Abstract
Objective: One of the suggested methods for lowering polymerization shrinkage and improving the marginal sealing of restorations is the simultaneous light polymerization of the adhesive system and the first layer of the composite material, i.e., the co-curing method. This study investigates how different adhesive polymerization techniques, adhesive systems, tooth section depths, tooth types, and sample aging affect dentin bond strength. Methodology: This experiment tests three adhesive systems, G-Premio Bond (GC), Clearfil SE Bond 2 (Kuraray), and Adper Single Bond 2 (3M ESPE), using two polymerization techniques, namely, separate composite polymerization and simultaneous curing of the composite (“co-curing”). A total of 480 dentin samples are prepared and assigned to 24 groups (3 adhesives × 2 curing methods × 4 aging times). The shear bond strength is measured after one month, three months, six months, and one year, using an UltraTester. The statistical analyses include an ANOVA and Weibull analysis. Results: The separate polymerization of the adhesive and composite shows a significantly higher bond strength than that achieved through co-curing. Significant differences (p < 0.001) exist among adhesives, with Clearfil SE Bond 2 showing the highest bond strength. The bond strength decreases over time. Occlusal dentin has a higher bond strength than radicular dentin. There is no statistically significant difference in the bond strength between the maxillary and mandibular third molars. After one and three months of aging, the experimental groups with the highest average bond strength do not show the highest level of material reliability. Conclusion: The co-curing technique consistently results in a lower bond strength across all the adhesive systems compared to conventional separate polymerization.
1. Introduction
The connecting of two separate surfaces through the attraction of atoms and molecules is known as adhesion. Adhesion systems act as mediators between hard dental tissues and restorative material, i.e., composite material [1]. Therefore, adhesion forms the cornerstone of modern dentistry, as the development of adhesion systems, composite materials, and light polymerization led to the abandonment of Black’s principles of preparation, i.e., the removal of hard dental tissues in order to achieve mechanical retention and stabilization [2]. Nevertheless, modern dentistry and composite materials had some undesirable properties that needed to be overcome in order to achieve long-term clinical success [3]. In polymer composite systems, the formation of a macromolecular chain network of monomers involved the conversion of intermolecular distances from 0.3 nm to 0.4 nm, under the influence of Van der Waals forces, to a length of about 0.15 nm, due to the formation of covalent bonds [4]. This is the background of polymerization shrinkage, a chemical reaction characteristic of resins and the consequent occurrence of closely correlated polymerization stress, one of the main reasons for the failure of dental composite materials [3]. Polymerization stress is a complex phenomenon that is determined by various factors, such as cavity geometry, material characteristics, layering, and the polymerization technique used in terms of the composite material [5,6,7].
Several clinical treatments and strategies were proposed to improve the marginal sealing of restorations and decrease the shrinkage caused by polymerization. One of the suggested techniques was “co-curing”, or light polymerization, of the first layer of the composite material and the adhesive system at the same time. In order to overcome oxygen inhibition of extremely thin adhesive layers, Unterbrink and Liebenberg investigated the use of flowable composites as filled adhesives. They accomplished this by combining a single-component adhesive, as a dentin primer, with a thin layer of flowable composite, as a filled adhesive. Both components were then cured together [8].
Even though there are not many studies on how co-curing affected the dentin shear bond strength, the prior research did not support the co-curing technique. Similar to Chapman et al., Viswanathan et al. found that co-curing resulted in noticeably reduced shear bond strengths in regard to dentin. They attributed the defect to two things: insufficient adhesive agent curing and contraction stress of the curing overlapping the composite material [9,10]. Data from the literature indicated that using a bulk-fill composite material might have resulted in successful co-curing. Compared to traditional composite materials, bulk-fill resin composites transfer the curing light significantly deeper, which reduces contraction stress [11,12,13,14].
The first study that was presented to scientifically analyze Unterbrink and Liebenberg’s proposed theory in vitro was published by Deliper et al. They studied whether microleakage was lower when using the co-curing method. They concluded that microleakage was not affected by the adhesive system or the technique used in terms of its placement; thus, their study contradicted the proposed theory [15]. In a recent study of microleakage, the results contradicted the findings of previous studies. The results showed that there were no statistically significant differences between the groups in which simultaneous light polymerization and separate light polymerization were performed. However, in the group in which the co-curing technique was tested, lower microleakage values were achieved. These findings confirmed that this is a valid technique that might be useful in certain clinical situations [16].
In order to provide consistent strength, marginal sealing, and clinical durability, the objective of adhesive treatments is to create and preserve a stable bond between the adhesive system and dentin for a certain number of years. Many clinical issues can be resolved by longer-lasting adhesive restorations [17,18,19,20]. Durability testing has to be a part of any effort to assess the efficacy of adhesive systems [21]. Since water is essential for deterioration, long-term storage in water is the most commonly used artificial aging method [22]. For a certain period of time, samples are kept in liquid at 37 °C [23]. A number of studies have reported significant reductions in shear bond strength as a result of various test regimes, even after relatively short periods of storage [24,25,26,27].
Despite the different adhesive strategies, the number of clinical steps, and the variety of commercial products available on the market, all enamel–dentin adhesive systems contain similar components. Adhesive systems primarily consist of monomers with hydrophilic and hydrophobic groups. The first group increases the wetting ability of hard dental tissues, while the other enables interaction and copolymerization with the restorative material. Adhesive systems are also chemically composed of initiators, solvents, inhibitors or stabilizers, and occasionally, inorganic fillers [28]. Although manufacturers often do not wish to reveal the precise composition of adhesive systems on the market, particularly if they contain proprietary compounds, the ratio of chemicals contained in various adhesive systems was found to be the primary factor responsible for their variances [29].
High variability is the primary characteristic of dentin bond strength data. While factors such as sample preparation, material handling, storage conditions, experimental methodology, and test equipment design can influence bond strength test results, the primary source of variability lies in the inherent fragility of dental composites and adhesive systems. These materials are particularly susceptible to mechanical and chemical degradation, which can compromise their durability and performance in dental restorations. Their fragility stems from several factors, including mechanical weakness, progressive degradation, inadequate adhesion to tooth structures, and polymerization shrinkage [30].
In the case of brittle materials, one study found that the stress concentration changed based on the geometry of the sample; thus, the bond strength was not determined by the strength of the material but by the existing defects present in the sample. The stress concentration in brittle materials altered the sample’s geometry, ultimately resulting in defect-induced failure [31].
The previous research has provided the characteristics of the examined samples, not the material [32,33]. Thus, according to some authors, it is more acceptable to use the Weibull distribution function to estimate the likelihood of fracture at a given stress level (and vice versa) in order to describe brittle materials [34,35,36].
The first aim of this in vitro study was to examine whether different methods of polymerizing the adhesive system (conventional light polymerization of the adhesive system and composite material or co-curing light polymerization of the adhesive system and composite material) affected the bond strength of dentin. The second aim was to determine whether the aging of the samples affected the bond strength of dentin, to compare the bond strengths of different adhesive systems that were polymerized in the same way, to compare the bond strengths in the occlusal and radicular sections of dentin, and to examine whether the type of tooth affected the bond strength of dentin.
Based on the previously mentioned aims, we set five hypotheses: (1) the method of polymerization (conventional or co-curing light polymerization) does not significantly affect the bond strength of dentin, (2) aging of the samples does not significantly affect the bond strength of dentin, (3) there is no significant difference in the bond strength among different adhesive systems that are polymerized in the same way, (4) there is no significant difference in the bond strength between the occlusal and radicular sections of dentin, and (5) the type of tooth does not significantly affect the bond strength of dentin.
