Exploration of Alkaline Degumming Printing Techniques for Silk Gauze Fabric: Alkaline Boiling, Alkaline Steaming, and Alkaline Gel
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Preparation of Alkaline Water
2.2.2. Preparation of Alkaline Paste
2.2.3. Preparation of Alkaline Gel
2.2.4. Alkaline Boiling Degumming
2.2.5. Alkaline Steaming Degumming
2.2.6. Alkaline Gel Degumming
2.3. Testing Methods
2.3.1. Calculation of Degumming Rate
2.3.2. Water Retention Capacity Test
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
3. Results and Analysis
3.1. Mechanism of Alkaline Agent Degumming
3.2. Alkali Gel Printing Process
3.3. Comparison of Alkali Agent Degumming Processes
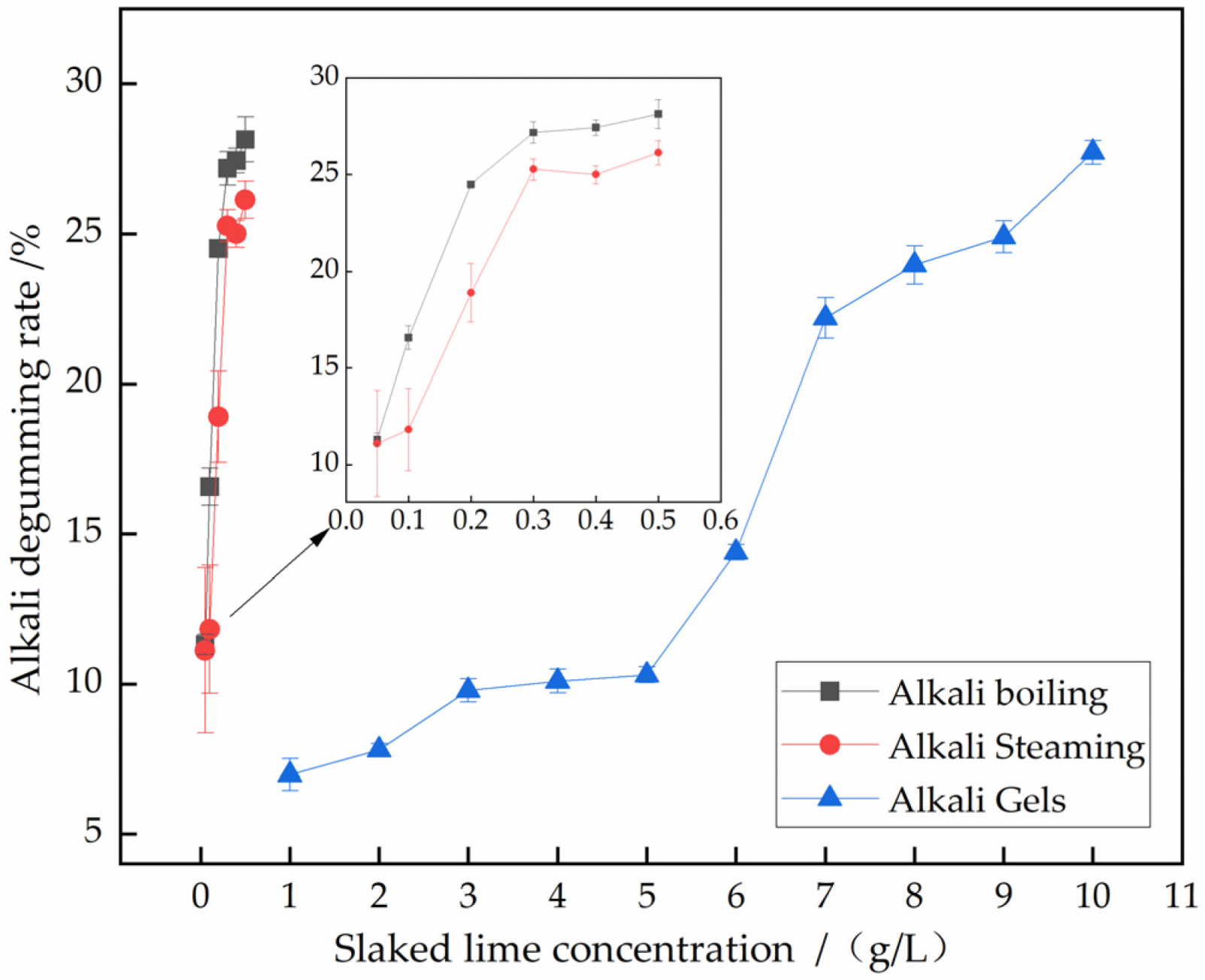
3.3.1. Effect of Alkali Agent Dosage on Degumming of Silk Gauze
3.3.2. Effect of Hot Pressing Temperature on Degumming of Silk Gauze
3.3.3. Effect of Hot Pressing Time on Degumming of Silk Gauze
3.4. Surface Morphology and Infrared Spectroscopy Analysis of Silk Gauze Under Different Alkaline Degumming Processes
3.4.1. Surface Morphology Comparison of Silk Gauze After Degumming with Different Alkaline Agents
3.4.2. Infrared Comparison of Silk Gauze After Degumming with Different Alkaline Agents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, M. Tang Dynasty Dyeing Techniques in Silk Textiles Unearthed in Turpan. Cult. Relic 1973, 10, 37–47+81. [Google Scholar]
- Zhao, F. A Comprehensive History of Chinese Silk; Suzhou University Press: Suzhou, China, 2005; pp. 230–231. [Google Scholar]
- Yang, J.; Cui, Y. Research on traditional dye safflower cake and its production technology. J. Silk 2017, 54, 73–80. [Google Scholar]
- Yu, S. Same Origin but Different Trends: A Comparative Study of Chinese Blue Calico and Japanese Komon. J. Zhejiang Univ. (Humanit. Soc. Sci.) 2024, 54, 100–111. [Google Scholar]
- Wang, L. Study on the Technical Characteristics and Design Application of the Tang Dynasty Printed Silk Unearthed from Astana Tomb. Art Des. Res. 2020, 1, 55–58. [Google Scholar]
- Zhang, H.; Wang, L. A study on the craft of “green hunting pattern printed silk” of Tang Dynasty unearthed in Astana. J. Donghua Univ. (Soc. Sci. Ed.) 2023, 23, 41–48. [Google Scholar]
- Zhu, P.; Wu, H.; Wu, H. Research on Traditional Silk Degumming Process and Color Deve-lopment of Alkaline Agent Printing. Acta Sericologica Sin. 2023, 5, 423–429. [Google Scholar]
