activity of green hydrothermal synthesized Ag@TiO2 nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 202, 111636. [Google Scholar] [CrossRef] [PubMed]
- ISO 10678:2010; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. ISO: Geneva, Switzerland, 2010. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/iso?c=046019 (accessed on 5 March 2025).
- ISO 27448:2009; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Self-Cleaning Performance of Semiconducting Photocatalytic Materials—Measurement of Water Contact Angle. ISO: Geneva, Switzerland, 2009. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/iso?c=053953 (accessed on 5 March 2025).
- ISO 22197-1:2016; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide. ISO: Geneva, Switzerland, 2016. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/iso?c=065416 (accessed on 5 March 2025).
- ISO 21702:2019; Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2019. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/iso?c=071365 (accessed on 5 March 2025).
- ISO 18061:2014; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Antiviral Activity of Semiconducting Photocatalytic Materials—Test Method Using Bacteriophage Q-Beta. ISO: Geneva, Switzerland, 2014. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/iso?c=061245 (accessed on 5 March 2025).
- Masar, M.; Ali, H.; Guler, A.C.; Urbanek, M.; Urbanek, P.; Hanulikova, B.; Pistekova, H.; Annusova, A.; Machovsky, M.; Kuritka, I. Multifunctional bandgap-reduced ZnO nanocrystals for photocatalysis, self-cleaning, and antibacterial glass surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130447. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, C.C.; Rahman, K.H.; Chen, K.C. A study on photodegradation of trichloroethylene using an optical fiber coated with different photocatalysts. Mater. Sci. Semicond. Process. 2023, 163, 107538. [Google Scholar] [CrossRef]
- Ajmal, Z.; Naciri, Y.; Ahmad, M.; Hsini, A.; Bouziani, A.; Laabd, M.; Raza, W.; Murtaza, A.; Kumar, A.; Ullah, S.; et al. Use of conductive polymer-supported oxide-based photocatalysts for efficient VOCs & SVOCs removal in gas/liquid phase. J. Environ. Chem. Eng. 2023, 11, 108935. [Google Scholar] [CrossRef]
- Guo, H.; Li, D.S.; Li, Q.; Qiu, G.X.; Hu, Y.; Liu, S.H. High-speed laser cladding (Fe,Cu)-dopped Ni-Co protective coatings for SOFC MICs: From long-term oxidation behavior to mechanical stability. Ceram. Int. 2024, 50, 48246–48256. [Google Scholar] [CrossRef]
- Izmirli, S.; Cavdar, S. Effect of film thickness on photodiode and photodetector performance of titanium dioxide (TiO2) prepared via solution assisted spin coating method. Appl. Surf. Sci. 2025, 688, 162187. [Google Scholar] [CrossRef]
- Skowronski, L.; Chorobinski, M. The effect of thickness and optical constants of the dielectric layer on the color behaviour of the glass/Ti/TiO2 decorative coatings. Thin Solid Film. 2019, 691, 137595. [Google Scholar] [CrossRef]
