Abstract
Heavy metal contamination of water is a critical environmental problem due to its toxicity and persistence in ecosystems. In this study, magnetic hydrogel spheres composed of carboxymethylated starch modified with poly(1-vinylimidazole) (CMS-g-PVI) and polyvinyl alcohol (PVA), combined with Fe3O4 nanoparticles, were synthesized and characterized to evaluate their efficiency in adsorbing metal ions such as Cu2+, Pb2+, and Cd2+. Structural characterization by FT-IR spectroscopy confirmed the successful integration of all functional components into the hydrogel matrix. Additionally, scanning electron microscopy (SEM) revealed a rough and porous surface morphology favorable for adsorption and an average bead diameter of 3.2 mm, influenced by the stirring rate during synthesis. Adsorption studies demonstrated maximum capacities of 82.4 mg·g−1 for Cu2+, 66.5 mg·g−1 for Pb2+, and 51.8 mg·g−1 for Cd2+, with optimal removal efficiencies at pH 6.2 and 5.7. From a theoretical perspective, density functional theory (DFT) calculations using the B3LYP/6-311+G(d,p) method allowed the optimization of molecular structures and analysis of electronic properties. The total dipole moment (TDM) of the CMS-g-PVI/PVA system reached 20.81 Debye. A significant reduction in the HOMO-LUMO energy gap was observed upon metal adsorption, with values of 0.0308 eV for Cu2+, 0.0175 eV for Pb2+, and 0.0235 eV for Cd2+, confirming strong interactions between the hydrogel matrix and the metal ions. The combined experimental and computational approach provides a comprehensive understanding of the adsorption mechanisms and supports the development of efficient materials for water decontamination.
1. Introduction
Water pollution with heavy metals represents one of the most worrying environmental problems today due to its toxicity, ecosystem persistence, and capacity for bioaccumulation in the food chain [1,2]. Industrial activity, mining, agriculture, and urban discharge have significantly contributed to the presence of elements such as lead (Pb), cadmium (Cd), copper (Cu), and zinc (Zn) in water bodies, generating adverse effects on both human health and aquatic ecosystems [3,4,5,6]. By not degrading naturally, these metals accumulate in the environment and can cause severe alterations in biodiversity, affecting the quality of drinking water and available water resources [7].
In response to this problem, various strategies have been developed to remove heavy metals from aqueous matrices, including membrane filtration, chemical precipitation, ion exchange, and adsorption [8,9,10,11,12,13]. Filtration processes such as reverse osmosis and ultrafiltration allow for the efficient retention of metal ions, although they have limitations associated with high operating costs and membrane obstruction [14,15]. On the other hand, chemical precipitation is based on converting metals into insoluble compounds by adding specific reagents, which, although practical, generates large volumes of sludge that are difficult to handle [13,16]. Ion exchange, which uses resins to exchange metal ions for others of lesser toxicity, is also used in the treatment of contaminated water; however, its efficiency depends on the nature of the metal to be removed and the regeneration capacity of the material used [17,18].
In this context, adsorption has been consolidated as a promising alternative due to its low cost, high efficiency, and applicability in removing metal contaminants, highlighting the use of natural biopolymers as adsorbents [19,20,21]. Among the biopolymers of natural origin, starch has aroused great interest due to its abundance, biodegradability, and ease of chemical modification, which makes it an attractive matrix for the adsorption of heavy metals [22]. However, starch in its native state presents limitations, such as low thermal and mechanical stability, which restricts its use in large-scale environmental applications [23]. To overcome these deficiencies, hybrid materials have been developed to modify starch by incorporating synthetic polymers, such as polyvinyl alcohol (PVA), to improve its structural and adsorption properties [24,25]. In recent years, the chemical modification of starch through grafting with functional polymers has proven to be an effective strategy to enhance its adsorption capacity and chemical stability. For instance, Haq et al. (2021) developed a biodegradable adsorbent based on carboxymethyl starch (CMS) grafted with poly(1-vinylimidazole), showing that increasing the density of imidazole rings on the starch backbone promotes hydrogen bonding and π–π interactions with aromatic compounds such as phenol [26]. These structural modifications not only improved the adsorption efficiency of CMS but also enhanced its surface morphology and thermal stability, key factors for sustainable environmental applications [26].
Similarly, Chen et al. (2018) synthesized a chelating resin by grafting poly(1-vinylimidazole) onto chloromethylated polystyrene using surface-initiated ATRP. The resulting material exhibited high adsorption capacities for heavy metal ions such as Cd(II), Ni(II), Pb(II), and Cu(II), primarily due to the high density of imidazole groups capable of forming stable coordination complexes [27]. The adsorption process followed a pseudo-second-order kinetic model, and the resin demonstrated excellent reusability and regeneration potential [27]. These findings support the use of CMS as a versatile platform for developing advanced adsorbent materials, especially when combined with functional polymers like poly(1-vinylimidazole), which introduce nitrogen-containing groups that can effectively interact with a broad range of pollutants, including both organic compounds and heavy metals.
PVA is a widely used polymer in environmental applications due to its water solubility, chemical stability, and ability to form three-dimensional structures with improved mechanical characteristics [28]. Combining starch with PVA has proven to be an effective strategy to increase water resistance, improve heavy metal adsorption, and facilitate the regeneration of the adsorbent material. Several studies have shown that incorporating PVA in starch matrices optimizes their structural stability and favors the interaction with metal ions through the formation of coordination bonds and electrostatic forces [24]. In parallel with the experimental development of these materials, theoretical/computational studies have allowed for a deeper understanding of the adsorption mechanisms at the molecular level, providing detailed information on the interaction between polymers and heavy metals [29,30]. Density functional theory (DFT) has established itself as an essential tool for analyzing adsorbent systems’ electronic and structural properties, allowing the optimization of materials and the prediction of their efficiency in removing contaminants [31]. In 2022, a study on Cd(II) ion remediation by starch-based activated carbon highlighted the importance of adsorption as an effective technique for heavy metal removal in aqueous media. This study demonstrated that activated carbon with a specific surface area of 1600 m2 g−1 can efficiently adsorb Cd(II), reaching a maximum adsorption capacity of 284 mg g−1 at pH 5.5–6. Furthermore, thermodynamic analysis indicated that the adsorption is a spontaneous and endothermic process, and density functional theory (DFT) calculations revealed a strong interaction between Cd(II) ions and the functional groups of the adsorbent [32]. Subsequently, in 2024, progress was made in functionalizing starch with Schiff bases to enhance Cu(II) adsorption. A new material, 2-hydrazinopyridine-modified dialdehyde starch (HYD-DAS), showed an adsorption capacity of 195.75 mg g−1 and a removal efficiency of 98.63%. Kinetics and thermodynamics studies reveal that adsorption follows a pseudo-second-order model and fits the Langmuir isotherm, indicating monolayer adsorption. Furthermore, DFT calculations and X-ray photoelectron spectroscopy (XPS) analysis confirm that Cu(II) mainly interacts with the nitrogen atoms of the Schiff base and hydrazinopyridine ring, providing strong stability to the system [33]. The combination of experimental and computational studies offers a comprehensive approach for designing new materials, contributing to the search for sustainable and efficient solutions for water decontamination [34,35]. In this context, the present theoretical/computational study focuses on modifying starch with PVA for the removal of metal ions in aqueous matrices, aiming to evaluate its adsorbent capacity and understand the molecular mechanisms that govern its interaction with heavy metals. Through an interdisciplinary approach, we seek to contribute to developing advanced materials to mitigate heavy metal contamination, providing viable and environmentally sustainable alternatives for wastewater treatment
2. Materials and Methods
2.1. Experimental Part
2.1.1. Materials
PVA with a hydrolysis degree of 97% and an average molecular weight of 50,000 was purchased from Aldrich Chemical, Milwaukee, WI, USA. In carrying out this research, several high purity reagents were used, without the need for additional purification procedures. Starch, ClCH2COOH, NaOH, HCl and an ammonia solution were obtained, as well as glutaraldehyde (GA), obtained from Merck (Darmstadt, Germany) in an aqueous solution with a concentration of 24% by weight.
