Thermodynamic Analysis on Complex Oxides Formed by Aerodynamic Heating for Ultrahigh-Temperature Ceramic Matrix Composites
Abstract
1. Introduction
2. Experimental Procedure
2.1. Material
2.2. Arc-Wind Tunnel Testing
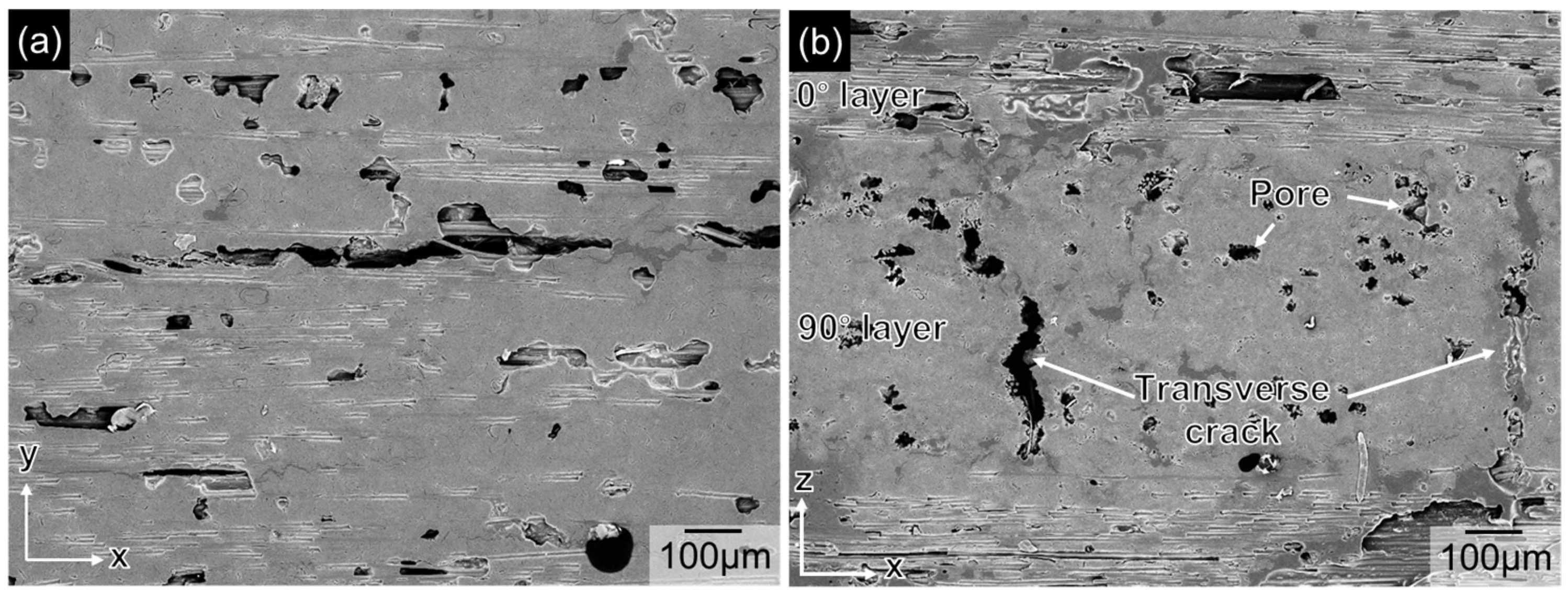
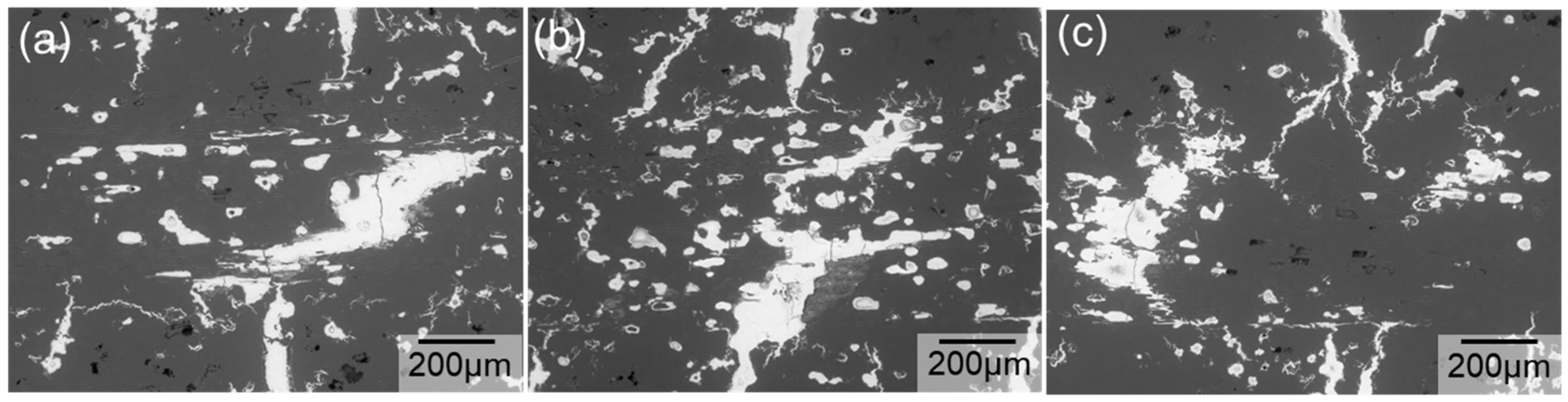
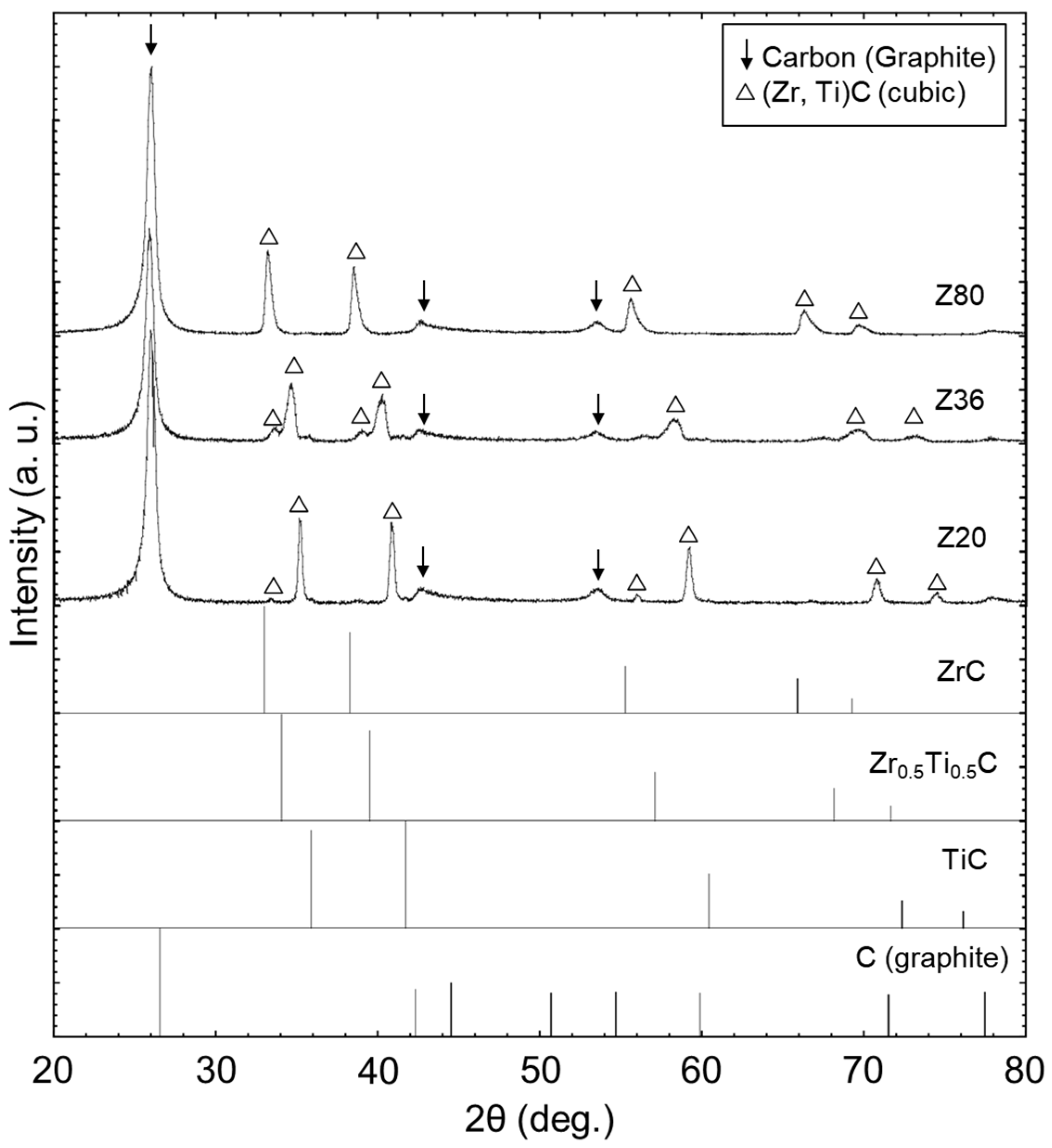
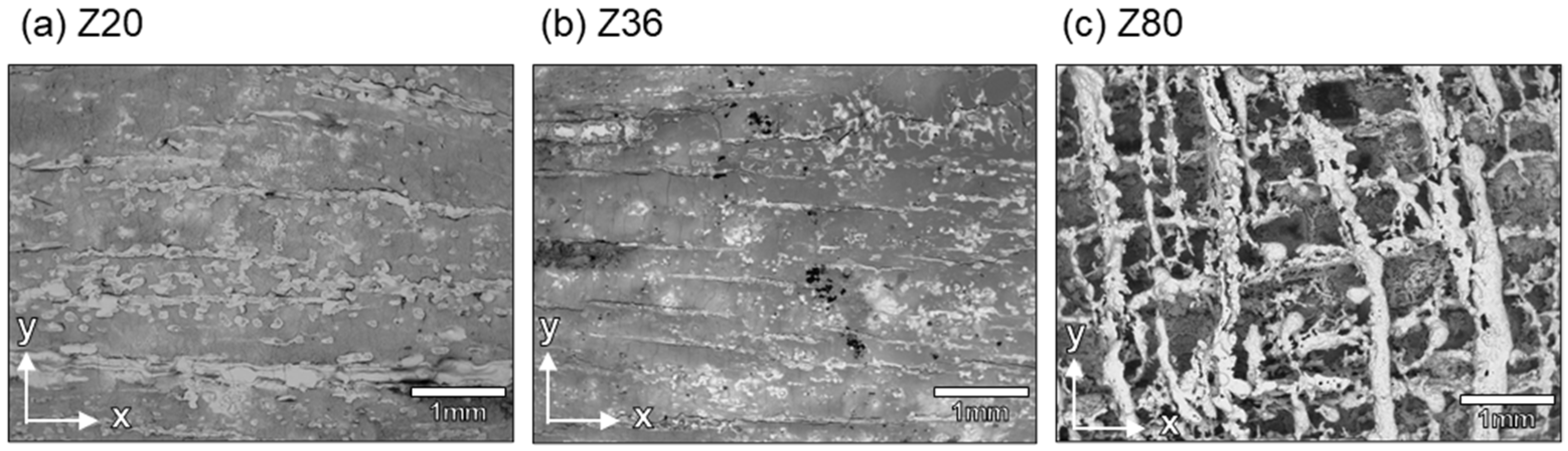
3. Results
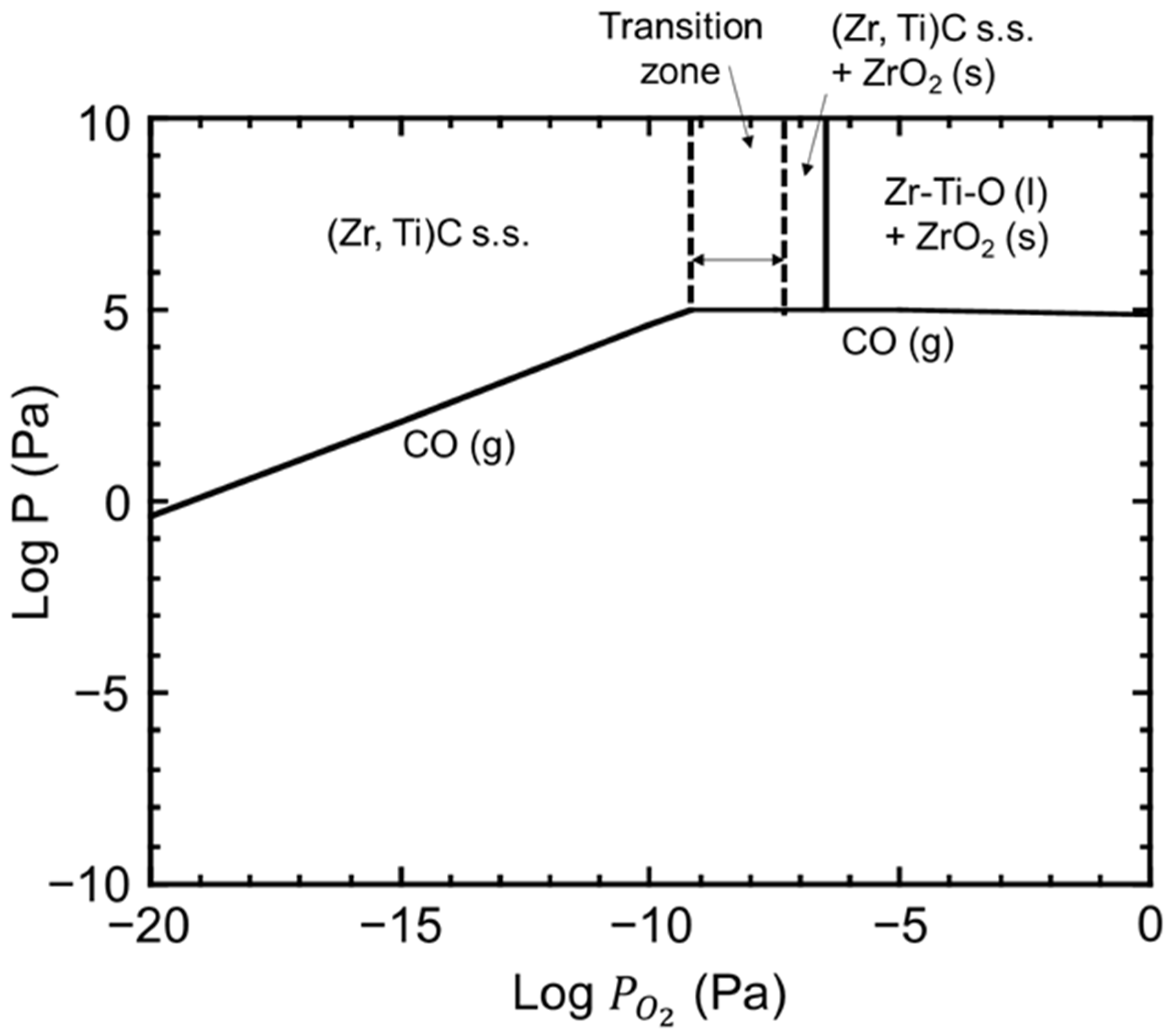
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UHTCs | Ultrahigh-temperature ceramics |
| UHTCMCs | Ultrahigh-temperature ceramic matrix composites |
| C/UHTCMCs | Carbon-fiber-reinforced UHTCMCs |
| RMI | Reactive melt infiltration |
| C/C | Carbon-fiber-reinforced carbon composites |
| ISAS | Institute of Space and Astronautical Science |
| JAXA | Japan Aerospace Exploration Agency |
| SEM | Scanning electron microscope |
| EDS | Energy-dispersive spectroscopy |
| XRD | X-ray diffraction |
| s.s. | Solid solution |
References
- Levine, S.R.; Opila, E.J.; Halbig, M.C.; Kiser, J.D.; Singh, M.; Salem, J.A. Evaluation of Ultra-High Temperature Ceramics for Aeropropulsion Use. J. Eur. Ceram. Soc. 2002, 22, 2757–2767. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.X.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; et al. Advances in Ultra-High Temperature Ceramics, Composites, and Coatings. J. Adv. Ceram. 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Sziroczak, D.; Smith, H. A Review of Design Issues Specific to Hypersonic Flight Vehicles. Prog. Aerosp. Sci. 2016, 84, 1–28. [Google Scholar] [CrossRef]
- Wuest, W. Review of German Work on Controlled Re-Entry Technology. Prog. Aerosp. Sci. 1983, 20, 217–318. [Google Scholar] [CrossRef]