- Yuan, Y.; Niu, Y.; Xu, H.; Fang, C.; Liu, W.; Song, B.; Li, M.; Shao, G.; Lu, H.; Wang, H.; et al. High efficiency removal of organic pollutant via the synergistic effect between adsorption and photocatalysis of rGO/TiO2/g-C3N4 composite aerogel. Ceram. Int. 2025, 51, 8965–8979. [Google Scholar] [CrossRef]
- Karamat, A.; Khan, M.I.; Mujtaba, A.; Atif, M.; Zar Muaawia, A.; Flores-Valenzuela, J.; Ali, B.; Shahid, W.; Hussain, S. Revolutionizing photocatalysis: Synergistic enhancement of structural, optical, and photodegradation properties in Be-doped V2O5 nanoparticles for efficient methylene blue removal. Results Chem. 2024, 8, 101600. [Google Scholar] [CrossRef]
- Ye, J.; Tian, D.; Song, W.; Lin, H.; Fan, Q.; Li, X. Preparation of magnetic ZnO/ZnFe2O4/CCA for highly efficient removal of methylene blue by synergistic adsorption-photocatalysis under visible light irradiation. J. Alloys Compd. 2025, 1010, 177808. [Google Scholar] [CrossRef]
- Liyanaarachchi, H.; Thambiliyagodage, C.; Liyanaarachchi, C.; Samarakoon, U. Efficient photocatalysis of Cu doped TiO2/g-C3N4 for the photodegradation of methylene blue. Arab. J. Chem. 2023, 16, 104749. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Wang, X.; Hu, N.; Li, Y.; Li, C.; Meng, Y.; An, Y. Synergistic effect of B-TiO2 and MIL-100(Fe) for high-efficiency photocatalysis in methylene blue degradation. Appl. Surf. Sci. 2021, 561, 149969. [Google Scholar] [CrossRef]
