Physicochemical Studies of Opoka as a Raw Material Component of Sodium Silicate Mixture for Subsequent Synthesis of Foam Glass Material Based on It
Abstract
1. Introduction
- -
- mineral wool products (65%);
- -
- glass wool materials (9%);
- -
- polystyrene foam and other foam plastics (21%).
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- -
- all the conducted physical and chemical studies of the studied opoka contribute to the complementarity of each other, and in most cases, they coincide and confirm the obtained results by correlating;
- -
- using chemical analysis, the interval of the content of basic silicon oxide (SiO2) in the studied opoka was established, which is presented in the range of 69–80%, contributing to the use of opoka as a raw material for the production of foam glass material;
- -
- based on the X-ray phase analysis, the phase composition of the studied opoka was established with pronounced quartz peaks, the main diagnostic lines of which are the values of 3.3485, 4.2704, and 4.1329 A;
- -
- as a result of X-ray energy-dispersive elemental microanalysis of the opoka, its elemental composition was established, which is represented by the sum of O2, Si, and Al by 91.98%, Fe by 2.27%, and various impurities by 5.75%;
- -
- the experimentally obtained sample of foam glass material consists of 93.37% Si, Al, Mg, and Na oxides, has a porous structure with a pore size of 2–5 microns, an average density of 375 kg/m3, thermal conductivity of 0.063 W/(m °C) at 25 °C, and noise absorption of −51.6 Db, and, according to GOST 33949-2016, foam glass products are heat-insulating for buildings and structures;
- -
- the results obtained from the conducted complex of modern physical and chemical studies of opoka determine the role of opoka as the main raw material for obtaining a sodium silicate mixture and further synthesis of foam glass material based on this mixture with the possibility of eliminating the energy-intensive and labor-intensive process of high-temperature cooking and granulation of complex multicomponent glass mass and obtaining in the future an ideal inexpensive local building material used for heat and sound insulation of housing construction, increasing the level and quality of life of the population.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latyntseva, E.A.; Podoinikova, Y.R.; Bezrukova, T.A.; Murtazina, A.A. The influence of raw materials on the properties of foam glass and the development prospects. Constr. Mater. Prod. 2020, 3, 44–48. [Google Scholar]
- Zhimalov, A.A.; Nikishonkova, O.A.; Spiridonov, Y.A.; Kosobudskii, I.D. Physical-chemical studies of gaizes as alternative raw materials for the production of foam glass and foam materials. Glass Ceram. 2019, 75, 387–390. [Google Scholar] [CrossRef]
- Gol’tsman, B.M.; Yatsenko, E.A.; Komunzhieva, N.Y.; Gerashchenko, V.S. Synthesis of foam glass based on natural silica raw material. Tekh. Tekhnol. Silik. 2019, 26, 102–105. [Google Scholar]
- Storozhenko, G.I.; Kazantseva, L.K. Granulated foam-glass crystal materials based on silica rocks of the Southern Urals. Stroit. Mater. 2020, 1–2, 78–81. [Google Scholar] [CrossRef]
- Ardakani, A.; Yazdani, M. The relation between particle density and static elastic moduli of lightweight expanded clay aggregates. Appl. Clay Sci. 2014, 93–94, 28–34. [Google Scholar] [CrossRef]
- Han, L.; Li, F.; Zhang, H.; Dong, L.; Pei, Y.; Zhu, Q.; Wu, W.; Jia, Q.; Zhang, S. Low-temperature preparation of porous diatomite ceramics via direct gel-casting using melamine and boric acid as cross-linker and sintering agent. Ceram. Int. 2019, 45, 24469–24473. [Google Scholar] [CrossRef]
- Suvorova, O.V.; Manakova, N.K.; Makarov, D.V. Use of bulk industrial wastes in the production of glass foam materials. Glass Ceram. 2021, 7, 384–389. [Google Scholar] [CrossRef]
- Bobrov, Y.L.; Ovcharenko, E.G.; Shoikhet, B.M.; Petukhova, E.Y. Thermal Insulation Materials and Structures; Infra-M: Moscow, Russia, 2003; 268p. [Google Scholar]
- Anufriev, D.P. New Building Materials and Products. Regional Peculiarities of Production; DIA: Moscow, Russia, 2014. [Google Scholar]
- Available online: https://izron.ru/articles/voprosy-sovremennykh-tekhnicheskikh-nauk-svezhiy-vzglyad-i-novye-resheniya-sbornik-nauchnykh-trudov-/sektsiya-10-stroitelstvo-i-arkhitektura-spetsialnost-05-23-00/evropeyskiy-opyt-utepleniya-naruzhnykh-sten-zdaniy-dlya-povysheniya-energoefektivnosti-zhilogo-fonda/ (accessed on 4 September 2024).