- Talmy, I.G.; Zaykoski, J.A.; Opeka, M.M. High-Temperature Chemistry and Oxidation of ZrB2 Ceramics Containing SiC, Si3N4, Ta5Si3, and TaSi2. J. Am. Ceram. Soc. 2008, 91, 2250–2257. [Google Scholar] [CrossRef]
- Savino, R.; De Stefano Fumo, M.; Paterna, D.; Serpico, M. Aerothermodynamic Study of UHTC-Based Thermal Protection Systems. Aerosp. Sci. Technol. 2005, 9, 151–160. [Google Scholar] [CrossRef]
- Wuchina, E.; Opila, E.; Opeka, M.; Fahrenholtz, W.; Talmy, I. UHTCs: Ultra-High Temperature Ceramic Materials for Extreme Environment Applications. Electrochem. Soc. Interface 2007, 16, 30–36. [Google Scholar]
- Zoli, L.; Vinci, A.; Galizia, P.; Gutièrrez-Gonzalez, C.F.; Rivera, S.; Sciti, D. Is Spark Plasma Sintering Suitable for the Densification of Continuous Carbon Fibre—UHTCMCs? J. Eur. Ceram. Soc. 2020, 40, 2597–2603. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Cinibulk, M.K.; Opeka, M. Modeling and Evaluating the Environmental Degradation of UHTCs under Hypersonic Flow. In Ultra-High Temperature Ceramics: Materials for Extreme Environment Applications; Wiley: Hoboken, NJ, USA, 2014; pp. 267–290. ISBN 9781118700. [Google Scholar] [CrossRef]
- Borrelli, R.; Riccio, A.; Tescione, D.; Gardi, R.; Marino, G. Thermo-Structural Behaviour of an UHTC Made Nose Cap of a Reentry Vehicle. Acta Astronaut. 2009, 65, 442–456. [Google Scholar] [CrossRef]
- Pulci, G.; Tului, M.; Tirillò, J.; Marra, F.; Lionetti, S.; Valente, T. High Temperature Mechanical Behavior of UHTC Coatings for Thermal Protection of Re-Entry Vehicles. J. Therm. Spray Technol. 2011, 20, 139–144. [Google Scholar] [CrossRef]
- Inoue, R.; Arai, Y.; Kubota, Y.; Kogo, Y.; Goto, K. Oxidation of ZrB₂ and Its Composites: A Review. J. Mater. Sci. 2018, 53, 14885–14906. [Google Scholar] [CrossRef]
