Perspective on Sustainable Solutions for Mitigating Off-Gassing of Volatile Organic Compounds in Asphalt Composites
Abstract
1. Introduction
1.1. Assessment of the Physicochemical Properties of Biochar, Activated Carbon, and Carbon Nanotubes
1.2. Comparative Environmental Impact: Activated Carbon vs. Biochar
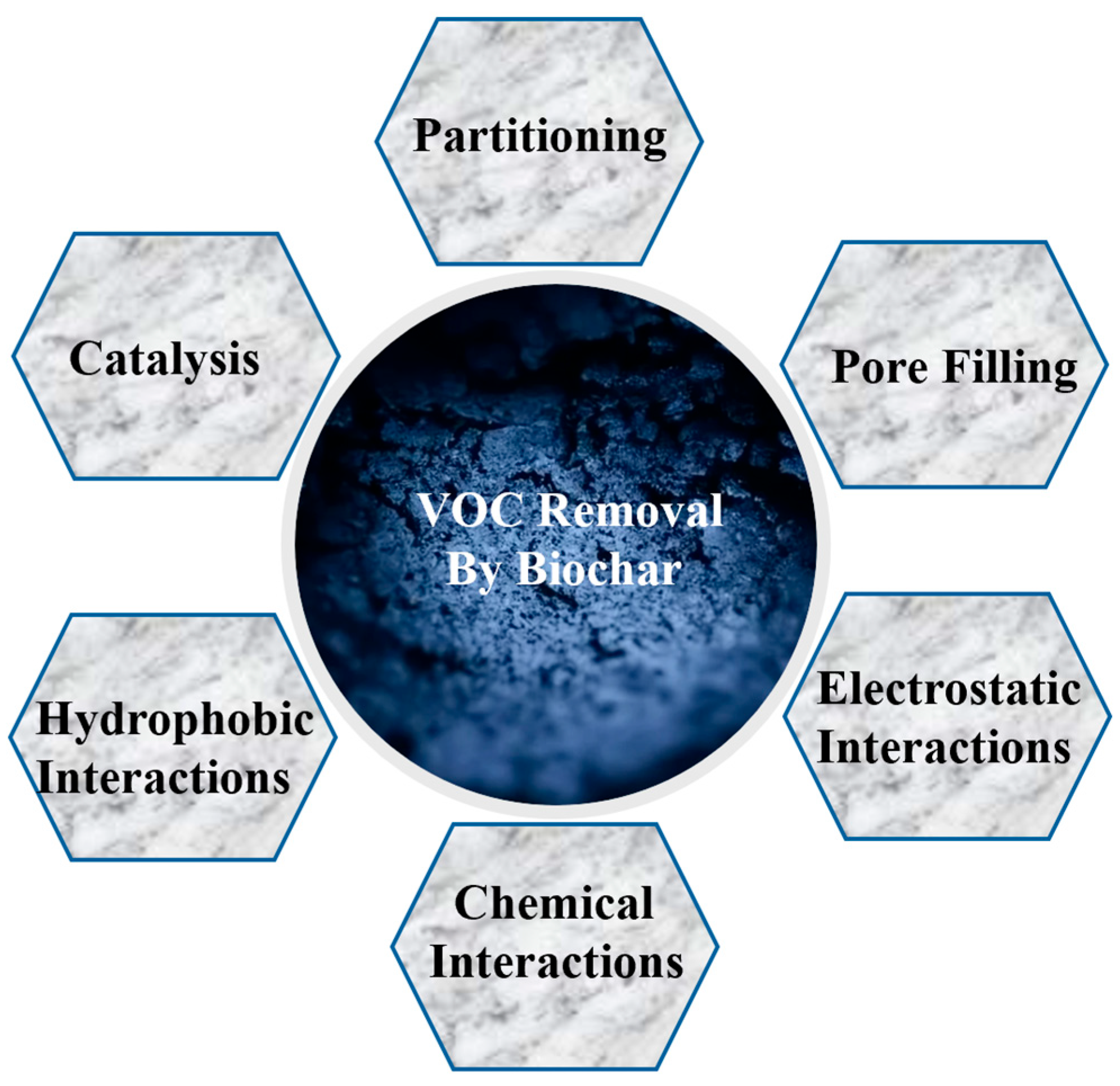
2. Biochar’s Multi-Faceted Mechanisms in Capturing Air Pollutants
- Atmospheric Aerosols and Greenhouse Gases
3. Asphalt Emissions: A Critical Non-Combustion Contributor to Air Pollution
The Main Components of Asphalt VOCs
4. Asphalt Aging: Evaporation of Lightweight Components
5. Adsorption Performance of Biochar in Asphalt: Impact on Air Quality and Asphalt Durability
Biochar’s Impact on Asphalt: Delaying Aging and Enhancing Performance
6. Biochar as a VOC Adsorbent in Asphalt
Biochar as a Carbon Negative Adsorbent
7. Research Gaps and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Gaunt, J.L.; Lehmann, J. Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ. Sci. Technol. 2008, 42, 4152–4158. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Blenis, N.; Hue, N.; Maaz, T.M.; Kantar, M. Biochar production, modification, and its uses in soil remediation: A review. Sustainability 2023, 15, 3442. [Google Scholar] [CrossRef]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, economical, and climate-related aspects of biochar production technologies: A literature review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Mehta, R.; Tiwari, A.; Sinha, A.; Bakshi, H.S.; Chellappa, V.; Drewnowski, J. Waste to energy: A review of biochar production with emphasis on mathematical modelling and its applications. Heliyon 2023, 9, e14873. [Google Scholar] [CrossRef]
- Park, K.-B.; Chae, D.-Y.; Fini, E.H.; Kim, J.-S. Pyrolysis of biomass harvested from heavy-metal contaminated area: Characteristics of bio-oils and biochars from batch-wise one-stage and continuous two-stage pyrolysis. Chemosphere 2024, 355, 141715. [Google Scholar] [CrossRef]
- Dandamudi, K.P.R.; Murdock, T.; Lammers, P.J.; Deng, S.; Fini, E.H. Production of functionalized carbon from synergistic hydrothermal liquefaction of microalgae and swine manure. Resour. Conserv. Recycl. 2021, 170, 105564. [Google Scholar] [CrossRef]
- Karnati, S.R.; Høgsaa, B.; Zhang, L.; Fini, E.H. Developing carbon nanoparticles with tunable morphology and surface chemistry for use in construction. Constr. Build. Mater. 2020, 262, 120780. [Google Scholar] [CrossRef]
- Mandal, S.; Kunhikrishnan, A.; Bolan, N.; Wijesekara, H.; Naidu, R. Application of biochar produced from biowaste materials for environmental protection and sustainable agriculture production. In Environmental Materials and Waste; Elsevier: Amsterdam, The Netherlands, 2016; pp. 73–89. [Google Scholar]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Huang, T.; Lu, H.; Wu, X.-L.; Chen, Z.; Yang, H.; Wang, S.; Tang, Z.; Li, Z.; Hu, B. Biochar-based materials in environmental pollutant elimination, H2 production and CO2 capture applications. Biochar 2023, 5, 42. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Ahmad, M.; Usman, A.R.; Akanji, M.; Rafique, M.I. Advances in pyrolytic technologies with improved carbon capture and storage to combat climate change. Environ. Clim. Plant Veg. Growth 2020, 535–575. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B.; Zhang, M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B.; Wang, S. Carbon dioxide capture using various metal oxyhydroxide–biochar composites. Chem. Eng. J. 2016, 283, 826–832. [Google Scholar] [CrossRef]
- Yang, W.; Li, Y.; Shi, S.; Chen, H.; Shan, Y.; Liu, Y. Mercury removal from flue gas by magnetic iron-copper oxide modified porous char derived from biomass materials. Fuel 2019, 256, 115977. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide-biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total Environ. 2020, 711, 134847. [Google Scholar] [CrossRef]
- Tan, G.; Sun, W.; Xu, Y.; Wang, H.; Xu, N. Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour. Technol. 2016, 211, 727–735. [Google Scholar] [CrossRef]
- Zou, H.; Zhao, J.; He, F.; Zhong, Z.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Yu, F.; Bashir, M.A.; et al. Ball milling biochar iron oxide composites for the removal of chromium (Cr(VI)) from water: Performance and mechanisms. J. Hazard. Mater. 2021, 413, 125252. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Choi, J.-C.; Ko, J.H.; Park, Y.-K.; Park, S.H.; Jeong, K.-E.; Kim, S.-S.; Jeon, J.-K. The low-temperature SCR of NO over rice straw and sewage sludge derived char. Chem. Eng. J. 2010, 156, 321–327. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; Hu, Y.; Feng, Y.; Dai, X. Preparation of activated sewage sludge char for low temperature De-NOx and its CO emission inhibition. Chemosphere 2020, 251, 126330. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Chaukura, N.; Wenga, T.; Mtisi, M. Biochars as media for air pollution control systems: Contaminant removal, applications and future research directions. Sci. Total Environ. 2021, 753, 142249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, B.; Theng, B.K.; Lee, X.; Zhang, X.; Chen, M.; Xu, P. Removal performance, mechanisms, and influencing factors of biochar for air pollutants: A critical review. Biochar 2022, 4, 30. [Google Scholar] [CrossRef]
