Advances in Dental Implants: A Review of In Vitro and In Vivo Testing with Nanoparticle Coatings
Abstract
1. Introduction
2. Nanoparticles in Dental Implant Coatings
2.1. Types of Nanoparticles and Their Mechanism of Action
2.1.1. Inorganic Nanoparticles
Silver
Gold
Zinc Oxide
Strontium
Titanium Dioxide
Hydroxyapatite
2.1.2. Carbon-Based Nanoparticles
Carbon Nanotubes
Nanodiamonds
2.2. Advantages of Nanoparticles Used in Implant Coatings
3. Thin Coating Techniques for Nanoparticle Integration
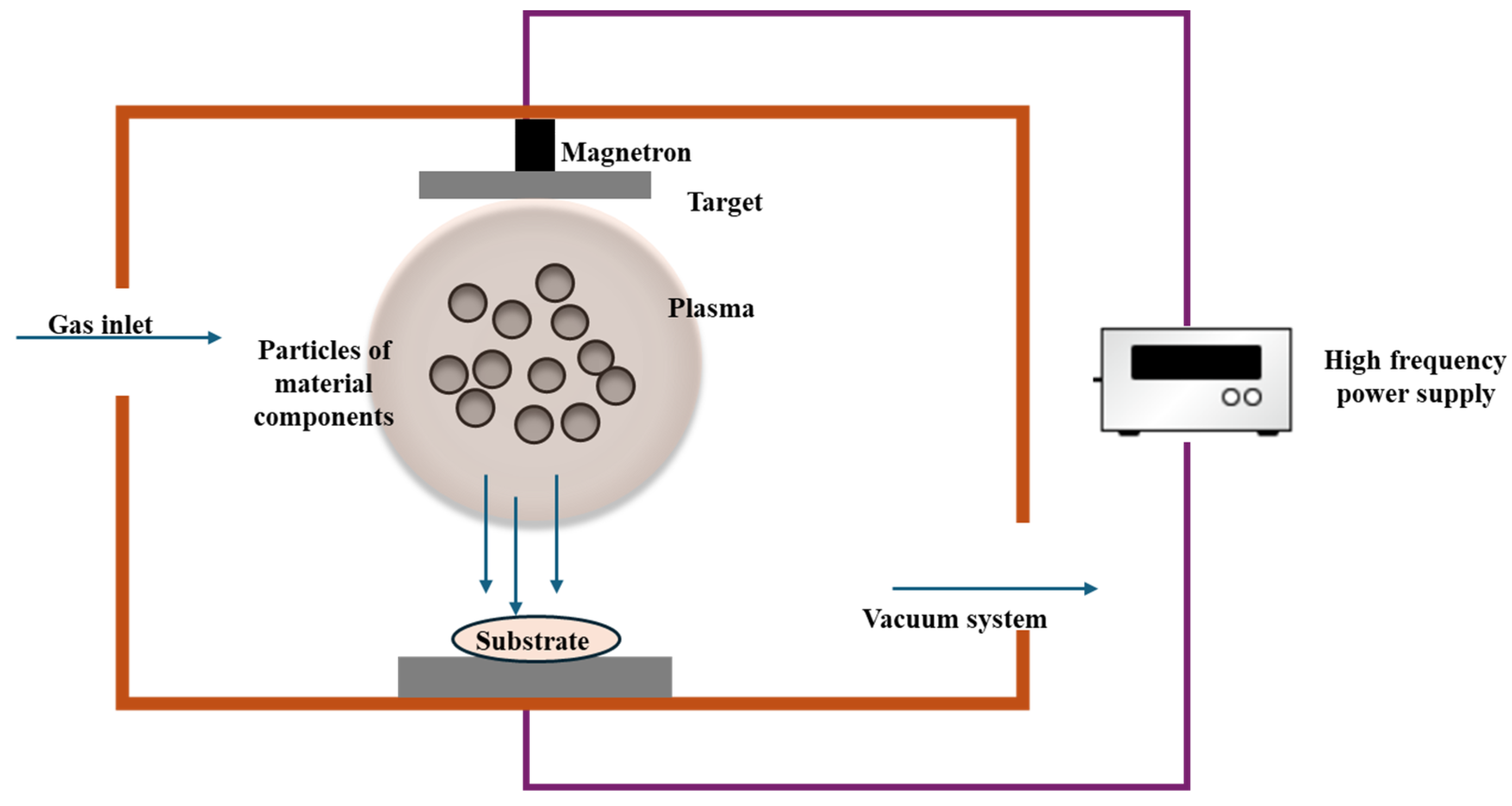
3.1. Physical Vapor Deposition
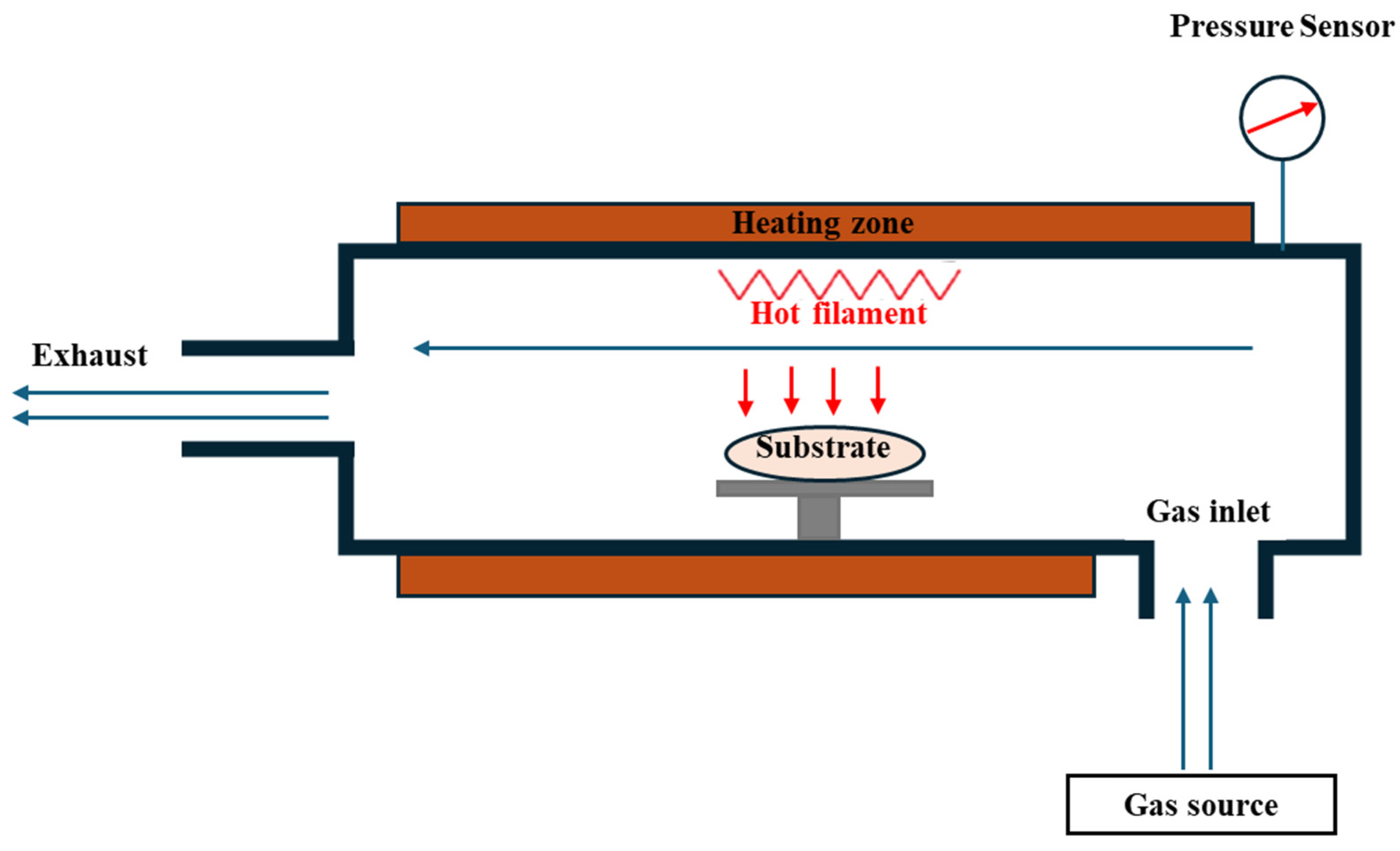
3.2. Chemical Vapor Deposition
3.3. Sol-Gel Method
3.4. Alkali Surface Treatment
3.5. Acid Etching
3.6. Anodization
3.7. Electrospinning
3.8. Overview of Thin Coating Techniques for Nanoparticle Integration
4. In Vitro and In Vivo Testing of Nanoparticle-Coated Implants
5. Emerging Trends and Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pandey, C.; Rokaya, D.; Bhattarai, B.P. Contemporary Concepts in Osseointegration of Dental Implants: A Review. BioMed Res. Int. 2022, 2022, 6170452. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.; Leung, K.C.; Chu, C.-H. Common Medical and Dental Problems of Older Adults: A Narrative Review. Geriatrics 2021, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Chopra, D.; Kocak-Oztug, N.A.; Verron, E. Fit and forget: The future of dental implant therapy via nanotechnology. Adv. Drug Deliv. Rev. 2023, 199, 114900. [Google Scholar] [CrossRef]
- Thomas, B.; Ramesh, A. Nanotechnology in Dental Implantology. In Nanomaterials in Dental Medicine; Springer: Singapore, 2023; pp. 159–175. [Google Scholar]
- WHO. World Health Organization (WHO)-Global Oral Health Status Report. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 9 December 2024).