- Opila, E.; Levine, S.; Lorincz, J. Oxidation of ZrB2- and HfB2-Based Ultra-High Temperature Ceramics: Effect of Ta Additions. J. Mater. Sci. 2004, 39, 5969–5977. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G. The ZrB2 Volatility Diagram. J. Am. Ceram. Soc. 2005, 88, 3509–3512. [Google Scholar] [CrossRef]
- Peng, F.; Speyer, R.F. Oxidation Resistance of Fully Dense ZrB2 with SiC, TaB2, and TaSi2 Additives. J. Am. Ceram. Soc. 2008, 91, 1489–1494. [Google Scholar] [CrossRef]
- Silvestroni, L.; Meriggi, G.; Sciti, D. Oxidation Behavior of ZrB2 Composites Doped with Various Transition Metal Silicides. Corros. Sci. 2014, 83, 281–291. [Google Scholar] [CrossRef]
- Karlsdottir, S.N.; Halloran, J.W.; Henderson, C.E. Convection Patterns in Liquid Oxide Films on ZrB2-SiC Composites Oxidized at a High Temperature. J. Am. Ceram. Soc. 2007, 90, 2863–2867. [Google Scholar] [CrossRef]
- Shugart, K.; Liu, S.; Craven, F.; Opila, E. Determination of Retained B2O3 Content in ZrB2-30 Vol% SiC Oxide Scales. J. Am. Ceram. Soc. 2015, 98, 287–295. [Google Scholar] [CrossRef]
- Zhang, S.C.; Fahrenholtz, W.G.; Hilmas, G.E. Oxidation of ZrB2 and ZrB2-SiC Ceramics with Tungsten Additions. ECS Trans. 2009, 16, 137–145. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Myers, D.L. Active Oxidation of SiC. Oxid. Met. 2011, 75, 1–25. [Google Scholar] [CrossRef]
- Jacobson, N.; Harder, B.; Myers, D. Oxidation Transitions for SiC Part I. Active-to-Passive Transitions. J. Am. Ceram. Soc. 2013, 96, 838–844. [Google Scholar] [CrossRef]
- Harder, B.; Jacobson, N.; Myers, D. Oxidation Transitions for SiC Part II. Passive-to-Active Transitions. J. Am. Ceram. Soc. 2012, 96, 606–612. [Google Scholar] [CrossRef]