- Available online: https://stat.gov.kz/ru/industries/business-statistics/stat-inno-build/publications/14121/ (accessed on 5 September 2024).
- Murtazaev, S.-A.Y.; Salamanova, M.S. High-strength concrete with graded aggregates from the waste recycling rocks. Sustain. Dev. Mt. Territ. 2015, 1, 23–28. [Google Scholar]
- Report on the Cellular Concrete Market by 2030. Available online: https://exactitudeconsultancy.com/ru/ (accessed on 6 September 2024).
- Chen, Z.; Wang, H.; Wang, M.; Wu, W.; Liu, L.; Wang, X. Simulation and experimental investigation on one-step process for recovery of valuable metals and preparation of clean mineral wool from red mud. J. Clean. Prod. 2022, 380, 134982. [Google Scholar] [CrossRef]
- Yessenbayev, B.A.; Kolesnikov, A.S.; Naukenova, A.S.; Shapalov, S.K.; Ramatullaeva, L.I.; Ivakhniyuk, G.K. Analysis of the impact of bauxite dumps on the environment and public health. MIAB Min. Inf. Anal. Bull. 2024, 3, 55–69. [Google Scholar]
- Zhakipbaev, B.E.; Spiridonov, Y.A.; Sigaev, V.N. Use of rocks to obtain foam glass. Glass Ceram. 2013, 70, 155–157. [Google Scholar] [CrossRef]
- Kulikova, E.Y.; Balovtsev, S.V.; Skopintseva, O.V. Complex estimation of geotechnical risks in mine and underground construction. Sustain. Dev. Mt. Territ. 2023, 15, 7–16. [Google Scholar] [CrossRef]
- Donayev, A.; Kolesnikov, A.; Shapalov, S.; Sapargaliyeva, B.; Ivakhniyuk, G. Studies of waste from the mining and metallurgical industry, with the determination of its impact on the life of the population. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Geol. Tech. Sci. 2022, 4, 55–68. [Google Scholar] [CrossRef]
- Bedov, A.I.; Gabitov, A.I.; Domarova, E.V.; Kolesnikov, A.S. Investigation of the stress-strain state of domical masonry vaults. Constr. Mater. Prod. 2023, 6, 6. [Google Scholar] [CrossRef]
- Zhangabay, N.; Sapargaliyeva, B.; Suleimenov, U.; Abshenov, K.; Utelbayeva, A.; Kolesnikov, A.; Baibolov, K.; Fediuk, R.; Arinova, D.; Duissenbekov, B.; et al. Analysis of Stress-Strain State for a Cylindrical Tank Wall Defected Zone. Materials 2022, 15, 5732. [Google Scholar] [CrossRef]
- Taghizadeh, S.; Niknejad, A.; Maccioni, L.; Concli, F. Investigating the Mechanical Behavior and Energy Absorption Characteristics of Empty and Foam-Filled Glass/Epoxy Composite Sections under Lateral Indentation. Materials 2024, 17, 3847. [Google Scholar] [CrossRef]
- Gerasimova, E.B.; Melnikova, L.A.; Loseva, A.V. Ecological safety of construction in single-industry town. Constr. Mater. Prod. 2023, 6, 59–78. [Google Scholar]
- Zhangabay, N.; Giyasov, A.; Ybray, S.; Tursunkululy, T.; Kolesnikov, A. Field thermovision study of externsl enclosure for multi-storey residential building under climatic conditions of Northern Kazakhstan. Constr. Mater. Prod. 2024, 7, 1–21. [Google Scholar] [CrossRef]
- Kulikova, E.Y.; Konyukhov, D.S. Accident risk monitoring in underground space development. MIAB Min. Inf. Anal. Bull. 2022, 1, 97–103. [Google Scholar] [CrossRef]
- Filin, A.E.; Kurnosov, I.Y.; Kolesnikova, L.A.; Ovchinnikova, T.I.; Kolesnikov, A.S. Description of The Methodology for Conducting an Experiment on Dust Deposition of Mining and Metallurgical Production. Ugol 2022, 9, 67–72. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Z.; Wang, H. Atomic-Scale Insights into the Effects of the Foaming Degree on the Glass–Ceramic Matrix Derived from Waste Glass and Incineration Bottom Ash. Materials 2024, 17, 2820. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.d.S.; Fernandes, T.F.d.S.; Rossignolo, J.A. Production of Glass Foam in a Microwave Oven Using Agro-Industrial Waste as Raw Material. Buildings 2024, 14, 1643. [Google Scholar] [CrossRef]