2. Materials and Methods
2.1. Collection and Preparation of Samples
This research was conducted on 222 intact third molars with completed root formation that were extracted for justified reasons. After extraction, the soft deposits were removed from the teeth, and the teeth were stored at room temperature in a 1% chloramine solution (KEFO, Sisak, Croatia). The teeth were used within three months after extraction. In order to create a flat dentin substrate for the sample, a mid-crown section was made using a low-speed saw (IsoMet, Buehler; Lake Bluff, IL, USA) with a diamond blade at 300 rpm with continuous water cooling. The mid-crown section produced two dentin slabs known as the “occlusal” and “radicular” parts. If the surface area of these slabs was sufficient to hold several composite samples, they were further sectioned. The average number of dentin slabs per tooth was 2.16. Before making a section, each tooth was marked as maxillary or mandibular.
The dentin samples were embedded in acrylate resin (Technovit 4004, Kulzer, Hanau, Germany) using an Ultradent mold (Ultradent Products, South Jordan, UT, USA). In order to create a flat adhesive area, the dentin surface was polished with waterproof silicon carbide paper, grit 4000 (Buehler, Dusseldorf, Germany), followed by polishing with professional silicone granules of 1.0 μm, 0.3 μm, and 0.05 μm (Buehler, Dusseldorf, Germany). The polishing process was carried out using a polishing machine (Minitech 250, Presi, France). After that, the samples were washed in distilled water and immediately used for the adhesive procedure. The materials used in this research were orthophosphoric acid, three different adhesive systems, and a bulk-fill flowable composite (Table 1). Dental adhesives morphologies are typically assessed using SEM (Scanning Electron Microscopy). G-Premio Bond (GC) is a universal adhesive (one-bottle, light-cured). It forms a relatively thin hybrid layer with well-infiltrated resin tags. It shows consistent nanoleakage resistance due to the MDP monomer. It is compatible with self-etch, total-etch, and selective-etch techniques [37]. Clearfil SE Bond 2 (Kuraray) is a self-etch adhesive (two-step). It produces a uniform hybrid layer with a well-defined transition to dentin with strong interaction with hydroxyapatite due to the 10-MDP monomer and well-developed resin tags and lateral branches in dentinal tubules [38]. Adper Single Bond 2 (3M ESPE) is a total-etch adhesive (one-bottle, ethanol-based). It forms a thicker adhesive layer compared to self-etch adhesives and produces long and well-penetrated resin tags into dentinal tubules. It also exhibits a distinct hybrid layer with high demineralization depth and possible nanoleakage in cases of incomplete solvent evaporation [39].

Table 1.
Materials used in this research according to the manufacturer’s specifications.
2.2. Experimental Groups
A total of 480 dentine samples were prepared and randomly distributed into 24 test groups. Two methods of application, i.e., light polymerization of the adhesive system and composite material, were carried out using three different adhesive systems, and the bond strength was tested over four time periods (Figure 1). The number of samples per experimental group was n = 20, and care was taken to ensure that only one slab from one tooth was in the same experimental group. The minimum sample size for ANOVA was calculated using G*power software 3.1.9.7. Taking into account the medium-sized effect (Cohen’s f = 0.25), the significance level of 0.05, the statistical power of 0.90 (that is, 90%), and 24 experimental groups, it was necessary to include a minimum of 480 samples in this research, that is, 20 per group, as it was performed.
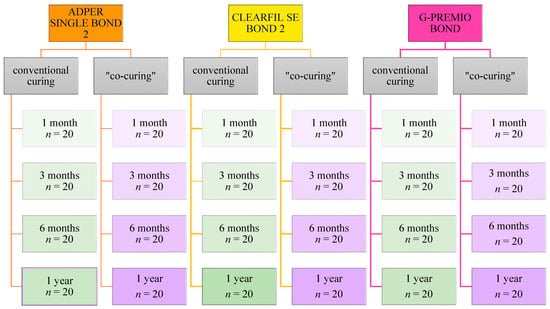
Figure 1.
Flowchart showing the distribution of samples into test groups.
2.3. Adhesive Procedure
Following the distribution of the dentin samples into experimental groups, the dentin surface was gradually allowed to air dry until there was no more obvious wetness present. The adhesive region was defined using a polymer adhesive tape with a hole diameter of 2.4 mm, and a thickness of 0.2 mm was used to define the adhesive area. For each adhesive, two methods of application were used. The first approach, i.e., the conventional method, implied the adhesive system was light-cured in accordance with the manufacturer’s instructions before applying the composite material. G-Premio Bond, a universal adhesive system, was applied as self-etch. Following the polymerization of the adhesive system, a plastic mold (Ultradent Products, South Jordan, UT, USA) was used to make composite cylinders with an inner diameter of 2.38 mm and a height of 2.0 mm on the adhesive surface. The composite material was polymerized with light for 20 s (Figure 2).
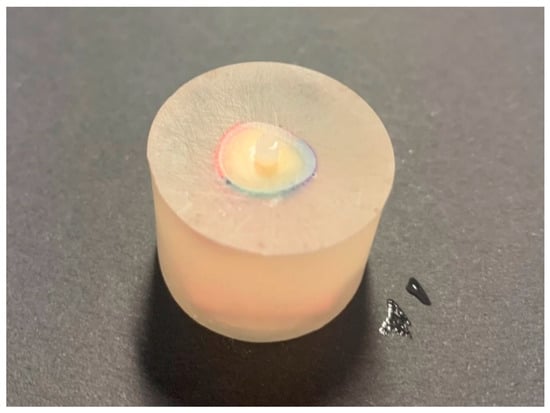
Figure 2.
Dentin slice with a composite cylinder before shear bond strength testing.
The second method involved co-curing, or light polymerization, of the adhesive system and the additional composite material at the same time. Although adhesive systems were applied in accordance with the manufacturer’s recommendations, they were not light-cured immediately after application. The adhesive system was gradually air-dried but not immediately light-cured after application in a single layer. As in previous studies, the adhesive agent was light-cured with composite material for 40 s [9,40].
For both methods, light polymerization was carried out at a distance of 1 mm using a polymerization lamp (Bluphase Style, Ivoclar Vivadent; Schaan, Liechtenstein, serial number: 1120006563) with a high light intensity of 1100 mW/cm2, which was measured with an LED light radiometer called the Bluephase Meter II (Ivoclar Vivadent, Schaan, Liechtenstein, serial number: 000012). After removing the mold and the adhesive polymer tape that limited the area of adhesion, the samples were stored in distilled water in an incubator (INEL, Zagreb, Croatia) at 37 °C for a period of 1 month, 3 months, 6 months, and 1 year.
2.4. Shear Bond Strength Testing
After aging for one month, three months, six months, and one year, the samples were subjected to bond strength testing. An UltraTester (Ultradent Products, SAS Institute Inc., Cary, NC, USA) was used to test the bond strength of the samples. The UltraTester contains a specific jig that comes into contact with a larger surface area of the sample compared to other shear tests. The holding device surrounded half of the sample and was located at the junction between the surface of the dentine sample and the composite material. The test was carried out by loading the sample at a constant speed of 1 mm/min until the adhesive bond failed, i.e., until the composite cylinders detached from the dentine surface. The measurement was carried out according to ISO 29022:2013 (Figure 3). Shear bond strength values were calculated according to the equation: σ = F/A, where σ (MPa)—shear bond strength, F (N)—breaking force, i.e., the maximum force at which a fracture occurred, and A (mm2)—bond area.