- Smialek, J.L.; Robinson, R.C.; Opila, E.J.; Fox, D.S.; Jacobson, N.S. SiC and Si3N4 Recession Due to SiO2 Scale Volatility under Combustor Conditions. Adv. Compos. Mater. 1999, 8, 33–45. [Google Scholar] [CrossRef]
- Han, J.; Hu, P.; Zhang, X.; Meng, S.; Han, W. Oxidation-Resistant ZrB2-SiC Composites at 2200 °C. Compos. Sci. Technol. 2008, 68, 799–806. [Google Scholar] [CrossRef]
- Vaughn, W.L.; Maahs, H.G. Active-to-Passive Transition in the Oxidation of Silicon Carbide and Silicon Nitride in Air. J. Am. Ceram. Soc. 1990, 73, 1540–1543. [Google Scholar] [CrossRef]
- Arai, Y.; Inoue, R.; Goto, K.; Kogo, Y. Carbon Fiber Reinforced Ultra-High Temperature Ceramic Matrix Composites: A Review. Ceram. Int. 2019, 45, 14481–14489. [Google Scholar] [CrossRef]
- Makurunje, P.; Middleburgh, S.C.; Lee, W.E. Addressing High Processing Temperatures in Reactive Melt Infiltration for Multiphase Ceramic Composites. J. Eur. Ceram. Soc. 2023, 43, 183–197. [Google Scholar]
- Sciti, D.; Silvestroni, L.; Monteverde, F.; Vinci, A.; Zoli, L. Introduction to H2020 Project C3HARME–next Generation Ceramic Composites for Combustion Harsh Environment and Space. Adv. Appl. Ceram. 2018, 117, s70–s75. [Google Scholar] [CrossRef]
- Binner, J.; Porter, M.; Baker, B.; Zou, J.; Venkatachalam, V.; Diaz, V.R.; D’Angio, A.; Ramanujam, P.; Zhang, T.; Murthy, T.S.R.C. Selection, Processing, Properties and Applications of Ultra-High Temperature Ceramic Matrix Composites, UHTCMCs—A Review. Int. Mater. Rev. 2020, 65, 389–444. [Google Scholar] [CrossRef]
- Tang, S.; Deng, J.; Wang, S.; Liu, W. Comparison of Thermal and Ablation Behaviors of C/SiC Composites and C/ZrB2-SiC Composites. Corros. Sci. 2009, 51, 54–61. [Google Scholar] [CrossRef]