- Sri Shalini, S.; Palanivelu, K.; Ramachandran, A.; Raghavan, V. Biochar from biomass waste as a renewable carbon material for climate change mitigation in reducing greenhouse gas emissions—A review. Biomass Convers. Biorefin. 2021, 11, 2247–2267. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Müller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Wang, Y.; Wang, L.; Sun, Y.; Liu, L. Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capture Sci. Technol. 2022, 4, 100059. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W. Factors determining the potential of biochar as a carbon capturing and sequestering construction material: Critical review. J. Mater. Civ. Eng. 2017, 29, 04017086. [Google Scholar] [CrossRef]
- Zhou, X.; Moghaddam, T.B.; Chen, M.; Wu, S.; Adhikari, S. Biochar removes volatile organic compounds generated from asphalt. Sci. Total Environ. 2020, 745, 141096. [Google Scholar] [CrossRef]
- Mousavi, M.; Aldagari, S.; Crocker, M.S.; Ackerman-Biegasiewicz, L.K.; Fini, E.H. Iron-rich biochar to adsorb volatile organic compounds emitted from asphalt-surfaced areas. ACS Sustain. Chem. Eng. 2023, 11, 2885–2896. [Google Scholar] [CrossRef]
- Pahlavan, F.; Aldagari, S.; Park, K.B.; Kim, J.S.; Fini, E.H. Bio-Carbon as a Means of Carbon Management in Roads. Adv. Sustain. Syst. 2023, 7, 2300054. [Google Scholar] [CrossRef]
- Khare, P.; Machesky, J.; Soto, R.; He, M.; Presto, A.A.; Gentner, D.R. Asphalt-related emissions are a major missing nontraditional source of secondary organic aerosol precursors. Sci. Adv. 2020, 6, eabb9785. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jia, M.; Zhang, X.; Wang, Z.; Liu, Y.; Yang, J.; Yang, B.; Sun, Y.; Wang, H.; Ma, H. Laboratory investigation on fumes generated by different modified asphalt binders. Transp. Res. Part D Transp. Environ. 2023, 121, 103828. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Q.; Wang, F.; Xie, J.; Li, Y.; Li, J.; Wu, S. Emission behavior, environmental impact and priority-controlled pollutants assessment of volatile organic compounds (VOCs) during asphalt pavement construction based on laboratory experiment. J. Hazard. Mater. 2020, 398, 122904. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Yan, Y.; Al-Aly, Z. Burden of cause-specific mortality associated with PM2. 5 air pollution in the United States. JAMA Netw. Open 2019, 2, e1915834. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Y.; Di, Q.; Choirat, C.; Wang, Y.; Koutrakis, P.; Zanobetti, A.; Dominici, F.; Schwartz, J.D. Short term exposure to fine particulate matter and hospital admission risks and costs in the Medicare population: Time stratified, case crossover study. BMJ 2019, 367, l6258. [Google Scholar] [CrossRef]
- Sahoo, B.M.; Ravi Kumar, B.V.; Banik, B.K.; Borah, P. Polyaromatic hydrocarbons (PAHs): Structures, synthesis and their biological profile. Curr. Org. Synth. 2020, 17, 625–640. [Google Scholar] [CrossRef]
- Mousavi, M.; Martis, V.; Fini, E.H. Inherently functionalized carbon from algae to adsorb precursors of secondary organic aerosols in noncombustion sources. ACS Sustain. Chem. Eng. 2021, 9, 14375–14384. [Google Scholar] [CrossRef]
- Mousavi, M.; Kaur, H.; Ackerman-Biegasiewicz, L.K.; Park, K.-B.; Kim, J.-S.; Fini, E.H. Efficiency of Metal-Incorporated Carbonaceous Adsorbents in the Presence of Phenolic Compounds. ACS Appl. Eng. Mater. 2024, 2, 1526–1541. [Google Scholar] [CrossRef]
- Mousavi, M.; Park, K.-B.; Kim, J.-S.; Fini, E.H. Metal-rich biochar as an asphalt modifier to improve sustainability and reduce VOC emissions. Sustain. Mater. Technol. 2024, 40, e00903. [Google Scholar] [CrossRef]
- Ma, F.; Dai, J.; Fu, Z.; Li, C.; Wen, Y.; Jia, M.; Wang, Y.; Shi, K. Biochar for asphalt modification: A case of high-temperature properties improvement. Sci. Total Environ. 2022, 804, 150194. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Mamaghani, Z.G.; Hawboldt, K.A.; MacQuarrie, S. Adsorption of CO2 using biochar-review of the impact of gas mixtures and water on adsorption. J. Environ. Chem. Eng. 2023, 11, 109643. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A review of non-soil biochar applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. A review of the next-generation biochar production from waste biomass for material applications. Sci. Total Environ. 2023, 904, 167171. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Gcb Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Lu, Y.; Gu, K.; Shen, Z.; Tang, C.-S.; Shi, B.; Zhou, Q. Biochar implications for the engineering properties of soils: A review. Sci. Total Environ. 2023, 888, 164185. [Google Scholar] [CrossRef]