- Wu, H.; Zhou, J.; Zhu, P.; Li, J.; Li, Y. An Exploration of Alkaline Degumming in the Printing and Dyeing Process of Silk Gauze. Polymers 2024, 16, 2926. [Google Scholar]
- Ling, X.; Lin, H.; Huang, J. Research Progress of Scouring Methods and Technologies of Silk. Sci. Seric. 2013, 39, 1186–1192. [Google Scholar]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Batara, B.; Steven, S.; Mulyana, M.; Saputra, A.S.; Hutahaean, A.C.; Yemensia, E.V.; Soekotjo, E.S.A.; Abidin, A.Z.; Graha, H.P.R. Recent Advances, Applications, and Challenges in Superabsorbent Polymers to Support Water Sustainability. J. Appl. Polym. Sci. 2025, 142, 11. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; Chapter IX Gels and Networks. [Google Scholar]
- Li, Y.; Qu, G.; Zhang, H.; Xie, L.; Zhang, Y.-F. pH-Responsive removal of dyes from wastewater using MXene composited L-Cysteine-grafted HEMA hydrogel: Dynamics, selectivity, regeneration and mechanism. Chem. Eng. Sci. 2024, 300, 120648. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Shi, Z.; Jiang, W.; Liu, X.; Ni, Q.-Q. Degumming of raw silk via steam treatment. J. Clean. Prod. 2018, 203, 492–497. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, P.; Huang, L.; Zhang, L. Preparation and printing properties of sodium polyacrylate/CMC composite paste. J. Zhejiang Sci-Tech Univ. (Nat. Sci.) 2015, 33, 439–446. [Google Scholar]
- Hao, B.; Shao, J.; Wang, L. Application of monochloromethazine-modified guar gum as thickener in the penetration printing of silk fabric. Silk 2018, 4, 7–12. [Google Scholar]
- Zhou, J.; Wang, H.; Xiang, L.; Cheng, J. Study on crosslinking condensation reaction of FCC Slurry. J. Wuhan Inst. Tech. 2011, 23, 4–10. [Google Scholar]
- Syromiatnikova, V.; Gupta, S.; Zhuravleva, M.; Masgutova, G.; Zakirova, E.; Aimaletdinov, A.; Rizvanov, A.; Salafutdinov, I.; Naumenko, E.; Bit, A. Engineered GO-Silk Fibroin-Based Hydrogel for the Promotion of Collagen Synthesis in Full-Thickness Skin Defect. J. Compos. Sci. 2023, 7, 186. [Google Scholar] [CrossRef]
- Wang, S.; Hu, X.; Yang, Z.; Qu, D. Preparation and properties of polyacrylic acid/silk fibroin composite hydrogel. J. Guangxi Univ. Sci. Technol. 2024, 1, 2–13. [Google Scholar]