- Vinci, A.; Zoli, L.; Sciti, D.; Melandri, C.; Guicciardi, S. Understanding the Mechanical Properties of Novel UHTCMCs through Random Forest and Regression Tree Analysis. J. Mater. Des. 2018, 145, 97–107. [Google Scholar] [CrossRef]
- Servadei, F.; Zoli, L.; Galizia, P.; Vinci, A.; Sciti, D. Development of UHTCMCs via Water Based ZrB₂ Powder Slurry Infiltration and Polymer Infiltration and Pyrolysis. J. Eur. Ceram. Soc. 2020, 40, 5076–5084. [Google Scholar] [CrossRef]
- Inoue, R.; Yang, J.M.; Kakisawa, H.; Kagawa, Y. Mixed-Mode Fracture Criterion of Short Carbon Fiber- Dispersed SiC Matrix Composite. J. Ceram. Sci. Technol. 2017, 8, 223–232. [Google Scholar] [CrossRef]
- Inoue, R.; Yang, J.M.; Kakisawa, H.; Kagawa, Y. Mode I Fracture Toughness of Short Carbon Fiber-Dispersed SiC Matrix Composite Fabricated by Melt Infiltration Process. Ceram. Int. 2013, 39, 8341–8346. [Google Scholar] [CrossRef]
- Schulte-Fischedick, J.; Zern, A.; Mayer, J.; Rü Hle, M.; Frieß, M.; Krenkel, W.; Kochendö Rfer, R. The Morphology of Silicon Carbide in C/C-SiC Composites. Mater. Sci. Eng. 2002, A332, 146–152. [Google Scholar]
- Inoue, R.; Arai, Y.; Kubota, Y.; Goto, K.; Kogo, Y. Oxidation Behavior of Carbon Fiber-Dispersed ZrB₂-SiC-ZrC Triple Phase Matrix Composites in an Oxyhydrogen Torch Environment. Ceram. Int. 2018, 44, 8387–8396. [Google Scholar] [CrossRef]
- Inoue, R.; Arai, Y.; Kubota, Y.; Goto, K.; Kogo, Y. Development of Short- and Continuous Carbon Fiber-Reinforced ZrB₂-SiC-ZrC Matrix Composites for Thermal Protection Systems. Ceram. Int. 2018, 44, 15859–15867. [Google Scholar] [CrossRef]
- Schneider, J.M. How High Is the Entropy in High Entropy Ceramics? J. Appl. Phys. 2021, 130, 150903. [Google Scholar]
