Highly Sensitive Titanium-Based MXene-Reduced Graphene Oxide Composite for Efficient Electrochemical Detection of Cadmium and Copper Ions in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ti3C2Tx-rGO Nanocomposite
2.3. Ti3C2Tx-rGO Nanocomposite Characterization
2.4. Electrochemical Detection of Heavy Metals
3. Result and Discussion
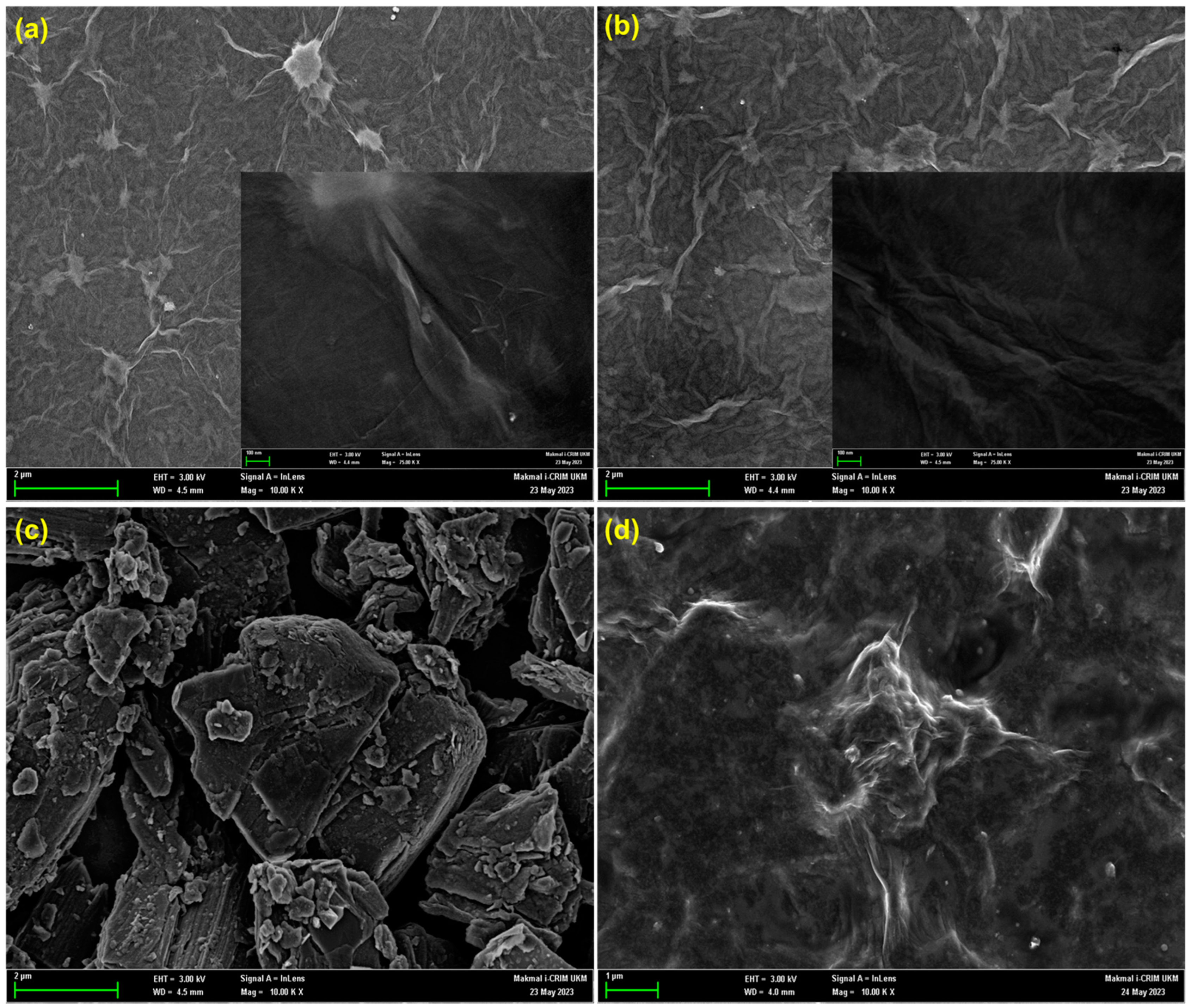
3.1. Characterization
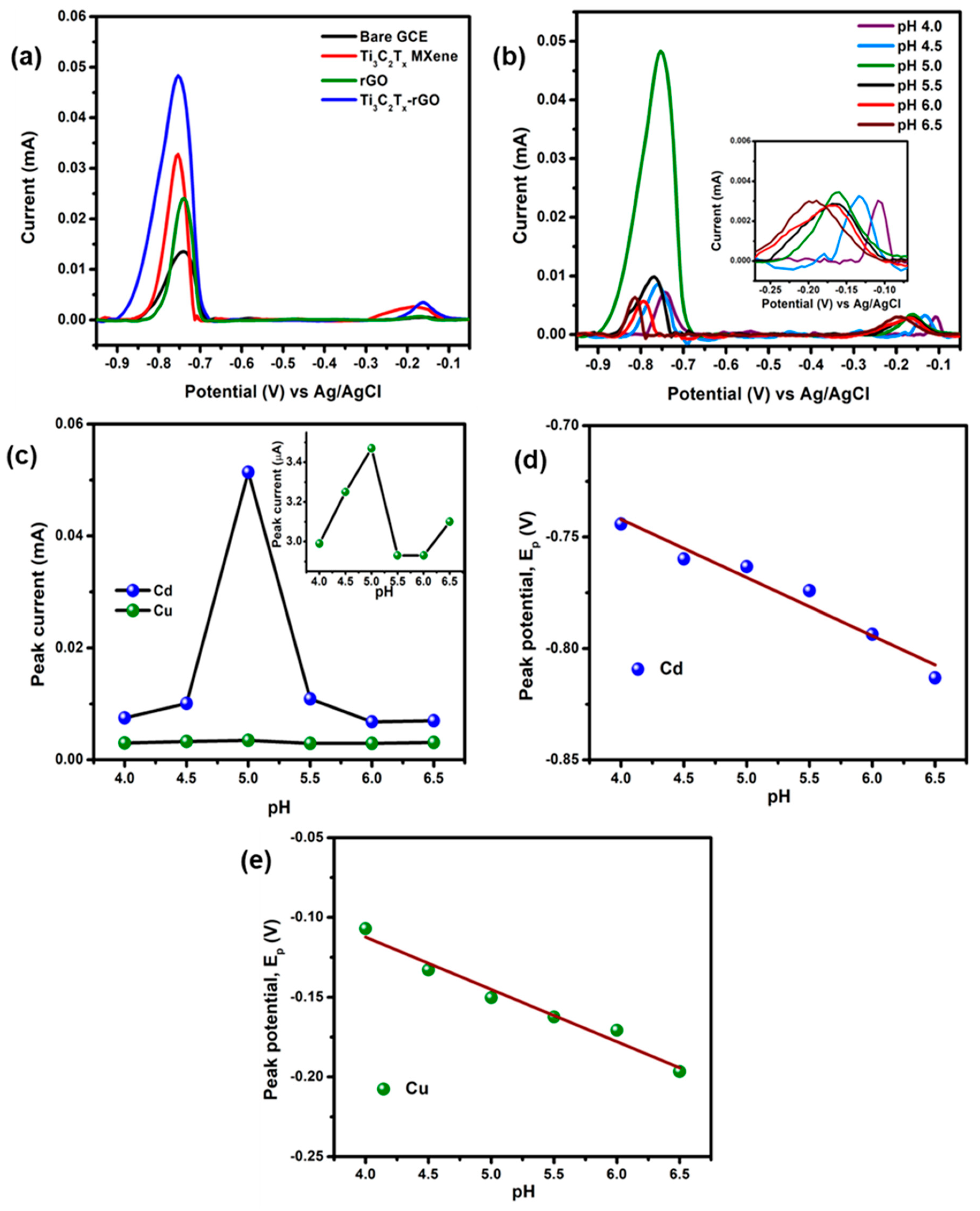
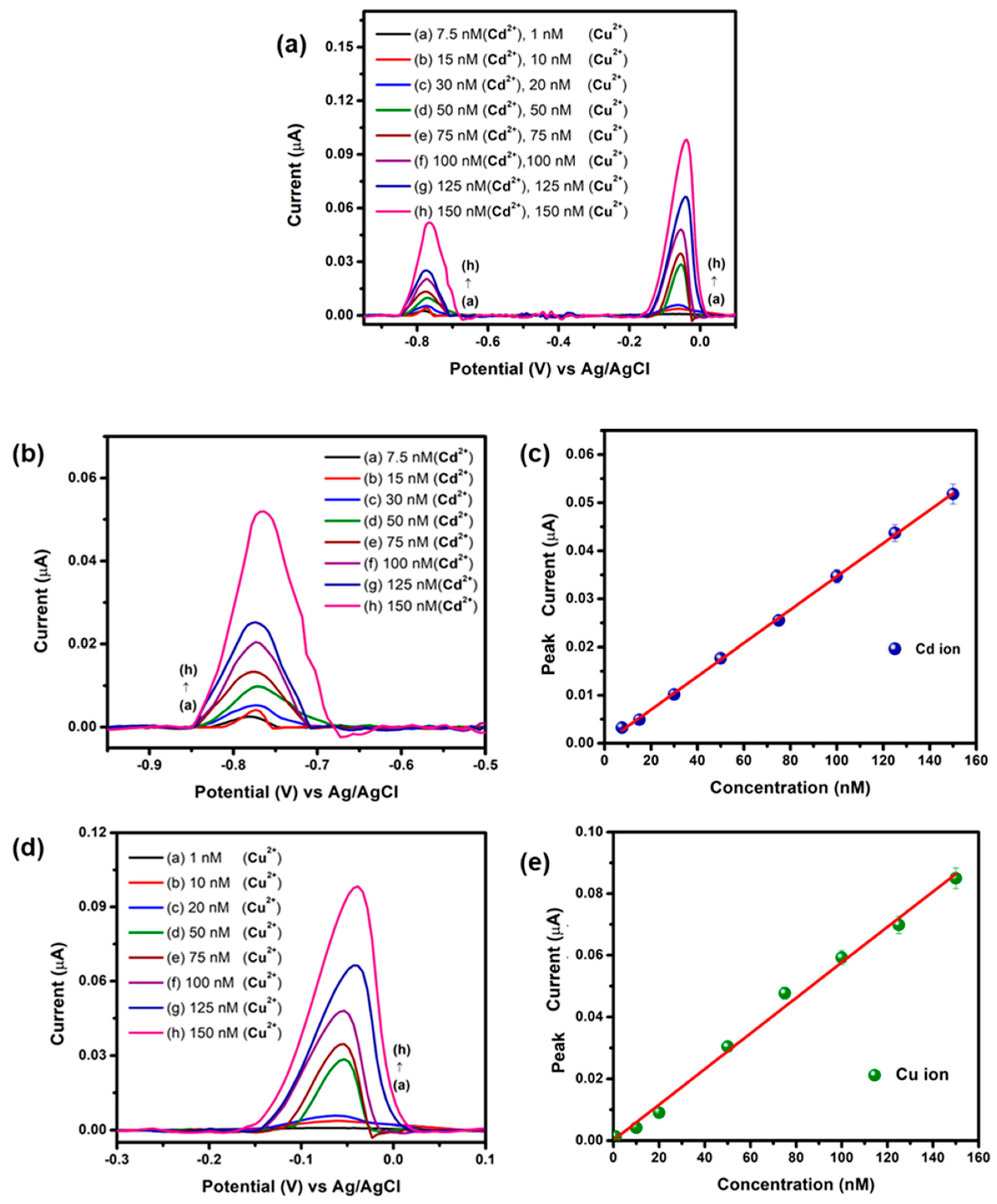
3.2. Electrochemical Detection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water–an electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Q.; Zhou, Y.; Ma, Q.; Zhu, L.; Ai, S. Electrochemical behavior of catechol, resorcinol and hydroquinone at graphene-chitosan composite film modified glassy carbon electrode and their simultaneous determination in water samples. Electrochim. Acta 2011, 56, 2748–2753. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Rahman, M.M.; Xu, G.-R.; Kim, S.; Lee, J.-J. Highly sensitive and simultaneous determination of hydroquinone and catechol at poly (thionine) modified glassy carbon electrode. Electrochim. Acta 2011, 56, 5266–5271. [Google Scholar] [CrossRef]
- Yi, Y.; Zhao, Y.; Zhang, Z.; Wu, Y.; Zhu, G. Recent developments in electrochemical detection of cadmium. Trends Environ. Anal. Chem. 2022, 33, e00152. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Miran, W.; Nawaz, M.; Jang, J.; Mahmoud, K.A.; Lee, D.S. Two-dimensional Ti3C2Tx MXene nanosheets for efficient copper removal from water. ACS Sustain. Chem. Eng. 2017, 5, 11481–11488. [Google Scholar] [CrossRef]
- Yi, Y.; Ma, Y.; Ai, F.; Xia, Y.; Lin, H.; Zhu, G. Novel methodology for anodic stripping voltammetric sensing of heavy-metal ions using Ti 3 C 2 T x nanoribbons. Chem. Commun. 2021, 57, 7790–7793. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef]