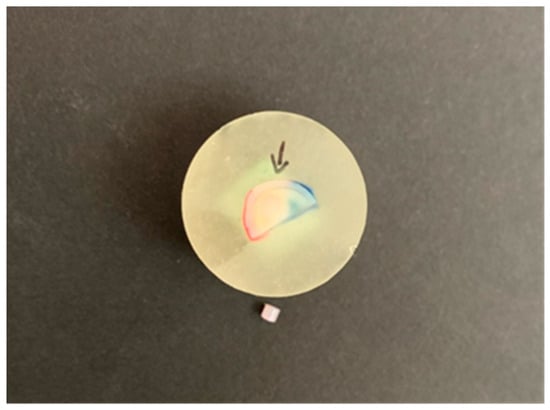
Figure 3.
Broken composite cylinder after shear bond strength testing. Arrow shows the dentin bond area.
2.5. Statistical Analysis
Descriptive data analysis was used to summarize the measurement results, which included the calculation of arithmetic means and associated variability indicators (standard deviation, coefficient of variation). To assess the effect of treatment on bond strength, a multifactor analysis of variance (ANOVA) was used, in which the following factors were included: type of treatment (i.e., polymerization technique of a certain adhesive system), time point of measurement (1, 3, 6, or 12 months after treatment), dentin section (occlusal or radicular) and tooth type (maxillary or mandibular). In addition to the main effects, an interaction between treatment type and time point of measurement was included in the initial model. The interaction was not statistically significant (p = 0.814) and was excluded from the final model. Levene’s test rejected the null hypothesis about the homogeneity of the variance in the measured bond strength values between the different materials (p < 0.001). Accordingly, in the specification of the ANOVA model, the variance for each material was modeled separately. The results were analyzed at a significance level of 0.05, at which the statistical power of the test was satisfactory (80%) to detect medium-sized effects (Cohen’s f = 0.25). The original p-values of the planned comparisons between factor levels within the ANOVA model were corrected using the Bonferroni–Holm correction for multiple comparisons in order to reduce the probability of “false positive” results, i.e., rejection of the true null hypothesis. The analysis was performed in the SAS 9.4. System software package (SAS Institute Inc., Cary, NC, USA).
The first step in reliability analysis was ranking the samples based on the measured bond strength. Natural logarithms of bond strength were plotted along a Weibull graph’s horizontal axis. The following equation was used to calculate the probability of failure (Pf) for each sample from a set of n samples: Pf = (i − 0.5)/n, where i is the ranking number in ascending order of bond strength data (weakest rank 1, strongest rank N), while n is the total number of samples inside the experimental group. The double natural logarithm of [1/(1 − Pf)] was plotted on the vertical axis. The displayed data points were then utilized to fit a function using maximum likelihood estimation. The Weibull modulus was represented by the obtained slope. The form parameter, also known as the Weibull modulus, was used to quantify the variation in the bond strength measurement. A larger dispersion of faults and less consistent bond strength were indicated by a lower value of m. A steeper slope of the fit line, or a larger value of m, indicated more reliability. Characteristic bond strength, also known as the scale parameter, was the second parameter of the Weibull distribution. Its value was obtained from the x-axis where the probability of failure (Pf) on the y-axis equals 63.2%. The data move to the left, toward the lower values of ln (strength) on the x-axis, as the scale parameter decreases [41].
3. Results
The mean values and the 95% confidence interval for the bond strength of these groups of samples are shown graphically in Figure 4. The highest average bond strength was measured in the Clearfil SE Bond 2 group one month after treatment; it was 38.48 MPa for the conventional method of applying the adhesive system and 29.90 MPa for the method of simultaneous light polymerization.
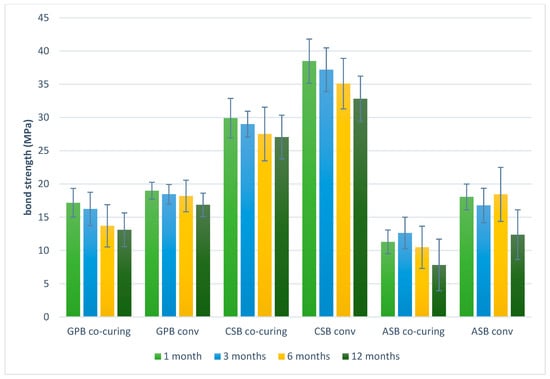
Figure 4.
Graphic representation of mean values and 95% confidence intervals of the bond strength obtained by the shear test. GPB co-curing: G-Premio Bond co-curing, GPB conv: G-Premio Bond conventional, CSB co-curing: Clearfil SE Bond 2 co-curing, CSB conv: Clearfil SE Bond 2 conventional, ASB co-curing: Adper Single Bond 2 co-curing, ASB conv: Adper Single Bond 2 conventional.
The lowest average bond strength was measured in the Adper Single Bond 2 co-curing group 12 months after treatment; the value was 7.83 MPa. The lowest measurement variability was recorded in the G-Premio Bond group using the conventional method, which amounted to 14% according to the coefficient of variation. A decrease in average bond strength values over time was observed in all experimental groups.
The results obtained by analysis of variance revealed significant differences in all the examined factors (aging time, adhesive system and method of polymerization, and part of dentin), except for the location of the teeth (maxillary or mandibular). A significant difference between the conventional and co-curing methods was observed across all the examined adhesive systems. Higher average bond strength values were observed with the conventional method of polymerization of the adhesion systems. The largest average difference of 7.5 MPa was recorded with Clearfil SE Bond 2, while the smallest difference was recorded with G-Premio Bond (3.1 MPa).
Between all the different experimental groups in which the adhesive systems were applied and polymerized by the same method (conventional or “co-curing”), with the exception of Adper Single Bond 2 and G-Premio Bond by the conventional method, a statistically significant difference was observed. Although G-Premio Bond exhibited a higher average bond strength compared to Adper Single Bond 2, the difference was not demonstrated in the group comparison (p = 0.054). Clearfil SE Bond 2 showed the highest average bond strength in both the methods of application and polymerization of the adhesive system. After 6 months, the bond strength decreased compared to 1 month (p = 0.044). An additional decrease in the bond strength occurred after 12 months, and the bond strength was significantly lower compared to 1 (p < 0.001), 3 (p < 0.001), and 6 months (p = 0.044). The average bond strength after 1 month was 3.7 MPa higher than the bond strength after 12 months. The smallest difference in the average bond strength was recorded after one month and 3 months (0.66 MPa) and was not statistically significant (p = 0.370).
In the occlusal parts of the dentin, on average, a statistically significantly higher bond strength was recorded compared to the radicular parts of the dentin (while controlling for the effects of other factors—the method of applying adhesive systems, the aging of the samples, and the type of tooth) (p = 0.033). Between the maxillary and mandibular teeth, no difference in bond strength was recorded (while controlling for the effects of other factors—methods of applying adhesive systems, aging of samples, and dentin sections) (p = 0.999)
The highest Weibull modulus after 1 month was achieved in the G-Premio Bond conventional group, after 3 months in the Clearfil SE Bond 2 co-curing group, and after 6 and 12 months in the Clearfil SE Bond 2 conventional group, as indicated by the results of the MLE estimation. The least reliable experimental group was Adper Single Bond 2 co-curing, with the lowest Weibull modulus at all time points (according to the MLE estimation) (Figure 5, Figure 6, Figure 7 and Figure 8).
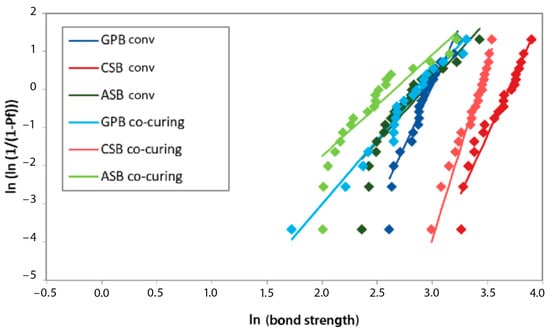
Figure 5.