- Cheng, L.; Zhang, L.; Yue, L.; Ling, J.; Fan, M.; Yang, D.; Huang, Z.; Niu, Y.; Liu, J.; Zhao, J.; et al. Expert consensus on dental caries management. Int. J. Oral Sci. 2022, 14, 17. [Google Scholar] [CrossRef]
- Meyer, F.; Schulze zur Wiesche, E.; Amaechi, B.T.; Limeback, H.; Enax, J. Caries Etiology and Preventive Measures. Eur. J. Dent. 2024, 18, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Xiang, Z.; Wakade Rohan, S.; Ribeiro Apoena, A.; Hu, W.; Bittinger, K.; Simon-Soro, A.; Kim, D.; Li, J.; Krysan Damian, J.; Liu, Y.; et al. Human Tooth as a Fungal Niche: Candida albicans Traits in Dental Plaque Isolates. mBio 2023, 14, e0276922. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Siow, D.S.F.; Goh, E.X.J.; Ong, M.M.A.; Preshaw, P.M. Risk factors for tooth loss and progression of periodontitis in patients undergoing periodontal maintenance therapy. J. Clin. Periodontol. 2023, 50, 61–70. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Al-Rafee, M.A. The epidemiology of edentulism and the associated factors: A literature Review. J. Fam. Med. Prim. Care 2020, 9, 1841–1843. [Google Scholar] [CrossRef] [PubMed]
- Bohner, L.; Hanisch, M.; Kleinheinz, J.; Jung, S. Dental implants in growing patients: A systematic review. Br. J. Oral Maxillofac. Surg. 2019, 57, 397–406. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Freag, P.; Fakhrzadeh, A.; Saghiri, A.M.; Eid, J. Current technology for identifying dental implants: A narrative review. Bull. Natl. Res. Cent. 2021, 45, 7. [Google Scholar] [CrossRef]
- Soe, Z.C.; Wahyudi, R.; Mattheos, N.; Lertpimonchai, A.; Everts, V.; Tompkins, K.A.; Osathanon, T.; Limjeerajarus, C.N.; Limjeerajarus, N. Application of nanoparticles as surface modifiers of dental implants for revascularization/regeneration of bone. BMC Oral Health 2024, 24, 1175. [Google Scholar] [CrossRef] [PubMed]
- Overmann, A.L.; Aparicio, C.; Richards, J.T.; Mutreja, I.; Fischer, N.G.; Wade, S.M.; Potter, B.K.; Davis, T.A.; Bechtold, J.E.; Forsberg, J.A.; et al. Orthopaedic osseointegration: Implantology and future directions. J. Orthop. Res. 2020, 38, 1445–1454. [Google Scholar] [CrossRef]
- Panchal, M.; Khare, S.; Khamkar, P.; Suresh Bhole, K. Dental implants: A review of types, design analysis, materials, additive manufacturing methods, and future scope. Mater. Today: Proc. 2022, 68, 1860–1867. [Google Scholar] [CrossRef]
- Hanif, A.; Qureshi, S.; Sheikh, Z.; Rashid, H. Complications in implant dentistry. Eur. J. Dent. 2017, 11, 135–140. [Google Scholar] [CrossRef]
- Dutta, S.R.; Passi, D.; Singh, P.; Atri, M.; Mohan, S.; Sharma, A. Risks and complications associated with dental implant failure: Critical update. Natl. J. Maxillofac. Surg. 2020, 11, 14–19. [Google Scholar] [CrossRef]
- Sotova, C.; Yanushevich, O.; Kriheli, N.; Grigoriev, S.; Evdokimov, V.; Kramar, O.; Nozdrina, M.; Peretyagin, N.; Undritsova, N.; Popelyshkin, E.; et al. Dental Implants: Modern Materials and Methods of Their Surface Modification. Materials 2023, 16, 7383. [Google Scholar] [CrossRef]
- Kandavalli, S.R.; Wang, Q.; Ebrahimi, M.; Gode, C.; Djavanroodi, F.; Attarilar, S.; Liu, S. A Brief Review on the Evolution of Metallic Dental Implants: History, Design, and Application. Front. Mater. 2021, 8, 646383. [Google Scholar] [CrossRef]
- Eftekhar Ashtiani, R.; Alam, M.; Tavakolizadeh, S.; Abbasi, K. The Role of Biomaterials and Biocompatible Materials in Implant-Supported Dental Prosthesis. Evid.-Based Complement. Altern. Med. Ecam 2021, 2021, 3349433. [Google Scholar] [CrossRef] [PubMed]
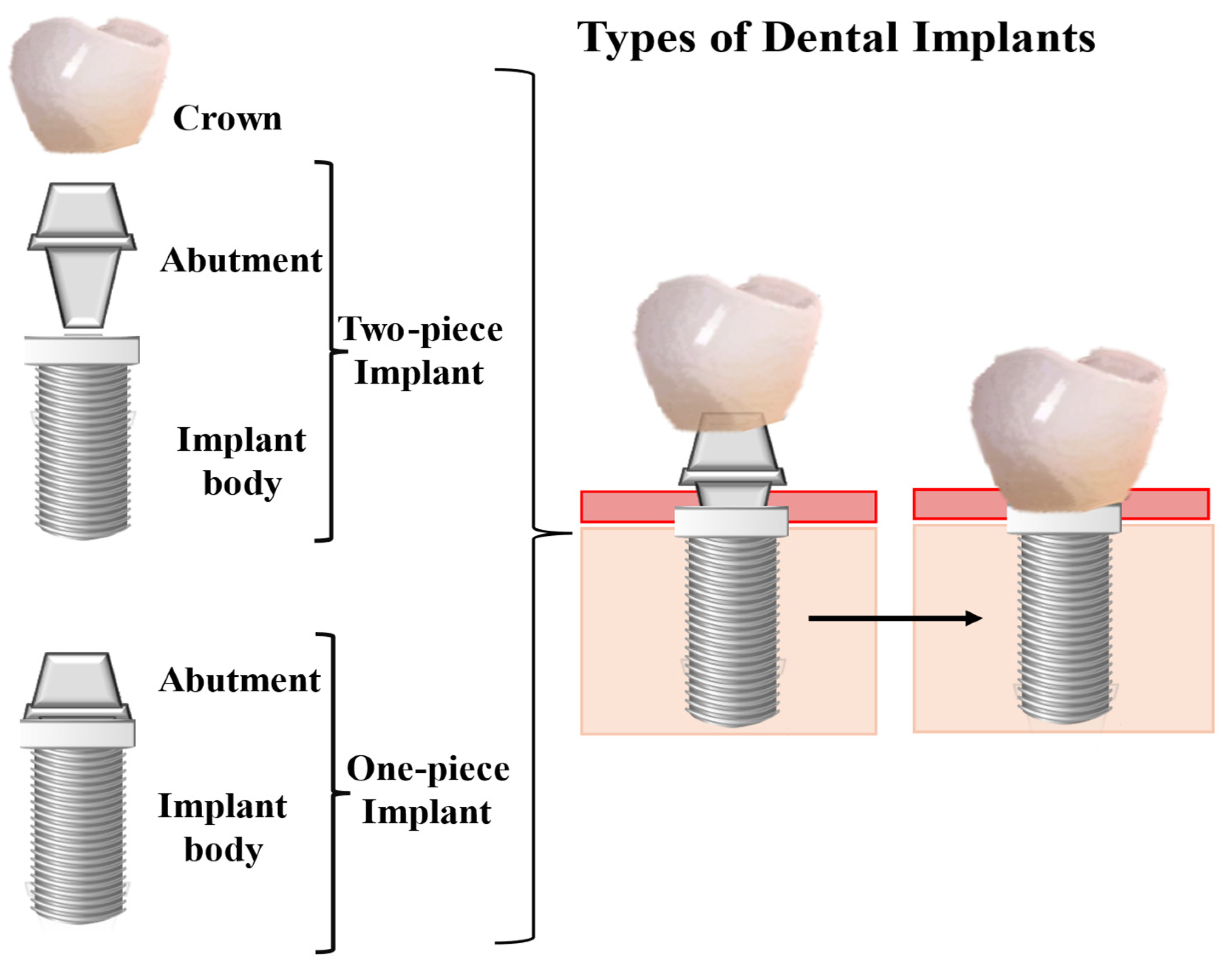
- Gamper, F.B.; Benic, G.I.; Sanz-Martin, I.; Asgeirsson, A.G.; Hämmerle, C.H.F.; Thoma, D.S. Randomized controlled clinical trial comparing one-piece and two-piece dental implants supporting fixed and removable dental prostheses: 4- to 6-year observations. Clin. Oral Implant. Res. 2017, 28, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Durrani, F.; Nahid, R.; Pandey, S.; Singh, P.; Pandey, A. One-piece implants: Careful approach for complex rehabilitation. Natl. J. Maxillofac. Surg. 2021, 12, 266–270. [Google Scholar] [CrossRef]
- de Oliveira Limírio, J.P.J.; Lemos, C.A.A.; de Luna Gomes, J.M.; Minatel, L.; Alves Rezende, M.C.R.; Pellizzer, E.P. A clinical comparison of 1-piece versus 2-piece implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2020, 124, 439–445. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Inchingolo, A.D.; Mancini, A.; Annicchiarico, C.; Inchingolo, F.; Dipalma, G.; et al. Surface Coatings of Dental Implants: A Review. J. Funct. Biomater. 2023, 14, 287. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2020, 39, 167–172. [Google Scholar] [CrossRef]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Kunrath, M.F.; Garaicoa-Pazmino, C.; Giraldo-Osorno, P.M.; Haj Mustafa, A.; Dahlin, C.; Larsson, L.; Asa’ad, F. Implant surface modifications and their impact on osseointegration and peri-implant diseases through epigenetic changes: A scoping review. J. Periodontal Res. 2024, 59, 1095–1114. [Google Scholar] [CrossRef]
- El-Banna, A.; Bissa, M.W.; Khurshid, Z.; Zohaib, S.; Asiri, F.Y.I.; Zafar, M.S. 4-Surface modification techniques of dental implants. In Dental Implants; Zafar, M.S., Khurshid, Z., Khan, A.S., Najeeb, S., Sefat, F., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 49–68. [Google Scholar]
- AlMaimouni, Y.K.; Benrashed, M.A.; Alyousef, N.I.; Shah, A.T.; Khan, A.S. 6-Bioactive glass coated dental implants. In Dental Implants; Zafar, M.S., Khurshid, Z., Khan, A.S., Najeeb, S., Sefat, F., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 93–115. [Google Scholar]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, G.; Li, J.J. Advances in implant surface modifications to improve osseointegration. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
- Mordorski, B.; Landriscina, A.; Friedman, A. Chapter 3-An Overview of Nanomaterials in Dermatology. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 31–46. [Google Scholar]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, T.; Schmitzer, E.; Suresh, A.P.; Acharya, A.P. Immune response differences in degradable and non-degradable alloy implants. Bioact. Mater. 2023, 24, 153–170. [Google Scholar] [CrossRef]
- Rafikova, G.; Piatnitskaia, S.; Shapovalova, E.; Chugunov, S.; Kireev, V.; Ialiukhova, D.; Bilyalov, A.; Pavlov, V.; Kzhyshkowska, J. Interaction of Ceramic Implant Materials with Immune System. Int. J. Mol. Sci. 2023, 24, 4200. [Google Scholar] [CrossRef]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef]
- Hakim, L.K.; Yari, A.; Nikparto, N.; Mehraban, S.H.; Cheperli, S.; Asadi, A.; Darehdor, A.A.; Nezaminia, S.; Dortaj, D.; Nazari, Y.; et al. The current applications of nano and biomaterials in drug delivery of dental implant. BMC Oral Health 2024, 24, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gulati, K.; Li, Z.; Di, P.; Liu, Y. Dental Implant Nano-Engineering: Advances, Limitations and Future Directions. Nanomaterials 2021, 11, 2489. [Google Scholar] [CrossRef]
- Vasiliu, S.; Racovita, S.; Gugoasa, I.A.; Lungan, M.-A.; Popa, M.; Desbrieres, J. The Benefits of Smart Nanoparticles in Dental Applications. Int. J. Mol. Sci. 2021, 22, 2585. [Google Scholar] [CrossRef]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymer 2022, 14, 864. [Google Scholar] [CrossRef]
