Aluminum-Modified Graphene Oxide Composite Adsorbent for Humic Acid Removal from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GO-Al
2.3. Analytical Determinations
2.4. Adsorption Experiments
2.4.1. Equilibrium Experiments
2.4.2. Kinetics Experiments
2.5. Thermodynamics
2.6. Characterization Techniques
2.7. Regeneration Study
3. Results and Discussion
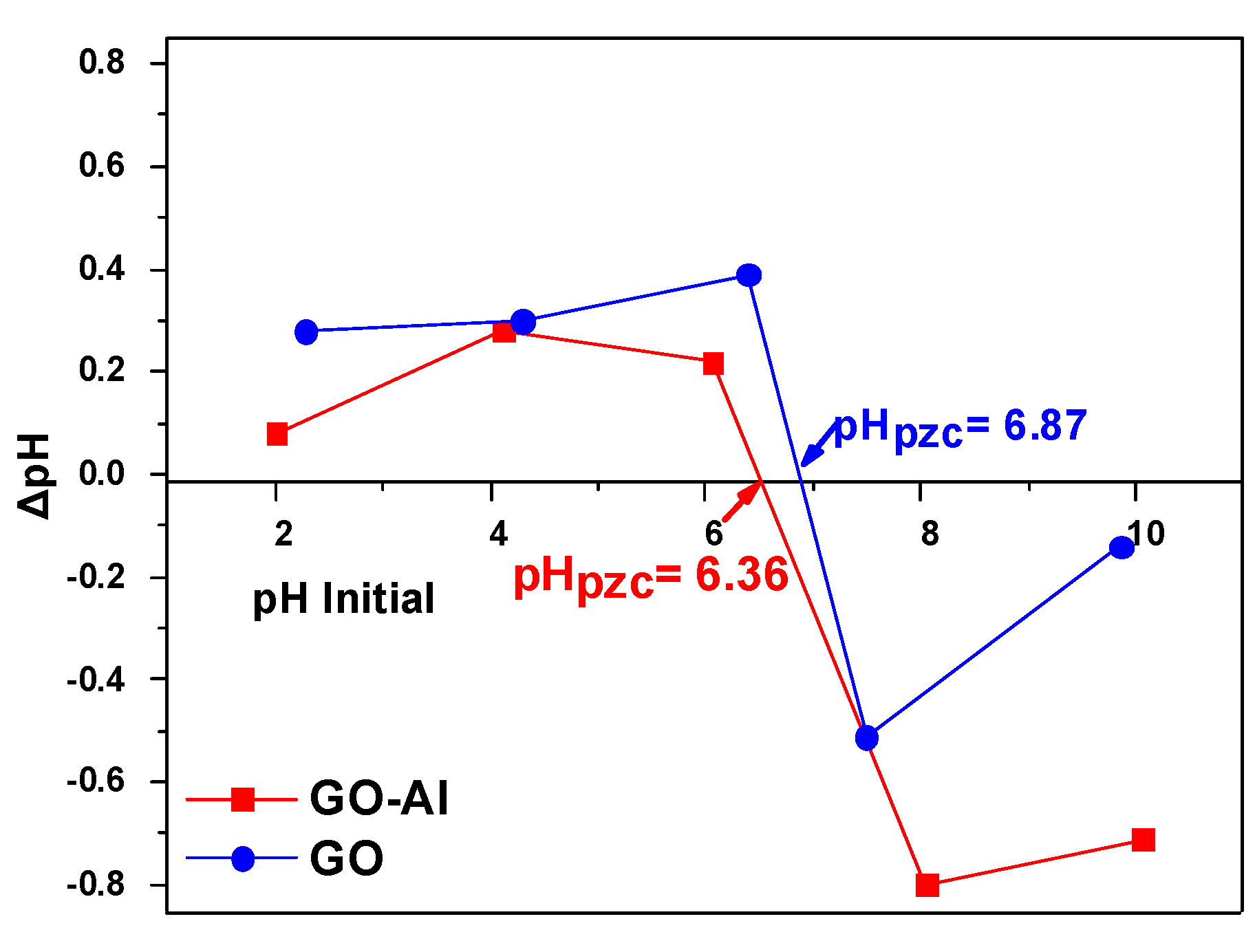
3.1. Effect of Experimental pH Solution
3.2. Effect of Dosage
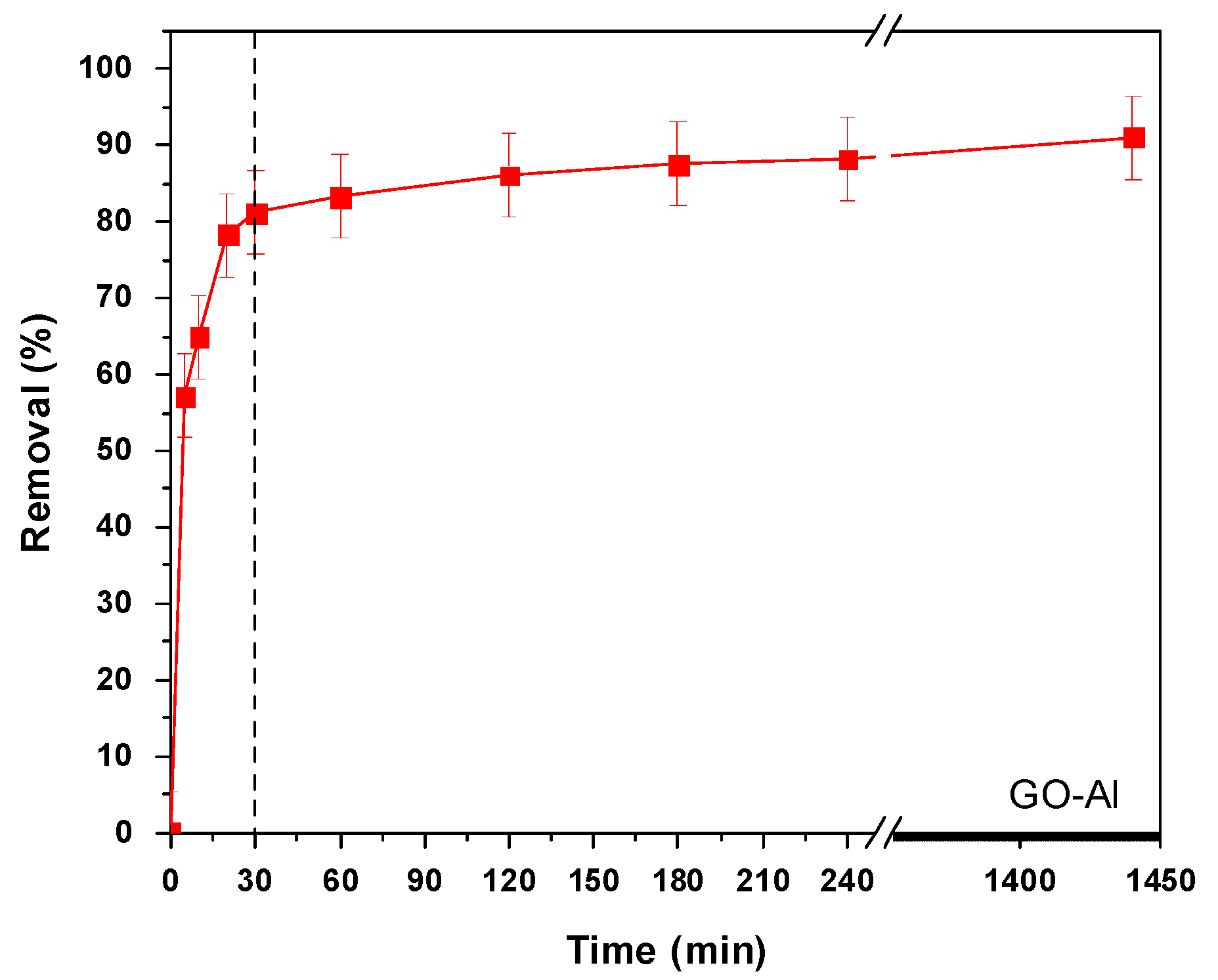
3.3. Effect of Contact Time—Kinetics
3.4. Adsorption Isotherms
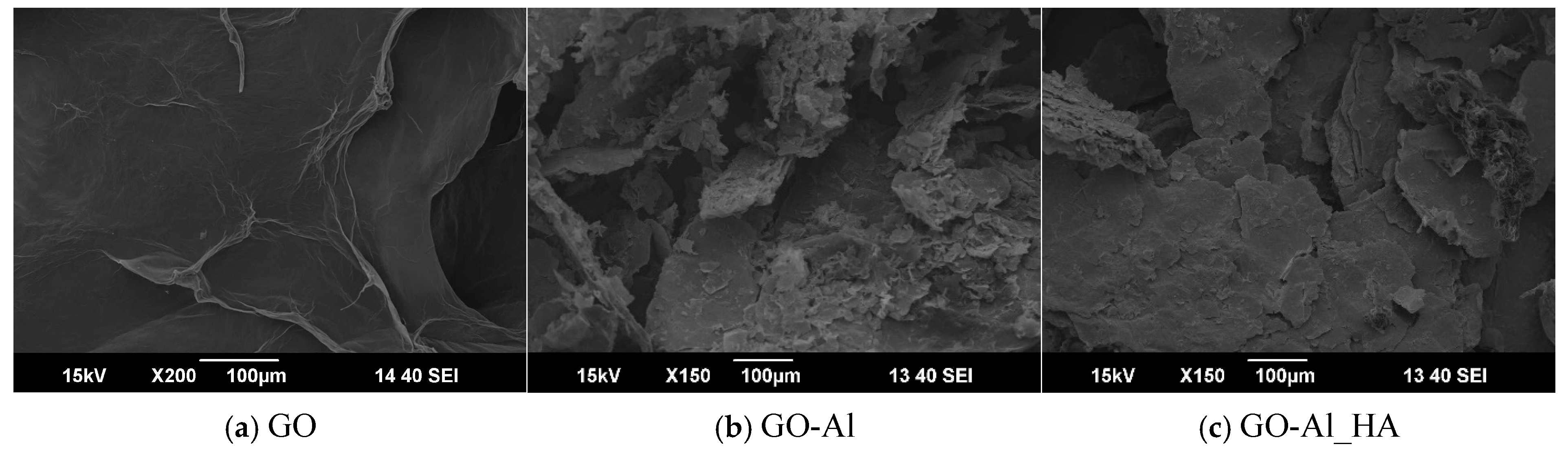
3.5. SEM Images of GO-Al
3.6. Thermodynamics
3.7. Regeneration Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahato, J.K.; Gupta, S.K. Exceptional adsorption of different spectral indices of natural organic matter (NOM) by using cerium oxide nanoparticles (CONPs). Environ. Sci. Pollut. Res. 2021, 28, 45496–45505. [Google Scholar] [CrossRef] [PubMed]
- Dayarathne, H.N.P.; Angove, M.J.; Aryal, R.; Abuel-Naga, H.; Mainali, B. Removal of natural organic matter from source water: Review on coagulants, dual coagulation, alternative coagulants, and mechanisms. J. Water Process Eng. 2021, 40, 101820. [Google Scholar] [CrossRef]
- Alomar, T.; Qiblawey, H.; Almomani, F.; Al-Raoush, R.I.; Han, D.S.; Ahmad, N.M. Recent advances on humic acid removal from wastewater using adsorption process. J. Water Process Eng. 2023, 53, 103679. [Google Scholar] [CrossRef]
- Kulikowska, D.; Klik, B.K.; Gusiatin, Z.M.; Hajdukiewicz, K. Characteristics of humic substances from municipal sewage sludge: A case study. Desalin. Water Treat. 2019, 144, 57–64. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, B.; Guo, K.; Gao, Y.; Yue, Q.; Gao, B. Coagulation performance and mechanism of different hydrolyzed aluminum species for the removal of composite pollutants of polyethylene and humic acid. J. Hazard. Mater. 2024, 465, 133076. [Google Scholar] [CrossRef]