Essential inorganic compounds necessary for the chemical interactions involved were incorporated, including 1-vinylimidazole (VI), copper sulfate pentahydrate (Cu(SO4)2·5H2O), cadmium chloride dodecahydrate (CdCl2·12H2O), and lead nitrate (Pb(NO3)2). Additionally, ferric (FeCl3·6H2O) and ferrous (FeCl2·7H2O) chlorides were added, all of which were supplied by Fluka (Morristown, NJ, USA). In the grafting process of poly(1-vinylimidazole) (PVI) onto carboxymethyl starch (CMS), potassium persulfate (KPS), supplied by Aldrich (Milwaukee, WI, USA), was used as the initiator.
2.1.2. Elaboration of Carboxymethylated Starch (CMS)
The experimental procedure was carried out as follows: 4.5 g of NaOH, 5 g of starch, 5 g of ClCH2COOH, and 25 mL of distilled H2O were combined in a 100 mL flask. The mixture was kept under magnetic stirring at 65 °C for 2.5 h. It was then neutralized using a 12% by weight acetic acid solution. The product obtained was precipitated with ethanol, then filtered and dried in a vacuum oven at 65 °C for 5 h.
2.1.3. Preparation of Fe3O4 Nanoparticles
The preparation of Fe3O4 nanoparticles was performed by a conventional co-precipitation method. Briefly, 12 mmol of ferric chloride (FeCl3 6H2O) and six mmol of ferrous chloride (FeCl2 7H2O) were dissolved in 25 mL of deionized water, and the mixture was stirred at 90 °C. Then, 100 mL of a 1.5 M ammonium hydroxide (NH4OH) solution was slowly added under an N2 atmosphere until reaching a pH of 11, and the mixture remained stirring for 1 h [36]. The black precipitate formation was extracted using a magnet and washed multiple times with deionized water.
2.1.4. Synthesis of Carboxymethyl Starch-g-Polyvinylimidazole (CMS-g-PVI)
To perform the copolymerization, a homogeneous solution was prepared by diluting 1.5 g of CMS in 60 mL of distilled water in a three-necked flask and then subjecting it to magnetic stirring for a period of 20 min. Then, 4.5 g of 1-vinylimidazole (VI) was incorporated and the combination was subjected to a flow of argon for 40 min to remove the existing oxygen. After completion of this procedure, the temperature was raised to 65 °C and a KPS solution was added, functioning as a reaction initiator. The copolymerization was carried out under these conditions over a time span of 3.5 h. The final product was deposited in acetone, subsequently separated through filtration, and subjected to a strict purification process. The copolymerization took place under these circumstances for a period of 3.5 h. The acquired product was deposited in acetone, then separated through filtration and subjected to a stringent purification process, which involved constant washing with hot methanol for 24 h.
The synthesis of CMS-g-PVI and CMS from starch is represented in Figure 1. For the estimation of the grafting yield, the following equation was used:
in which W1 and W2 represent the masses of CMS before and after the grafting reaction, respectively. The grafting yield for the PVI-modified carboxymethyl starch was 200% (±16.3).
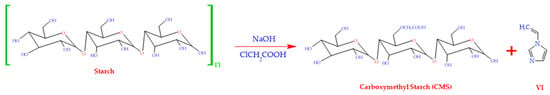
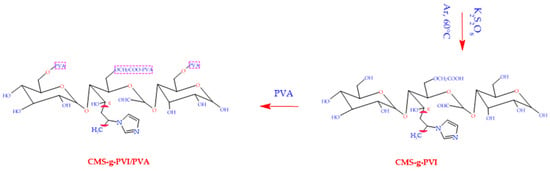
Figure 1.
Synthetic process for the preparation of CMS, CMS-g-PVI, and CMS-g-PVI/PVA.
2.1.5. Preparation of CMS-g-PVI/PVA/Fe3O4 Magnetic Hydrogel Beads
To prepare the magnetic hydrogel, a 5% PVA solution was initially prepared by dissolving the polymer in distilled H2O at 80 °C [37]. On the other hand, 2 g of CMS-g-PVI was added to 40 mL of distilled H2O to obtain a homogeneous and transparent solution. Both solutions were carefully merged to ensure uniform integration. Next, Fe3O4 nanoparticles were incorporated into the mixture and subjected to vigorous stirring for 24 h, promoting the creation of a firm gel. This solution was then injected using a syringe and needle into a medium consisting of boric acid and acetone, which was constantly stirred at 300 rpm. As a result, spheres were generated immediately and were left to stand in the solution for a full day for stabilization. To reinforce the hydrogel structure through a chemical crosslinking process, 1.5 mL of GA and 1 mL of HCl (1 M) were added, and the mixture was incubated at 55 °C for eight hours. The magnetic spheres produced were then efficiently separated by an external magnet, ensuring the expulsion of any reagent that was not absorbed in the reaction. Finally, the microspheres were washed with distilled water and then dried in a vacuum oven at 50 °C.
2.1.6. Characterization
The hydrogel beads enriched with magnetic nanoparticles (m-CVPs) were analyzed through various analytical methods to analyze their structure and functionalities. To establish the existence of various chemical units in their composition, a spectroscopic study was performed using Fourier-transform infrared spectroscopy (FT-IR). The data were collected in a range of 300 to 4000 cm−1, using potassium bromide tablets as the basis for the measurement. Scanning electron microscopy (SEM) analysis was performed using a Quanta 250 instrument (FEI Company, Bogotá, Colombia) was used to examine the surface morphology of the m-CVP beads, providing information about their structure at the micro level.