- Li, D.; Dai, D.; Xiong, G.; Lan, S.; Zhang, C. Composite Nanocoatings of Biomedical Magnesium Alloy Implants: Advantages, Mechanisms, and Design Strategies. Adv. Sci. 2023, 10, 2300658. [Google Scholar] [CrossRef]
- Hossain, N.; Islam, M.A.; Chowdhury, M.A.; Alam, A. Advances of nanoparticles employment in dental implant applications. Appl. Surf. Sci. Adv. 2022, 12, 100341. [Google Scholar] [CrossRef]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Waiezi, S.; Malek, N.; Asraf, M.H.; Sani, N.S. Preparation, characterization, and antibacterial activity of green-biosynthesised silver nanoparticles using Clinacanthus nutans extract. Biointerface Res. Appl. Chem. 2023, 13, 171. [Google Scholar]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- El Shanshoury, A.E.-R.R.; Sabae, S.Z.; El Shouny, W.A.; Elsaied, H.E.; Badr, H.M.; Abo-Shady, A.M. Biomimetic Synthesis of Silver Nanoparticles Using New Aquatic Species of Bacillus, Alcaligenes, and Paenibacillus and their Potential Antibiofilm Activity against Biofilm-Forming Escherichia coli. Lett. Appl. NanoBioSci. 2023, 12. [Google Scholar] [CrossRef]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef]
- da Cruz, M.K.; Morais, T.M.; Trevisani, D.M. Clinical assessment of the oral cavity of patients hospitalized in an intensive care unit of an emergency hospital. Rev. Bras. De Ter. Intensiv. 2014, 26, 379–383. [Google Scholar] [CrossRef]
- Rivas Caldas, R.; Le Gall, F.; Revert, K.; Rault, G.; Virmaux, M.; Gouriou, S.; Héry-Arnaud, G.; Barbier, G.; Boisramé, S. Pseudomonas aeruginosa and Periodontal Pathogens in the Oral Cavity and Lungs of Cystic Fibrosis Patients: A Case-Control Study. J. Clin. Microbiol. 2015, 53, 1898–1907. [Google Scholar] [CrossRef]
- Yu, Y.M.; Lu, Y.P.; Zhang, T.; Zheng, Y.F.; Liu, Y.S.; Xia, D.D. Biomaterials science and surface engineering strategies for dental peri-implantitis management. Mil. Med. Res. 2024, 11, 29. [Google Scholar] [CrossRef]
- Jongrungsomran, S.; Pissuwan, D.; Yavirach, A.; Rungsiyakull, C.; Rungsiyakull, P. The Integration of Gold Nanoparticles into Dental Biomaterials as a Novel Approach for Clinical Advancement: A Narrative Review. J. Funct. Biomater. 2024, 15, 291. [Google Scholar] [CrossRef]
- Heo, D.N.; Ko, W.-K.; Lee, H.R.; Lee, S.J.; Lee, D.; Um, S.H.; Lee, J.H.; Woo, Y.-H.; Zhang, L.G.; Lee, D.-W.; et al. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J. Colloid Interface Sci. 2016, 469, 129–137. [Google Scholar] [CrossRef]
- Zhan, X.; Yan, J.; Tang, H.; Xia, D.; Lin, H. Antibacterial Properties of Gold Nanoparticles in the Modification of Medical Implants: A Systematic Review. Pharmaceutics 2022, 14, 2654. [Google Scholar] [CrossRef]
- Su, C.; Huang, K.; Li, H.-H.; Lu, Y.-G.; Zheng, D.-L. Antibacterial Properties of Functionalized Gold Nanoparticles and Their Application in Oral Biology. J. Nanomater. 2020, 2020, 5616379. [Google Scholar] [CrossRef]
- Samsulkahar, N.F.; Hadi, A.A.; Shamsuddin, M.; Nik, N.A.N. Biosynthesis of Gold Nanoparticles Using Strobilanthes crispa Aqueous Leaves Extract and Evaluation of Its Antibacterial Activity. Biointerface Res. Appl. Chem. 2023, 13, 63. [Google Scholar]
- Tian, E.-K.; Wang, Y.; Ren, R.; Zheng, W.; Liao, W. Gold Nanoparticle: Recent Progress on Its Antibacterial Applications and Mechanisms. J. Nanomater. 2021, 2021, 2501345. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.; Sowmya, S.; Augustine, D.; Alamoudi, A.; Zidane, B.; Mohammad Albar, N.H.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef]
- Moradpoor, H.; Safaei, M.; Mozaffari, H.R.; Sharifi, R.; Imani, M.M.; Golshah, A.; Bashardoust, N. An overview of recent progress in dental applications of zinc oxide nanoparticles. RSC Adv. 2021, 11, 21189–21206. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Nahidh, M.; Khalaf, M.K.; Marrapodi, M.M.; Cicciù, M.; Minervini, G. Antimicrobial Effect of Zinc Oxide Nanoparticle Coating on Titanium 6 Aluminum 4 Vanadium (Ti-6Al-4V)-Fixed Orthodontic Retainer Substrate. Eur. J. Gen. Dent. 2024. [Google Scholar] [CrossRef]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat- Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C 2020, 108, 110319. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, M. Application of strontium-based nanoparticles in medicine and environmental sciences. Nanotechnol. Environ. Eng. 2021, 6, 25. [Google Scholar] [CrossRef]
- Alshammari, H.; Bakitian, F.; Neilands, J.; Andersen, O.Z.; Stavropoulos, A. Antimicrobial Properties of Strontium Functionalized Titanium Surfaces for Oral Applications, A Systematic Review. Coatings 2021, 11, 810. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Mahmoud, N.; Abdou, Y.; El-Husseiny, F. Novel strontium borate modified henchs bioglass synthesis and characterization for bone replacement. Biointerface Res. Appl. Chem. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Yang, W.-E.; Huang, H.-H. Multiform TiO2 nano-network enhances biological response to titanium surface for dental implant applications. Appl. Surf. Sci. 2019, 471, 1041–1052. [Google Scholar] [CrossRef]
- Satyanarayana, C.P.; Raju, L.S.; Raju, L.R.; Dondapati, S.; Dumpala, R.; Buradagunta, R.S. Comparative investigations on the bioactivity of surface grain refined titanium and surface oxidized titanium for biomedical implant applications. Biointer. Res. Appl. Chem. 2023, 13, 1–10. [Google Scholar]
- Bokobza, L. On the Use of Nanoparticles in Dental Implants. Materials 2024, 17, 3191. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Zhang, S.; Liu, H. Role of TiO2 Nanotubes on the Surface of Implants in Osseointegration in Animal Models: A Systematic Review and Meta-Analysis. J. Prosthodont. 2020, 29, 501–510. [Google Scholar] [CrossRef]
- Tan, G.; Xu, J.; Chirume, W.M.; Zhang, J.; Zhang, H.; Hu, X. Antibacterial and Anti-Inflammatory Coating Materials for Orthopedic Implants: A Review. Coatings 2021, 11, 1401. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef]
- Renuka, R.R.; Julius, A.; Karunakaran, H.; Samrot, A.V.; Deenadhayalan, R.; Nagarajan, S.; Soju, D. In vitro free radical scavenging effects of Titanium dioxide Nanoparticles (TiO2 NPs) biosynthesized using stem extract of Cissus quadrangularis. Lett. Appl. NanoBioSci. 2024, 13. [Google Scholar] [CrossRef]
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Maleki Dizaj, S. A short view on nanohydroxyapatite as coating of dental implants. Biomed. Pharmacother. 2018, 105, 553–557. [Google Scholar] [CrossRef]
- Salahuddin, N.; Ibrahim, E.M.; El-Kemary, M. Different methods for preparation of hydroxyapatite nanostructures. Biointer. Res. Appl. Chem. 2023, 13, 236. [Google Scholar]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental Applications of Systems Based on Hydroxyapatite Nanoparticles—An Evidence-Based Update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Gayathri, V.S.; Sowmya, S.V.; Augustine, D.; Alamoudi, A.; Zidane, B.; Hassan Mohammad Albar, N.; Bhandi, S. Nanohydroxyapatite in dentistry: A comprehensive review. Saudi Dent. J. 2023, 35, 741–752. [Google Scholar] [CrossRef]