- Lin, M.; Li, F.; Li, X.; Rong, X.; Oh, K. Biochar-clay, biochar-microorganism and biochar-enzyme composites for environmental remediation: A review. Environ. Chem. Lett. 2023, 21, 1837–1862. [Google Scholar] [CrossRef]
- Shan, R.; Han, J.; Gu, J.; Yuan, H.; Luo, B.; Chen, Y. A review of recent developments in catalytic applications of biochar-based materials. Resour. Conserv. Recycl. 2020, 162, 105036. [Google Scholar] [CrossRef]
- Xiong, X.; Iris, K.; Cao, L.; Tsang, D.C.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Liu, S.; Zeng, G.; Hu, X.; Hu, X.; Guo, Z.; Tan, X.; Wang, L.; Wu, Z. Adsorption of estrogen contaminants by graphene nanomaterials under natural organic matter preloading: Comparison to carbon nanotube, biochar, and activated carbon. Environ. Sci. Technol. 2017, 51, 6352–6359. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, C.; Xu, G.; Ma, Y.; Liu, X.; Zhang, Y. Depolymerization of lignin via a non-precious Ni–Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, B.; Majumder, A.; Gupta, A.K.; Nimbhorkar, S.K. A comprehensive review on the synthesis, performance, modifications, and regeneration of activated carbon for the adsorptive removal of various water pollutants. J. Environ. Chem. Eng. 2021, 9, 106177. [Google Scholar] [CrossRef]
- Bouchelta, C.; Medjram, M.S.; Bertrand, O.; Bellat, J.-P. Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis 2008, 82, 70–77. [Google Scholar] [CrossRef]
- Hayashi, J.I.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Hayashi, J.I.; Horikawa, T.; Takeda, I.; Muroyama, K.; Ani, F.N. Preparing activated carbon from various nutshells by chemical activation with K2CO3. Carbon 2002, 40, 2381–2386. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Alhashimi, H.A.; Aktas, C.B. Life cycle environmental and economic performance of biochar compared with activated carbon: A meta-analysis. Resour. Conserv. Recycl. 2017, 118, 13–26. [Google Scholar] [CrossRef]
- Clurman, A.M.; Rodríguez-Narvaez, O.M.; Jayarathne, A.; De Silva, G.; Ranasinghe, M.I.; Goonetilleke, A.; Bandala, E.R. Influence of surface hydrophobicity/hydrophilicity of biochar on the removal of emerging contaminants. Chem. Eng. J. 2020, 402, 126277. [Google Scholar] [CrossRef]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Rehman, M.Z.U.; Al-Wabel, M.I. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Kwon, S.; Lu, Y. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): Attenuation of surface activity by humic and fulvic acids. Environ. Sci. Technol. 2006, 40, 7757–7763. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Cho, H.-H.; Poster, D.L.; Ball, W.P. Evidence for a pore-filling mechanism in the adsorption of aromatic hydrocarbons to a natural wood char. Environ. Sci. Technol. 2007, 41, 1212–1217. [Google Scholar] [CrossRef]
- Regkouzas, P.; Diamadopoulos, E. Adsorption of selected organic micro-pollutants on sewage sludge biochar. Chemosphere 2019, 224, 840–851. [Google Scholar] [CrossRef]
- Xiao, F.; Pignatello, J.J. π+–π Interactions between (Hetero) aromatic Amine cations and the graphitic surfaces of pyrogenic carbonaceous materials. Environ. Sci. Technol. 2015, 49, 906–914. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Sherrill, C.D. Energy component analysis of π interactions. Acc. Chem. Res. 2013, 46, 1020–1028. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, D.; Pan, B.; Peng, J. Contribution of hydrophobic effect to the sorption of phenanthrene, 9-phenanthrol and 9, 10-phenanthrenequinone on carbon nanotubes. Chemosphere 2017, 168, 739–747. [Google Scholar] [CrossRef]
- Chen, J.; Chen, W.; Zhu, D. Adsorption of nonionic aromatic compounds to single-walled carbon nanotubes: Effects of aqueous solution chemistry. Environ. Sci. Technol. 2008, 42, 7225–7230. [Google Scholar] [CrossRef]
- Pignatello, J.; Mitch, W.A.; Xu, W. Activity and reactivity of pyrogenic carbonaceous matter toward organic compounds. Environ. Sci. Technol. 2017, 51, 8893–8908. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, M.A.H.; Sun, L.; Asadullah, M.; Belhaj, D. Sorption of hydrophobic organic contaminants on functionalized biochar: Protagonist role of π-π electron-donor-acceptor interactions and hydrogen bonds. J. Hazard. Mater. 2018, 360, 270–278. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Bi, L.; Zhu, X.; Li, Y.; Wang, S. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on π–π electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757. [Google Scholar] [CrossRef]
- Tong, Y.; McNamara, P.J.; Mayer, B.K. Adsorption of organic micropollutants onto biochar: A review of relevant kinetics, mechanisms and equilibrium. Environ. Sci. Water Res. Technol. 2019, 5, 821–838. [Google Scholar] [CrossRef]
- Miao, Q.; Bi, E.; Li, B. Roles of polar groups and aromatic structures of biochar in 1-methyl-3-octylimidazolium chloride ionic liquid adsorption: pH effect and thermodynamics study. Environ. Sci. Pollut. Res. 2017, 24, 22265–22274. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Lyu, H.; Zhao, Q.; Jiang, L.; Liu, L. Ball-milled biochar for galaxolide removal: Sorption performance and governing mechanisms. Sci. Total Environ. 2019, 659, 1537–1545. [Google Scholar] [CrossRef]
- Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Study of hexane adsorption on activated carbons with differences in their surface chemistry. Molecules 2018, 23, 476. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B. Interactions of aluminum with biochars and oxidized biochars: Implications for the biochar aging process. J. Agric. Food Chem. 2014, 62, 373–380. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y.; Zhang, D.; Pan, B. pH-dependent KOW provides new insights in understanding the adsorption mechanism of ionizable organic chemicals on carbonaceous materials. Sci. Total Environ. 2018, 618, 269–275. [Google Scholar] [CrossRef]
- Rosales, E.; Meijide, J.; Pazos, M.; Sanromán, M.A. Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresour. Technol. 2017, 246, 176–192. [Google Scholar] [CrossRef]
- Kanellopoulos, P.G.; Verouti, E.; Chrysochou, E.; Koukoulakis, K.; Bakeas, E. Primary and secondary organic aerosol in an urban/industrial site: Sources, health implications and the role of plastic enriched waste burning. J. Environ. Sci. 2021, 99, 222–238. [Google Scholar] [CrossRef]
- Ghosh, S.; Rabha, R.; Chowdhury, M.; Padhy, P.K. Source and chemical species characterization of PM10 and human health risk assessment of semi-urban, urban and industrial areas of West Bengal, India. Chemosphere 2018, 207, 626–636. [Google Scholar] [CrossRef]
- Heald, C.; Henze, D.; Horowitz, L.; Feddema, J.; Lamarque, J.F.; Guenther, A.; Hess, P.; Vitt, F.; Seinfeld, J.; Goldstein, A. Predicted change in global secondary organic aerosol concentrations in response to future climate, emissions, and land use change. J. Geophys. Res. Atmos. 2008, 113, 1–16. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.; Donahue, N.; Prevot, A.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef]
- Ervens, B.; Turpin, B.; Weber, R. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmos. Chem. Phys. 2011, 11, 11069–11102. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Raja, J.D.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Devi, S.M.; Rathika, B. A complete review on biochar: Production, property, multifaceted applications, interaction mechanism and computational approach. Fuel 2021, 292, 120243. [Google Scholar] [CrossRef]
- Su, Y.; Li, S.; Jiang, H.; Duan, B.; Liu, M.; Zhang, Y. Sex-specific physiological and growth responses to elevated temperature and CO2 concentration in Chinese seabuckthorn (Hippophae rhamnoides subsp. sinensis Rousi). Acta Physiol. Plant. 2023, 45, 53. [Google Scholar] [CrossRef]
- Olivier, J.G.; Van Aardenne, J.A.; Dentener, F.J.; Pagliari, V.; Ganzeveld, L.N.; Peters, J.A. Recent trends in global greenhouse gas emissions: Regional trends 1970–2000 and spatial distributionof key sources in 2000. Environ. Sci. 2005, 2, 81–99. [Google Scholar] [CrossRef]
- Wiedinmyer, C.; Akagi, S.; Yokelson, R.J.; Emmons, L.; Al-Saadi, J.; Orlando, J.; Soja, A. The Fire INventory from NCAR (FINN): A high resolution global model to estimate the emissions from open burning. Geosci. Model Dev. 2011, 4, 625–641. [Google Scholar] [CrossRef]
- Tian, H.; Lu, C.; Ciais, P.; Michalak, A.M.; Canadell, J.G.; Saikawa, E.; Huntzinger, D.N.; Gurney, K.R.; Sitch, S.; Zhang, B. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 2016, 531, 225–228. [Google Scholar] [CrossRef]
- Van Der Werf, G.R.; Randerson, J.T.; Giglio, L.; Van Leeuwen, T.T.; Chen, Y.; Rogers, B.M.; Mu, M.; Van Marle, M.J.; Morton, D.C.; Collatz, G.J. Global fire emissions estimates during 1997–2016. Earth Syst. Sci. Data 2017, 9, 697–720. [Google Scholar] [CrossRef]
- Armour, K.; Forster, P.; Storelvmo, T.; Collins, W.; Dufresne, J.-L.; Frame, D.; Lunt, D.; Mauritsen, T.; Palmer, M.; Watanabe, M.; et al. The Earth’s energy budget, climate feedbacks, and climate sensitivity. In Proceedings of the AGU Fall Meeting 2021, New Orleans, LA, USA, 13–17 December 2021. [Google Scholar] [CrossRef]
- Meinshausen, M.; Vogel, E.; Nauels, A.; Lorbacher, K.; Meinshausen, N.; Etheridge, D.M.; Fraser, P.J.; Montzka, S.A.; Rayner, P.J.; Trudinger, C.M. Historical greenhouse gas concentrations for climate modelling (CMIP6). Geosci. Model Dev. 2017, 10, 2057–2116. [Google Scholar] [CrossRef]
- Nisbet, E.G.; Manning, M.; Dlugokencky, E.; Fisher, R.; Lowry, D.; Michel, S.; Myhre, C.L.; Platt, S.M.; Allen, G.; Bousquet, P. Very strong atmospheric methane growth in the 4 years 2014–2017: Implications for the Paris Agreement. Glob. Biogeochem. Cycles 2019, 33, 318–342. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Huang, S.; Yuan, H. Study on asphalt volatile organic compounds emission reduction: A state-of-the-art review. J. Clean. Prod. 2021, 318, 128596. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Q.; Wang, F.; Cui, P.; Xie, J.; Li, J.; Wu, S.; Barbieri, D.M. Comparative assessment of asphalt volatile organic compounds emission from field to laboratory. J. Clean. Prod. 2021, 278, 123479. [Google Scholar] [CrossRef]