Weibull diagram of bond strength measurements after 1 month of aging. GPB co-curing: G-Premio Bond co-curing, GPB conv: G-Premio Bond conventional, CSB co-curing: Clearfil SE Bond 2 co-curing, CSB conv: Clearfil SE Bond 2 conventional, ASB co-curing: Adper Single Bond 2 co-curing, ASB conv: Adper Single Bond 2 conventional.
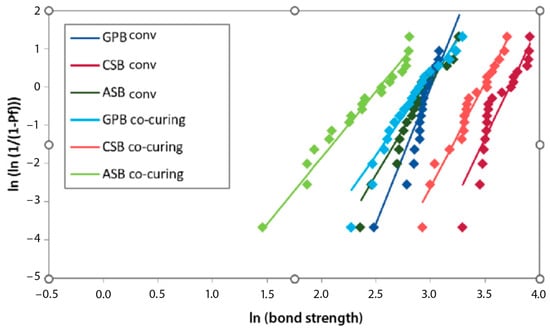
Figure 6.
Weibull diagram of bond strength measurements after 3 months of aging. GPB co-curing: G-Premio Bond co-curing, GPB conv: G-Premio Bond conventional, CSB co-curing: Clearfil SE Bond 2 co-curing, CSB conv: Clearfil SE Bond 2 conventional, ASB co-curing: Adper Single Bond 2 co-curing, ASB conv: Adper Single Bond 2 conventional.
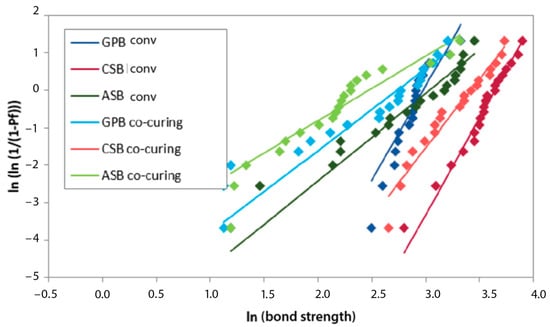
Figure 7.
Weibull diagram of bond strength measurements after 6 months of aging. GPB co-curing: G-Premio Bond co-curing, GPB conv: G-Premio Bond conventional, CSB co-curing: Clearfil SE Bond 2 co-curing, CSB conv: Clearfil SE Bond 2 conventional, ASB co-curing: Adper Single Bond 2 co-curing, ASB conv: Adper Single Bond 2 conventional.
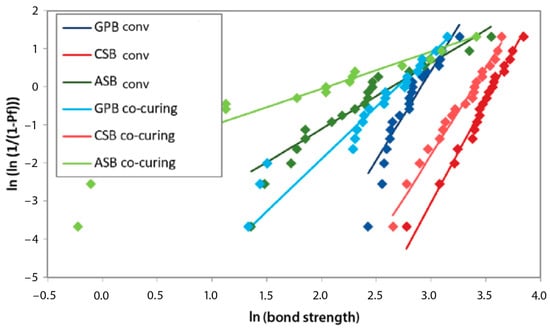
Figure 8.
Weibull diagram of bond strength measurements after 12 months of aging. GPB co-curing: G-Premio Bond co-curing, GPB conv: G-Premio Bond conventional, CSB co-curing: Clearfil SE Bond 2 co-curing, CSB conv: Clearfil SE Bond 2 conventional, ASB co-curing: Adper Single Bond 2 co-curing, ASB conv: Adper Single Bond 2 conventional.
Within a given experimental group, the mean strength and characteristic strength followed a similar pattern of variation over aging time. The highest average bond strength values were in the same experimental groups as the characteristic bond strength. The Weibull modulus, i.e., the shape parameter, decreased during aging in all experimental groups, except for Clearfil SE Bond 2. In the Clearfil SE Bond 2 co-curing group, the Weibull modulus increased after 3 months compared to 1 month (5.47 to 9.22). In the same experimental group, the greatest decline in reliability occurred between three and six months. In the Clearfil SE Bond 2 conventional group, the smallest difference in the Weibull modulus over time was observed.
In the descriptive statistics, the Clearfil SE Bond 2 conventional experimental group exhibited the highest average value at all time points. The same applies to the characteristic strength. In the reliability analysis, the lowest variability after one month of aging was recorded with G-Premio Bond conventional, after 3 months with Clearfil SE Bond 2 co-curing, and after 6 and 12 months with Clearfil SE Bond 2 conventional. Lower values of the shape parameter showed a wider distribution of bond strength values per tested experimental group, signifying reduced reproducibility and reliability. Adper Single Bond 2, in all experimental groups, proved to be the material with the least reliability.
In Figure 9, a consistent drop in reliability can be observed with the aging of the samples. In the experimental group, Clearfil SE Bond 2, applied and polymerized by the co-curing method, showed a jump after 3 months with a subsequent decline. The least pronounced drop in reliability over time was observed with Clearfil SE Bond 2 using the conventional method.
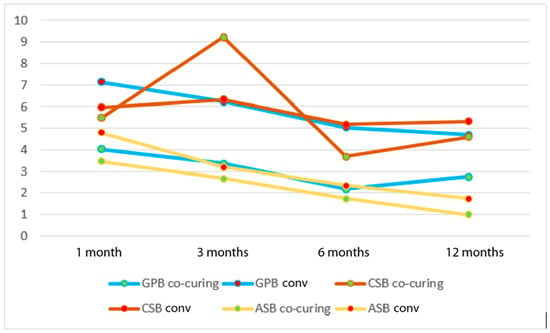
Figure 9.
Weibull modulus as a function of different methods of application of the adhesive system and aging time. GPB co-curing: G-Premio Bond co-curing, GPB conv: G-Premio Bond conventional, CSB co-curing: Clearfil SE Bond 2 co-curing, CSB conv: Clearfil SE Bond 2 conventional, ASB co-curing: Adper Single Bond 2 co-curing, ASB conv: Adper Single Bond 2 conventional.
4. Discussion
The aim of this in vitro study was to investigate the effects of different polymerization methods of the adhesive system (conventional light polymerization vs. co-curing light polymerization with composite material) on the bond strength of dentin. Additionally, this study aimed to evaluate the impact of sample aging, compare the bond strengths of different adhesive systems polymerized using the same method, assess bond strength differences between occlusal and radicular dentin, and determine whether tooth type influences dentin bond strength.
Since a statistically significant difference was observed in all experimental groups between the conventional curing and co-curing light polymerization of the adhesive system and the composite material, the first null hypothesis that the method of polymerization (conventional or co-curing light polymerization) does not significantly affect the bond strength of dentin was rejected. Despite variations in methodology and evaluation criteria, the shear bond strength results align with findings from similar studies. In a recent study, where the bond strength was tested by conventional curing and co-curing light polymerization of the universal adhesive system and composite cement, lower bond strength values were also recorded for the co-curing method [42]. As an explanation, the adhesive system’s exposure to light for polymerization is limited due to the weakening and dispersion of light energy during the simultaneous passage of polymerization light through the adhesive system and the composite material. This ultimately results in unfavorable polymerization of the adhesive system and the formation of an unstable bond that is likely to remain in the form of a gel [42]. Unfavorable polymerization of the adhesive system refers to a situation where the polymerization process of an adhesive system does not occur optimally, leading to weak adhesive, reduced durability, and compromised mechanical properties. This can happen due to various factors, including incomplete polymerization, oxygen inhibition layer, polymerization shrinkage, or incompatibility with composite material [43].
The simultaneous light polymerization of the adhesive system and composite material hinders the penetration of resin monomers into the dentinal tubules and their lateral branches due to composite material shrinkage. Since a complete interaction of resin extensions is not formed, an insufficiently strong bond is created [44]. In addition, it is possible that the layer of the adhesive system in these groups was insufficiently polymerized due to the incorporation of filler particles, as any component added to the resin mixture can potentially affect the polymerization reaction and the final properties of the adhesive system [28].