2.1.7. Heavy Metal Adsorption Test
Adsorption experiments were carried out to evaluate the ability of m-CVP to remove heavy metals found in aqueous solutions. For this purpose, solutions were prepared that included copper (Cu2+), lead (Pb2+), and cadmium (Cd2+) ions, with initial concentrations ranging from 30 to 250 mg L−1. To improve the retention procedure of these contaminants, the pH of the solutions was modified to suitable levels by adding nitric acid (0.1 M HNO3) or sodium hydroxide (0.1 M NaOH) solutions. Additionally, initial tests were carried out to establish the most effective pH levels in the adsorption process.
For each experiment, different amounts of m-CVP were incorporated into the heavy metal contaminated solutions, ensuring a constant adsorbent concentration of 1 g L−1. The mixtures were subjected to agitation at 200 rpm under a controlled temperature of 25 °C, with contact times varying between 1 and 48 h, allowing the adsorption process to reach equilibrium. During the test, samples were collected regularly to calculate the residual concentration of metals in the solution. Quantification of metal ions was carried out using atomic absorption spectroscopy, which facilitated the calculation of the amount of pollutant retained based on the variation between the initial and final concentrations. To determine the efficiency of the procedure, the percentage of removal of each metal (R%) and the adsorption capacity (Qe, mg g−1) were established by applying the corresponding equations. The influence of the contact time and the initial concentration of the metal on the adsorption efficiency was also analyzed. These analyses were key to understanding the potential of m-CVP beads as a viable and sustainable alternative for the remediation of heavy metal-contaminated waters.
The numbers and (mg L−1) represent the concentrations of heavy metals in the initial solution and in the aqueous phase after the adsorption process. Additionally, V(L) represents the volume of the aqueous phase, while m refers to the weight of the adsorbent.
2.2. Computational Details
In the exploration of novel tactics for environmental restoration, starch alteration has emerged as an encouraging tactic for the removal of heavy metals in aquatic environments. Its mixture with PVA significantly increases its adsorption ability, improving the retention of contaminants such as Cu, Pb, and Cd. To understand the nature of these interactions at the molecular scale, theoretical calculations were carried out using the GAUSSIAN 16 program [38], based on density functional theory (DFT). By structural optimization and studying the electronic features using the B3LYP method [39,40] and the 6-311+(d,p) basis set, the local energy minima in the modeled structures were determined, providing essential data for the development of new water purification tactics. In the field of computational chemistry, selecting the appropriate predictive method is essential to describe molecular features and analyze chemical processes. The accuracy of the calculations and their computational feasibility must be kept in balance, which is why theoretical strategies such as B3LYP/6-311+(d,p), B3LYP/6-31++(d,p), and M06-2X/6-311+(d,p) have been used. Based on density functional theory, these methods have been strategically selected due to their ability to offer reliable results in describing electronic structures. In particular, B3LYP/6-311+(d,p) has proven to be a robust tool, providing an optimal balance between accuracy and computational cost.
Computer simulations facilitated the identification of key electronic features, such as the total dipole moment (TDM) of the studied structures and the HOMO-LUMO band gap energies. Additionally, the limiting molecular electrostatic potential (MPEM) was determined for each structure, providing a comprehensive insight into the charge partitioning in the molecules and, consequently, a deeper understanding of their reactivity and adsorbent potential.
3. Results and Discussion
3.1. Characterization of m-CVP Pearls
The synthesis process of m-CVP hydrogel microspheres follows a detailed mechanistic scheme, depicted in Figure 1. These microspheres were obtained from the instantaneous gelation of a solution composed of PVA, carboxymethylated starch grafted with polyvinylimidazole (CMS-g-PVI) and Fe3O4 nanoparticles in a boric acid environment. Subsequently, chemical cross-linking was performed by the addition of glutaraldehyde (GA), ensuring the formation of a stable and functional structure.
To analyze the chemical composition and structural modifications of the materials, Fourier-transform infrared spectroscopy (FT-IR) was performed on starch and the modified composite system MCS-g-PVI/PVA y CMS-g-PVI/PVA/Fe3O4 (Figure 2).
Figure 2.
FTIR spectral profile of the resulting materials.
The FTIR spectrum of native starch exhibited characteristic absorption bands corresponding to its polysaccharide structure. A broad band around 3430 cm−1 was attributed to the O–H stretching vibrations, indicating the presence of an extensive hydrogen bonding network. Additionally, the band observed near 2930 cm−1 was assigned to C–H stretching vibrations, while the absorption around 1020 cm−1 was ascribed to C–O–C stretching vibrations associated with the glycosidic bonds in the starch backbone. These features are consistent with the structure of unmodified starch. Following chemical modification, significant spectral changes were observed in the CMS-g-PVI/PVA system. A new absorption band appeared around 3120 cm−1, corresponding to the stretching vibrations of C=C–H and N=C–H bonds, characteristic of the imidazole ring in poly(1-vinylimidazole) (PVI). Furthermore, additional peaks at approximately 2860 and 2940 cm−1 were assigned to C–H stretching vibrations, confirming the presence of both PVI and polyvinyl alcohol (PVA) chains. The bands observed near 1500 and 1550 cm−1 were attributed to C–C and N–C stretching vibrations within the heterocyclic structure. Signals around 770 and 670 cm−1 were related to C–H bending and C–N stretching vibrations, respectively, supporting the successful grafting of functional polymers onto the starch matrix. Finally, the FTIR spectrum of the CMS-g-PVI/PVA/Fe3O4 nanocomposite revealed further modifications resulting from the incorporation of magnetic nanoparticles. The spectral region below 800 cm−1 showed prominent bands between 400 and 700 cm−1, characteristic of Fe–O stretching vibrations from magnetite (Fe3O4). The emergence of these new absorption bands confirms the effective integration of the magnetic component into the polymeric matrix [41,42,43,44,45].
Morphological characterization of the m-CVP beads showed a spherical structure with an average diameter of 3.2 mm (Figure 3a). The stirring rate during the synthesis influenced the final size, as a higher stirring regime resulted in smaller-diameter beads. Scanning electron microscopy (SEM) analysis revealed a rough and highly porous surface (Figure 3a,b), which favors adsorption of contaminants.
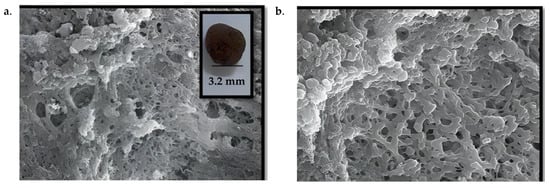
Figure 3.