- Gbajabiamila, A.T.; Elemike, E.E.; Akpeji, B.H.; Bawa, H.A.; Hossain, I.; Pere, C.; Anekwe-Nwekeaku, O.J. Enhanced solar-assisted degradation of Sunset Yellow dye using plant-mediated Ag-TiO2/Na-BNT nanocomposite: Synthesis, characterization, and performance optimization. J. Hazard. Mater. Adv. 2025, 18, 100682. [Google Scholar] [CrossRef]
- Lin, K.; Wang, Z.; Hu, Z.; Luo, P.; Yang, X.; Zhang, X.; Rafiq, M.; Huang, F.; Cao, Y. Amino-functionalised conjugated porous polymers for improved photocatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 19087–19093. [Google Scholar] [CrossRef]
- Zhu, H.; Cai, S.; Liao, G.; Gao, Z.F.; Min, X.; Huang, Y.; Jin, S.; Xia, F. Recent Advances in Photocatalysis Based on Bioinspired Superwettabilities. ACS Catal. 2021, 11, 14751–14771. [Google Scholar] [CrossRef]
- Garlisi, C.; Palmisano, G. Radiation-free superhydrophilic and antifogging properties of e-beam evaporated TiO2 films on glass. Appl. Surf. Sci. 2017, 420, 83–93. [Google Scholar] [CrossRef]
- Bibi, Z.; Ali, M.; Abohashrh, M.; Ahmad, I.; Khan, H.; Ali, M.; Akbar, F.; Ahmad, N.; Iqbal, A.; Ullah, F.; et al. Biologically Synthesized Silver Nanoparticles Efficiently Control Plant Pathogenic Bacteria-Erwinia carotovora and Ralstonia solanacearum. Inorganics 2023, 11, 309. [Google Scholar] [CrossRef]

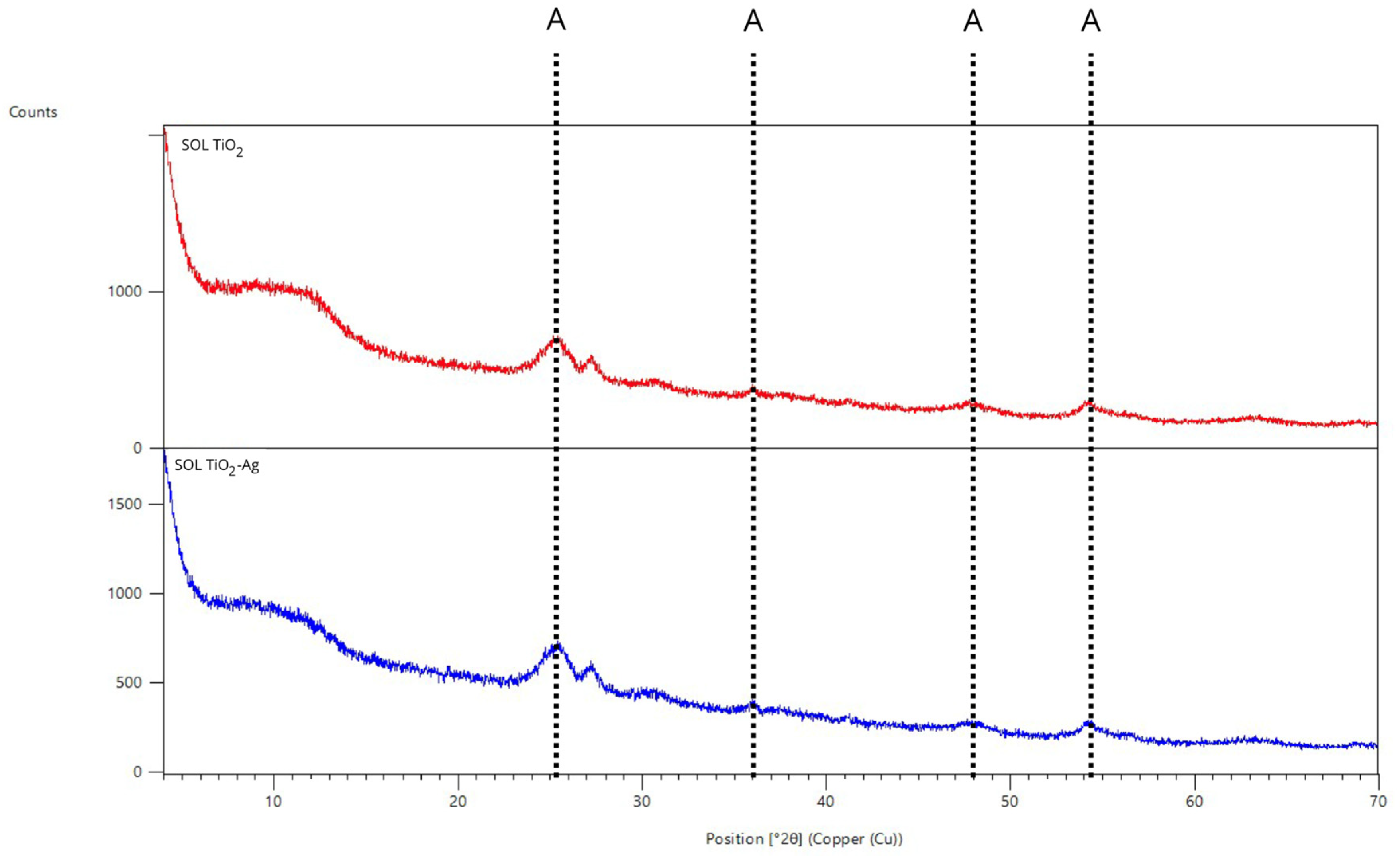