- Filipovic, S.; Obradovic, N.; Hilmas, G.E.; Fahrenholtz, W.G.; Brenner, D.W.; Maria, J.P.; Wolfe, D.E.; Zurek, E.; Campilongo, X.; Curtarolo, S. A Super-Hard High Entropy Boride Containing Hf, Mo, Ti, V, and W. J. Am. Ceram. Soc. 2024, 107, 4430–4435. [Google Scholar] [CrossRef]
- Arai, Y.; Saito, M.; Samizo, A.; Inoue, R.; Nishio, K.; Kogo, Y. Material Design Using Calculation Phase Diagram for Refractory High-Entropy Ceramic Matrix Composites. Int. J. Appl. Ceram. Technol. 2024, 21, 2702–2711. [Google Scholar] [CrossRef]
- Peters, A.B.; Zhang, D.; Chen, S.; Ott, C.; Oses, C.; Curtarolo, S.; McCue, I.; Pollock, T.; Prameela, S.E. Materials Design for Hypersonics. Nat. Commun. 2023, 15, 3328. [Google Scholar] [CrossRef]
- Divilov, S.; Eckert, H.; Hicks, D.; Oses, C.; Toher, C.; Friedrich, R.; Esters, M.; Mehl, M.J.; Zettel, A.C.; Lederer, Y.; et al. Disordered Enthalpy–Entropy Descriptor for High-Entropy Ceramics Discovery. Nature 2024, 625, 66–73. [Google Scholar] [CrossRef]
- Arai, Y.; Saito, M.; Samizo, A.; Inoue, R.; Nishio, K.; Kogo, Y. Hot-Corrosion of Refractory High-Entropy Ceramic Matrix Composites Synthesized by Alloy Melt-Infiltration. Ceram. Int. 2021, 47, 31740–31748. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-Entropy Ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Ye, B.; Wen, T.; Chu, Y. High-temperature Oxidation Behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C High-Entropy Ceramics in Air. J. Am. Ceram. Soc. 2020, 103, 500–507. [Google Scholar] [CrossRef]
- Zou, L.; Wali, N.; Yang, J.-M.; Bansal, N.P. Microstructural Development of a Cf/ZrC Composite Manufactured by Reactive Melt Infiltration. J. Eur. Ceram. Soc. 2010, 30, 1527–1535. [Google Scholar] [CrossRef]
- Zou, L.; Wali, N.; Yang, J.M.; Bansal, N.P.; Yan, D. Microstructural Characterization of a Cf/ZrC Composite Manufactured by Reactive Melt Infiltration. Int. J. Appl. Ceram. Technol. 2011, 8, 329–341. [Google Scholar] [CrossRef]