- Xia, Y.; Ma, Y.; Wu, Y.; Yi, Y.; Lin, H.; Zhu, G. Free-electrodeposited anodic stripping voltammetry sensing of Cu (II) based on Ti3C2Tx MXene/carbon black. Microchim. Acta 2021, 188, 377. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, Y.; Ai, F.; Yi, Y.; Liu, T.; Lin, H.; Zhu, G. N and P co-doped MXenes nanoribbons for electrodeposition-free stripping analysis of Cu (II) and Hg (II). J. Hazard. Mater. 2022, 425, 127974. [Google Scholar] [CrossRef]
- Maity, A.; Sui, X.; Tarman, C.R.; Pu, H.; Chang, J.; Zhou, G.; Ren, R.; Mao, S.; Chen, J. Pulse-driven capacitive lead ion detection with reduced graphene oxide field-effect transistor integrated with an analyzing device for rapid water quality monitoring. ACS Sens. 2017, 2, 1653–1661. [Google Scholar] [CrossRef]
- Hamsawahini, K.; Sathishkumar, P.; Ahamad, R.; Yusoff, A.R.M. A sensitive, selective and rapid determination of lead (II) ions in real-life samples using an electrochemically reduced graphene oxide-graphite reinforced carbon electrode. Talanta 2015, 144, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Hossain, M.; Park, J.Y. A fully integrated and miniaturized heavy-metal-detection sensor based on micro-patterned reduced graphene oxide. Sci. Rep. 2016, 6, 33125. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, K.; Wang, X.; Jia, Y.; Sun, B.; Luo, T.; Meng, F.; Jin, Z.; Lin, D.; Shen, W. Enhanced adsorption of cadmium ions by 3D sulfonated reduced graphene oxide. Chem. Eng. J. 2015, 262, 1292–1302. [Google Scholar] [CrossRef]
- Mohanadas, D.; Azman, N.H.N.; Abdullah, J.; Endot, N.A.; Sulaiman, Y. Bifunctional ternary manganese oxide/vanadium oxide/reduced graphene oxide as electrochromic asymmetric supercapacitor. Ceram. Int. 2021, 47, 34529–34537. [Google Scholar] [CrossRef]
- Mohanadas, D.; Azman, N.H.N.; Sulaiman, Y. A bifunctional asymmetric electrochromic supercapacitor with multicolor property based on nickel oxide/vanadium oxide/reduced graphene oxide. J. Energy Storage 2022, 48, 103954. [Google Scholar] [CrossRef]
- Sengupta, A.; Rao, B.B.; Sharma, N.; Parmar, S.; Chavan, V.; Singh, S.K.; Kale, S.; Ogale, S. Comparative evaluation of MAX, MXene, NanoMAX, and NanoMAX-derived-MXene for microwave absorption and Li ion battery anode applications. Nanoscale 2020, 12, 8466–8476. [Google Scholar] [CrossRef]