- Thenmozhi, R.B.; Suresh, R.; Srividhya, B.; Baskaran, P.; Subramanian, R. Effect of Sugarcane Juice Stabilized Synthesis of Hydroxyapatite Nanoparticles, Characterization and Morphology Studies. Biointerface Res. Appl. Chem. 2023, 13, 261. [Google Scholar]
- Vijay, R.; Mendhi, J.; Prasad, K.; Xiao, Y.; MacLeod, J.; Ostrikov, K.K.; Zhou, Y. Carbon Nanomaterials Modified Biomimetic Dental Implants for Diabetic Patients. Nanomaterials 2021, 11, 2977. [Google Scholar] [CrossRef]
- Abo-Neima, S.E.; Motaweh, H.A.; Elsehly, E.M. Antimicrobial activity of functionalised carbon nanotubes against pathogenic microorganisms. IET Nanobiotechnol. 2020, 14, 457–464. [Google Scholar] [CrossRef]
- Teh, S.J.; Lai, C.W. 5-Carbon nanotubes for dental implants. In Applications of Nanocomposite Materials in Dentistry; Asiri, A.M., Inamuddin, M.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 93–105. [Google Scholar]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.C.; Wong, E.H. An Overview of Antimicrobial Properties of Carbon Nanotubes-Based Nanocomposites. Adv. Pharm. Bull. 2022, 12, 449–465. [Google Scholar] [CrossRef]
- Mengesha, A.E.; Youan, B.B.C. 8-Nanodiamonds for drug delivery systems. In Diamond-Based Materials for Biomedical Applications; Narayan, R., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 186–205. [Google Scholar]
- Kausar, A. 2-Carbonaceous nanofillers in polymer matrix. In Polymeric Nanocomposites with Carbonaceous Nanofillers for Aerospace Applications; Kausar, A., Ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 23–53. [Google Scholar]
- Taymour, N.; Haque, M.A.; Atia, G.A.N.; Mohamed, S.Z.; Rokaya, D.; Bajunaid, S.M.; Soliman, M.M.; Shalaby, H.K.; Barai, P.; Roy, M.; et al. Nanodiamond: A Promising Carbon-Based Nanomaterial for Therapeutic and Regenerative Dental Applications. ChemistrySelect 2024, 9, e202401328. [Google Scholar] [CrossRef]
- Adel, M.; Keyhanvar, P.; Zare, I.; Tavangari, Z.; Akbarzadeh, A.; Zahmatkeshan, M. Nanodiamonds for tissue engineering and regeneration. J. Drug Deliv. Sci. Technol. 2023, 90, 105130. [Google Scholar] [CrossRef]
- Patel, B.; Duran-Martinez, A.C.; Gurman, P.; Auciello, O.; Barao, V.; Campbell, S.; Sukotjo, C.; Mathew, M.T. Ultrananocrystalline diamond coatings for the dental implant: Electrochemical nature. Surf. Innov. 2017, 5, 106–117. [Google Scholar] [CrossRef]
- Auciello, O.; Renou, S.; Kang, K.; Tasat, D.; Olmedo, D. A Biocompatible Ultrananocrystalline Diamond (UNCD) Coating for a New Generation of Dental Implants. Nanomaterials 2022, 12, 782. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef]
- Amirtharaj Mosas, K.K.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent Advancements in Materials and Coatings for Biomedical Implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Yin, J.; Yang, Q.; Yu, Y.; Chen, L. Long-term application of silver nanoparticles in dental restoration materials: Potential toxic injury to the CNS. J. Mater. Sci. Mater. Med. 2023, 34, 52. [Google Scholar] [CrossRef]
- Niżnik, Ł.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krośniak, A.; Kapka-Skrzypczak, L.; Jurowski, K. Gold Nanoparticles (AuNPs)-Toxicity, Safety and Green Synthesis: A Critical Review. Int. J. Mol. Sci. 2024, 25, 4057. [Google Scholar] [CrossRef]
- Fujihara, J.; Nishimoto, N. Review of Zinc Oxide Nanoparticles: Toxicokinetics, Tissue Distribution for Various Exposure Routes, Toxicological Effects, Toxicity Mechanism in Mammals, and an Approach for Toxicity Reduction. Biol. Trace Elem. Res. 2024, 202, 9–23. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Gong, H.; Wu, C.; Wang, B.; Chen, W.; Hu, J.; Xiang, H.; Zhang, K.; Sun, M. Neurotoxicity of Titanium Dioxide Nanoparticles: A Comprehensive Review. Int. J. Nanomed. 2023, 18, 7183–7204. [Google Scholar] [CrossRef]
- Nguyen, N.P.; Dang, N.T.; Doan, L.; Nguyen, T.T. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Mallineni, S.K.; Sakhamuri, S.; Kotha, S.L.; AlAsmari, A.; AlJefri, G.H.; Almotawah, F.N.; Mallineni, S.; Sajja, R. Silver Nanoparticles in Dental Applications: A Descriptive Review. Bioengineering 2023, 10, 327. [Google Scholar] [CrossRef]
- Bolenwar, A.; Reche, A.; Dhamdhere, N.; Rathi, S. Applications of Silver Nanoparticles in Dentistry. Cureus 2023, 15, e44090. [Google Scholar] [CrossRef]
- Wang, N.; Fuh, J.Y.H.; Dheen, S.T.; Senthil Kumar, A. Functions and applications of metallic and metallic oxide nanoparticles in orthopedic implants and scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 160–179. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Santhosh, P.; Genova, J.; Chamati, H. Review Green Synthesis of Gold Nanoparticles: An Eco-Friendly Approach. Chemistry 2022, 4, 345–369. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Simakin, A.V.; Smirnova, V.V.; Uvarov, O.V.; Ivashkin, P.I.; Kucherov, R.N.; Ivanov, V.E.; Bruskov, V.I.; Sevostyanov, M.A.; Baikin, A.S.; et al. Bacteriostatic and Cytotoxic Properties of Composite Material Based on ZnO Nanoparticles in PLGA Obtained by Low Temperature Method. Polymers 2022, 14, 49. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide-From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Anandan, D.; Jaiswal, A.K. Synthesis methods of hydroxyapatite and biomedical applications: An updated review. J. Aust. Ceram. Soc. 2024, 60, 663–679. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Ganta, D.D.; Hirpaye, B.Y.; Raghavanpillai, S.K.; Menber, S.Y. Green Synthesis of Hydroxyapatite Nanoparticles Using Monoon longifolium Leaf Extract for Removal of Fluoride from Aqueous Solution. J. Chem. 2022, 2022, 4917604. [Google Scholar] [CrossRef]
- Ong, J.L.; Chan, D.C.N.; Bessho, K. HA Coatings on Dental Implants. In Biomaterials Engineering and Devices: Human Applications: Volume 2. Orthopedic, Dental, and Bone Graft Applications; Wise, D.L., Trantolo, D.J., Lewandrowski, K.-U., Gresser, J.D., Cattaneo, M.V., Yaszemski, M.J., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 49–60. [Google Scholar]
- Nasar, A. 8-Hydroxyapatite and its coatings in dental implants. In Applications of Nanocomposite Materials in Dentistry; Asiri, A.M., Inamuddin, M.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 145–160. [Google Scholar]
- Family, R.; Solati-Hashjin, M.; Namjoy Nik, S.; Nemati, A. Surface modification for titanium implants by hydroxyapatite nanocomposite. Casp. J. Intern. Med. 2012, 3, 460–465. [Google Scholar]
- Motskin, M.; Wright, D.M.; Muller, K.; Kyle, N.; Gard, T.G.; Porter, A.E.; Skepper, J.N. Hydroxyapatite nano and microparticles: Correlation of particle properties with cytotoxicity and biostability. Biomaterials 2009, 30, 3307–3317. [Google Scholar] [CrossRef] [PubMed]
- Maher, N.; Mahmood, A.; Fareed, M.A.; Kumar, N.; Rokaya, D.; Zafar, M.S. An updated review and recent advancements in carbon-based bioactive coatings for dental implant applications. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Hassan, S.; Nadeem, A.Y.; Qaiser, H.; Kashif, A.S.; Ahmed, A.; Khan, K.; Altaf, A. A review of carbon-based materials and their coating techniques for biomedical implants applications. Carbon. Lett. 2023, 33, 1171–1188. [Google Scholar] [CrossRef]