- Hu, P.; Li, H.; Tan, Y.; Adeleye, A.S.; Hao, T. Enhanced electrochemical treatment of humic acids and metal ions in leachate concentrate: Experimental and molecular mechanism investigations. J. Hazard. Mater. 2024, 462, 132774. [Google Scholar] [CrossRef]
- Tan, S.; Long, K.; Chen, W.; Liu, H.; Liang, S.; Zhang, Q. Synergistic oxidation of humic acid treated by H2O2/O3 activated by CuCo/C with high efficiency and wide pH range. J. Environ. Manag. 2024, 358, 120896. [Google Scholar] [CrossRef]
- Mahmud, N.A.C.; Saufi, S.M.; Abu Seman, M.N.; Takriff, M.S.; Ang, W.L. Effect of cellulose nanocrystals and carboxylated multiwalled carbon nanotubes on performance of polyethersulfone membrane for humic acid removal. Chem. Eng. Res. Des. 2024, 201, 185–193. [Google Scholar] [CrossRef]
- Zulfikar, M.A.; Afrita, S.; Wahyuningrum, D.; Ledyastuti, M. Preparation of Fe3O4-chitosan hybrid nano-particles used for humic acid adsorption. Environ. Nanotechnol. Monit. Manag. 2016, 6, 64–75. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Maroulas, K.N.; Theologis, D.; Katsoyiannis, I.A.; Kyzas, G.Z. Comparison of Modified Peels: Natural Peels or Peels-Based Activated Carbons for the Removal of Several Pollutants Found in Wastewaters. C-J. Carbon Res. 2024, 10, 22. [Google Scholar] [CrossRef]
- Xu, M.; Tremblay, P.L.; Joya, M.B.; Kaka, A.Z.H.; Kollah, E.S.; Mwansa, B.K.; Wang, W.; Liu, Y.; Xing, X.; Qiu, F.; et al. An efficient Bi2MoO6 adsorbent with a positive surface and abundant oxygen vacancies for the removal of humic acid contaminants. J. Environ. Chem. Eng. 2024, 12, 113296. [Google Scholar] [CrossRef]