- Li, X.; Qian, Y.; Liu, T.; Cao, F.; Zang, Z.; Sun, X.; Sun, S.; Niu, Q.; Wu, J. Enhanced lithium and electron diffusion of LiFePO4 cathode with two-dimensional Ti3C2 MXene nanosheets. J. Mater. Sci. 2018, 53, 11078–11090. [Google Scholar] [CrossRef]
- Fang, H.; Pan, Y.; Yin, M.; Pan, C. Enhanced visible light photocatalytic activity of CdS with alkalized Ti3C2 nano-sheets as co-catalyst for degradation of rhodamine B. J. Mater. Sci. Mater. Electron. 2019, 30, 14954–14966. [Google Scholar] [CrossRef]
- Mohanadas, D.; Abdah, M.A.A.M.; Azman, N.H.N.; Ravoof, T.B.; Sulaiman, Y. Facile synthesis of PEDOT-rGO/HKUST-1 for high performance symmetrical supercapacitor device. Sci. Rep. 2021, 11, 11747. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X.; Zhong, X.; Liu, Y.; Zhu, G.; Xu, X.; Chen, K. One-pot solvothermal preparation of magnetic reduced graphene oxide-ferrite hybrids for organic dye removal. Carbon 2012, 50, 2337–2346. [Google Scholar] [CrossRef]
- Wang, S.-X.; Maimaiti, H.; Xu, B.; Awati, A.; Zhou, G.-B.; Cui, Y.-D. Synthesis and visible-light photocatalytic N2/H2O to ammonia of ZnS nanoparticles supported by petroleum pitch-based graphene oxide. Appl. Surf. Sci. 2019, 493, 514–524. [Google Scholar] [CrossRef]
- Gohari-Bajestani, Z.; Akhlaghi, O.; Yürüm, Y.; Yürüm, A. Synthesis of anatase TiO2 with exposed (001) facets grown on N-doped reduced graphene oxide for enhanced hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 6096–6103. [Google Scholar] [CrossRef]
- Huang, M.; Yu, J.; Hu, Q.; Su, W.; Fan, M.; Li, B.; Dong, L. Preparation and enhanced photocatalytic activity of carbon nitride/titania (001 vs. 101 facets)/reduced graphene oxide (g-C3N4/TiO2/rGO) hybrids under visible light. Appl. Surf. Sci. 2016, 389, 1084–1093. [Google Scholar] [CrossRef]
- Xu, J.; Liang, Q.; Li, Z.; Osipov, V.Y.; Lin, Y.; Ge, B.; Xu, Q.; Zhu, J.; Bi, H. Rational Synthesis of Solid-State Ultraviolet B Emitting Carbon Dots via Acetic Acid-Promoted Fractions of sp3 Bonding Strategy. Adv. Mater. 2022, 34, 2200011. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, S.; Zhang, J. The chemical composition and bonding structure of B–C–N–H thin films deposited by reactive magnetron sputtering. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2009, 41, 865–871. [Google Scholar] [CrossRef]