- Kang, M.S.; Jang, H.J.; Lee, S.H.; Lee, J.E.; Jo, H.J.; Jeong, S.J.; Kim, B.; Han, D.-W. Potential of Carbon-Based Nanocomposites for Dental Tissue Engineering and Regeneration. Materials 2021, 14, 5104. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-based nanomaterials: Synthesis and prospective applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Safin Kaosar Saad, K.; Saba, T.; Bin Rashid, A. Application of PVD coatings in medical implantology for enhanced performance, biocompatibility, and quality of life. Heliyon 2024, 10, e35541. [Google Scholar] [CrossRef]
- Escorcia-Díaz, D.; García-Mora, S.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. Advancements in Nanoparticle Deposition Techniques for Diverse Substrates: A Review. Nanomaterials 2023, 13, 2586. [Google Scholar] [CrossRef]
- Geyao, L.; Yang, D.; Wanglin, C.; Chengyong, W. Development and application of physical vapor deposited coatings for medical devices: A review. Procedia CIRP 2020, 89, 250–262. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Chapter 5-Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 121–139. [Google Scholar]
- Noč, L.; Jerman, I. Review of the spectrally selective (CSP) absorber coatings, suitable for use in SHIP. Sol. Energy Mater. Sol. Cells 2022, 238, 111625. [Google Scholar] [CrossRef]
- Farooq, S.A.; Raina, A.; Mohan, S.; Arvind Singh, R.; Jayalakshmi, S.; Irfan Ul Haq, M. Nanostructured Coatings: Review on Processing Techniques, Corrosion Behaviour and Tribological Performance. Nanomaterials 2022, 12, 1323. [Google Scholar] [CrossRef]
- Nikolaidis, P. Analysis of Green Methods to Synthesize Nanomaterials. In Green Synthesis of Nanomaterials for Bioenergy Applications; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 125–144. [Google Scholar]
- Kumar, L.; Shrivastava, P.; Panda, D.; Ghosh, A.; Syed, N. Tribology and Characterization of Surface Coatings; Ahmed, S., Dakre, V.S., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2021; ISBN 9781119818786. [Google Scholar]
- Baptista, A.; Silva, F.J.G.; Porteiro, J.; Míguez, J.L.; Pinto, G.; Fernandes, L. On the Physical Vapour Deposition (PVD): Evolution of Magnetron Sputtering Processes for Industrial Applications. Procedia Manuf. 2018, 17, 746–757. [Google Scholar] [CrossRef]
- Palani, S.; Michael, E.G.; Desta, M.; Atnaw, S.M.; Banoth, R.; Kolanji, S. Physical Vapor Deposition Coating Process in Biomedical Applications: An Overview. In Sustainable Advanced Manufacturing and Materials Processing; CRC Press: Boca Raton, FL, USA, 2022; pp. 67–93. [Google Scholar]
- Xu, Y.; Zhang, J.; Liang, F.; Yin, M.; He, M. Investigation of magnetron sputtered nano-silver coating on titanium surface with micro-nanostructure. Surf. Interfaces 2023, 38, 102770. [Google Scholar] [CrossRef]
- Juan, L.; Zhimin, Z.; Anchun, M.; Lei, L.; Jingchao, Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int. J. Nanomed. 2010, 5, 261–267. [Google Scholar] [CrossRef]
- Fialho, L.; Grenho, L.; Fernandes, M.H.; Carvalho, S. Porous tantalum oxide with osteoconductive elements and antibacterial core-shell nanoparticles: A new generation of materials for dental implants. Mater. Sci. Eng. C 2021, 120, 111761. [Google Scholar] [CrossRef]
- Garg, R.; Gonuguntla, S.; Sk, S.; Iqbal, M.S.; Dada, A.O.; Pal, U.; Ahmadipour, M. Sputtering thin films: Materials, applications, challenges and future directions. Adv. Colloid. Interface Sci. 2024, 330, 103203. [Google Scholar] [CrossRef]
- Simon, A.H. Chapter 7-Sputter Processing. In Handbook of Thin Film Deposition, 4th ed.; Seshan, K., Schepis, D., Eds.; William Andrew Publishing: New York, NY, USA, 2018; pp. 195–230. [Google Scholar]
- Carlsson, J.-O.; Martin, P.M. Chapter 7-Chemical Vapor Deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 314–363. [Google Scholar]
- Xie, L.; Abliz, D.; Li, D. 7.07-Thin Film Coating for Polymeric Micro Parts. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Oxford, UK, 2014; pp. 157–170. [Google Scholar]
- Fuentes, G.G. Chapter 20-Surface Engineering and Micro-manufacturing. In Micromanufacturing Engineering and Technology, 2nd ed.; Qin, Y., Ed.; William Andrew Publishing: Boston, MA, USA, 2015; pp. 459–486. [Google Scholar]
- Long, S.; Zhu, J.; Jing, Y.; He, S.; Cheng, L.; Shi, Z. A Comprehensive Review of Surface Modification Techniques for Enhancing the Biocompatibility of 3D-Printed Titanium Implants. Coatings 2023, 13, 1917. [Google Scholar] [CrossRef]
- Saba, T.; Saad, K.S.K.; Rashid, A.B. Precise surface engineering: Leveraging chemical vapor deposition for enhanced biocompatibility and durability in biomedical implants. Heliyon 2024, 10, e37976. [Google Scholar] [CrossRef]
- Zhang, Q.; Sando, D.; Valanoor, N. Chemical Route derived Bismuth Ferrite Thin films and Nanomaterials. J. Mater. Chem. C 2016, 4, 4092–4124. [Google Scholar] [CrossRef]
- Fraga, M.; Pessoa, R.; Maciel, H.; Massi, M. Recent Developments on Silicon Carbide Thin Films for Piezoresistive Sensors Applications. In Silicon Carbide—Materials, Processing and Applications in Electronic Devices; Intechopen: London, UK, 2011; pp. 369–388. [Google Scholar]
- Rifai, A.; Tran, N.; Lau, D.W.; Elbourne, A.; Zhan, H.; Stacey, A.D.; Mayes, E.L.; Sarker, A.; Ivanova, E.P.; Crawford, R.J.; et al. Polycrystalline diamond coating of additively manufactured titanium for biomedical applications. ACS Appl. Mater. Interfaces 2018, 10, 8474–8484. [Google Scholar] [CrossRef]
- Zanurin, A.; Johari, N.A.; Alias, J.; Mas Ayu, H.; Redzuan, N.; Izman, S. Research progress of sol-gel ceramic coating: A review. Mater. Today Proc. 2022, 48, 1849–1854. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F. Surface modifications of titanium implants by coating with bioactive and biocompatible poly (ε-caprolactone)/SiO2 hybrids synthesized via sol–gel. Arab. J. Chem. 2018, 11, 1126–1133. [Google Scholar] [CrossRef]
- Pilliar, R.M. 6-Sol–gel surface modification of biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Wen, C., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 185–217. [Google Scholar]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Blanco, I.; Piccolella, S.; Pacifico, S. Use of the Sol–Gel Method for the Preparation of Coatings of Titanium Substrates with Hydroxyapatite for Biomedical Application. Coatings 2020, 10, 203. [Google Scholar] [CrossRef]
- Veeman, D.; Shree, M.V.; Sureshkumar, P.; Jagadeesha, T.; Natrayan, L.; Ravichandran, M.; Paramasivam, P. Sustainable Development of Carbon Nanocomposites: Synthesis and Classification for Environmental Remediation. J. Nanomater. 2021, 2021, 5840645. [Google Scholar] [CrossRef]
- Patel, P.; Gundloori, R. A review on electrospun nanofibers for multiple biomedical applications. Polym. Adv. Technol. 2022, 34, 44–63. [Google Scholar] [CrossRef]
- Nhlapo, N.; Dzogbewu, T.C.; de Smidt, O. Nanofiber Polymers for Coating Titanium-Based Biomedical Implants. Fibers 2022, 10, 36. [Google Scholar] [CrossRef]
- Shahi, R.G.; Albuquerque, M.T.P.; Münchow, E.A.; Blanchard, S.B.; Gregory, R.L.; Bottino, M.C. Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 2017, 105, 354–363. [Google Scholar] [CrossRef]
- Chris, J.M.; Jonathan, P.W.; Chris, J.W. Electrospinning of Functional Nanofibers for Regenerative Medicine: From Bench to Commercial Scale. In Novel Aspects of Nanofibers; Tong, L., Ed.; IntechOpen: Rijeka, Croatia, 2018; p. 6. [Google Scholar]