In this study, the polymerization time for simultaneous light polymerization was set to 40 s, according to most other studies, as well as assuming that manufacturers recommend a minimum polymerization of 20 s for composite resin and 10–20 s for adhesive systems. Extending the polymerization time remains an option since the use of a light source with adequate radiation power and the use of an exposure time exceeding the manufacturer’s recommendations have been shown to improve the degree of conversion and reduce the permeability of enamel–dentin adhesive systems, contributing to an improvement in their in vitro performance [45,46,47,48]. Larger deviations in the average bond strength between different experimental groups can perhaps be explained by the formulation of adhesive systems. G-Premio Bond, which does not contain HEMA monomer, has the smallest average difference [49]. Although the studies do not support co-curing [50,51], different study methodologies could be the reason for the differences in the results. Therefore, we recommend that future research should attempt to standardize the method.
The bond strength curve, obtained from previous measurement results, decreases mainly after 100 days or 6 months, and a certain bond strength is retained after long-term storage in water [23,50,51]. These findings are consistent with the results of this study, where reduced bond strength was noted at 6 months compared to 1 month, with a further decline at 12 months. Therefore, the second hypothesis that the aging of the samples does not significantly affect the bond strength of dentin was rejected. One possible explanation for this gradual decline in binding efficacy is the deterioration of adhesive bond components brought on by storage in water [52]. Two main mechanisms through which hybrid layer degradation takes place are described in the literature: hydrolysis and enzymatic degradation [51,53]. In this study, aging was not associated with the adhesive strategy. Demineralized collagen fibers are susceptible to time-dependent hydrolytic degradation by water, regardless of the adhesive strategy [54]. Most of the current knowledge about adhesive joints comes from laboratory studies. The question of whether these laboratory results are somehow related or can predict clinical performance remains questionable. Apart from a few weak relationships, most attempts to correlate laboratory and clinical data are inconclusive. Most studies evaluating the bond strength of dentin have been performed over short periods of time, generally 24 h periods. However, changes in pH, temperature, masticatory loads, and chemical attacks commonly occur in the oral cavity and affect the resin–tooth bond over months and years [2].
Due to variations in protocols and different formulations of primers and adhesives, it is expected that some will simply perform better than others. This is what was observed in this study as well as in others [2,55,56]. G-Premio Bond, Clearfil SE Bond 2, and Adper Single Bond 2, which were evaluated in this study, differ in generation, the number of clinical steps, solvents, pH, and partially in monomer composition. Between all the different experimental groups in which adhesive systems were applied and polymerized by the same method, with the exception of Adper Single Bond 2 and G-Premio Bond by the conventional method, a statistically significant difference was observed. Clearfil SE Bond 2’s strong and consistent adhesive ability with various dentin substrates has also been documented in earlier research [57,58,59]. Adper Single Bond 2 showed lower average bond strength values compared to the remaining materials. Potential errors that could lead to lower bond strength values of Adper Single Bond 2 compared to self-etching adhesives include too strong drying of dentin and excessive demineralization, which creates a hybridoid layer [17,60,61]. Therefore, the third hypothesis that there is no significant difference in bond strength among different adhesion systems that were polymerized in the same way was rejected. G-Premio Bond, Clearfil SE Bond 2, and Adper Single Bond 2 are commonly compared in dental research because they represent different categories of adhesive systems used in restorative dentistry. The comparison is typically made to evaluate their adhesive effectiveness, durability, and clinical performance.
Clearfil SE Bond 2, with a pH of ≈2, belongs to mild self-etching adhesive systems, while G-Premio Bond, with a pH of 1.5, is categorized as moderate [62]. With light-curing resin-based materials, there is concern that the lower pH may interfere with the activation reaction between the tertiary amine and camphorquinone, leading to a lower degree of conversion. A lower degree of conversion can weaken the mechanical properties of the layer of adhesive systems and thus negatively affect the durability of the joint [63]. Also, mild self-etching systems are thought to be able to establish a better chemical bond between specific carboxyl or phosphate groups of functional resin monomers (especially 10-MDP) and calcium in the residual hydroxyapatite crystals found around the collagen fibrils [64]. Despite the fact that the composition of Clearfil SE Bond 2 included HEMA and Bis-Gma, similar to Adper Single Bond 2, i.e., that the similarity with G-Premio Bond was based on the monomer 10-MDP, the inclusion of different ratios, co-monomers, catalysts and solvents probably led to large variations in the properties of the adhesive systems, which affected their reactivity with dentin and consequently their bond strength [29,63]. Adhesive systems consist of monomers that contain both hydrophilic and hydrophobic groups, each playing a crucial role in the adhesive process. The hydrophilic groups improve the wettability of hard dental tissues, ensuring better penetration and adaptation of the adhesive to the tooth surface. In contrast, the hydrophobic groups promote interaction and copolymerization with the restorative material, enhancing the strength and durability of the bond. Understanding these properties is essential, as they directly relate to how monomer modifications can optimize wettability and interfacial characteristics, ultimately improving the performance of composite materials in dental restorations [65].
In a more recent study, an adhesive system containing HEMA and 10-MDP monomers showed the highest bond strength values [66]. Although the Clearfil SE Bond 2 formulation contained this monomer, the 10-MDP present in the composition probably favored the mechanical properties of this group, showing a statistical difference compared to Adper Single Bond 2, whose formulation contained only the HEMA monomer. In the current research, the superior bond strength values of Clearfil SE Bond 2 may also be attributed to its new photoinitiator that improves its degree of conversion, leading to improved mechanical properties and higher bond strength [58,67,68]. Indeed, long-term laboratory [19,69] and clinical [70,71] studies have shown that the bond strength of two-component self-etching and three-component etch–rinse adhesive systems is better than that of simplified systems, which is consistent with the results of this research. It is important to note that the bond strength value cannot be considered a material property. There will always be confounding variables in every study, whether it is conducted in vitro or in vivo. These include the test tooth’s age, the storage medium and duration, the environmental and cultural characteristics of the individual from whom the tooth was taken, and the sample processing techniques. The statistical explanation of these concerns is challenging, if not impossible.
Due to its dependence on dentin water content and tubule width, bond strength is known to be dependent on dentin depth [72,73,74]. Two slabs, an occlusal and a radicular one, were obtained by making a mid-coronal cut in order to prepare the dentin substrate for adhesion. The thickness of the diamond cutting blade, which was 0.2 mm, determined the difference in dentin depth. Lower shear bond strength with increased dentin depth and permeability has been reported in most studies [74,75]. In our study, in the occlusal parts of the dentin, on average, a statistically significantly higher bond strength was recorded compared to the radicular parts of the dentin. Therefore, the hypothesis that there was no significant difference in bond strength between the occlusal and radicular sections of dentin was also rejected. The results of this research are, to a certain extent, comparable to those from the 2018 study by Par et al., since the samples were prepared in the same way. In the aforementioned study, there was no significant statistical difference in the bond strength between occlusal and radicular dentin sections, and it was suggested that the variability resulting from the small difference in dentin depth was negligible compared to other sources of variability [76].
In this study, there was no statistically significant difference between the obtained bond strengths of maxillary and mandibular third molars, so the fifth hypothesis that the type of tooth does not significantly affect the bond strength of dentin was therefore accepted. A possible explanation is that despite the anatomical and functional differences between the upper (maxillary) and lower (mandibular) arches, the enamel surface characteristics (e.g., mineral content, microstructure, and etch pattern after acid treatment) may be very similar between these teeth. This similarity suggests that when conventional adhesive protocols are applied, the resin infiltration and micromechanical retention processes occur to a similar degree in both maxillary and mandibular molars, resulting in comparable bond strengths [77]. Some investigations, which focused mostly on bond strength to enamel, found notable variations between the bond strength values of teeth in the upper and lower arches [78,79].