- Mohanadas, D.; Abdah, M.A.A.M.; Azman, N.H.N.; Abdullah, J.; Sulaiman, Y. A promising negative electrode of asymmetric supercapacitor fabricated by incorporating copper-based metal-organic framework and reduced graphene oxide. Int. J. Hydrogen Energy 2021, 46, 35385–35396. [Google Scholar] [CrossRef]
- Rebelo, S.L.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araújo, J.P.; Freire, C. Progress in the raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef]
- Lorencova, L.; Bertok, T.; Dosekova, E.; Holazova, A.; Paprckova, D.; Vikartovska, A.; Sasinkova, V.; Filip, J.; Kasak, P.; Jerigova, M. Electrochemical performance of Ti3C2Tx MXene in aqueous media: Towards ultrasensitive H2O2 sensing. Electrochim. Acta 2017, 235, 471–479. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Zhou, W.-T.; Song, W.-L.; Zhu, Q.-Q.; Xiong, H.-J.; Zhang, Y.; Cheng, S.; Luo, P.-F.; Lu, Y.-W. Terminal Groups-Dependent Near-Field Enhancement Effect of Ti3C2Tx Nanosheets. Nanoscale Res. Lett. 2021, 16, 60. [Google Scholar] [CrossRef]
- Genorio, B.; Harrison, K.L.; Connell, J.G.; Dražić, G.; Zavadil, K.R.; Markovic, N.M.; Strmcnik, D. Tuning the selectivity and activity of electrochemical interfaces with defective graphene oxide and reduced graphene oxide. ACS Appl. Mater. Interfaces 2019, 11, 34517–34525. [Google Scholar] [CrossRef]
- Gusain, M.; Nagpal, R. MXene for solar cells. In Solar Energy Harvesting, Conversion, and Storage; Elsevier: Amsterdam, The Netherlands, 2023; pp. 171–200. [Google Scholar]
- Zhou, Y.; Wang, Y.; Wang, Y.; Li, X. Humidity-enabled ionic conductive trace carbon dioxide sensing of nitrogen-doped Ti3C2Tx MXene/polyethyleneimine composite films decorated with reduced graphene oxide nanosheets. Anal. Chem. 2020, 92, 16033–16042. [Google Scholar] [CrossRef] [PubMed]