Morphological characterization of m-CVP beads: overview with digital photography: surface analysis by SEM ((a): low magnification, (b): high magnification).
3.1.1. Influence of pH on Adsorption
The pH of the solution is a determining factor in the efficiency of the adsorption process since it modulates the speciation of the metal ions, the surface charge of the adsorbent, and the degree of ionization of the functional groups responsible for the interaction with the contaminants. Figure 4a,b illustrate how the adsorption capacity of the m-CVP beads varies as a function of pH.
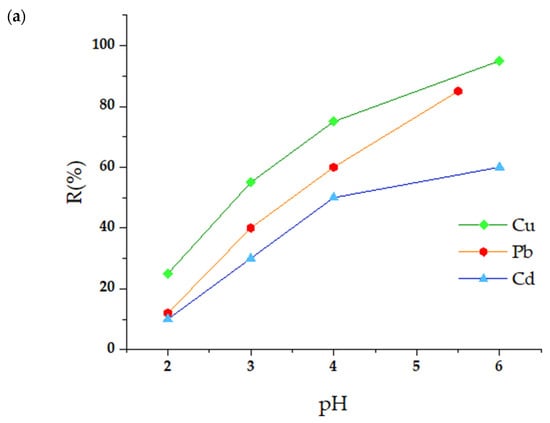
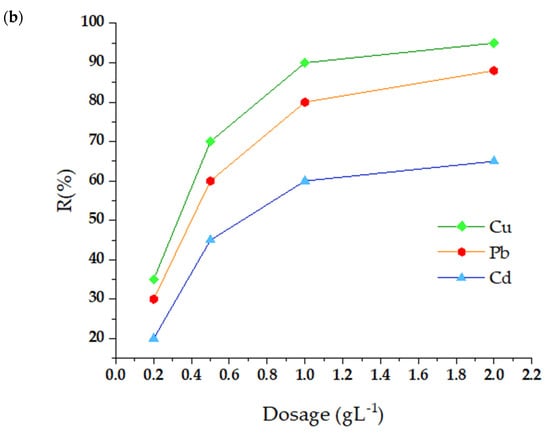
Figure 4.
Influence of pH (a) and adsorbent dose (b) on the adsorption of heavy metal ions by m-CVP beads.
The findings reveal that pH has a great impact on the adsorption of Cu2+, Pb2+, and Cd2+ since this element affects the solubility of the metals and the ionization state of the hydroxyl and imidazole groups found on the adsorbent surface. It was noted that as the pH increased, the efficiency in suppressing these cations also increased, reaching an ideal level before the creation of metal hydroxides began to restrict the adsorption process. At pH levels below 4, the retention of Cd2+, Pb2+, and Cu2+ was significantly reduced, indicating that an acidic medium complicates the adsorption of these contaminants. This occurs because functional groups are protonated on the surface of the m-CVP beads, leading to intense rivalry between protons (H+) and metal ions for active adsorption sites. On the other hand, as the pH increases, the functional groups lose protons and the adsorbent surface becomes negatively charged, which promotes electrostatic interaction with the metal cations. These findings are in agreement with previous research related to the adsorption of Pb2+ on macro-reticulated PVA (MR-PVA) beads. According to these findings, adsorption experiments were performed by modifying the pH to 5.7 for Pb2+ and 6.2 for Cu2+ and Cd2+. Under these ideal conditions, the removal efficiency for Cu2+, Pb2+, and Cd2+ reached 96.1%.
3.1.2. Effect of the Amount of Adsorbent
To determine the impact of the number of m-CVP beads on the adsorption of contaminants, tests were carried out with 50 mL solutions with an initial level of 20 mg L−1. Figure 4b represents the achievements obtained by employing various doses of the adsorbent on a scale from 0.2 to 2 g L−1. The figures revealed that the adsorption efficiency increased significantly with increasing adsorbent volume, reaching maximum removal levels of 72.5% for Cd2+, 98.9% for Cu2+, and 92.7% for Pb2+ when applying a dose of 2 g L−1 of m-CVP beads. However, a saturation point was identified at 1 g L−1, where an increase in adsorbent volume did not significantly increase contaminant removal. Thus, 1 g L−1 was established as the optimal dosage for future experiments. The increase in adsorption with a larger adsorbent volume is attributed to the greater availability of active sites and the increase in surface area, which promotes interaction with metal ions.
3.1.3. Adsorption Isotherms
To examine the correlation between the initial concentration of contaminants and the adsorption process, experiments were conducted in the range of 20 to 200 mg L−1, keeping the other experimental conditions unchanged (Figure 5). The findings indicated that, as the initial concentration of Pb2+, Cu2+, and Cd2+ increased, the percentage removal tended to decrease. This decrease is a result of the gradual saturation of the active zones in the m-CVP beads, which restricts the removal capacity in percentage terms. However, it was noted that the adsorption capacity (Qe) increased as the initial concentration of the contaminant increased. The maximum adsorption levels established were 82.4 mg g−1 for Cu2+, 66.5 mg g−1 for Pb2+, and 51.8 mg g−1 for Cd2+. It was found that Cu2+ showed a higher affinity for m-CVP beads compared to Pb2+ and Cd2+, which could be due to its lower ionic radius and higher charge density, elements that promote its interaction with the functional groups of the adsorbent.
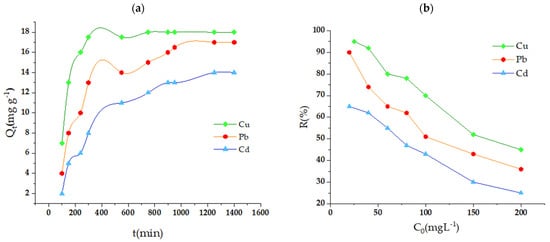
Figure 5.
Influence of initial heavy metal ion concentration (a) and contact time (b) on the adsorption process.
To model the adsorption behavior, the experimental data were fitted to the Langmuir and Freundlich isotherms [46,47]. The Langmuir model, which assumes monolayer adsorption on equivalent and homogeneous sites, was expressed by the equation:
where Qe is the amount of adsorbate retained (mg g−1), Ce is the equilibrium concentration (mg L−1), Qm is the maximum adsorption capacity (mg g−1), and KL is the Langmuir constant. The Freundlich model, which describes adsorption on heterogeneous and multilayer surfaces, was represented by:
where KF and n are Freundlich constants related to adsorption capacity and adsorption intensity (Figure 6). The correlation coefficients indicated that the Langmuir model showed a better fit, suggesting that adsorption occurs in a well-defined monocation. In addition, the separation factor RL, derived from KL, confirmed that adsorption on m-CVP beads is highly favorable. To gain a deeper understanding of the adsorption behavior of Cu2+, Pb2+, and Cd2+ ions onto the magnetic hydrogel beads (m-CVP), equilibrium data were fitted to the Langmuir and Freundlich isotherm models. The kinetic parameters derived from these fittings are summarized in Table 1, providing insight into the adsorption affinity, maximum capacity, and nature of the adsorbent surface.