- Xiu, M.; Wang, X.; Morawska, L.; Pass, D.; Beecroft, A.; Mueller, J.F.; Thai, P. Emissions of particulate matters, volatile organic compounds and polycyclic aromatic hydrocarbons from warm and hot asphalt mixes. J. Clean. Prod. 2020, 275, 123094. [Google Scholar] [CrossRef]
- Kriech, A.J.; Schreiner, C.A.; Osborn, L.V.; Riley, A.J. Assessing cancer hazards of bitumen emissions–a case study for complex petroleum substances. Crit. Rev. Toxicol. 2018, 48, 121–142. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Li, J.; Qin, Y.; Zhang, X.; Shan, B.; Liu, C. Emission characteristics, environmental impacts, and health risks of volatile organic compounds from asphalt materials: A state-of-the-art review. Energy Fuels 2024, 38, 4787–4802. [Google Scholar] [CrossRef]
- Špánik, I.; Machyňáková, A. Recent applications of gas chromatography with high-resolution mass spectrometry. J. Sep. Sci. 2018, 41, 163–179. [Google Scholar] [CrossRef]
- Zhou, B.; Gong, G.; Wang, C. Characteristics and assessment of volatile organic compounds from different asphalt binders in laboratory. Transp. Res. Part D Transp. Environ. 2023, 118, 103708. [Google Scholar] [CrossRef]
- Espinoza, J.; Medina, C.; Calabi-Floody, A.; Sánchez-Alonso, E.; Valdés, G.; Quiroz, A. Evaluation of reductions in fume emissions (VOCs and SVOCs) from warm mix asphalt incorporating natural zeolite and reclaimed asphalt pavement for sustainable pavements. Sustainability 2020, 12, 9546. [Google Scholar] [CrossRef]
- Mo, S.; Wang, Y.; Xiong, F.; Ai, C. Effects of asphalt source and mixing temperature on the generated asphalt fumes. J. Hazard. Mater. 2019, 371, 342–351. [Google Scholar] [CrossRef]
- Lin, S.; Hung, W.; Leng, Z. Air pollutant emissions and acoustic performance of hot mix asphalts. Constr. Build. Mater. 2016, 129, 1–10. [Google Scholar] [CrossRef]
- Mousavi, M.; Aldagari, S.; Fini, E.H. Adsorbing volatile organic compounds within bitumen improves colloidal stability and air quality. ACS Sustain. Chem. Eng. 2023, 11, 9581–9594. [Google Scholar] [CrossRef]
- Wang, F.; Li, N.; Hoff, I.; Wu, S.; Li, J.; Barbieri, D.M.; Zhang, L. Characteristics of VOCs generated during production and construction of an asphalt pavement. Transp. Res. Part D Transp. Environ. 2020, 87, 102517. [Google Scholar] [CrossRef]
- Boczkaj, G.; Przyjazny, A.; Kamiński, M. Characteristics of volatile organic compounds emission profiles from hot road bitumens. Chemosphere 2014, 107, 23–30. [Google Scholar] [CrossRef]
- Gasthauer, E.; Mazé, M.; Marchand, J.; Amouroux, J. Characterization of asphalt fume composition by GC/MS and effect of temperature. Fuel 2008, 87, 1428–1434. [Google Scholar] [CrossRef]
- Speight, J. Petroleum Asphaltenes-Part 1: Asphaltenes, resins and the structure of petroleum. Oil Gas Sci. Technol. 2004, 59, 467–477. [Google Scholar] [CrossRef]
- Andersen, S.I.; Speight, J.G. Petroleum resins: Separation, character, and role in petroleum. Pet. Sci. Technol. 2001, 19, 1–34. [Google Scholar] [CrossRef]
- Qin, Q.; Schabron, J.F.; Boysen, R.B.; Farrar, M.J. Field aging effect on chemistry and rheology of asphalt binders and rheological predictions for field aging. Fuel 2014, 121, 86–94. [Google Scholar] [CrossRef]
- Qu, X.; Liu, Q.; Guo, M.; Wang, D.; Oeser, M. Study on the effect of aging on physical properties of asphalt binder from a microscale perspective. Constr. Build. Mater. 2018, 187, 718–729. [Google Scholar] [CrossRef]
- Fernández-Gómez, W.D.; Rondón Quintana, H.; Reyes Lizcano, F. A review of asphalt and asphalt mixture aging: Una revisión. Ing. Investig. 2013, 33, 5–12. [Google Scholar] [CrossRef]
- Sirin, O.; Paul, D.K.; Kassem, E. State of the art study on aging of asphalt mixtures and use of antioxidant additives. Adv. Civ. Eng. 2018, 2018, 3428961. [Google Scholar] [CrossRef]
- Jiang, W.; Bao, R.; Lu, H.; Yuan, D.; Lu, R.; Sha, A.; Shan, J. Analysis of rheological properties and aging mechanism of bitumen after short-term and long-term aging. Constr. Build. Mater. 2021, 273, 121777. [Google Scholar] [CrossRef]
- Hofko, B.; Cannone Falchetto, A.; Grenfell, J.; Huber, L.; Lu, X.; Porot, L.; Poulikakos, L.; You, Z. Effect of short-term ageing temperature on bitumen properties. Road Mater. Pavement Des. 2017, 18 (Suppl. 2), 108–117. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Wang, S.; Xu, T. Thermal-oxidative aging mechanism of asphalt binder based on isothermal thermal analysis at the SARA level. Constr. Build. Mater. 2020, 255, 119349. [Google Scholar] [CrossRef]
- Tauste, R.; Moreno-Navarro, F.; Sol-Sánchez, M.; Rubio-Gámez, M. Understanding the bitumen ageing phenomenon: A review. Constr. Build. Mater. 2018, 192, 593–609. [Google Scholar] [CrossRef]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Ke, N.; Huang, J.; Zhu, C. The effect of different nanomaterials on the long-term aging properties of bitumen. Pet. Sci. Technol. 2015, 33, 388–396. [Google Scholar] [CrossRef]