- McCann, B. Removal of humic substances from water. Water 1999, 21, 32–33. [Google Scholar]
- Tigrine, Z.; Benhabiles, O.; Merabti, L.; Chekir, N.; Mellal, M.; Aoudj, S.; Abdeslam, N.A.; Tassalit, D.; Lebouachera, S.E.I.; Drouiche, N. Sustainable Activated Carbon from Agricultural Waste: A Study on Adsorption Efficiency for Humic Acid and Methyl Orange Dyes. Sustainability 2024, 16, 9308. [Google Scholar] [CrossRef]
- Jafari, S.; Javid, A.H.; Moniri, E.; Hassani, A.H.; Panahi, H.A. Efficient removal of humic acid from aqueous solutions using threedimensional/magnetic graphene oxide allylamine/allyl glycidyl ether: Optimization by Taguchi design method. Desalin. Water Treat. 2023, 292, 152–164. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Momeni, F.; Derakhshani, E.; Kamranifar, M. Humic acid removal efficiency from aqueous solutions using graphene and graphene oxide nanoparticles. Desalin. Water Treat. 2017, 100, 116–125. [Google Scholar] [CrossRef]
- Asghar, F.; Shakoor, B.; Fatima, S.; Munir, S.; Razzaq, H.; Naheed, S.; Butler, I.S. Fabrication and prospective applications of graphene oxide-modified nanocomposites for wastewater remediation. RSC Adv. 2022, 12, 11750–11768. [Google Scholar] [CrossRef]
- Ghulam, A.N.; Dos Santos, O.A.L.; Hazeem, L.; Backx, B.P.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef]
- Fei, L.; Chen, C.; Shen, L.; Zhang, Y.; Wang, B.; Xu, J.; Li, B.; Raza, S.; Lin, H. Graphene oxide assisted assembly of superhydrophilic MOF-based membrane with 2D/3D hybrid nanochannels for enhanced water purification. Chem. Eng. J. 2023, 460, 141694. [Google Scholar] [CrossRef]