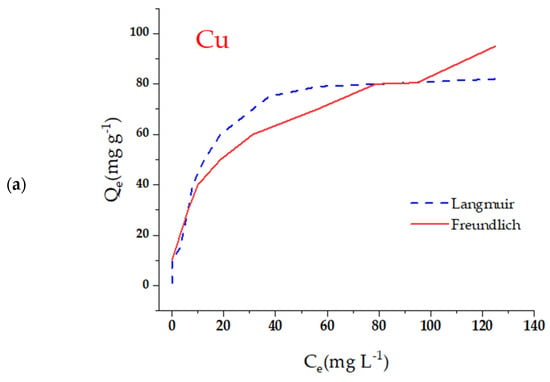
Figure 6.
Adsorption isotherms of the metal ions evaluated under the experimental conditions: (a) Cu; (b) Pb; (c) Cd. Note: initial concentration (C0) of 20–200 mg/L−1, adsorbent dose of 1 g/L−1, temperature of 25 °C, and contact time of 24 h.

Table 1.
Kinetic constants of heavy metal adsorption in m-CVP.
The Langmuir model, which assumes monolayer adsorption on a homogeneous surface, demonstrated a good fit with the experimental data, as evidenced by the high values of the theoretical maximum adsorption capacity (Qm). Among the tested ions, Cu2+ exhibited the highest Qm (141.49 mg/g), followed by Pb2+ (114.90 mg/g) and Cd2+ (80.17 mg/g). This trend indicates a stronger affinity of the m-CVP beads toward copper, possibly due to its smaller ionic radius and higher charge density, which enhance its interaction with the functional groups of the polymeric matrix. The Langmuir affinity constant (KL) values ranged from 0.0068 to 0.0094 L/mg, reflecting favorable but moderately strong adsorption for all metals.
Regarding the Freundlich model, the parameters KF and n were used to assess the adsorption intensity and surface heterogeneity. All metals showed n values greater than 1, confirming that the adsorption process is favorable. In particular, Cd2+ showed the highest n value (1.80), suggesting a more heterogeneous adsorption surface for this ion. Meanwhile, Cu2+ presented the highest KF value (3.38), reinforcing its higher affinity, as also seen in the Langmuir model.
Although a CMS-g-PVI/PVA system without nanoparticles was not directly evaluated in this study, the results obtained allow inferring the positive impact of the incorporation of Fe3O4 on the adsorption capacity. The formation of stable magnetic microspheres, the high removal efficiency obtained (up to 96.1% for Cu2+), and the maximum adsorption capacities higher than 80 mg g−1 evidence an outstanding performance of the final material. Moreover, from the computational approach, a remarkable electronic rearrangement was observed in the CMS-g-PVI/PVA system upon interaction with metal ions, suggesting a strong affinity between the adsorbent and the contaminants.
3.2. Computational Results
3.2.1. Method Selection
To determine the most efficient and reliable predictive method for the study of molecular characteristics, a simulation of starch and the CMS, CMS-g-PVI, and CMS-g-PVI/PVA systems was performed. In this research, different theoretical levels were used, selected with the aim of balancing the accuracy of the results with the computational cost linked to each calculation.
The comparative evaluation of these methods focused on two fundamental elements, the dipole moment and the electronic energy, both essential for the structural and electronic characterization of the molecules examined. The findings, presented in Table 2, revealed that the B3LYP/6-311+(d,p) level of theory provided high stability compared to the other models evaluated, in addition to showing a greater ability to anticipate molecular interactions and physical properties of the system under study.

Table 2.
Parameters evaluated for the theoretical levels.
3.2.2. Model Structure
Structural models were designed to represent its behavior in aqueous media to evaluate the effectiveness of starch in removing heavy metals. The base structure of these models was built from three starch units, which were considered essential to capture the essence of their interaction with metals. To realistically simulate the aquatic environment, each metal analyzed was accompanied by five water molecules (H2O), giving rise to the configuration M-5H2O, where “M” refers to the metals Cu, Pb, and Cd. The adsorption phenomenon describes the interaction between starch and hydrated metals. Depending on the nature of the bond, the metal bond could displace one or two water molecules. In cases where a single bond was formed with starch, one water molecule was displaced, while if the metal established two bonds, the interaction was mediated through two water molecules. The different configurations obtained are presented in Figure 7.
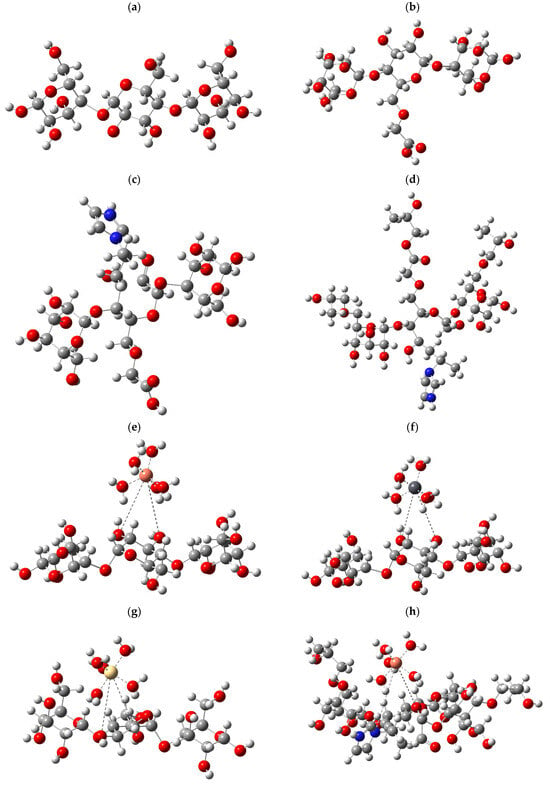
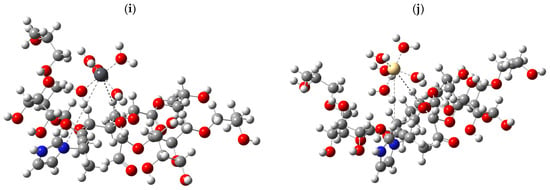
Figure 7.
B3LYP/6-311g+(d,p) calculated the optimized structure for (a) starch; (b) CMS; (c) CMS-g-PVI; (d) CMS-g-PVI/PVA; (e) starch + Cu; (f) starch + Pb; (g) starch + Cd; (h) CMS-g-PVI/PVA + Cu; (i) CMS-g-PVI/PVA + Pb; (j) CMS-g-PVI/PVA + Cd.