- Lu, X.; Soenen, H.; Sjövall, P.; Pipintakos, G. Analysis of asphaltenes and maltenes before and after long-term aging of bitumen. Fuel 2021, 304, 121426. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Lin, P.; Erkens, S.; Xiao, Y. Chemo-physical characterization and molecular dynamics simulation of long-term aging behaviors of bitumen. Constr. Build. Mater. 2021, 302, 124437. [Google Scholar] [CrossRef]
- Durrieu, F.; Farcas, F.; Mouillet, V. The influence of UV aging of a styrene/butadiene/styrene modified bitumen: Comparison between laboratory and on site aging. Fuel 2007, 86, 1446–1451. [Google Scholar] [CrossRef]
- Li, H.; Tong, P.; Zhang, X.; Lin, X.; Li, B. Influence of ultraviolet and oxygen coupling aging on rheological properties and functional group index of warm mix asphalt binder. Materials 2020, 13, 4216. [Google Scholar] [CrossRef]
- He, X.; Hochstein, D.; Ge, Q.; Ali, A.W.; Chen, F.; Yin, H. Accelerated aging of asphalt by UV photo-oxidation considering moisture and condensation effects. J. Mater. Civ. Eng. 2018, 30, 04017261. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Liu, Q.; Xie, J.; Li, H.; Dai, Y.; Li, C.; Nie, S.; Song, W. Aging effects of ultraviolet lights with same dominant wavelength and different wavelength ranges on a hydrocarbon-based polymer (asphalt). Polym. Test. 2019, 75, 64–75. [Google Scholar] [CrossRef]
- Hung, A.M.; Fini, E.H. Absorption spectroscopy to determine the extent and mechanisms of aging in bitumen and asphaltenes. Fuel 2019, 242, 408–415. [Google Scholar] [CrossRef]
- Fini, E.; Rajib, A.I.; Oldham, D.; Samieadel, A.; Hosseinnezhad, S. Role of chemical composition of recycling agents in their interactions with oxidized asphaltene molecules. J. Mater. Civ. Eng. 2020, 32, 04020268. [Google Scholar] [CrossRef]
- Mousavi, M.; Abdollahi, T.; Pahlavan, F.; Fini, E.H. The influence of asphaltene-resin molecular interactions on the colloidal stability of crude oil. Fuel 2016, 183, 262–271. [Google Scholar] [CrossRef]
- Nazari, H.; Naderi, K.; Nejad, F.M. Improving aging resistance and fatigue performance of asphalt binders using inorganic nanoparticles. Constr. Build. Mater. 2018, 170, 591–602. [Google Scholar] [CrossRef]
- Dong, W.; Ma, F.; Li, C.; Fu, Z.; Huang, Y.; Liu, J. Evaluation of anti-aging performance of biochar modified asphalt binder. Coatings 2020, 10, 1037. [Google Scholar] [CrossRef]
- Saadeh, S.; Al-Zubi, Y.; Katawal, P.; Zaatarah, B.; Fini, E. Biochar effects on the performance of conventional and rubberized HMA. Road Mater. Pavement Des. 2023, 24, 156–172. [Google Scholar] [CrossRef]
- Rajib, A.; Saadeh, S.; Katawal, P.; Mobasher, B.; Fini, E.H. Enhancing biomass value chain by utilizing biochar as a free radical scavenger to delay ultraviolet aging of bituminous composites used in outdoor construction. Resour. Conserv. Recycl. 2021, 168, 105302. [Google Scholar] [CrossRef]
- Rajib, A.; Fini, E.H. Inherently functionalized carbon from lipid and protein-rich biomass to reduce ultraviolet-induced damages in bituminous materials. ACS Omega 2020, 5, 25273–25280. [Google Scholar] [CrossRef]
- Ghasemi, H.; Yazdani, H.; Rajib, A.; Fini, E.H. Toward carbon-negative and emission-curbing roads to drive environmental health. ACS Sustain. Chem. Eng. 2022, 10, 1857–1862. [Google Scholar] [CrossRef]
- Celauro, C.; Teresi, R.; Dintcheva, N.T. Evaluation of anti-aging effect in biochar-modified bitumen. Sustainability 2023, 15, 10583. [Google Scholar] [CrossRef]
- Zhou, X.; Adhikari, S. Flow-induced crystallization of biochar in bio-asphalt under various aging conditions. Sci. Total Environ. 2019, 695, 133943. [Google Scholar] [CrossRef]
- Walters, R.C.; Fini, E.H.; Abu-Lebdeh, T. Enhancing asphalt rheological behavior and aging susceptibility using bio-char and nano-clay. Am. J. Eng. Appl. Sci. 2014, 7, 66–76. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Ji, J.; Wang, H. Viscoelastic properties, rutting resistance, and fatigue resistance of waste wood-based biochar-modified asphalt. Coatings 2022, 12, 89. [Google Scholar] [CrossRef]
- Yao, H.; You, Z.; Li, L.; Goh, S.W.; Lee, C.H.; Yap, Y.K.; Shi, X. Rheological properties and chemical analysis of nanoclay and carbon microfiber modified asphalt with Fourier transform infrared spectroscopy. Constr. Build. Mater. 2013, 38, 327–337. [Google Scholar] [CrossRef]
- Khattak, M.J.; Khattab, A.; Rizvi, H.R.; Zhang, P. The impact of carbon nano-fiber modification on asphalt binder rheology. Constr. Build. Mater. 2012, 30, 257–264. [Google Scholar] [CrossRef]