- Volokitina, I.; Sapargaliyeva, B.; Agabekova, A.; Syrlybekkyzy, S.; Volokitin, A.; Nurshakhanova, L.; Nurbaeva, F.; Kolesnikov, A.; Sabyrbayeva, G.; Izbassar, A.; et al. Increasing Strength and Performance Properties of Bimetallic Rods during Severe Plastic Deformation. Case Stud. Constr. Mater. 2023, 19, e02256. [Google Scholar] [CrossRef]
- Lyu, X.; Seo, Y.; Han, D.H.; Cho, S.; Kondo, Y.; Goto, T.; Sekino, T. Porous Lithium Disilicate Glass–Ceramics Prepared by Cold Sintering Process Associated with Post-Annealing Technique. Materials 2024, 17, 381. [Google Scholar] [CrossRef]
- Tihomirovs, P.; Kara De Maeijer, P.; Korjakins, A. Demolition Waste Glass Usage in the Construction Industry. Infrastructures 2023, 8, 182. [Google Scholar] [CrossRef]
- Smiljanić, S.; Hribar, U.; Spreitzer, M.; König, J. Water-Glass-Assisted Foaming in Foamed Glass Production. Ceramics 2023, 6, 1646–1654. [Google Scholar] [CrossRef]
- Klyuev, S.V.; Kashapov, N.F.; Radaykin, O.V.; Sabitov, L.S.; Klyuev, A.V.; Shchekina, N.A. The Reliability Coefficient for Fibre Concrete Material. Constr. Mater. Prod. 2022, 5, 2–51. [Google Scholar] [CrossRef]
- Melkonyan, R.G. Amorfnye Gornye Porody i Steklovarenie (Amorphous Rocks and Glass Melting); NIA-Priroda: Moscow, Russia, 2002. [Google Scholar]
- Klyuyev, S.V.; Guryanov, Y.V. External reinforcing of fiber concrete constructions by carbon fiber tapes. Mag. Civ. Eng. 2013, 36, 21–26. [Google Scholar] [CrossRef]
- Vaisman, Y.I.; Ketov, A.A.; Ketov, P.A. Production of foamed materials from synthesized silicate glasses. Russ. J. Appl. Chem. 2013, 86, 952–957. [Google Scholar] [CrossRef]
- Demin, A.M. Calculation of the properties of raw briquet for producing foam glass in the temperature range of preheating. Glass Phys. Chem. 2013, 39, 462–466. [Google Scholar] [CrossRef]
- Hu, F.; Wu, S.; Sun, Y. Hollow-Structured Materials for Thermal Insulation. Adv. Mater. 2019, 31, 1801001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ji, W.; Huang, Y.; Wei, Y.; Lu, J.; Liu, L.; Poon, C.S. Upcycling municipal solid wastes to self-foaming glass-ceramics by chemical additive-free and rapid low-temperature sintering. J. Clean. Prod. 2024, 444, 141261. [Google Scholar] [CrossRef]
- Petersen, R.R.; König, J.; Yue, Y. The viscosity window of the silicate glass foam production. J. Non-Cryst. Solids 2017, 456, 49–54. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, Y.; Huang, Y.; Ji, W.; Yio, M. Christopher Cheeseman, Lili Liu, Chi Sun Poon, Atomic-molecular perspectives on local high-temperature structure and transport properties of CaCO3-foamed glass. Constr. Build. Mater. 2024, 439, 137347. [Google Scholar] [CrossRef]
- Thomsen, L.; Jensen, L.R.; Yue, Y.; Østergaard, B. Crystallinity dependence of thermal and mechanical properties of glass-ceramic foams. J. Eur. Ceram. Soc. 2024, 44, 7936–7942. [Google Scholar] [CrossRef]
- GOST 33949-2016; Foam Glass Thermal Insulation Products for Buildings and Structures. Technical Specifications. Standartinform: Moscow, Russia, 2019.