The analysis of the change in bond strength over time revealed a drop in average bond strength after 6 months, with an additional drop in all experimental groups. The Weibull analysis showed the smallest difference in the Weibull modulus in the Clearfil SE Bond 2 conventional group, or rather, the smallest variability, which could mean that in this study, Clearfil SE Bond 2 was the most reliable over time. In the other experimental groups, the Weibull modulus decreased during aging. What is unusual is the increase in the shape parameter in the Clearfil SE Bond 2 co-curing group, i.e., the Weibull modulus after 3 months compared to 1 month (5.47 to 9.22). In the same experimental group, the greatest drop in reliability was recorded after 6 months compared to 3 months. A significant but transient increase in reliability after 3 months was also observed during the application of the Clearfil SE Bond 2 enamel–dentine adhesive system; however, this was also observed in the research by Par et al. [76] when using different bioactive composite materials. The reliability of adhesive systems varies greatly depending on their chemical composition, as shown in a study of the shear bond strength of 11 self-etch and etch–rinse adhesive systems, which reported Weibull moduli ranging from 2.1 to 8.3 [33].
An increase in reliability with aging is not expected, as bond strength is generally expected to decrease with aging or remain unchanged, as observed in conventional statistics. However, it is possible that plasticization occurred due to water absorption, and as a result, brittleness decreased, which made the adhesive system/composite interface more resistant to fracture [35]. The results of this experiment do not allow any clear conclusions on this issue. Although the highest characteristic bond strength at all time points was observed in the Clearfil SE Bond 2 conventional group, the Weibull modulus after 1 and 3 months was not the highest, indicating a higher degree of dispersion of the strength data. In other words, this showed that Clearfil SE Bond 2 was not the most reliable material in the measured conditions at the specified aging times.
Over time, water absorption can weaken the adhesive interface, leading to the hydrolysis of adhesive monomers and polymer chains. This breakdown reduces mechanical interlocking and chemical adhesive, ultimately lowering bond strength [80]. Also, if collagen fibrils in the dentin are not properly infiltrated and protected by the adhesive, they can degrade due to enzymatic activity (e.g., matrix metalloproteinases or MMPs). This process weakens the adhesive–dentin bond, contributing to a drop in reliability [81]. Over time, polymerization-induced stress relaxation and leaching of unreacted monomers can affect the cross-linking density of the adhesive. This structural instability reduces the bond’s durability, leading to progressive failure [82].
Since there is not yet an internationally recognized standardized test protocol for testing adhesive systems, completely different bond strength values for the same product can be found and published, depending on the method and the method of testing. For the purpose of standardization, some of the important recommended parameters for standardizing bond strength testing are a defined and limited adhesion area, sample aging of at least 6 months, and Weibull analysis with a minimum of 15 samples per group [41]. This research followed these suggestions in order to make it easier to compare tests in the future.
The limitations of this study are that it does not apply all the factors that could affect the material itself, such as chewing forces, temperature changes, and exposure to saliva. However, all the samples were exposed to the same experimental conditions. In addition, only representative commercial samples were used as materials, whereas a wide range of adhesive systems, as well as bulk-fill composite materials, are available for testing. According to the conditions in this research, an insight into the properties of the experimental materials was obtained, but all recorded values must be observed within the experimental conditions.
5. Conclusions
Under the limitations of this in vitro study, it was possible to conclude that the adhesive application method influences the shear bond strength of dentin. When compared to a conventional procedure, the co-curing methodology produced lower adhesive strength values in all of the adhesive systems examined. Furthermore, bond strength depended on the aging time as well as the various adhesive systems. The experimental groups with the highest average bond strength after some points of aging did not show the highest reliability of the material. Although the co-curing technique streamlined the workflow, the conventional adhesive application consistently yielded superior bond strength and reliability. Ongoing research is essential to optimize adhesive strategies, aiming to combine procedural efficiency with the highest standards of clinical performance. Also, future research could explore modifications to the co-curing method or the development of new adhesive formulations that might enhance bond strength and reliability, potentially making co-curing a more viable option in clinical settings.
Author Contributions
Conceptualization, J.V.B. and E.K.; methodology, J.V.B. and E.K.; software, J.V.B.; validation, J.V.B. and E.K.; formal analysis, J.V.B., E.K. and I.S.; investigation, J.V.B.; resources, Z.T.; data curation, J.V.B., E.K. and I.S.; writing—original draft, J.V.B.; writing—review and editing, J.V.B., E.K. and Z.T.; visualization, J.V.B.; supervision, E.K.; project administration, E.K.; funding acquisition Z.T.; validation, J.V.B. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was fully supported by the Croatian Science Foundation, project number IP-2019-04-6183.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
References
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar]
- Van Meerbeek, B.; Peumans, M.; Poitevin, A.; Mine, A.; Van Ende, A.; Neves, A.; De Munck, J. Relationship between bond-strength tests and clinical outcomes. Dent. Mater. 2010, 26, 100–121. [Google Scholar] [CrossRef]
- Soares, C.J.; Rodrigues, M.D.; Vilela, A.B.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization shrinkage stress of composite resins and resin cements—What do we need to know? Braz. Oral Res. 2017, 31, 62. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.-Y.; Kim, Y.-J.; Choi, N.-S.; Lee, I.-B. Polymerization shrinkage, modulus, and shrinkage stress related to tooth-restoration interfacial debonding in bulk-fill composites. J. Dent. 2015, 43, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Son, S.A.; Roh, H.M.; Hur, B.; Kwon, Y.H.; Park, J.K. The effect of resin thickness on polymerization characteristics of silorane-based composite resin. Restor. Dent. Endod. 2014, 39, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef]
- Al-Yousifany, N.N. Effects of flowable composite resin and curing method on microleakage. Al-Rafidain Dent. J. 2010, 10, 1–7. [Google Scholar] [CrossRef]
- Unterbrink, G.L.; Liebenberg, W.H. Flowable resin composites as “filled adhesives”: Literature review and clinical recommendations. Quintessence Int. 1999, 30, 249–257. [Google Scholar]