- Anegbe, B.; Ifijen, I.H.; Maliki, M.; Uwidia, I.E.; Aigbodion, A.I. Graphene oxide synthesis and applications in emerging contaminant removal: A comprehensive review. Environ. Sci. Eur. 2024, 36, 15. [Google Scholar] [CrossRef]
- Majumder, P.; Gangopadhyay, R. Evolution of graphene oxide (GO)-based nanohybrid materials with diverse compositions: An overview. RSC Adv. 2022, 12, 5686–5719. [Google Scholar] [CrossRef]
- Bharadwaj, P.; Kiran, G.R.; Acharyya, S.G. Remarkable performance of GO/ZnO nanocomposites under optimized parameters for remediation of Cd (II) from water. Appl. Surf. Sci. 2023, 626, 157238. [Google Scholar] [CrossRef]
- Song, J.; Wang, J.; Wang, D. Changes in the structural characteristics of EfOM during coagulation by aluminum chloride and the effect on the formation of disinfection byproducts. J. Environ. Manag. 2023, 326, 116850. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Guo, M.; Lu, F.; Huang, A.; Nie, Y.; Ma, J. Coagulation performance and mechanism analysis of humic acid by using covalently bonded coagulants: Effect of pH and matching mechanism of humic acid functional groups. Environ. Sci. Pollut. Res. 2024, 31, 22560–22575. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, E.; Fortier, I.; Courchesne, F.; Pepin, P.; Mortimer, J.; Gauvreau, D. Aluminum forms in drinking water and risk of Alzheimer’s disease. Environ. Res. 2000, 84, 234–246. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Debnath, S.; Maity, A.; Pillay, K. Impact of process parameters on removal of Congo red by graphene oxide from aqueous solution. J. Environ. Chem. Eng. 2014, 2, 260–272. [Google Scholar] [CrossRef]