- Yoo, D.-Y.; Kim, S.; Kim, M.-J.; Kim, D.; Shin, H.-O. Self-healing capability of asphalt concrete with carbon-based materials. J. Mater. Res. Technol. 2019, 8, 827–839. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Peng, X.; Liu, X.; Zhou, Y.; Peng, B.; Tang, L.; Luo, L.; Yao, B.; Deng, Y.; Tang, J.; Zeng, G. New insights into the activity of a biochar supported nanoscale zerovalent iron composite and nanoscale zero valent iron under anaerobic or aerobic conditions. RSC Adv. 2017, 7, 8755–8761. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Mosa, A.; Abdelrahman, H.; Niazi, N.K.; Antoniadis, V.; Shahid, M.; Song, H.; Kwon, E.E.; Rinklebe, J. Removal of toxic elements from aqueous environments using nano zero-valent iron-and iron oxide-modified biochar: A review. Biochar 2022, 4, 24. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Navarathna, C.M.; Gunatilake, S.R.; Crowley, M.; Anderson, R.; Mohan, D.; Perez, F.; Pittman Jr, C.U.; Mlsna, T. Fe3O4 nanoparticles dispersed on Douglas fir biochar for phosphate sorption. ACS Appl. Nano Mater. 2019, 2, 3467–3479. [Google Scholar] [CrossRef]
- Jung, K.-W.; Jeong, T.-U.; Kang, H.-J.; Ahn, K.-H. Characteristics of biochar derived from marine macroalgae and fabrication of granular biochar by entrapment in calcium-alginate beads for phosphate removal from aqueous solution. Bioresour. Technol. 2016, 211, 108–116. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, W.; Xu, H.; Li, S.; Peng, Y. Adsorption removal and reuse of phosphate from wastewater using a novel adsorbent of lanthanum-modified platanus biochar. Process Saf. Environ. Prot. 2020, 140, 221–232. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Gascó, G.; Palacios, T.; Paz-Ferreiro, J.; Méndez, A. Fe oxides-biochar composites produced by hydrothermal carbonization and pyrolysis of biomass waste. J. Anal. Appl. Pyrolysis 2020, 151, 104893. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, Y.; Tsang, D.C.; Iris, K.; Fan, J.; Clark, J.H.; Zhou, Y.; Cao, X.; Gao, B.; Ok, Y.S. A sustainable biochar catalyst synergized with copper heteroatoms and CO2 for singlet oxygenation and electron transfer routes. Green Chem. 2019, 21, 4800–4814. [Google Scholar] [CrossRef]
- Wurzer, C.; Mašek, O. Feedstock doping using iron rich waste increases the pyrolysis gas yield and adsorption performance of magnetic biochar for emerging contaminants. Bioresour. Technol. 2021, 321, 124473. [Google Scholar] [CrossRef]
- Ajmal, Z.; Muhmood, A.; Dong, R.; Wu, S. Probing the efficiency of magnetically modified biomass-derived biochar for effective phosphate removal. J. Environ. Manag. 2020, 253, 109730. [Google Scholar] [CrossRef]
- Alberto, D.R.; Repa, K.S.; Hegde, S.; Miller, C.W.; Trabold, T.A. Novel production of magnetite particles via thermochemical processing of digestate from manure and food waste. IEEE Magn. Lett. 2019, 10, 3504605. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.W.; Drikas, M.; Korshin, G.; Amal, R. Multi-wavelength spectroscopic and chromatography study on the photocatalytic oxidation of natural organic matter. Water Res. 2010, 44, 2525–2532. [Google Scholar] [CrossRef]
- Leppäkoski, L.; Marttila, M.P.; Uusitalo, V.; Levänen, J.; Halonen, V.; Mikkilä, M.H. Assessing the carbon footprint of biochar from willow grown on marginal lands in Finland. Sustainability 2021, 13, 10097. [Google Scholar] [CrossRef]
- Azzi, E.S.; Karltun, E.; Sundberg, C. Prospective life cycle assessment of large-scale biochar production and use for negative emissions in Stockholm. Environ. Sci. Technol. 2019, 53, 8466–8476. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, P.; Wang, C.; Tang, J.; Sun, H. Enhancement of persulfate activation by Fe-biochar composites: Synergism of Fe and N-doped biochar. Appl. Catal. B Environ. 2022, 303, 120926. [Google Scholar] [CrossRef]
- Xu, L.; Fu, B.; Sun, Y.; Jin, P.; Bai, X.; Jin, X.; Shi, X.; Wang, Y.; Nie, S. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application. Chem. Eng. J. 2020, 400, 125870. [Google Scholar] [CrossRef]
- Peng, X.; Wu, J.; Zhao, Z.; Wang, X.; Dai, H.; Li, Y.; Wei, Y.; Xu, G.; Hu, F. High efficiency degradation of tetracycline by peroxymonosulfate activated with Fe/NC catalysts: Performance, intermediates, stability and mechanism. Environ. Res. 2022, 205, 112538. [Google Scholar] [CrossRef]
- Guan, Z.; Zuo, S.; Yang, F.; Zhang, B.; Xu, H.; Xia, D.; Huang, M.; Li, D. The p and d hybridization interaction in Fe-NC boosts peroxymonosulfate non-radical activation. Sep. Purif. Technol. 2021, 258, 118025. [Google Scholar] [CrossRef]
- Carvalho, J.; Nascimento, L.; Soares, M.; Valério, N.; Ribeiro, A.; Faria, L.; Silva, A.; Pacheco, N.; Araújo, J.; Vilarinho, C. Life cycle assessment (LCA) of biochar production from a circular economy perspective. Processes 2022, 10, 2684. [Google Scholar] [CrossRef]
- Zhou, X.; Moghaddam, T.B.; Chen, M.; Wu, S.; Adhikari, S.; Xu, S.; Yang, C. Life cycle assessment of biochar modified bioasphalt derived from biomass. ACS Sustain. Chem. Eng. 2020, 8, 14568–14575. [Google Scholar] [CrossRef]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The costs and benefits of biochar production and use: A systematic review. J. Clean. Prod. 2023, 408, 137138. [Google Scholar] [CrossRef]
- Fini, E.H.; Pahlavan, F.; Vega, N.O.; Bibo, A.; Kaur, H.; Ghasemi, H.; Aldagari, S.; Hung, A.; Kannan, L.; Yazdani, H. Health impacts of asphalt emissions: Examining neurological risks and the need for long-term exposure mitigation. J. Hazard. Mater. 2025, 486, 136849. [Google Scholar] [CrossRef]