- Pazniak, A.; Bazhin, P.; Shplis, N.; Kolesnikov, E.; Shchetinin, I.; Komissarov, A.; Polcak, J.; Stolin, A.; Kuznetsov, D. Ti3C2Tx MXene characterization produced from SHS-ground Ti3AlC2. Mater. Des. 2019, 183, 108143. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Tran, N.M.; Noh, J.-S. Rice crust-like ZnO/Ti3C2Tx MXene hybrid structures for improved photocatalytic activity. Catalysts 2020, 10, 1140. [Google Scholar] [CrossRef]
- Yao, L.; Tian, X.; Cui, X.; Zhao, R.; Xiao, X.; Wang, Y. Partially oxidized Ti3C2Tx MXene-sensitive material-based ammonia gas sensor with high-sensing performances for room temperature application. J. Mater. Sci. Mater. Electron. 2021, 32, 27837–27848. [Google Scholar] [CrossRef]
- Ding, X.; Huang, Y.; Li, S.; Zhang, N.; Wang, J. FeNi3 nanoalloy decorated on 3D architecture composite of reduced graphene oxide/molybdenum disulfide giving excellent electromagnetic wave absorption properties. J. Alloys Compd. 2016, 689, 208–217. [Google Scholar] [CrossRef]
- Jin, L.; Chai, L.; Yang, W.; Wang, H.; Zhang, L. Two-dimensional titanium carbides (Ti3C2Tx) functionalized by poly (m-phenylenediamine) for efficient adsorption and reduction of hexavalent chromium. Int. J. Environ. Res. Public Health 2020, 17, 167. [Google Scholar] [CrossRef]
- Ma, W.; Yao, X.; Sun, D. Simultaneous electrochemical determination of dopamine, epinephrine and uric acid at silver doped poly-l-cysteine film electrode. Asian J. Chem 2013, 25, 6625–6634. [Google Scholar] [CrossRef]
- Mohanadas, D.; Tukimin, N.; Sulaiman, Y. Simultaneous electrochemical detection of hydroquinone and catechol using poly(3,4-ethylenedioxythiophene)/reduced graphene oxide/manganese dioxide. Synth. Met. 2019, 252, 76–81. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; El Hajjaji, S.; Szabό, T.; Trif, L.; Felhősi, I.; Abbi, K.; Labjar, N.; Harmouche, L.; Shaban, A. Biomass valorization of walnut shell into biochar as a resource for electrochemical simultaneous detection of heavy metal ions in water and soil samples: Preparation, characterization, and applications. Arab. J. Chem. 2022, 15, 104252. [Google Scholar] [CrossRef]