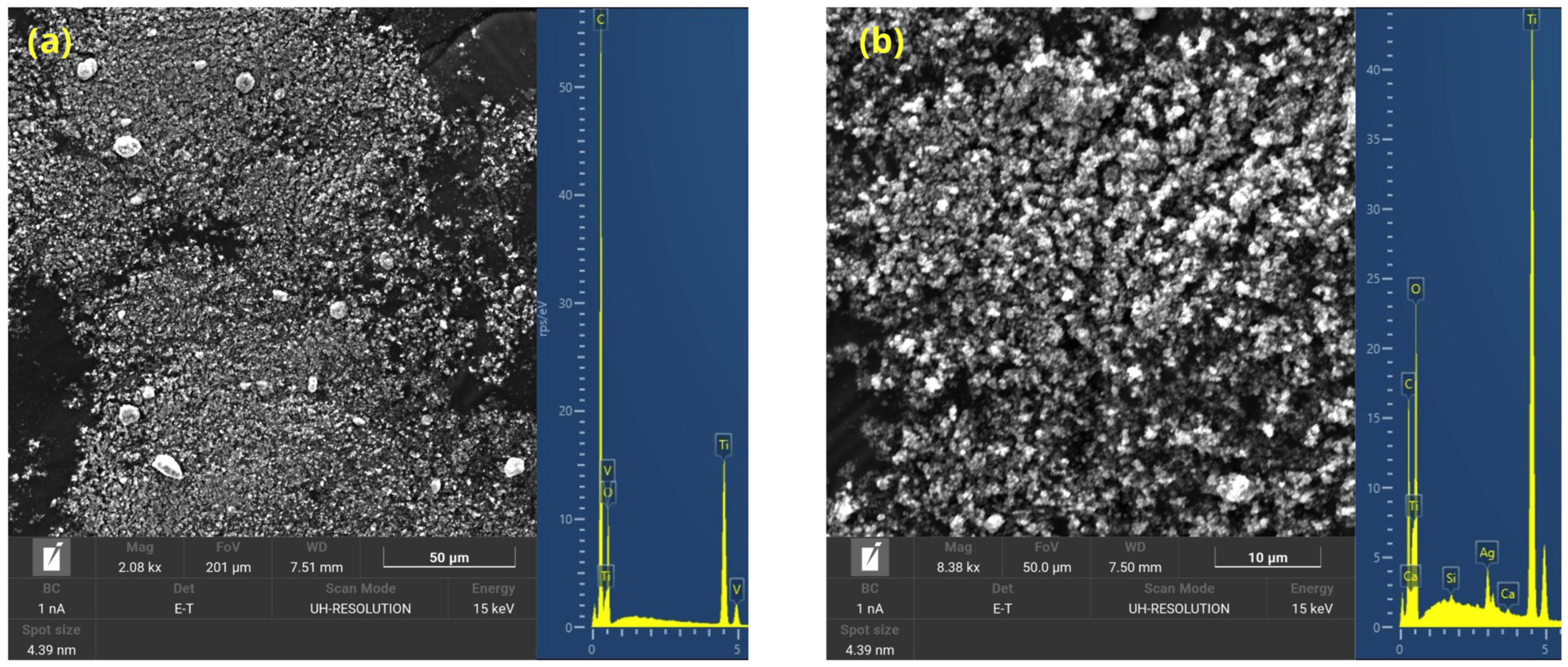
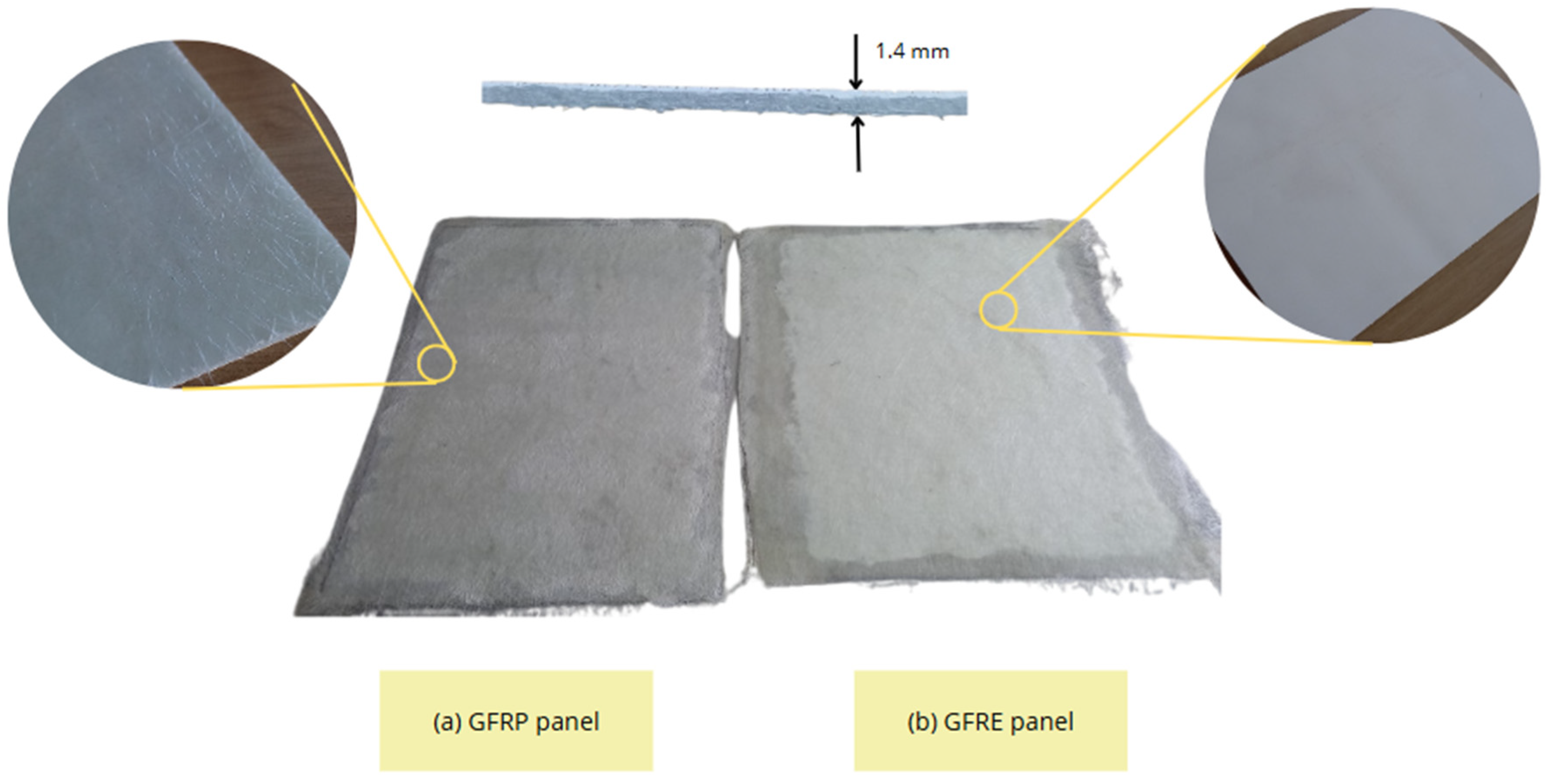






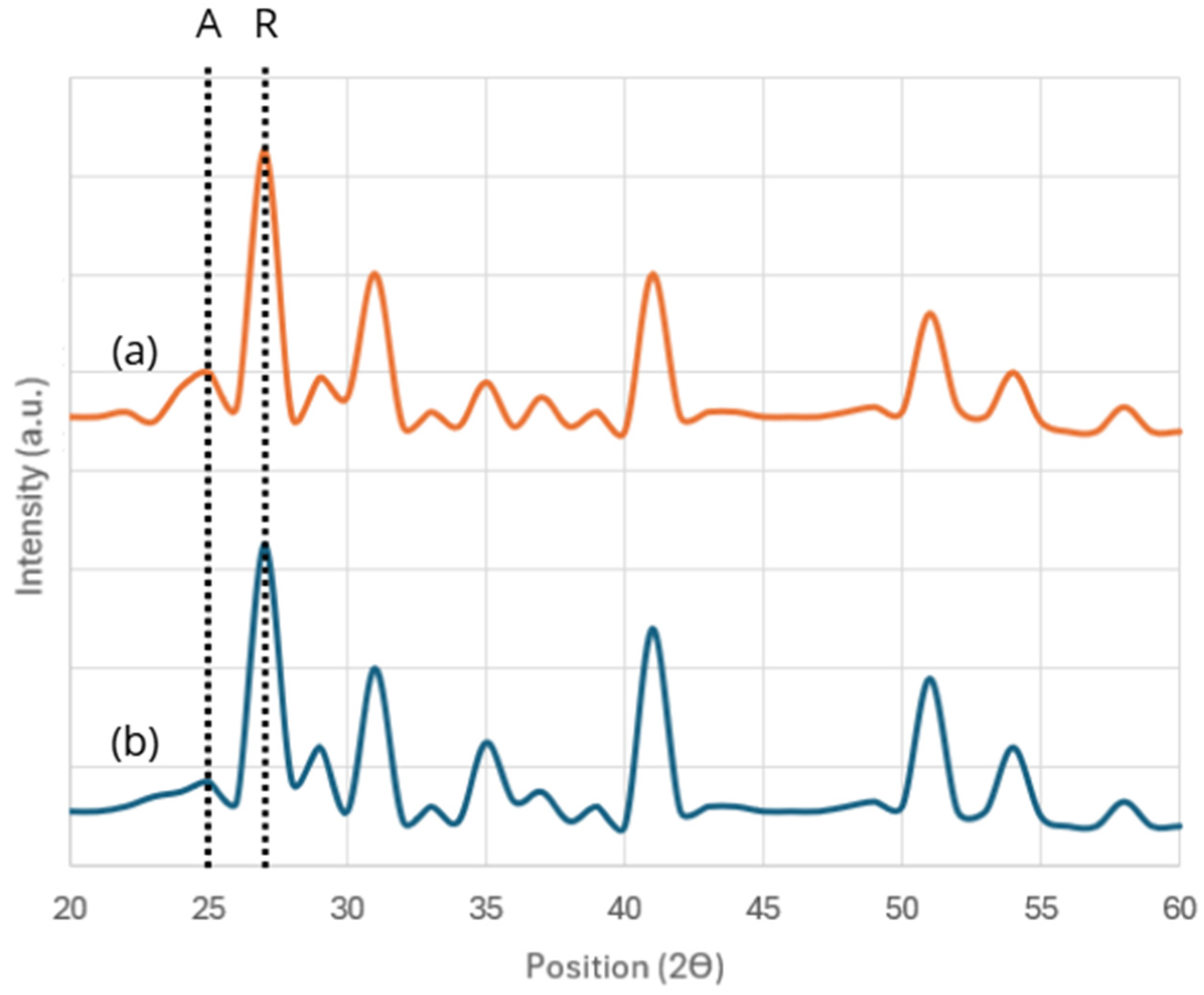









| Resin Type | Curing Temperature (°C) | Time Types | Waiting Time Range (Minutes) | Viscosity (cps) | Density (Kg/m3) |
|---|---|---|---|---|---|
| Polyester resin 5-1026 | 25 | Open time | 16–20 | 4000 (20 °C) | 1.109 |
| Hardening time | 32–37 | ||||
| Curing time | 1440 | ||||
| SikaBiresin CR75 | 20–22 | Open time | 10–15 | 1800 (25 °C) | 1.160 |
| Hardening time | 30–35 | ||||
| Curing time | 1440 |
| Parameter | Standard | Equipment |
|---|---|---|
| UV–Vis spectrophotometry | - | Spectrophotometer Shimadzu UV-1800 (Shimadzu, Kyoto, Japan) |
| UV-A spectrophotometry | - | Spectroradiometer Ramses ACC-UV (Trios GmbH, Rastede, Germany) |
| Diffuse reflectance UV–Vis | - | Spectrophotometer Agilent 8453 (Agilent Technologies Deutschland GmbH, Waldbronn, Germany) |
| Methylene blue (MB) | ISO 10678:2010 [61] | Spectrophotometer Shimadzu UV-1800 (Jenck S.A., Buenos Aires, Argentina) |
| Oleic acid (AO) | ISO 27448:2009 [62] | Krüss Easy Drop (Krüss GmbH, Hamburg, Germany) |
| TCE degradation | ISO 22197-1:2016 [63] | Spectrophotometer Shimadzu UV-1800 and Spectroradiometer Ramses ACC-UV (Trios GmbH, Rastede, Germany) |
| Ellipsometry | - | Spectroscopic ellipsometer GES-5E (Sopra Semilab, Budapest, Hungary) |
| DRX | - | X’Pert Pro PANalytical (Malvern Panalytical, Almelo, The Netherlands) |