From a computational perspective, the study was approached using density functional theory (DFT), the B3LYP calculation layer, and the 6-311+(d,p) basis set. In addition, key parameters such as the molecular electrostatic potential (MPEM) and the HOMO-LUMO band gap energy were calculated to understand the reactivity of the modeled systems. The results highlight that physical properties such as the total dipole moment density (TDM), the HOMO-LUMO band gap energy, and the MPEM are determining indicators of the chemical behavior of the evaluated compounds.
3.2.3. HOMO-LUMO Calculations
The HOMO-LUMO band-splitting energy and the total dipole moment (TDM) were calculated for the proposed starch and CMS-g-PVI/PVA structures in the presence of M-5H2O, where “M” corresponds to the metals Cu, Pb, and Cd. These calculations were performed using the same theoretical approach, allowing an accurate comparison of the electronic effects of the interaction with metals. Figure 8 illustrates the obtained HOMO-LUMO band splitting, highlighting the distribution of the orbitals within the molecule. The orbitals are uniformly distributed along the three structural units in the starch and CMS-g-PVI/PVA models without metals. However, when metals come into play, the electron density is predominantly concentrated around the metal atoms, evidencing an alteration in the electron distribution and, therefore, in the chemical properties of the system.
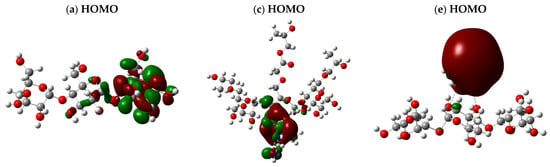
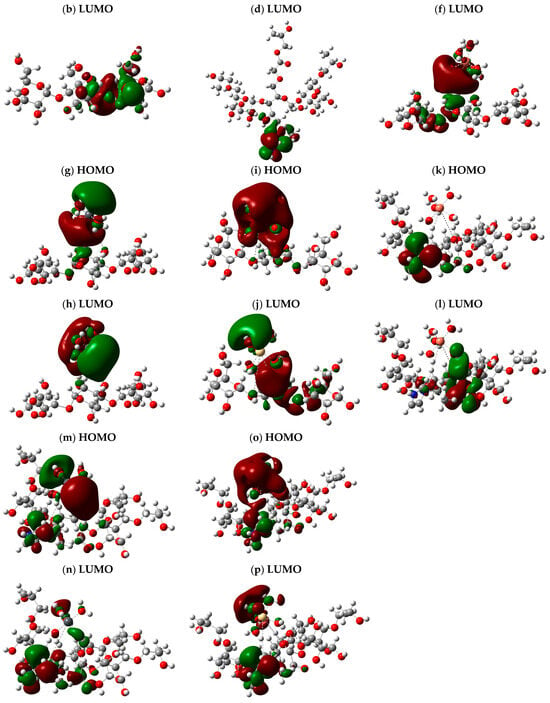
Figure 8.
HOMO-LUMO band energy with optimized structure calculated B3LYP/6-311+(d,p) for (a,b) starch; (c,d) CMS-g-PVI/PVA; (e,f) starch + Cu; (g,h) starch + Pb; (i,j) Starch + Cd; (k,l) CMS-g-PVI/PVA + Cu; (m,n) CMS-g-PVI/PVA + Pb; (o,p) CMS-g-PVI/PVA + Cd.
The analyzed structures revealed remarkable changes in their total dipole moment (TDM) and the HOMO-LUMO energy gap (ΔE), as observed in Table 3. In its original state, Al-starch presented an energy gap of 0.20884 eV and a TDM of 14.522835 Debye. However, incorporating transition metals triggered significant modifications in its electronic behavior. Upon interaction with starch, Cu drastically reduced the energy gap to 0.02988 eV, while the TDM experienced a slight increase to 14.857359 Debye, suggesting a moderate electronic interaction. On the other hand, the introduction of Pb produced a different behavior. Although the energy gap remained relatively close (0.03445 eV), the TDM shot up to 20.412808 Debye, indicating a much more pronounced electronic effect. Similarly, the starch + Cd system showed an increase in TDM to 18.647506 Debye and a ΔE of 0.03708 eV, evidencing the influence of Cd on the electronic reorganization of the structure.

Table 3.
Calculated TDM (Debye) and HOMO-LUMO band gap energies ∆E (eV) using B3LYP/6-311g+(d,p) for starch, starch + Cu, starch + Pb, starch + Cd, CMS-g-PVI/PVA, CMS-g-PVI/PVA + Cu, CMS-g-PVI/PVA + Pd, CMS-g-PVI/PVA + Cd.
As for the CMS-g-PVI/PVA polymer, it presented quite different characteristics from pure starch. Its energy gap was calculated to be −0.022602 eV, while its dipole moment reached 20.810176 Debye, suggesting a higher dipolar polarization. Adding Cu to this matrix reduced the energy gap to 0.03080 eV and decreased the TDM to 18.250570 Debye, hinting at a stabilizing effect. In contrast, Pb again made a considerable difference: it reduced the energy gap to 0.01752 eV. It raised the TDM to 25.290852 Debye, reaffirming its strong impact on the electronic configuration of the system. Cd also played a key role in modifying the CMS-g-PVI/PVA, reducing the energy gap to 0.0235 eV and raising the TDM to 23.333746 Debye. Although its influence was smaller than that of lead, it still demonstrated a significant effect on the electronic structure of the polymer.
3.2.4. Molecular Electrostatic Potential Map (MPEM)
The molecular electrostatic potential map (MPEM) is a powerful visual tool that allows one to explore the charge distribution within a molecule, revealing how the interaction between nuclei and electrons shapes its electrostatic environment. This analysis is represented by a color scale that ranges from red to blue, passing through intermediate shades such as orange, yellow, and green. In this palette, the red regions indicate a low electrostatic potential associated with a high electron density. At the same time, the blue areas reflect a higher potential, corresponding to regions with lower electron density. Understanding the MPEM distribution is essential to identify the active sites of a molecule and predict its ability to interact with other atoms or functional groups.
In Figure 9a, the MPEM contour of starch shows a marked red region, indicating a significant accumulation of electronic charge at specific strategic points. This high electron density suggests that the (OH) groups act as the main reactivity centers within the structure. Figure 9b, on the other hand, presents the MPEM of the CMS-g-PVI/PVA system in interaction with hydrated metals such as Cu, Pb, and Cd. In this case, the predominance of red zones on the molecular surface shows regions of high chemical activity, highlighting their crucial role in the adsorption of metals and suggesting a preferential affinity for these sites during the capture and retention process.
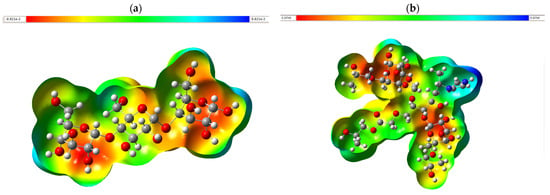
Figure 9.