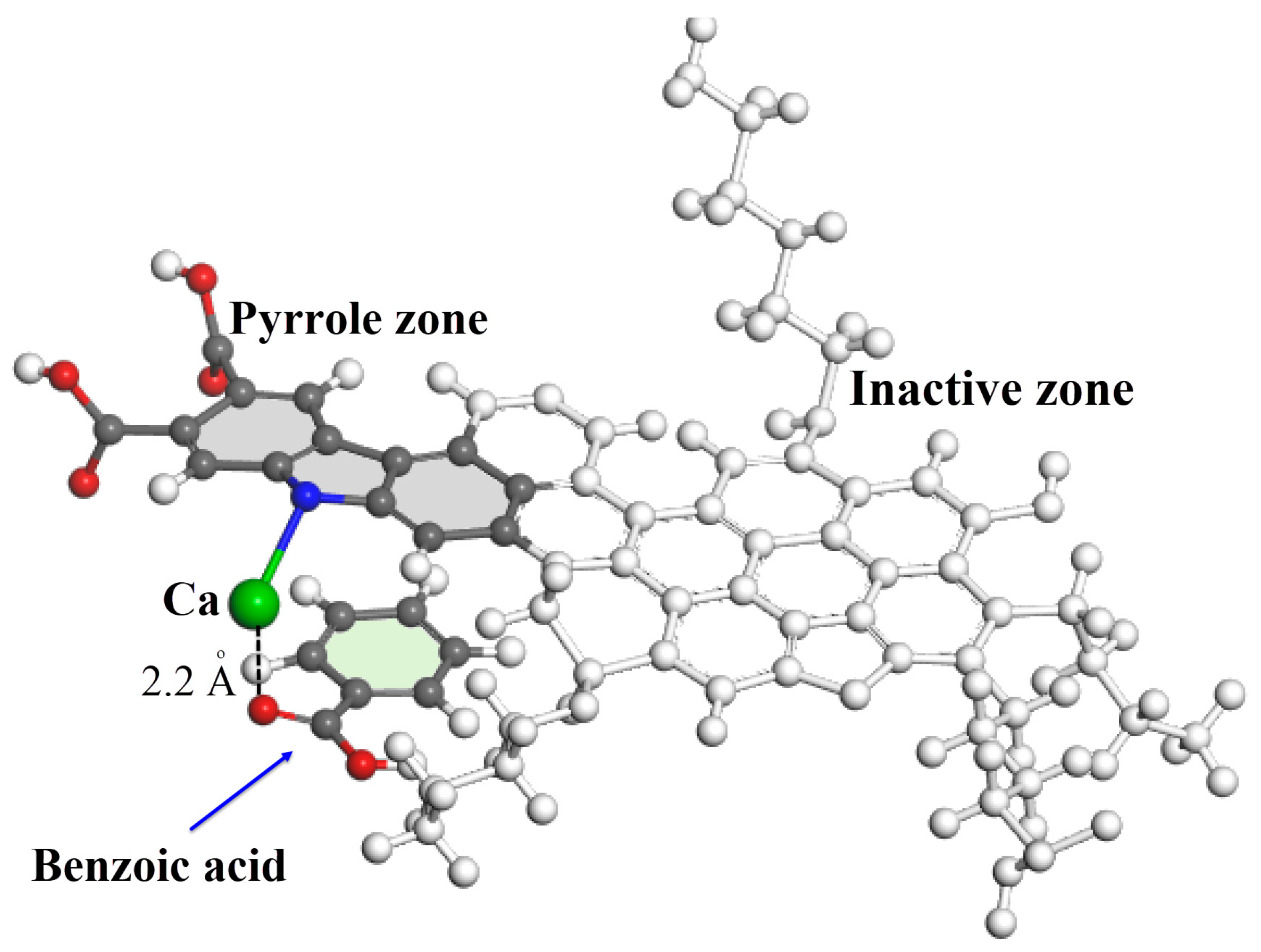
- Pahlavan, F.; Rajib, A.I.; Martis, V.; Fini, E.H. A nature-inspired design for sequestering polycyclic aromatic hydrocarbons in asphalt-surfaced areas. Sustain. Mater. Technol. 2024, 41, e01035. [Google Scholar] [CrossRef]
- Thengane, S.K.; Kung, K.; Hunt, J.; Gilani, H.R.; Lim, C.J.; Sokhansanj, S.; Sanchez, D.L. Market prospects for biochar production and application in California. Biofuels Bioprod. Biorefining 2021, 15, 1802–1819. [Google Scholar] [CrossRef]

| Property | BC1 | BC2 | MWCNT | SWCNT | PAC | GAC |
|---|---|---|---|---|---|---|
| Surface Area (m2/g) | 85 | 142 | 175 | 557 | 1255 | 1354 |
| Pore Volume (cm3/g) | 0.057 | 0.185 | 0.664 | 1.043 | 0.757 | 0.778 |
| Micropores (%) | 13.4 | 11.5 | 7.0 | 16.1 | 37.0 | 42.6 |
| Mesopores (%) | 75.7 | 69.8 | 65.4 | 77.8 | 61.9 | 56.4 |
| Macropores (%) | 10.9 | 18.7 | 27.6 | 6.1 | 1.1 | 1.0 |
| Oxygen Content (%) | 24.27 | 21.95 | 2.99 | 4.94 | 28.01 | 21.92 |
| pH PZC (pristine) | 3.3 | 4.1 | 7.5 | 6.7 | 3.2 | 4.1 |
| Material Category | Material Example | Energy Demand (MJ/kg) | Global Warming Potential (kg CO2eq/kg) |
|---|---|---|---|
| Activated carbon | Virgin (hard coal) | 44 | 3–11 |
| Olive waste-based | 170 | 11 | |
| Recycled | - | 1.2 | |
| Granular | 79.8 | 9.3 | |
| Fossil waste biochar | Dense refuse-derived fuel | 1.8 | −0.3 |
| Manure biochar | Poultry litter | 1.1 | −0.2 to −0.5 |
| Crop residue biochar | Barley, wheat, corn stover, straw | 1–3 | −0.9 to −2.1 |
| Food and paper waste biochar | Food waste, cardboard, paper sludge | 1.1–1.8 | −0.1 to −1.1 |
| Woody biomass biochar | Forestry, wood waste, poplar | 1.4–16 | −1.3 to −0.1 |
| Green/yard waste biochar | Green waste, yard waste | 1.8–3 | −1.1 to −0.3 |
| Energy crops biochar | Miscanthus, switchgrass | 1.4–11 | −3.5 to 0 |
| Sewage-based Biochar | Sewage sludge | 1.8 | −0.8 |
| Mixture Plant Process | Transportation Process | Paving Process | ||||
|---|---|---|---|---|---|---|
| 1 | Benzene | 1000.000 | 1,3-Butadiene | 1000.000 | Trichloroethylene | 1000.000 |
| 2 | 1,3-Butadiene | 963.555 | Trichloroethylene | 926.417 | 1,3-Butadiene | 712.823 |
| 3 | Toluene | 531.012 | Toluene | 568.961 | Toluene | 645.573 |
| 4 | Propionaldehyde | 381.854 | Propylene | 467.649 | Benzene | 645.573 |
| 5 | Propylene | 356.978 | Benzene | 452.333 | Propionaldehyde | 582.964 |
| 6 | m-/p-Xylene | 290.172 | 2,2,4-Trimethylpentane | 380.699 | m-/p-Xylene | 391.040 |
| 7 | 2,2,4-Trimethylpentane | 248.160 | Propionaldehyde | 342.968 | Propylene | 338.007 |
| 8 | Butanal | 229.527 | Ethylene | 290.900 | Butanal | 337.763 |
| 9 | Ethylene | 193.049 | m-/p-Xylene | 229.481 | 2,2,4-Trimethylpentane | 292.806 |
| 10 | Pentanal | 152.576 | Butanal | 200.007 | Ethylene | 228.143 |
| T (°C) | No. of VOCs | Some of the Main VOCs Identified | References | Year |
|---|---|---|---|---|
| 150 | 31 | benzene derivatives, alkanes (nonane, heptane, octane), alkenes (1-pentene, 2-methyl), alkynes (3-octyne, 5-methyl-) | Mousavi et al. [32,110] | 2023 |
| 160–180 | 14 | styrene, toluene, ethylbenzene, O/M-xylene, P-diethylbenzene | Zhou et al. [106] | 2023 |
| 120–180 | 10 | benzene, toluene, 1,3-butadiene, propionaldehyde | Li et al. [36] | 2020 |
| 155 | 81 | trichloromethane, heptane, octane, dimethyl heptane, nonane | Espinoza et al. [107] | 2020 |
| 165 | 34 | trichloromethane, benzene, toluene, etc. | Wang et al. [111] | 2020 |
| 165 | 77 | anthracene, fluorene, pyrene, etc. | Xiu et al. [101] | 2020 |
| 160 | 12 | 12 types of PAHs | Mo et al. [108] | 2019 |
| 175 | 41 | benzene, toluene, trichloromethane, ethylbenzene, etc.) | Lin et al. [109] | 2016 |
| 180 | 44 | toluene, N-butyraldehyde, ethane, etc. | Boczkaj et al. [112] | 2014 |
| 180 | 25 | xylene, anthracene, naphthalene, etc. | Gasthauer et al. [113] | 2008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, M.; Akbarzadeh, V.; Kazemi, M.; Deng, S.; Fini, E.H. Perspective on Sustainable Solutions for Mitigating Off-Gassing of Volatile Organic Compounds in Asphalt Composites. J. Compos. Sci. 2025, 9, 353. https://doi.org/10.3390/jcs9070353
Mousavi M, Akbarzadeh V, Kazemi M, Deng S, Fini EH. Perspective on Sustainable Solutions for Mitigating Off-Gassing of Volatile Organic Compounds in Asphalt Composites. Journal of Composites Science. 2025; 9(7):353. https://doi.org/10.3390/jcs9070353
Chicago/Turabian StyleMousavi, Masoumeh, Vajiheh Akbarzadeh, Mohammadjavad Kazemi, Shuguang Deng, and Elham H. Fini. 2025. "Perspective on Sustainable Solutions for Mitigating Off-Gassing of Volatile Organic Compounds in Asphalt Composites" Journal of Composites Science 9, no. 7: 353. https://doi.org/10.3390/jcs9070353
APA StyleMousavi, M., Akbarzadeh, V., Kazemi, M., Deng, S., & Fini, E. H. (2025). Perspective on Sustainable Solutions for Mitigating Off-Gassing of Volatile Organic Compounds in Asphalt Composites. Journal of Composites Science, 9(7), 353. https://doi.org/10.3390/jcs9070353