- Okamoto, H. The Si-Zr (Silicon-Zirconium) System. Bull. Alloy Phase Diagr. 1990, 11, 513–519. [Google Scholar] [CrossRef]
- Koide, N.; Marumo, T.; Arai, Y.; Hasegawa, M.; Nishimura, T.; Inoue, R. Degradation of Carbon Fiber-Reinforced Ultra-High-Temperature Ceramic Matrix Composites at Extremely High Temperature Using Arc-Wind Tunnel Tests. J. Mater. Sci. 2022, 57, 19785–19798. [Google Scholar] [CrossRef]
- Arai, Y.; Marumo, T.; Inoue, R. Use of Zr–Ti Alloy Melt Infiltration for Fabricating Carbon-Fiber-Reinforced Ultrahigh-Temperature Ceramic Matrix Composites. J. Compos. Sci. 2021, 5, 186. [Google Scholar] [CrossRef]
- Marumo, T.; Koide, N.; Arai, Y.; Nishimura, T.; Hasegawa, M.; Inoue, R. Characterization of Carbon Fiber-Reinforced Ultra-High Temperature Ceramic Matrix Composites Fabricated via Zr-Ti Alloy Melt Infiltration. J. Eur. Ceram. Soc. 2022, 42, 5208–5219. [Google Scholar] [CrossRef]
- Neuer, G. Spectral and Total Emissivity Measurements of Highly Emitting Materials. Int. J. Thermophys. 1995, 16, 257–265. [Google Scholar] [CrossRef]
- Warlimont, H. Ceramics. In Handbook of Materials Data; Warlimont, H., Martienssen, W., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 448–453. [Google Scholar]
- Duan, L.; Zhao, X.; Wang, Y. Comparative Ablation Behaviors of C/SiC-HfC Composites Prepared by Reactive Melt Infiltration and Precursor Infiltration and Pyrolysis Routes. Ceram. Int. 2017, 43, 16114–16120. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, K.; Cao, Z.; Zhu, H. Thermodynamic Study of Titanium Oxycarbide. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 3510–3514. [Google Scholar] [CrossRef]