- Chapman, J.L.; Burgess, O.; Holst, S.; Sadan, A.; Biatz, M.B. Pre-curing of self-etching bonding agents and its effect on the bond strengths of the resin composite to the dentin and enamel. Quintessence Int. 2007, 38, 637–641. [Google Scholar]
- Viswanathan, R.; Shashibhushan, K.K.; Subba Reddy, V.V. Short communication: Pre- and co-curing effect of adhesives on shear bond strengths of composite resins to primary enamel and dentine: An in vitro study. Eur. Arch. Paediatr. Dent. 2011, 12, 308–311. [Google Scholar] [CrossRef]
- Bucuta, S.; Ilie, N. Light transmittance and micro-mechanical properties of bulk fill vs. conventional resin-based composites. Clin. Oral. Investig. 2014, 18, 1991–2000. [Google Scholar] [PubMed]
- Savadi Oskoee, S.; Bahari, M.; Jafari Navimipour, E.; Ajami, A.A.; Ghiasvand, N.; Savadi Oskoee, A. Factors affecting marginal integrity of class II bulk-fill composite resin restorations. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Farahat, F.; Daneshkazemi, A.R.; Hajiahmadi, Z. The effect of bulk depth and irradiation time on the surface hardness and degree of cure of bulk-fill composites. J. Dent. Biomater. 2016, 3, 284–291. [Google Scholar]
- Abdelaziz, K.M.; Saleh, A.A. Influence of adhesive-composite application modalities on their bonding to tooth structure and resistance of the performed restorations to failure. J. Dent. Sci. 2018, 13, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Deliperi, S.; Bardwell, D.N.; Papathanasiou, A.; Kastali, S.; García-Godoye, F. Microleakage of a microhybrid composite resin using three different adhesive placement techniques. J. Adhes. Dent. 2004, 6, 135–139. [Google Scholar]
- Pinto, M.V.; Pires, S.; Marto, C.M.; Amaro, I.; Coelho, A.; Sousa, J.; Ferreira, M.M.; Botelho, M.F.; Carrilho, E.; Abrantes, A.M.; et al. Micro-leakage study of a bulk fill over an uncured adhesive system. J. Compos. Sci. 2023, 7, 40. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef]
- Maravic, T.; Mazzoni, A.; Comba, A.; Scotti, N.; Checchi, V.; Breschi, L. How stable is dentin as a substrate for bonding? Curr. Oral. Health Rep. 2017, 4, 248–257. [Google Scholar] [CrossRef]
- Carvalho, R.M.; Manso, A.P.; Geraldeli, S.; Tay, F.R.; Pashley, D.H. Durability of bonds and clinical success of adhesive restorations. Dent. Mater. 2012, 28, 72–86. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ohno, H.; Sano, H. In vitro degradation of resin-bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials 2003, 24, 3795–3803. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.D.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Kitasako, Y.; Burrow, M.F.; Nikaido, T.; Tagami, J. The influence of storage solution on dentin bond durability of resin cement. Dent. Mater. 2000, 16, 1–6. [Google Scholar] [CrossRef]
- Kitasako, Y.; Burrow, M.F.; Katahira, N.; Nikaido, T.; Tagami, J. Shear bond strengths of three resin cements to dentine over 3 years in vitro. J. Dent. 2001, 29, 139–144. [Google Scholar] [CrossRef]
- Giannini, M.; Seixas, C.A.; Reis, A.F.; Pimenta, L.A. Six-month storage-time evaluation of one-bottle adhesive systems to dentin. J. Esthet. Restor. Dent. 2003, 15, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Ashi, T.; Eid, A.; Maguina, L.; Zghal, J.; Sekayan, N.; Bourgi, R.; Hardan, L.; Sauro, S.; Haikel, Y.; et al. Does adhesive layer thickness and tag length influence short/long-term bond strength of universal adhesive systems? An in-vitro study. Appl. Sci. 2021, 11, 2635. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Azad, E.; Atai, M.; Zandi, M.; Shokrollahi, P.; Solhi, L. Structure-properties relationships in dental adhesives: Effect of initiator, matrix monomer structure, and nano-filler incorporation. Dent. Mater. 2018, 34, 1263–1270. [Google Scholar] [CrossRef]
- Heintze, S.D.; Rousson, V.; Mahn, E. Bond strength tests of dental adhesive systems and their correlation with clinical results: A meta-analysis. Dent. Mater. 2015, 31, 423–434. [Google Scholar] [CrossRef]
- Xuewu, L.; Zhengqi, L.; Lihong, D.; Bin, L. Study of microstructure evolution and fatigue crack extension properties of 42CrMo steel strengthened by induction hardening. JMR&T 2025, 35, 3887–3901. [Google Scholar]
- Lu, C.; Danzer, R.; Fischer, F.D. Fracture statistics of brittle materials: Weibull or normal distribution. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2002, 65, 067102. [Google Scholar] [CrossRef] [PubMed]
- Bradna, P.; Vrbova, R.; Dudek, M.; Roubickova, A.; Housova, D. Comparison of bonding performance of self-etching and etch-and-rinse adhesives on human dentin using reliability analysis. J. Adhes. Dent. 2008, 10, 423–429. [Google Scholar]
- McCabe, J.F.; Carrick, T.E. A statistical approach to the mechanical testing of dental materials. Dent. Mater. 1986, 2, 139–142. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef]
- Sensi, L.G.; Marson, F.C.; Monteiro, S., Jr.; Baratieri, L.N.; Caldeira de Andrada, M.A. Flowable composites as “filled adhesives”: A microleakage study. J. Contemp. Dent. Pract. 2004, 5, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://campaigns-gceurope.com/g-premio-bond/?lang=hr (accessed on 1 March 2025).
- Available online: https://kuraraydental.com/product/clearfil-se-bond-2/ (accessed on 1 March 2025).
- Available online: https://multimedia.3m.com/mws/media/1480232O/adper-single-bond-2-ifu.pdf40 (accessed on 1 March 2025).
- Quinn, J.B.; Quinn, G.D. A practical and systematic review of Weibull statistics for reporting strengths of dental materials. Dent. Mater. 2010, 26, 135–147. [Google Scholar] [CrossRef]
- Abedin, F.; Ye, Q.; Camarda, K.; Spencer, P. Impact of light intensity on the polymerization kinetics and network structure of model hydrophobic and hydrophilic methacrylate based dental adhesive resin. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 104, 1666–1678. [Google Scholar] [CrossRef]
- Son, S.A.; Kim, J.H.; Seo, D.G.; Park, J.K. Effect of dentin surface conditions and curing mode of resin cement on the dentin bond strength. Dent. Mater. J. 2024, 43, 469–476. [Google Scholar] [CrossRef]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2019, 35, 1–22. [Google Scholar] [CrossRef]
- Breschi, L.; Cadenaro, M.; Antoniolli, F.; Sauro, S.; Biasotto, M.; Prati, C.; Tay, F.R.; Di Lenarda, R. Polymerization kinetics of dental adhesives cured with LED: Correlation between extent of conversion and permeability. Dent. Mater. 2007, 23, 1066–1072. [Google Scholar] [CrossRef]
- Cadenaro, M.; Antoniolli, F.; Sauro, S.; Tay, F.R.; Di Lenarda, R.; Prati, C.; Biasotto, M.; Contardo, L.; Breschi, L. Degree of conversion and permeability of dental adhesives. Eur. J. Oral. Sci. 2005, 113, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Breschi, L.; Rueggeberg, F.A.; Suchko, M.; Grodin, E.; Agee, K.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent. Mater. 2009, 25, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Roulet, J.F.; Heintze, S.D. Parameters influencing increase in pulp chamber temperature with light-curing devices: Curing lights and pulpal flow rates. Oper. Dent. 2010, 35, 353–361. [Google Scholar] [CrossRef]
- Leprince, J.G.; Palin, W.M.; Hadis, M.A.; Devaux, J.; Leloup, G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent. Mater. 2013, 29, 139–156. [Google Scholar] [CrossRef]