Molecular electrostatic potential map with B3LYP/6-311+(d,p) calculated optimized structure for (a) starch; (b) MCS-g-PVI/PVA.
4. Conclusions
This analysis has corroborated the feasibility and effectiveness of hydrogel magnetic beads designed with PVI- and PVA-modified carboxymethylated starch (CMS-g-PVI/PVA) for the adsorption of heavy metals in water solutions. The mixture of experimental methods and computational calculations enabled a thorough examination of the interaction mechanisms between the engineered material and metal ions, demonstrating a high removal capacity for Cu2+, Pb2+, and Cd2+. From an experimental point of view, the findings indicated that the use of artificial polymers and magnetic nanoparticles enhances their structural stability and significantly improves their adsorption ability. It was established that elements such as pH, adsorbent volume and initial metal concentration play a crucial role in the removal efficiency, achieving ideal adsorption levels exceeding 96% in the case of Cu. Additionally, the isotherm study evidenced an appropriate fit to the Langmuir model, which indicates a single layer adsorption process with uniform locations.
From the computational perspective, DFT made it possible to describe the electronic essence of the material and its relationship with heavy metals. Through experiments performed with the B3LYP functional and the 6-311+(d,p) basis set, the molecular structures of starch and its derivatives were improved, providing essential data about the energetic stability, TDM, and HOMO-LUMO energy band gap. The inclusion of metals was found to cause remarkable alterations in the electronic arrangement of the adsorbent material, indicating a positive interaction between metal ions and functional groups found on the hydrogel surface. The results of the MPEM calculations facilitated the identification of the areas with the highest charge density in the adsorbent structure, highlighting the involvement of the hydroxyl and nitrogen groups in metal capture. Additionally, the HOMO-LUMO energy gap study showed that the existence of metal ions considerably decreases the electronic stability of the system, corroborating the intense interaction between the adsorbent and the contaminants. It was established that Pb2+ causes the most prominent modifications in the electronic restructuring of the material, with a significant increase in the TDM and a decrease in the energy gap, indicating a highly efficient adsorption.
Author Contributions
Conceptualization, J.A.H.F., J.A.P.P. and C.A.T.T.; Methodology, J.A.H.F.; Software, J.A.H.F.; Validation, J.A.H.F., J.A.P.P. and C.A.T.T.; Formal analysis, J.A.H.F.; Investigation, J.A.H.F., J.A.P.P. and C.A.T.T.; Resources, J.A.H.F.; Data curation, J.A.H.F. and J.A.P.P.; Writing—original draft, J.A.H.F. and J.A.P.P.; Writing—review & editing, J.A.H.F., J.A.P.P. and C.A.T.T.; Visualization, J.A.H.F.; Supervision, J.A.H.F.; Project administration, J.A.H.F.; Funding acquisition, J.A.H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the University of Cartagena for its support in terms of equipment and materials for the development of this work. We dedicate this scientific breakthrough to LiCorSo primarily to inspire the principal investigator to develop new aspects of science and technology. Inspiration requires a unique gift.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Jaiswal, A.; Verma, A.; Jaiswal, P. Detrimental Effects of Heavy Metals in Soil, Plants, and Aquatic Ecosystems and in Humans. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 183–197. [Google Scholar] [CrossRef]
- Chan, W.S.; Routh, J.; Luo, C.; Dario, M.; Miao, Y.; Luo, D.; Wei, L. Metal accumulations in aquatic organisms and health risks in an acid mine-affected site in South China. Environ. Geochem. Health 2021, 43, 4415–4440. [Google Scholar] [CrossRef] [PubMed]
- Barwick, M.; Maher, W. Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia. Mar. Environ. Res. 2003, 56, 471–502. [Google Scholar] [CrossRef]
- Davis, A.P.; Shokouhian, M.; Ni, S. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere 2001, 44, 997–1009. [Google Scholar] [CrossRef]
- Rekha, R. Trace Metals in the Aquatic Environment and its Effect on Aquatic Life and Human Body. Int. J. Sci. Res. 2023, 12, 789–795. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Zhou, T.; Li, X. Removal of chelated heavy metals from aqueous solution: A review of current methods and mechanisms. Sci. Total Environ. 2019, 678, 253–266. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2018, 17, 729–754. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Nazaripour, M.; Reshadi, M.A.M.; Mirbagheri, S.A.; Nazaripour, M.; Bazargan, A. Research trends of heavy metal removal from aqueous environments. J. Environ. Manag. 2021, 287, 112322. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Livinalli, N.F.; Silvestre, W.P.; Duarte, J.; Peretti, I.; Baldasso, C. Study of reverse osmosis performance for manganese and iron removal from raw freshwater. Chem. Eng. Commun. 2023, 210, 1961–1971. [Google Scholar] [CrossRef]
- Hoslett, J.; Massara, T.M.; Malamis, S.; Ahmad, D.; Van Den Boogaert, I.; Katsou, E.; Ahmad, B.; Ghazal, H.; Simons, S.; Wrobel, L.; et al. Surface water filtration using granular media and membranes: A review. Sci. Total Environ. 2018, 639, 1268–1282. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Vaaramaa, K.; Lehto, J. Removal of metals and anions from drinking water by ion exchange. Desalination 2003, 155, 157–170. [Google Scholar] [CrossRef]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Krstić, V.; Urošević, T.; Pešovski, B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Manzoor, K.; Ahmad, M.; Ahmad, S.; Ikram, S. Synthesis, Characterization, Kinetics, and Thermodynamics of EDTA-Modified Chitosan-Carboxymethyl Cellulose as Cu(II) Ion Adsorbent. ACS Omega 2019, 4, 17425–17437. [Google Scholar] [CrossRef]