- Zhu, L.; Lin, J.; Pei, L.; Luo, Y.; Li, D.; Huang, Z. Recent Advances in Environmentally Friendly and Green Degumming Processes of Silk for Textile and Non-Textile Applications. Polymers 2022, 14, 659. [Google Scholar] [CrossRef]
- Cao, T.; Zhang, Y. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Q.; Pan, P.; Fang, M.; Zhang, Y.; Yang, S.; Li, M.; Liu, Y. Comparative Study of the Preparation of High-Molecular-Weight Fibroin by Degumming Silk with Several Neutral Proteases. Polymers 2023, 15, 3383. [Google Scholar] [CrossRef]
- Khan, M.R.; Tsukada, M.; Gotoh, Y.; Morikawa, H.; Freddi, G.; Shiozaki, H. Physical properties and dyeability of silk fibers degummed with citric acid. Bioresour. Technol. 2010, 101, 8439–8445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L. Dyeing process and performance of persimmon lacquer for silk fabric. Silk 2021, 5, 1–7. [Google Scholar]
- Huang, J.; Yang, B.; Chen, D. Experimental study on hydrochloric acid-steam degumming process of silk knitted fabrics. Silk 2020, 6, 40–44. [Google Scholar]
- Ming, J.F.; Pan, F.K.; Zuo, B.Q. Influence factors analysis on the formation of silk I structure. Int. J. Biol. Macromol. 2015, 75, 398–401. [Google Scholar] [CrossRef]






| Item | Concentration/% | Water Retention/mm | Viscosity/mPa∙s | ||
|---|---|---|---|---|---|
| Sodium polyacrylate | 4 | Distilled Water | 5 g/L Alkaline Water | Distilled Water | 5 g/L Alkaline Water |
| 0.9 | 0 | 9473 | 68,285 | ||
| Alkaline Agent Amount g/L | Alkaline Boiling Degumming Rate/% | Alkaline Steaming Degumming Rate/% | Alkaline Gel Degumming Rate/% |
|---|---|---|---|
| 0.05 | 11.32 ± 0.34 | 11.11 ± 2.75 | \ |
| 0.1 | 16.57 ± 0.62 | 11.82 ± 2.13 | \ |
| 0.2 | 24.51 ± 0.18 | 18.90 ± 1.52 | \ |
| 0.3 | 27.17 ± 0.55 | 25.27 ± 0.54 | \ |
| 0.4 | 27.43 ± 0.41 | 25.00 ± 0.45 | \ |
| 0.5 | 28.13 ± 0.75 | 26.13 ± 0.62 | \ |
| 1 | \ | \ | 6.98 ± 0.53 |
| 2 | \ | \ | 7.81 ± 0.21 |
| 3 | \ | \ | 9.79 ± 0.39 |
| 4 | \ | \ | 10.10 ± 0.39 |
| 5 | \ | \ | 10.31 ± 0.26 |
| 6 | \ | \ | 14.38 ± 0.26 |
| 7 | \ | \ | 22.19 ± 0.68 |
| 8 | \ | \ | 23.96 ± 0.64 |
| 9 | \ | \ | 24.90 ± 0.53 |
| 10 | \ | \ | 27.71 ± 0.39 |
| Hot Pressing Temperature/°C | Alkaline Boiling Degumming Rate/% | Alkaline Steaming Degumming Rate/% | Alkaline Gel Degumming Rate/% |
|---|---|---|---|