- Ivanov, K.S. Preparation and properties of foam glass-ceramic from diatomite. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2018, 33, 273. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Klimova, L.V.; Yatsenko, L.A. Peculiarities of foam glass synthesis from natural silica-containing raw materials. J. Therm. Anal. Calorim. 2020, 142, 119–127. [Google Scholar] [CrossRef]
- da Silva, R.C.; Kubaski, E.T.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Tebcherani, S.M. Foam glass using sodium hydroxide as foaming agent: Study on the reaction mechanism in soda–lime glass matrix. J. Non-Cryst. Solids 2019, 511, 177. [Google Scholar] [CrossRef]
- Amirhosseinia, B.A.; Mirkazemia, S.M.; Ghanbaria, H. Effect of temperature and water glass addition on the microstructure and physical properties of soda–lime foam glass. Glass Phys. Chem. 2021, 47, 83. [Google Scholar]
- Petersen, R.R.; König, J.; Iversen, N.; Østergaard, M.B.; Yue, Y. The foaming mechanism of glass foams prepared from the mixture of Mn3O4, carbon and CRT panel glass. Ceram. Int. 2021, 47, 2839. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Hao, P.; Shi, Y.; Xu, Y.; Ding, X. Study on factors affecting properties of foam glass made from waste glass. J. Renew. Mater. 2021, 9, 237. [Google Scholar] [CrossRef]
- Karandashova, N.S.; Goltsman, B.M.; Yatsenko, E.A. Analysis of influence of foaming mixture components on structure and properties of foam glass. IOP Conf. Ser. Mater. Sci. Eng. 2017, 262, 012020. [Google Scholar] [CrossRef]
- Jiang, C.; Huang, S.; Zhang, X.; Cheng, X. Tailoring pore structure and properties of waste derived ceramic foams for lightweight construction. RSC Adv. 2019, 9, 36308. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.-N.; Xu, J.; Su, Z.-G.; Ma, N.; Zhang, X.-Y.; Xi, X.-Q.; Yang, J.-L. Lightweight and high-strength glass foams prepared by a novel green spheres hollowing technique. Ceram. Int. 2016, 42, 2370. [Google Scholar] [CrossRef]
- Gol’tsman, B.M.; Yatsenko, E.A. Modern Methods for Foaming of Glass and Silicate Raw Materials: Review and Analysis. Theor. Found Chem. Eng. 2022, 56, 678–686. [Google Scholar] [CrossRef]
- Zang, J.; Pan, C.; Hu, Y.; Qu, S. Preparation of ceramsite using dehydrated silt soil and its performance on compressive strength of ceramsite concrete block. Sustainability 2023, 15, 9134. [Google Scholar] [CrossRef]
- Zhangabay, N.; Suleimenov, U.; Utelbayeva, A.; Kolesnikov, A.; Baibolov, K.; Imanaliyev, K.; Moldagaliyev, A.; Karshyga, G.; Duissenbekov, B.; Fediuk, R.; et al. Analysis of a Stress-Strain State of a Cylindrical Tank Wall Vertical Field Joint Zone. Buildings 2022, 12, 1445. [Google Scholar] [CrossRef]
- Kulikova, A.A.; Kovaleva, A.M. Use of tailings of enrichment for laying of the developed space of mines. MIAB Min. Inf. Anal. Bull. 2021, 144–154. [Google Scholar] [CrossRef]
- Zhangabay, N.; Giyasov, A.; Bakhbergen, S.; Tursunkululy, T.; Kolesnikov, A. Thermovision study of a residential building under climatic conditions of South Kazakhstan in a cold period. Constr. Mater. Prod. 2024, 7, 1. [Google Scholar] [CrossRef]