- Kumar Sharma, G.; Rachel James, N. Electrospinning: The Technique and Applications. In Recent Developments in Nanofibers Research; Khan, M., Chelladurai, S.J.S., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Pouponneau, P.; Perrey, O.; Brunon, C.; Grossiord, C.; Courtois, N.; Salles, V.; Alves, A. Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants. Polymers 2020, 12, 66. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Sun, P.; Zhou, C.; Liu, Y.; Fang, Z.Z. An overview of TiFe alloys for hydrogen storage: Structure, processes, properties, and applications. J. Energy Storage 2023, 68, 107772. [Google Scholar] [CrossRef]
- Doll, G.L. Chapter Three-Rolling bearing mechanics. In Rolling Bearing Tribology; Doll, G.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 35–86. [Google Scholar]
- Wen, Q.; Fu, H.; Cui, R.-d.; Chen, H.-Z.; Ji, R.-H.; Tang, L.-B.; Yan, C.; Mao, J.; Dai, K.-H.; Zhang, X.-H.; et al. Recent advances in interfacial modification of zinc anode for aqueous rechargeable zinc ion batteries. J. Energy Chem. 2023, 83, 287–303. [Google Scholar] [CrossRef]
- Devasia, R.; Painuly, A.; Devapal, D.; Sreejith, K.J. 22-Continuous fiber reinforced ceramic matrix composites. In Fiber Reinforced Composites; Joseph, K., Oksman, K., George, G., Wilson, R., Appukuttan, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 669–751. [Google Scholar]
- Zuo, D.; Tian, G.; Li, X.; Chen, D.; Shu, K. Recent progress in surface coating of cathode materials for lithium ion secondary batteries. J. Alloys Compd. 2017, 706, 24–40. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Chemical Vapor Deposition (CVD): Principles, Technology, and Applications; Noyes Publications/William Andrew Publishing: Norwich, NY, USA, 1999. [Google Scholar]
- Grivas, C. Optically pumped planar waveguide lasers, Part I: Fundamentals and fabrication techniques. Prog. Quantum Electron. 2011, 35, 159–239. [Google Scholar] [CrossRef]
- Rahmani, E. Preparation and Characterization of Thin Films by Sol-Gel Method. In Thin Films-Growth, Characterization and Electrochemical Applications; Sarf, F., Yakar, E., Karaduman Er, I., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Niranjan, C.A.; Raghavendra, T.; Rao, M.P.; Siddaraju, C.; Gupta, M.; Jain, V.K.S.; Aishwarya, R. Magnesium alloys as extremely promising alternatives for temporary orthopedic implants–A review. J. Magnes. Alloys 2023, 11, 2688–2718. [Google Scholar] [CrossRef]
- Wang, X.; Hsiao, B.S. Electrospun nanofiber membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. [Google Scholar] [CrossRef]
- Ramazani, S.; Karimi, M. Investigating the influence of temperature on electrospinning of polycaprolactone solutions. e-Polymers 2014, 14, 323–333. [Google Scholar] [CrossRef]
- Hellmann, C.; Belardi, J.; Dersch, R.; Greiner, A.; Wendorff, J.H.; Bahnmueller, S. High Precision Deposition Electrospinning of nanofibers and nanofiber nonwovens. Polymer 2009, 50, 1197–1205. [Google Scholar] [CrossRef]
- Al-Hazeem, N.Z. Nanofibers and Electrospinning Method. In Novel Nanomaterials-Synthesis and Applications; Kyzas, G.Z., Mitropoulos, A.C., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Othman, S.S.; El-Waseif, A.A.-E.; Hameed, M.A.; Abbas, Q.A. Antimicrobial behavior of nanocoated orthodontic micro-implants: An in vitro study. J. Orthod. Sci. 2024, 13, 2. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Zaręba, T.; Szaraniec, B.; Pałka, K.; Mielczarek, A.; Menaszek, E.; Tyski, S. In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery. Int. J. Nanomed. 2017, 12, 4285–4297. [Google Scholar] [CrossRef]
- de Lima Cavalcanti, J.H.; Matos, P.C.; Depes de Gouvêa, C.V.; Carvalho, W.; Calvo-Guirado, J.L.; Aragoneses, J.M.; Pérez-Díaz, L.; Gehrke, S.A. In Vitro Assessment of the Functional Dynamics of Titanium with Surface Coating of Hydroxyapatite Nanoparticles. Materials 2019, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Oleshko, O.; Liubchak, I.; Husak, Y.; Korniienko, V.; Yusupova, A.; Oleshko, T.; Banasiuk, R.; Szkodo, M.; Matros-Taranets, I.; Kazek-Kęsik, A.; et al. In Vitro Biological Characterization of Silver-Doped Anodic Oxide Coating on Titanium. Materials 2020, 13, 4359. [Google Scholar] [CrossRef] [PubMed]
- Łapaj, Ł.; Woźniak, W.; Markuszewski, J. Osseointegration of hydroxyapatite coatings doped with silver nanoparticles: Scanning electron microscopy studies on a rabbit model. Folia Morphol. 2019, 78, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Srivastava, A.; Bhati, P.; Chaturvedi, M.; Patil, V.; Kunnoth, S.; Kumari, N.; Arya, V.; Pandya, M.; Agarwal, M.; et al. Enhanced osseointegration of drug eluting nanotubular dental implants: An in vitro and in vivo study. Bioact. Mater. 2023, 28, 432–447. [Google Scholar] [CrossRef]
- Çayır Bozoğlu, Ü.; Kiremitçi, A.; Yurtsever, M.Ç.; Gümüşderelioğlu, M. Peek dental implants coated with boron-doped nano-hydroxyapatites: Investigation of in-vitro osteogenic activity. J. Trace Elem. Med. Biol. 2022, 73, 127026. [Google Scholar] [CrossRef]
- Yao, L.; Wang, H.; Li, L.; Cao, Z.; Dong, Y.; Yao, L.; Lou, W.; Zheng, S.; Shi, Y.; Shen, X.; et al. Development and evaluation of osteogenesis and antibacterial properties of strontium/silver-functionalized hierarchical micro/nano-titanium implants. Mater. Des. 2022, 224, 111425. [Google Scholar] [CrossRef]
- Al-Noaman, A.; Rawlinson, S.C.F. A bioactive and anti-bacterial nano-sized zirconium phosphate/GO (nZrP/GO) composite: Potential use as a coating for dental implants? Dent. Mater. 2024, 40, e72–e81. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Y.; Yao, L.; Liu, C.; Al-Bishari, A.M.; Ru Yie, K.H.; Zhang, H.; Liu, J.; Wu, G. Nanoporous tantalum coated zirconia implant improves osseointegration. Ceram. Int. 2020, 46, 17437–17448. [Google Scholar] [CrossRef]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Tang, W.; Fischer, N.G.; Kong, X.; Sang, T.; Ye, Z. Hybrid coatings on dental and orthopedic titanium implants: Current advances and challenges. BMEMat 2024, 2, e12105. [Google Scholar] [CrossRef]
- Duraccio, D.; Mussano, F.; Faga, M. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]

| Nanoparticle Type | Synthesis Methods | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| AgNPs | Chemical methods: Chemical reduction Electrochemical Microemulsion Photoreduction Physical methods: Evaporation–condensation Laser ablation Gamma irradiation Lithography Biological methods: Bacteria Fungi Plant extract | High antimicrobial activity against bacteria, fungi, and viruses Antibiofilm Anti-inflammatory effect Osteoconductive activity | Ag+ ions released correspond with the toxicity of AgNPs At high concentrations, it can provoke neurotoxicity Might cause decreased mitochondrial activity in a variety of cell types Can trigger oxidative stress, DNA damage, and inflammation High tendency to accumulate in tissues | [98,102,103,104,105,106,107] |