- Sheth, Y.; Dharaskar, S.; Khalid, M.; Sonawane, S. An environment friendly approach for heavy metal removal from industrial wastewater using chitosan based biosorbent: A review. Sustain. Energy Technol. Assess. 2020, 43, 100951. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rawat, K.; Bhadauria, V.; Singh, H. Recent trends in the application of modified starch in the adsorption of heavy metals from water: A review. Carbohydr. Polym. 2021, 269, 117763. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, H.; Wang, L.; Abdin, Z.U.; Chen, Y.; Wang, J.; Zhou, W.; Yang, X.; Khan, R.U.; Zhang, H.; et al. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015, 5, 67459–67474. [Google Scholar] [CrossRef]
- Lemma, E.; Kiflie, Z.; Kassahun, S.K. Adsorption of Cr (VI) ion from aqueous solution on acrylamide—Grafted starch (Coccinia abyssinicca)—PVA/PVP/chitosan/graphene oxide blended hydrogel: Isotherms, kinetics, and thermodynamics studies. Sep. Sci. Technol. 2022, 58, 241–256. [Google Scholar] [CrossRef]
- Ounkaew, A.; Kasemsiri, P.; Kamwilaisak, K.; Saengprachatanarug, K.; Mongkolthanaruk, W.; Souvanh, M.; Pongsa, U.; Chindaprasirt, P. Polyvinyl Alcohol (PVA)/Starch Bioactive Packaging Film Enriched with Antioxidants from Spent Coffee Ground and Citric Acid. J. Polym. Environ. 2018, 26, 3762–3772. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Mehmood, S.; Haroon, M.; Amin, B.; Fahad, S.; Uddin, M.A.; Shen, D. Synthesis of carboxymethyl starch grafted polyvinyl imidazole (CMS-g-PVIs) and their role as an absorbent for the removal of phenol. Environ. Eng. Res. 2020, 26, 200327. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, W.; Yang, X.; Li, Y. Efficient removal of heavy metal ions from aqueous solution by a novel poly (1-vinylimidazole) chelate resin. Polym. Bull. 2018, 76, 1081–1097. [Google Scholar] [CrossRef]
- Abid, Z.; Hakiki, A.; Boukoussa, B.; Launay, F.; Hamaizi, H.; Bengueddach, A.; Hamacha, R. Preparation of highly hydrophilic PVA/SBA-15 composite materials and their adsorption behavior toward cationic dye: Effect of PVA content. J. Mater. Sci. 2019, 54, 7679–7691. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, Z.; Li, H.; Wu, M.; Zhao, Q.; Pan, B. An electron-scale comparative study on the adsorption of six divalent heavy metal cations on MnFe2O4@CAC hybrid: Experimental and DFT investigations. Chem. Eng. J. 2019, 381, 122656. [Google Scholar] [CrossRef]
- Sellaoui, L.; Hessou, E.P.; Badawi, M.; Netto, M.S.; Dotto, G.L.; Silva, L.F.O.; Tielens, F.; Ifthikar, J.; Bonilla-Petriciolet, A.; Chen, Z. Trapping of Ag+, Cu2+, and Co2+ by faujasite zeolite Y: New interpretations of the adsorption mechanism via DFT and statistical modeling investigation. Chem. Eng. J. 2020, 420, 127712. [Google Scholar] [CrossRef]
- Sellaoui, L.; Mendoza-Castillo, D.; Reynel-Ávila, H.; Ávila-Camacho, B.; Díaz-Muñoz, L.; Ghalla, H.; Bonilla-Petriciolet, A.; Lamine, A.B. Understanding the adsorption of Pb2+, Hg2+ and Zn2+ from aqueous solution on a lignocellulosic biomass char using advanced statistical physics models and density functional theory simulations. Chem. Eng. J. 2019, 365, 305–316. [Google Scholar] [CrossRef]
- Melhi, S.; Jan, S.U.; Khan, A.A.; Badshah, K.; Ullah, S.; Bostan, B.; Selamoglu, Z. Remediation of Cd (II) Ion from an Aqueous Solution by a Starch-Based Activated Carbon: Experimental and Density Functional Theory (DFT) Approach. Crystals 2022, 12, 189. [Google Scholar] [CrossRef]
- Liang, L.; Han, M.; Liu, Y.; Huang, C.; Leng, Y.; Zhang, Y.; Cai, X. Schiff base functionalized dialdehyde starch for enhanced removal of Cu (II): Preparation, performances, DFT calculations. Int. J. Biol. Macromol. 2024, 268, 131424. [Google Scholar] [CrossRef]
- Tahini, H.A.; Tan, X.; Smith, S.C. Computational Materials Science: Discovering and Accelerating Future Technologies. Adv. Theory Simul. 2019, 2, 1900023. [Google Scholar] [CrossRef]
- Jie, Y.; Wen-ren, C.; Manurung, R.M.; Ganzeveld, K.J.; Heeres, H.J. Exploratory Studies on the Carboxymethylation of Cassava Starch in Water-miscible Organic Media. Starch—Stärke 2004, 56, 100–107. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Z.; Xing, J.; Liu, H. Preparation and characterization of amino–silane modified superparamagnetic silica nanospheres. J. Magn. Magn. Mater. 2003, 270, 1–6. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Q.; Pan, Y.; Li, Y.; Huang, Z.; Li, M.; Xiao, H. Functionalized porous magnetic cellulose/Fe3O4 beads prepared from ionic liquid for removal of dyes from aqueous solution. Int. J. Biol. Macromol. 2020, 163, 309–316. [Google Scholar] [CrossRef]
- Frisch, M.J. Revisión B. 01; Gaussiano. Inc.: Wallingford, CT, USA, 2018. [Google Scholar]
- Giroday, T.; Montero-Campillo, M.M.; Mora-Diez, N. Thermodynamic stability of PFOS: M06-2X and B3LYP comparison. Comput. Theor. Chem. 2014, 1046, 81–92. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuß, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Komulainen, S.; Verlackt, C.; Pursiainen, J.; Lajunen, M. Oxidation and degradation of native wheat starch by acidic bromate in water at room temperature. Carbohydr. Polym. 2012, 93, 73–80. [Google Scholar] [CrossRef]
- Kara, A. Poly(ethylene glycol dimethacrylate-n-vinyl imidazole) beads for heavy metal removal. J. Hazard. Mater. 2004, 106, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ajji, Z.; Ali, A.M. Separation of copper ions from iron ions using PVA-g-(acrylic acid/N-vinyl imidazole) membranes prepared by radiation-induced grafting. J. Hazard. Mater. 2009, 173, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Niksefat, M.; Rahimi, J.; Hajizadeh, Z. Design and preparation of Fe3O4 @PVA polymeric magnetic nanocomposite film and surface coating by sulfonic acid via in situ methods and evaluation of its catalytic performance in the synthesis of dihydropyrimidines. BMC Chem. 2019, 13, 19. [Google Scholar] [CrossRef]
- Llanos, J.H.; Tadini, C.C. Preparation and characterization of bio-nanocomposite films based on cassava starch or chitosan, reinforced with montmorillonite or bamboo nanofibers. Int. J. Biol. Macromol. 2017, 107, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Duwiejuah, A.B.; Cobbina, S.J.; Bakobie, N. Review of Eco-Friendly Biochar Used in the Removal of Trace Metals on Aqueous Phases. Int. J. Environ. Bioremediation Biodegrad. 2017, 5, 27–40. [Google Scholar] [CrossRef]
- Freundlich, H. Of the adsorption of gases. Section II. Kinetics and energetics of gas adsorption. Introductory paper to section II. Trans. Faraday Soc. 1932, 28, 195–201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).