- Huang, H.; Chen, T.; Liu, X.; Ma, H. Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal. Chim. Acta 2014, 852, 45–54. [Google Scholar] [CrossRef]
- Tang, Y.-Z.; Gin, K.Y.; Aziz, M. The relationship between pH and heavy metal ion sorption by algal biomass. Adsorpt. Sci. Technol. 2003, 21, 525–537. [Google Scholar] [CrossRef]
- Qu, N.; Zhu, D.; Chan, K.C.; Lei, W. Pulse electrodeposition of nanocrystalline nickel using ultra narrow pulse width and high peak current density. Surf. Coat. Technol. 2003, 168, 123–128. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, B.; Hou, H.; Huang, Z.; Zeinu, K.M.; Huang, L.; Yuan, X.; Guo, D.; Hu, J.; Yang, J. Alkaline intercalation of Ti3C2 MXene for simultaneous electrochemical detection of Cd (II), Pb (II), Cu (II) and Hg (II). Electrochim. Acta 2017, 248, 46–57. [Google Scholar] [CrossRef]
- Lv, X.; Pei, F.; Feng, S.; Wu, Y.; Chen, S.-M.; Hao, Q.; Lei, W. Facile synthesis of protonated carbon nitride/Ti3C2Tx nanocomposite for simultaneous detection of Pb2+ and Cd2+. J. Electrochem. Soc. 2020, 167, 067509. [Google Scholar] [CrossRef]
- Zhang, X.; An, D.; Bi, Z.; Shan, W.; Zhu, B.; Zhou, L.; Yu, L.; Zhang, H.; Xia, S.; Qiu, M. Ti3C2-MXene@ N-doped carbon heterostructure-based electrochemical sensor for simultaneous detection of heavy metals. J. Electroanal. Chem. 2022, 911, 116239. [Google Scholar] [CrossRef]
- He, Y.; Ma, L.; Zhou, L.; Liu, G.; Jiang, Y.; Gao, J. Preparation and application of bismuth/MXene nano-composite as electrochemical sensor for heavy metal ions detection. Nanomaterials 2020, 10, 866. [Google Scholar] [CrossRef]





| No. | Material | Heavy MetalDetected | LOD (nM) | Linear Range of Detection (μM) | Reference |
|---|---|---|---|---|---|
| 1 | alk-Ti3C2 | Cu2+ | 39.00 | 0.1–1.4 μM | [44] |
| Cd2+ | 82.00 | 0.1–1.4 μM | |||
| 2 | H–C3N4/Ti3C2Tx | Cd2+ | 1.00 | 0.5–1.5 μM | [45] |
| Pb2+ | 0.60 | 0.5–1.5 μM | |||
| 3 | Ti3C2@N-C | Cd2+ | 2.25 | 0.1–4 μM | [46] |
| Pb2+ | 1.10 | 0.05–2 μM | |||
| 4 | BiNPs/Ti3C2Tx | Cd2+ | 12.4 | 0.08–0.8 μM | [47] |
| Pb2+ | 10.8 | 0.06–0.6 μM | |||
| 5 | Ti3C2Tx-rGO | Cd2+ | 0.31 | 7.5–150 nM | This work |