| AuNPs | Chemical methods: Chemical reduction Sol-gel Turkevich Method Brust–Schiffrin method Electrochemical method Seeding growth method Physical methods: Laser ablation Ultrasonication Pyrolysis Nanolithography Biological methods: Bacteria Fungi Plant extract | Favorable antimicrobial effect Antioxidant and anti-inflammatory activities with less toxicity than other metal NPs Drug or gene delivery Enhancement of bone-related cell adhesion, proliferation, and differentiation | Long-term biocompatibility studies have not been performed Smaller NPs can accumulate in various organs, such as the liver, spleen, and brain Cytotoxicity and genotoxicity at a smaller size | [107,108,109,110] |
| ZnO-NPs | Chemical methods: Sol-gel method Chemical deposition Precipitation Solvothermal and hydrothermal methods Microwave-assisted synthesis Microemulsion Physical methods: Laser ablation Arc plasma Physical vapor deposition Ultrasonic irradiation Biological methods: Plant Microorganisms Algae | High antibacterial efficiency at low concentrations Antifungal effect Relatively low cost Enhance the mechanical strength of dental composites Improve implant osseointegration Enhance the osteoblast proliferation Prevent implants’ premature corrosion | Have deleterious effects on several key organs, including the lungs, kidneys, liver, CNS, reproductive system In animal models influenced fetal development Cause cell apoptosis, necrosis, genotoxic effects | [107,111,112,113,114] |
| HAp | Wet methods: Hydrothermal method Hydrolysis Mechanochemical method Precipitation Mechanochemical method Emulsion Sol-gel Dry methods: Solid state Mechanochemical High-temperature method: Combustion Pyrolysis Biological methods: Plant | Effective in the osseointegration of implants Favors absorption of proteins, adhesion, and proliferation of bone cells Enhance bone healing Enhance the bone–implant interfacial strength Considered harmless to the cell environment Similar chemical and crystallographic structures to those of the human bone Can immobilize proteins and growth factors | The particle size influences toxicity; smaller particles may damage some cells, while larger ones do not Some disadvantages include brittleness, low tensile strength, and fracture toughness Delamination of coating can produce marginal bone resorption and incompatibility with antibiotic incorporation | [42,115,116,117,118,119,120,121] |
| Carbon-based materials | Physical methods: Arc discharge Laser ablation Arc discharge method Chemical methods: Chemical vapor deposition technique Plasma-enhanced chemical vapor deposition Hydrothermal method | Toxicity can be reduced by chemical functionalization Can promote a suitable surface for bone growth Have tunable chemical, physical, and biological properties Enhance the growth of osteoblasts High antibacterial efficiency | Their toxicity is related to cellular uptake that is influenced by shape, size, and aspect ratio Their toxicity is related to impurities remaining during the synthesis or purification stage In high concentrations, they are toxic Produce inflammation High cost of production | [88,122,123,124,125,126] |
| Technique | Layer Thickness (µm) | Deposition Temperature (°C) | Uniformity | Cost | Process Complexity | Refs. |
|---|---|---|---|---|---|---|
| PVD | 1–5 | 100–600 | High precision | High costs | Complex | [163,164,165] |
| CVD | 1–1000 | 800–1200 | High precision | High costs | Complex | [166,167,168] |
| Sol-gel | <1 | <100 | Good precision | Low-cost | Simple | [169,170,171] |
| Electrospinning | <0.5 | 25–104 | High precision | Low-cost | Simple | [172,173,174,175] |
| Testing Stages | Substrate | Material | Study aim | Observations | Ref. |
|---|---|---|---|---|---|
| In vitro | Titanium alloy (TiAl6V4) micro-implants | TiO2 and ZnO NPs | Evaluation of antimicrobial efficiency of TiO2 and ZnO nanoparticles (NPs) when used as a coating for orthodontic micro-implants | The 30 implants were divided into 3 groups according to the coating method and the materials used for coating, as follows: control group without coatings, TiO2-coated group by direct current (DC) spattering method, TiO2-ZnO-coated group by DC spattering method (TiO2), ZnO by vacuum laser. Antibacterial tests were performed on Staphylococcus aureus, Streptococcus mutans, and Porphyromonas gingivalis strains. This study demonstrated the importance of improving the surface of orthodontic microimplants by coating them with TiO2 and ZnO NPs to prevent biofilm formation. | [176] |
| In vitro/in vivo | Titanium disks | AuNPs | Evaluation of an osseointegrated titanium implant coated with gold nanoparticles to promote bone regeneration | The titanium implant surface was chemically treated with (3-Mercaptopropyl) trimethoxysilane (MPTMS) and an immobilized AuNP (Ti-AuNPs) layer on their surfaces by Au-S bonding. The in vitro results revealed that Ti-AuNPs improve osteogenic differentiation by increasing mRNA expression of osteogenic differentiation-specific genes in human adipose-derived stem cells (ADSCs). The in vivo data demonstrated that Ti-AuNPs had a considerable effect on osseous interface formation in New Zealand rabbit models. In vitro and in vivo experiments revealed that Ti-AuNPs can be used as osseo-integration-inducing dental implants to produce an osseous contact and maintain nascent bone development. | [61] |
| In vitro | Titanium disks | AgNPs | Determination of the antimicrobial potential efficacy of nanosilver-doped titanium biomaterials | The Tollens reaction was used to integrate silver nanoparticles into titanium disks across different periods. The antibacterial activity was further assessed using disk diffusion assays for microorganisms often recovered from the peri-implant biofilm: Streptococcus mutans, Streptococcus mitis, Streptococcus oralis, Streptococcus sanguis, Porphyromonas gingivalis, Staphylococcus aureus, and Escherichia coli. Cytotoxicity was assessed in vitro using a genuine human osteoblast cell culture. After 48 h of exposure, these surfaces were considerably hazardous to all of the bacteria tested. A concentration of 0.05 ppm was adequate to inhibit both Gram-positive and Gram-negative bacteria, with the latter being substantially more sensitive to silver ions. The nanosilver on the titanium gives an antibacterial action associated with the microorganisms involved in peri-implantitis. However, after the exposure of human osteoblasts to 0.1 ppm of silver ions, a significant decrease in cell viability was observed after 72 h. | [177] |
| In vitro | Titanium surfaces | HAp-NPs | Evaluation of the effects of coating titanium surfaces with HAp nanoparticles on cell behavior and osseointegration in vitro, comparing smooth-surfaced and HAp-activated implants | The test was carried out on two groups: the mach group, in which the titanium surface was mechanically machined without additional treatments, and the nano group, in which the titanium surface was coated with HAp-NPs. For surface testing, osteoblast cell culture (MC3T3-E1) was used to assess cell adhesion, viability, and differentiation. Osteoblast cells showed significantly higher viability on nano compared to mach surfaces. Cells in the nano group have a more stable adhesion, covering the surface evenly. The nano group showed more intense mineralization after 28 days of culture, indicated by denser calcium accumulation. Nanoscale HA-activated surfaces significantly stimulate the adhesion and differentiation of osteoblasts due to their increased roughness and favorable chemical composition. | [178] |