| 40 | \ | \ | 7.4 ± 0.39 |
| 50 | \ | \ | 11.88 ± 0.68 |
| 60 | 20.28 ± 0.24 | 2.82 ± 2.11 | 16.56 ± 0.26 |
| 70 | 24.54 ± 0.11 | 3.01 ± 1.17 | 23.65 ± 0.39 |
| 80 | 27.12 ± 0.35 | 6.21 ± 1.46 | 23.96 ± 0.64 |
| 85 | 31.47 ± 0.16 | 10.61 ± 2.62 | 23.98 ± 0.32 |
| 90 | 34.01 ± 0.38 | 12.88 ± 1.31 | 22.5 ± 0.26 |
| 95 | 38.35 ± 0.52 | 18.79 ± 1.05 | 22.13 ± 0.45 |
| 100 | 42.14 ± 0.85 | 25.15 ± 0.26 | 21.77 ± 0.53 |
| 110 | \ | \ | 21.35 ± 0.82 |
| 120 | \ | \ | 21.25 ± 0.88 |
| 130 | \ | \ | 20.62 ± 0.68 |
| Hot Pressing Time | Alkaline Boiling Degumming Rate/% | Alkaline Steaming Degumming Rate/% | Alkaline Gel Degumming Rate/% |
|---|---|---|---|
| 10 s | \ | \ | 14.79 ± 0.82 |
| 30 s | \ | \ | 22.81 ± 0.26 |
| 50 s | \ | \ | 26.88 ± 0.26 |
| 70 s | \ | \ | 25.52 ± 0.39 |
| 90 s | \ | \ | 22.92 ± 0.39 |
| 110 s | \ | \ | 21.46 ± 0.15 |
| 130 s | \ | \ | 21.15 ± 0.97 |
| 150 s | 21.25 ± 0.51 | ||
| 15 min | 25.34 ± 0.56 | 16.54 ± 2.60 | \ |
| 30 min | 27.12 ± 0.35 | 20.00 ± 0.94 | \ |
| 45 min | 31.24 ± 0.81 | 21.43 ± 0.51 | \ |
| 60 min | 35.14 ± 0.62 | 22.53 ± 1.20 | \ |
| 75 min | 39.32 ± 1.17 | 23.72 ± 0.89 | \ |
| 90 min | 43.16 ± 0.62 | 24.41 ± 0.52 | \ |
| 120 min | 52.37 ± 0.54 | 25.27 ± 0.54 | \ |
| 150 min | 60.31 ± 0.78 | 25.54 ± 1.31 | \ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhou, J.; Li, Y. Exploration of Alkaline Degumming Printing Techniques for Silk Gauze Fabric: Alkaline Boiling, Alkaline Steaming, and Alkaline Gel. J. Compos. Sci. 2025, 9, 158. https://doi.org/10.3390/jcs9040158
Wu H, Zhou J, Li Y. Exploration of Alkaline Degumming Printing Techniques for Silk Gauze Fabric: Alkaline Boiling, Alkaline Steaming, and Alkaline Gel. Journal of Composites Science. 2025; 9(4):158. https://doi.org/10.3390/jcs9040158
Chicago/Turabian StyleWu, Huihui, Jiali Zhou, and Yufeng Li. 2025. "Exploration of Alkaline Degumming Printing Techniques for Silk Gauze Fabric: Alkaline Boiling, Alkaline Steaming, and Alkaline Gel" Journal of Composites Science 9, no. 4: 158. https://doi.org/10.3390/jcs9040158
APA StyleWu, H., Zhou, J., & Li, Y. (2025). Exploration of Alkaline Degumming Printing Techniques for Silk Gauze Fabric: Alkaline Boiling, Alkaline Steaming, and Alkaline Gel. Journal of Composites Science, 9(4), 158. https://doi.org/10.3390/jcs9040158