| Cu2+ | 0.18 | 1–150 nM |
| Stability Period | Peak Current Retention (%) | |
|---|---|---|
| Cd2+ | Cu2+ | |
| 1 Week | 98.19% | 99.81% |
| 2 Week | 98.61% | 99.48% |
| 3 Week | 99.89% | 98.49% |
| 4 Week | 97.86% | 98.01% |
| Sample | Added (nM) | Obtained (nM) | Recovery (%) | |||
|---|---|---|---|---|---|---|
| Cd2+ | Cu2+ | Cd2+ | Cu2+ | Cd2+ | Cu2+ | |
| 1 | 60 | 60 | 58.4 | 58.9 | 97.3% | 98.2% |
| 2 | 80 | 80 | 78.1 | 79.3 | 97.6% | 99.1% |
| 3 | 100 | 100 | 98.9 | 99.5 | 98.9% | 99.5% |
| Sample | Added (nM) | Obtained (nM) | Recovery (%) | |||
|---|---|---|---|---|---|---|
| Cd2+ | Cu2+ | Cd2+ | Cu2+ | Cd2+ | Cu2+ | |
| 1 | 60 | 60 | 58.7 | 57.6 | 97.8% | 96.0% |
| 2 | 80 | 80 | 78.2 | 78.3 | 97.8% | 97.9% |
| 3 | 100 | 100 | 99 | 98.8 | 99.0% | 98.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanadas, D.; Rohani, R.; Abdul Rahman, S.F.; Mahmoudi, E.; Sulaiman, Y. Highly Sensitive Titanium-Based MXene-Reduced Graphene Oxide Composite for Efficient Electrochemical Detection of Cadmium and Copper Ions in Water. J. Compos. Sci. 2025, 9, 232. https://doi.org/10.3390/jcs9050232
Mohanadas D, Rohani R, Abdul Rahman SF, Mahmoudi E, Sulaiman Y. Highly Sensitive Titanium-Based MXene-Reduced Graphene Oxide Composite for Efficient Electrochemical Detection of Cadmium and Copper Ions in Water. Journal of Composites Science. 2025; 9(5):232. https://doi.org/10.3390/jcs9050232
Chicago/Turabian StyleMohanadas, Dharshini, Rosiah Rohani, Siti Fatimah Abdul Rahman, Ebrahim Mahmoudi, and Yusran Sulaiman. 2025. "Highly Sensitive Titanium-Based MXene-Reduced Graphene Oxide Composite for Efficient Electrochemical Detection of Cadmium and Copper Ions in Water" Journal of Composites Science 9, no. 5: 232. https://doi.org/10.3390/jcs9050232
APA StyleMohanadas, D., Rohani, R., Abdul Rahman, S. F., Mahmoudi, E., & Sulaiman, Y. (2025). Highly Sensitive Titanium-Based MXene-Reduced Graphene Oxide Composite for Efficient Electrochemical Detection of Cadmium and Copper Ions in Water. Journal of Composites Science, 9(5), 232. https://doi.org/10.3390/jcs9050232