| In vitro | Ti cylindrical samples | Calcium-phosphate-based solution doped with AgNPs | Development of a functional coating on titanium (Ti) implants using a calcium-phosphate-based solution doped with AgNPs while evaluating the structural and chemical properties, biocompatibility, and antibacterial efficacy | AgNPs were synthesized with cubic morphology, and then electrolytic oxidation by mesh was performed using a solution containing the nanoparticles, nitrilotriacetic acid (NTA), and calcium–phosphate compounds. Cell adhesion and proliferation were performed on the U2OS cell line, while antibacterial assays were performed on the S. aureus strain. A porous surface with a silver-enriched ceramic layer was obtained. Cell adhesion and proliferation were significantly higher on AgNP-treated surfaces. AgNP-doped samples effectively inhibited bacterial adhesion and biofilm formation within 6 h. Combining silver and calcium phosphates created an environment favorable for osteogenic cell growth while providing antibacterial protection. | [179] |
| In vivo | Titanium alloy (TiAl6V4) implants | HAp and AgNPs | Examination of the osseointegration of AgNP-doped HAp coatings compared to conventional coatings using a rabbit experimental model | New Zealand white rabbits (12) each received two femur implants, one with conventional HAp and one with AgNPs-doped HAp. It was observed that the bone structure formed was similar between both implant types. The bone-to-implant contact was 52% for conventional HAp and 50.5% for HAp with AgNPs, with no statistically significant differences. AgNPs offer a potentially prolonged antimicrobial effect without interfering with bone formation. | [180] |
| In vitro/in vivo | Ti6Al4V ELI (Extra-low interstitial) alloy implants | TiO2-NTs | Exploration of enhanced osseointegration of nanostructured-modified titanium nanotube-coated and Simvastatin-loaded nanotube-coated dental implants | TiO2 nanotubes were created by electrochemical anodization and then loaded with Simvastatin using an ultrasonic immersion method. In vitro testing was performed on osteoblastic cell lines (MG-63) to assess cell viability, proliferation, and differentiation. In vivo testing was performed by using implants on rabbits for osseointegration using micro-CT analysis, histopathology, and torsion strength tests. In vitro tests demonstrated that at concentrations of 0.01 μM and 1 μM, the biocompatibility of the materials was very good, and they stimulated osteoblast differentiation. Also, bone mineralization was significantly better on drug-loaded surfaces compared to unloaded ones. In vivo tests showed that the coatings generated accelerated bone tissue development and great integration of implant coated and loaded with Simvastatin. Nanotubular surfaces showed improved cell adhesion and proliferation compared to smooth or acid-etched surfaces. | [181] |
| In vitro | Polyether ether ketone (PEEK) dental implant | Boron-doped nano-hydroxyapatites (B-nHAp) | Surface modification of PEEK implants with boron-doped nanostructured hydroxyapatite to improve implant bioactivity | The MTT study showed higher cell proliferation on PEEK implants treated with SPEEK sulfuric acid and SPEEK-B-nHAp compared to untreated PEEK. The cells attached better and formed a denser extracellular matrix on SPEEK-B-nHAp. ALP activity was significantly higher on SPEEK and SPEEK-B-nHAp. | [182] |
| In vitro/in vivo | Titanium implants | AgNPs and SrTiO3NPs | Development and evaluation of titanium implants with Ag and strontium titanate (SrTiO3) functional layers, highlighting their antibacterial and osteogenesis properties | Layered surfaces were obtained on titanium implants combining AgNPs and SrTiO3NPs to improve osseointegration and reduce the risk of peri-implant infections. Thus, sandwich layering improved the surface structure, combining micro- and nanometric structures. The SrTiO3 layer reduced the release of Ag ions by 30% and 15% on days 4 and 7, maintaining the antibacterial effects without affecting osteogenic cells. In vitro tests demonstrated improved osteoblast differentiation (increased ALP activity and mineralization). Antibacterial efficiency of approximately 93% against Staphylococcus aureus and 88% against Escherichia coli. In vivo tests of SrTiO3/Ag-layered implants showed significant increases in new bone formation compared to the control group. | [183] |
| In vitro | PEEK disks | Nano-dimensional zirconium phosphate (nZrP) and graphene oxide (GO)-based coating | Investigation of nano-dimensional zirconium phosphate (nZrP) and graphene oxide (GO)-based coatings of PEEK for the enhancement of hydrophilicity bioactivity, as well as antibacterial activity | nZrP/GO reduced the number of E. coli and S. aureus colonies 2-fold compared to untreated PEEK. Viability of MG-63 osteoblast and gingival fibroblast cells remained above 70% after 72 h, demonstrating the absence of cytotoxicity. After 28 days of immersion in SBF (Simulated body fluid), apatite crystals formed on the nZrP/GO surface, indicating bioactivity. | [184] |
| In vitro/in vivo | Zirconia implant | Nanoporous tantalum | Coating investigation of implant surfaces with a uniform layer of tantalum nanoporous to evaluate the surface topography, chemical composition of tantalum layer adhesion strength, hydrophilicity, and surface roughness, as well as the bioactivity and osseointegration of the TaNS layer | ZrO₂/TaNS showed significantly higher protein uptake, promoting enhanced cell adhesion. MC3T3-E1 osteoblast cells attached more rapidly and exhibited enhanced proliferation and differentiation on the surface covered with TaNS. The expression of osteogenic genes (RunX2, ALP, COL-1, OSX, OCN, OPG) was increased, indicating better osteogenic differentiation. Significantly greater bone formation around TaNS-coated implants was observed in animal models. | [185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehner, A.M.G.; Moldoveanu, E.-T.; Niculescu, A.-G.; Bîclesanu, F.C.; Pangică, A.M.; Grumezescu, A.M.; Croitoru, G.-A. Advances in Dental Implants: A Review of In Vitro and In Vivo Testing with Nanoparticle Coatings. J. Compos. Sci. 2025, 9, 140. https://doi.org/10.3390/jcs9030140
Rehner AMG, Moldoveanu E-T, Niculescu A-G, Bîclesanu FC, Pangică AM, Grumezescu AM, Croitoru G-A. Advances in Dental Implants: A Review of In Vitro and In Vivo Testing with Nanoparticle Coatings. Journal of Composites Science. 2025; 9(3):140. https://doi.org/10.3390/jcs9030140
Chicago/Turabian StyleRehner (Costache), Ana Maria Gianina, Elena-Theodora Moldoveanu, Adelina-Gabriela Niculescu, Florentina Cornelia Bîclesanu, Anna Maria Pangică, Alexandru Mihai Grumezescu, and George-Alexandru Croitoru. 2025. "Advances in Dental Implants: A Review of In Vitro and In Vivo Testing with Nanoparticle Coatings" Journal of Composites Science 9, no. 3: 140. https://doi.org/10.3390/jcs9030140
APA StyleRehner, A. M. G., Moldoveanu, E.-T., Niculescu, A.-G., Bîclesanu, F. C., Pangică, A. M., Grumezescu, A. M., & Croitoru, G.-A. (2025). Advances in Dental Implants: A Review of In Vitro and In Vivo Testing with Nanoparticle Coatings. Journal of Composites Science, 9(3), 140. https://doi.org/10.3390/jcs9030140