- Langmuir, I. The Evaporation, Condensation and Reflection of Molecules and the Mechanism of Adsorption. Phys. Rev. 1916, 8, 149–176. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Juang, R.-S.; Chen, M.-L. Application of the Elovich Equation to the Kinetics of Metal Sorption with Solvent-Impregnated Resins. Ind. Eng. Chem. Res. 1997, 36, 813–820. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Christodoulou, E.; Bikiaris, D.N. Basic dye removal with sorption onto low-cost natural textile fibers. Processes 2018, 6, 166. [Google Scholar] [CrossRef]
- Mullick, A.; Neogi, S. Acoustic cavitation induced synthesis of zirconium impregnated activated carbon for effective fluoride scavenging from water by adsorption. Ultrason. Sonochem. 2018, 45, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.L.; Krusnamurthy, P.A.; Nakajima, H.; Rashid, S.A. Adsorptive, kinetics and regeneration studies of fluoride removal from water using zirconium-based metal organic frameworks. RSC Adv. 2020, 10, 18740–18752. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.H.; Saha, B.; Streat, M. Determination of Point Zero Charge (PZC) of Homemade Charcoals of Shorea Robusta (Sakhuwa) and Pinus Roxburghii (Salla). Int. J. Eng. Res. Technol. 2020, 9, 153–155. [Google Scholar]
- Derakhshani, E.; Naghizadeh, A. Optimization of humic acid removal by adsorption onto bentonite and montmorillonite nanoparticles. J. Mol. Liq. 2018, 259, 76–81. [Google Scholar] [CrossRef]
- Heidarizad, M.; Şengör, S.S. Synthesis of graphene oxide/magnesium oxide nanocomposites with high-rate adsorption of methylene blue. J. Mol. Liq. 2016, 224, 607–617. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Turki, T.; Hamdouni, A.; Enesca, A. Fluoride Adsorption from Aqueous Solution by Modified Zeolite—Kinetic and Isotherm Studies. Molecules 2023, 28, 4076. [Google Scholar] [CrossRef]