| SEM-EDX | - | Microscope Carl Zeiss Merlin (Carl Zeiss AG, Oberkochen, Germany) |
| Antimicrobial properties | ISO 21702:2019 [64] ISO 18061:2014 [65] | Spectroradiometer Ramses ACC-UV (Trios GmbH, Rastede, Germany) |
| GFRE | GFRP |
|---|---|
 79.0° ± 3.6 |  78.2° ± 2.9 |
| Sample | WCA0 | WCAf |
|---|---|---|
| GFRE—TiO2-Ag |  60.2° ± 4.2 |  10.3° ± 3.4 |
| GFRP—TiO2-Ag |  58.4° ± 4.0 |  9.7° ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Expósito, J.J.V.; Picazo Camilo, E.; Corpas Iglesias, F.A. Synthesis and Evaluation of a Photocatalytic TiO2-Ag Coating on Polymer Composite Materials. J. Compos. Sci. 2025, 9, 383. https://doi.org/10.3390/jcs9080383
Expósito JJV, Picazo Camilo E, Corpas Iglesias FA. Synthesis and Evaluation of a Photocatalytic TiO2-Ag Coating on Polymer Composite Materials. Journal of Composites Science. 2025; 9(8):383. https://doi.org/10.3390/jcs9080383
Chicago/Turabian StyleExpósito, Juan José Valenzuela, Elena Picazo Camilo, and Francisco Antonio Corpas Iglesias. 2025. "Synthesis and Evaluation of a Photocatalytic TiO2-Ag Coating on Polymer Composite Materials" Journal of Composites Science 9, no. 8: 383. https://doi.org/10.3390/jcs9080383
APA StyleExpósito, J. J. V., Picazo Camilo, E., & Corpas Iglesias, F. A. (2025). Synthesis and Evaluation of a Photocatalytic TiO2-Ag Coating on Polymer Composite Materials. Journal of Composites Science, 9(8), 383. https://doi.org/10.3390/jcs9080383