- Vukelja, J.; Klarić Sever, E.; Sever, I.; Jukić Krmek, S.; Tarle, Z. Effect of conventional adhesive application or co-curing technique on dentin bond strength. Materials 2021, 14, 7664. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yang, H.; Guo, J.; Chen, X.; Zhang, W.; Huang, C. Effects of different artificial ageing methods on the degradation of adhesive-dentine interfaces. J. Dent. 2014, 42, 1577–1585. [Google Scholar] [CrossRef]
- Hashimoto, M.; Fujita, S.; Nagano, F.; Ohno, H.; Endo, K. Ten-years degradation of resin-dentin bonds. Eur. J. Oral. Sci. 2010, 118, 404–410. [Google Scholar] [CrossRef]
- Makishi, P.; André, C.B.; Ayres, A.P.A.; Martins, A.L.; Giannini, M. Effect of storage time on bond strength and nanoleakage expression of universal adhesives bonded to dentin and etched enamel. Oper. Dent. 2016, 41, 305–317. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tay, F.R.; Ohno, H.; Sano, H.; Kaga, M.; Yiu, C.; Kumagai, H.; Kudou, Y.; Kubota, M.; Oguchi, H. SEM and TEM analysis of water degradation of human dentinal collagen. J. Biomed. Mater. Res. 2003, 66, 287–298. [Google Scholar] [CrossRef]
- Sezinando, A.; Luque-Martinez, I.; Muñoz, M.A.; Reis, A.; Loguercio, A.D.; Perdigão, J. Influence of a hydrophobic resin coating on the immediate and 6-month dentin bonding of three universal adhesives. Dent. Mater. 2015, 31, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.; Swift, E.J., Jr.; Nagaoka, H.; Chung, Y.; Bartholomew, W.; Braswell, K.M.; Pereira, P.N. Two-year bond strengths of “all-in-one” adhesives to dentine. J. Dent. 2012, 40, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; Van Ende, A.; Van Meerbeek, B.; De Munck, J. Bonding effectiveness of a new “multi-mode” adhesive to enamel and dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.F.M.A.; Saikaew, P.; Alam, A.; Sun, J.; Carvalho, R.M.; Sano, H. Effects of double application of contemporary self-etch adhesives on their bonding performance to dentin with clinically relevant smear layers. J. Adhes. Dent. 2019, 21, 59–66. [Google Scholar]
- Sato, K.; Hosaka, K.; Takahashi, M.; Ikeda, M.; Tian, F.; Komada, W.; Nakajima, M.; Foxton, R.; Nishitani, Y.; Pashley, D.H.; et al. Dentin bonding durability of two-step self-etch adhesives with improved degree of conversion of adhesive resins. J. Adhes. Dent. 2017, 19, 31–37. [Google Scholar]
- Sato, K.; Hosaka, K.; Takahashi, M.; Ikeda, M.; Tian, F.; Komada, W.; Nakajima, M.; Foxton, R.; Nishitani, Y.; Pashley, D.H.; et al. The effect of dentine surface preparation and reduced application time of adhesive on bonding strength. J. Dent. 2016, 47, 63–70. [Google Scholar]
- Cardoso, M.V.; de Almeida Neves, A.; Mine, A.; Coutinho, E.; Van Landuyt, K.; De Munck, J.; Van Meerbeek, B. Current aspects on bonding effectiveness and stability in adhesive dentistry. Aust. Dent. J. 2011, 56, 31–44. [Google Scholar] [CrossRef]
- Lenzi, T.L.; Soares, F.Z.M.; de Oliveira Rocha, R. Does bonding approach influence the bond strength of universal adhesive to dentin of primary teeth? J. Clin. Pediatr. Dent. 2017, 41, 214–218. [Google Scholar] [CrossRef]
- Chen, C.; Niu, L.N.; Xie, H.; Zhang, Z.Y.; Zhou, L.Q.; Jiao, K.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Bonding of universal adhesives to dentine—Old wine in new bottles? J. Dent. 2015, 43, 525–536. [Google Scholar] [CrossRef]
- Silva e Souza, M.H., Jr.; Carneiro, K.G.; Lobato, M.F.; Silva e Souza, P.D.; Góes, M.F. Adhesive systems: Important aspects related to their composition and clinical use. J. Appl. Oral. Sci. 2010, 18, 207–214. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Li, X.; Ma, C.; Shi, T.; Yang, H.; Zhang, C.; Qi, W.; Li, C.; Liu, R.; He, W.; Liu, Y. Waterborne robust superhydrophobic PFDTES@TiO2-PU coating with stable corrosion resistance, long-term environmental adaptability, and delayed icing functions on Al–Li alloy. JMR&T. 2024, 32, 3357–3370. [Google Scholar]
- Pimentel de Oliveira, R.; de Paula, B.L.; Ribeiro, M.E.; Alves, E.; Costi, H.T.; Silva, C. Evaluation of the bond strength of self-etching adhesive systems containing HEMA and 10-MDP monomers. Int. J. Dent. 2022, 2022, 5756649. [Google Scholar] [CrossRef]
- Sadek, F.T.; Goracci, C.; Cardoso, P.E.C.; Tay, F.R.; Ferrari, M. Microtensile bond strength of current dentin adhesives measured immediately and 24 hours after application. J. Adhes. Dent. 2005, 7, 297–302. [Google Scholar]
- Van Landuyt, K.L.; Mine, A.; De Munck, J.; Jaecques, S.; Peumans, M.; Lambrechts, P.; Van Meerbeek, B. Are one-step adhesives easier to use and better performing? Multifactorial assessment of contemporary one-step self-etching adhesives. J. Adhes. Dent. 2009, 11, 175–190. [Google Scholar] [PubMed]
- De Munck, J.; Mine, A.; Poitevin, A.; Van Ende, A.; Cardoso, M.V.; Van Landuyt, K.L.; Peumans, M.; Van Meerbeek, B. Meta-analytical review of parameters involved in dentin bonding. J. Dent. Res. 2012, 91, 351–357. [Google Scholar] [CrossRef]
- Heintze, S.D.; Ruffieux, C.; Rousson, V. Clinical performance of cervical restorations—A meta-analysis. Dent. Mater. 2010, 26, 993–1000. [Google Scholar] [CrossRef]
- Peumans, M.; Kanumilli, P.; De Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent. Mater. 2005, 21, 864–881. [Google Scholar] [CrossRef]
- Leloup, G.; D’Hoore, W.; Bouter, D.; Degrange, M.; Vreven, J. Meta-analytical review of factors involved in dentin adherence. J. Dent. Res. 2001, 80, 1605–1614. [Google Scholar] [CrossRef]
- Perdigao, J. Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent. Mater. 2010, 26, 24–37. [Google Scholar] [CrossRef]
- Singh, K.; Naik, R.; Hegde, S.; Damda, A. Shear bond strength of superficial, intermediate and deep dentin in vitro with recent generation self-etching primers and single nano composite resin. J. Int. Oral. Health 2015, 7, 28–32. [Google Scholar] [PubMed]
- Triolo, P.T.; Swift, E.J., Jr. Shear bond strengths of ten dentin adhesive systems. Dent. Mater. 1992, 8, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Dentin bond strength of experimental composites containing bioactive glass: Changes during aging for up to 1 year. J. Adhes. Dent. 2018, 20, 325–334. [Google Scholar] [PubMed]
- Fares, M.H. Shear Bond Strength of resin composite materials to dentin: The effect of the direction of application of the adhesive system: A comparison between maxillary and mandibular teeth. Dent. J. Adv. 2023, 5, 52–58. [Google Scholar] [CrossRef]
- Hobson, R.S.; McCabe, J.F.; Hogg, S.D. Bond strength to surface enamel for different tooth types. Dent. Mater. 2001, 17, 184–189. [Google Scholar] [CrossRef]
- Oztürk, B.; Malkoç, S.; Koyutürk, A.E.; Catalbas, B.; Ozer, F. Influence of different tooth types on the bond strength of two orthodontic adhesive systems. Eur. J. Orthod. 2008, 30, 407–412. [Google Scholar] [CrossRef]
- Mohammadamin, E.; Qiang, Y.; Anil, M.; Tamerler, C.; Spencer, P. Autonomous-strengthening adhesive provides hydrolysis-resistance and enhanced mechanical properties in wet conditions. Molecules 2022, 27, 5505. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.; Breschi, L.; Mazzoni, A.; Tersariol, I.; Geraldeli, S. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. 2012, 29, 116–135. [Google Scholar] [CrossRef]
- Sarikaya, R.; Song, L.; Ye, Q.; Misr, A.; Tamerler, C.; Spencer, P. Evolution of network structure and mechanical properties in autonomous-strengthening dental adhesive. Polymers 2020, 12, 2076. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).