- Gkika, D.A.; Mitropoulos, A.C.; Kyzas, G.Z. Why reuse spent adsorbents? The latest challenges and limitations. Sci. Total Environ. 2022, 822, 153612. [Google Scholar] [CrossRef]


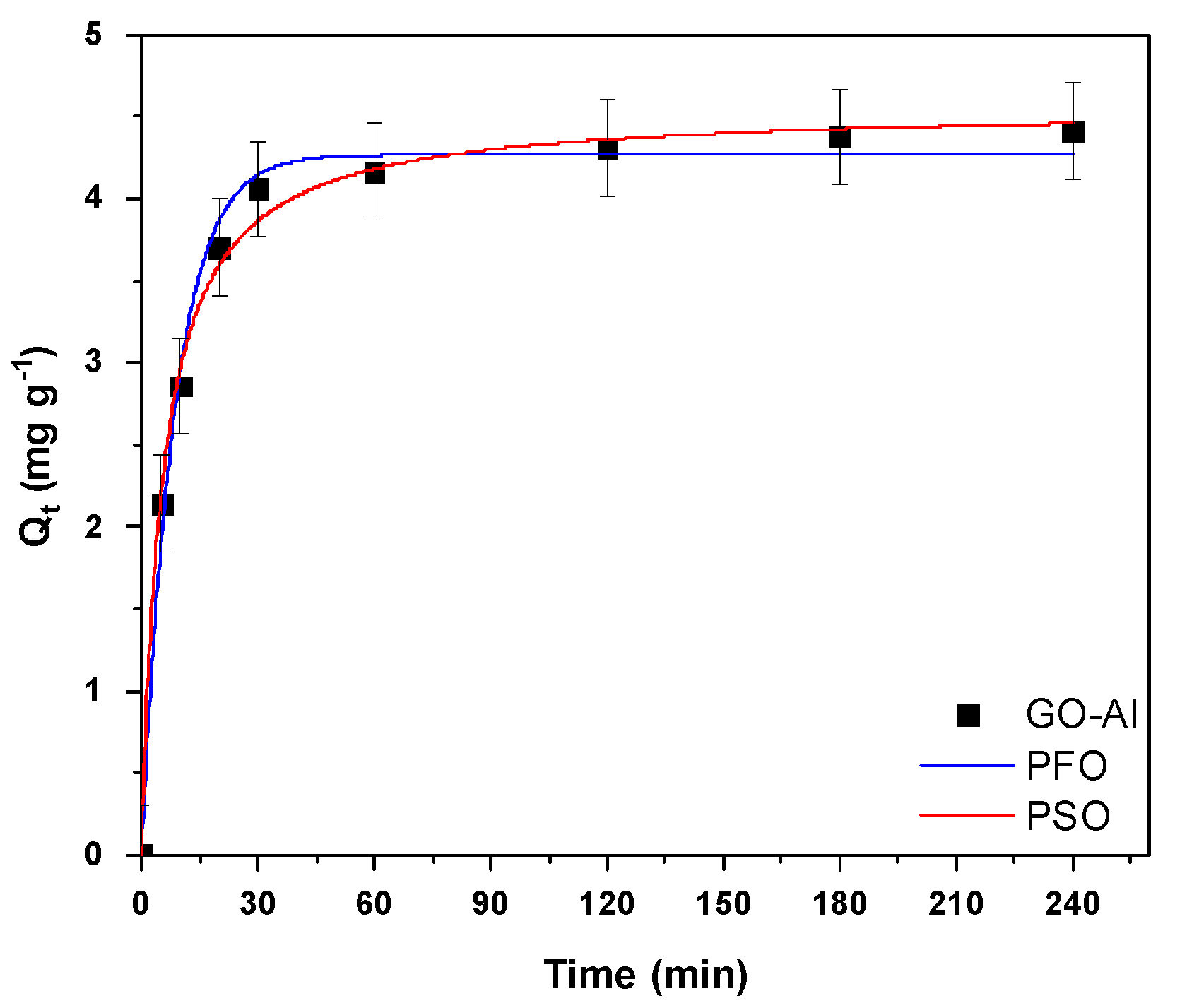


| Pseudo-First Order Model (PFO). | |||
| Qe.exp (mg g−1) | k1 (min−1) | Qe.cal (mg g−1) | R2 |
| 4.55 | 0.1175 | 4.27 | 0.9906 |
| Pseudo-Second Order model (PSO) | |||
| Qe.exp (mg g−1) | k2 (L (mg∙min)−1) | Qe.cal (mg g−1) | R2 |
| 4.55 | 0.0408 | 4.56 | 0.9952 |
| Langmuir Isotherm Model | ||
| Qm (mg g−1) | KL (L mg−1) | R2 |
| 5.91 | 0.3655 | 0.9584 |
| Freundlich isotherm model | ||
| 1/n | KF (mg g−1) (L mg−1)1/n | R2 |
| 0.2867 | 2.09 | 0.9087 |
| Adsorbent | T (K) | ∆G° (kJ mol−1) | ∆H° (kJ mol−1) | ∆S° (kJ mol−1∙K−1) | R2 |
| GO-Al | 298 | −3.752 | 15.507 | 0.0646 | 0.9997 |
| 303 | −4.075 | ||||
| 313 | −4.721 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolkou, A.K.; Katsoyiannis, I.A.; Kyzas, G.Z. Aluminum-Modified Graphene Oxide Composite Adsorbent for Humic Acid Removal from Water. J. Compos. Sci. 2025, 9, 327. https://doi.org/10.3390/jcs9070327
Tolkou AK, Katsoyiannis IA, Kyzas GZ. Aluminum-Modified Graphene Oxide Composite Adsorbent for Humic Acid Removal from Water. Journal of Composites Science. 2025; 9(7):327. https://doi.org/10.3390/jcs9070327
Chicago/Turabian StyleTolkou, Athanasia K., Ioannis A. Katsoyiannis, and George Z. Kyzas. 2025. "Aluminum-Modified Graphene Oxide Composite Adsorbent for Humic Acid Removal from Water" Journal of Composites Science 9, no. 7: 327. https://doi.org/10.3390/jcs9070327
APA StyleTolkou, A. K., Katsoyiannis, I. A., & Kyzas, G. Z. (2025). Aluminum-Modified Graphene Oxide Composite Adsorbent for Humic Acid Removal from Water. Journal of Composites Science, 9(7), 327. https://doi.org/10.3390/jcs9070327