- Gasparrini, C.; Chater, R.J.; Horlait, D.; Vandeperre, L.; Lee, W.E. Zirconium Carbide Oxidation: Kinetics and Oxygen Diffusion through the Intermediate Layer. J. Am. Ceram. Soc. 2018, 101, 2638–2652. [Google Scholar] [CrossRef]
- Kucheryavaya, A.; Lenčéš, Z.; Šajgalík, P.; Harmuth, H. Zirconium Oxycarbides and Oxycarbonitrides: A Review. Int. J. Appl. Ceram. Technol. 2023, 20, 541–562. [Google Scholar] [CrossRef]
- Réjasse, F.; Rapaud, O.; Trolliard, G.; Masson, O.; Maître, A. Experimental Investigation and Thermodynamic Evaluation of the C-O-Zr Ternary System. RSC Adv. 2016, 6, 100122–100135. [Google Scholar] [CrossRef]
- Rao, G.A.R.; Venugopal, V. Kinetics and Mechanism of the Oxidation of ZrC. J. Alloys Compd. 1994, 206, 237–242. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuganezawa, M.; Arai, Y.; Inoue, R. Thermodynamic Analysis on Complex Oxides Formed by Aerodynamic Heating for Ultrahigh-Temperature Ceramic Matrix Composites. J. Compos. Sci. 2025, 9, 87. https://doi.org/10.3390/jcs9020087
Tsuganezawa M, Arai Y, Inoue R. Thermodynamic Analysis on Complex Oxides Formed by Aerodynamic Heating for Ultrahigh-Temperature Ceramic Matrix Composites. Journal of Composites Science. 2025; 9(2):87. https://doi.org/10.3390/jcs9020087
Chicago/Turabian StyleTsuganezawa, Mizuki, Yutaro Arai, and Ryo Inoue. 2025. "Thermodynamic Analysis on Complex Oxides Formed by Aerodynamic Heating for Ultrahigh-Temperature Ceramic Matrix Composites" Journal of Composites Science 9, no. 2: 87. https://doi.org/10.3390/jcs9020087
APA StyleTsuganezawa, M., Arai, Y., & Inoue, R. (2025). Thermodynamic Analysis on Complex Oxides Formed by Aerodynamic Heating for Ultrahigh-Temperature Ceramic Matrix Composites. Journal of Composites Science, 9(2), 87. https://doi.org/10.3390/jcs9020087