- Skopintseva, O.V.; Ganova, S.D.; Buzin, A.A.; Fedotova, V.P. Measures to reduce dusting during loading and transportation of solid mineral resources. Gorn. Zhurnal 2019, 12, 76–79. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Lun, H.; Wang, Q. Analysis of the Influence of Shell Sand Content on the Performance of Ceramisite Lightweight Aggregate Concrete. Buildings 2024, 14, 986. [Google Scholar] [CrossRef]
- Kulikova, E.Y.; Balovtsev, S.V. Risk control system for the construction of urban underground structures. IOP Conf. Ser. Mater. Sci. Eng. 2020, 962, 042020. [Google Scholar] [CrossRef]
- Zhangabay, N.; Suleimenov, U.; Utelbayeva, A.; Buganova, S. Experimental research of the stress-strain state of prestressed cylindrical shells taking into account temperature effects. Case Stud. Constr. Mater. 2022, 18, e01776. [Google Scholar] [CrossRef]
- Kocherov, E.N.; Kolesnikov, A.S.; Mamitova, A.D. Research of clay raw materials of South Kazakhstan for thermal insulating keramzit production. Environmental technologies and engineering for sustainable development. IOP Conf. Ser. Earth Environ. Sci. 2023, 1284, 012003. [Google Scholar] [CrossRef]
- Taimasov, B.T.; Sarsenbayev, B.K.; Khudyakova, T.M.; Kolesnikov, A.S.; Zhanikulov, N.N. Development and testing of low-energy-intensive technology of receiving sulphate-resistant and road portlandcement. Eurasian Chem.-Technol. J. 2017, 19, 347–355. [Google Scholar] [CrossRef]
- Kocherov, Y.; Kolesnikov, A.; Makulbekova, G.; Mamitova, A.; Ramatullaeva, L.; Medeshev, B.; Kolesnikova, O. Investigation of the Physico-Chemical and Mechanical Properties of Expanded Ceramsite Granules Made on the Basis of Coal Mining Waste. J. Compos. Sci. 2024, 8, 306. [Google Scholar] [CrossRef]
- Kolesnikov, A.S.; Kenzhibaeva, G.S.; Botabaev, N.E.; Kutzhanova, A.N.; Iztleuov, G.M.; Suigenbaeva, A.Z.; Ashirbekov, K.A.; Kolesnikova, O.G. Thermodynamic Modeling of Chemical and Phase Transformations in a Waelz Process-Slag—Carbon System. Refract. Ind. Ceram. 2020, 61, 289–292. [Google Scholar] [CrossRef]
- Makulbekova, G.; Kocherov, Y.; Pivovarov, O.; Zhakipbayev, B.; Shapalov, S.; Kenzhaliyeva, G. Efficient and fire-resistant light expanded-clay granulates for heat insulation via heat treatment of bentonite clays with industrial wastes. ARPN J. Eng. Appl. Sci. 2021, 16, 2709–2721. [Google Scholar]
- Davraz, M.; Koru, M.; Bayrakçi, H.C.; Yusufoğlu, Y. The effect of opacifier properties on thermal conductivity of vacuum insulation panel with fumed silica. J. Therm. Anal. Calorim. 2020, 142, 1377–1386. [Google Scholar] [CrossRef]
- Kolesnikov, A.S.; Sapargaliyeva, B.O.; Bychkov, A.Y.; Alferyeva, Y.O.; Syrlybekkyzy, S.; Altybaeva, Z.K.; Nurshakhanova, L.K.; Seidaliyeva, L.K.; Suleimenova, B.S.; Zhidebayeva, A.E.; et al. Thermodynamic Modeling of the Formation of the Main Minerals of Cement Clinker and Zinc Fumes in the Processing of Toxic Technogenic Waste of the Metallurgical Industry. Rasayan J. Chem. 2022, 15, 2181–2187. [Google Scholar] [CrossRef]
- Köning, J.; Petersen, R.R.; Yue, Y. Influence of the glass–calcium carbonate mixture’s characteristics on the foaming process and the properties of the foam glass. J. Eur. Ceram. Soc. 2014, 34, 1591–1598. [Google Scholar] [CrossRef]
- Bondar, D.; Vinai, R. Chemical and Microstructural Properties of Fly Ash and Fly Ash/Slag Activated by Waste Glass-Derived Sodium Silicate. Crystals 2022, 12, 913. [Google Scholar] [CrossRef]
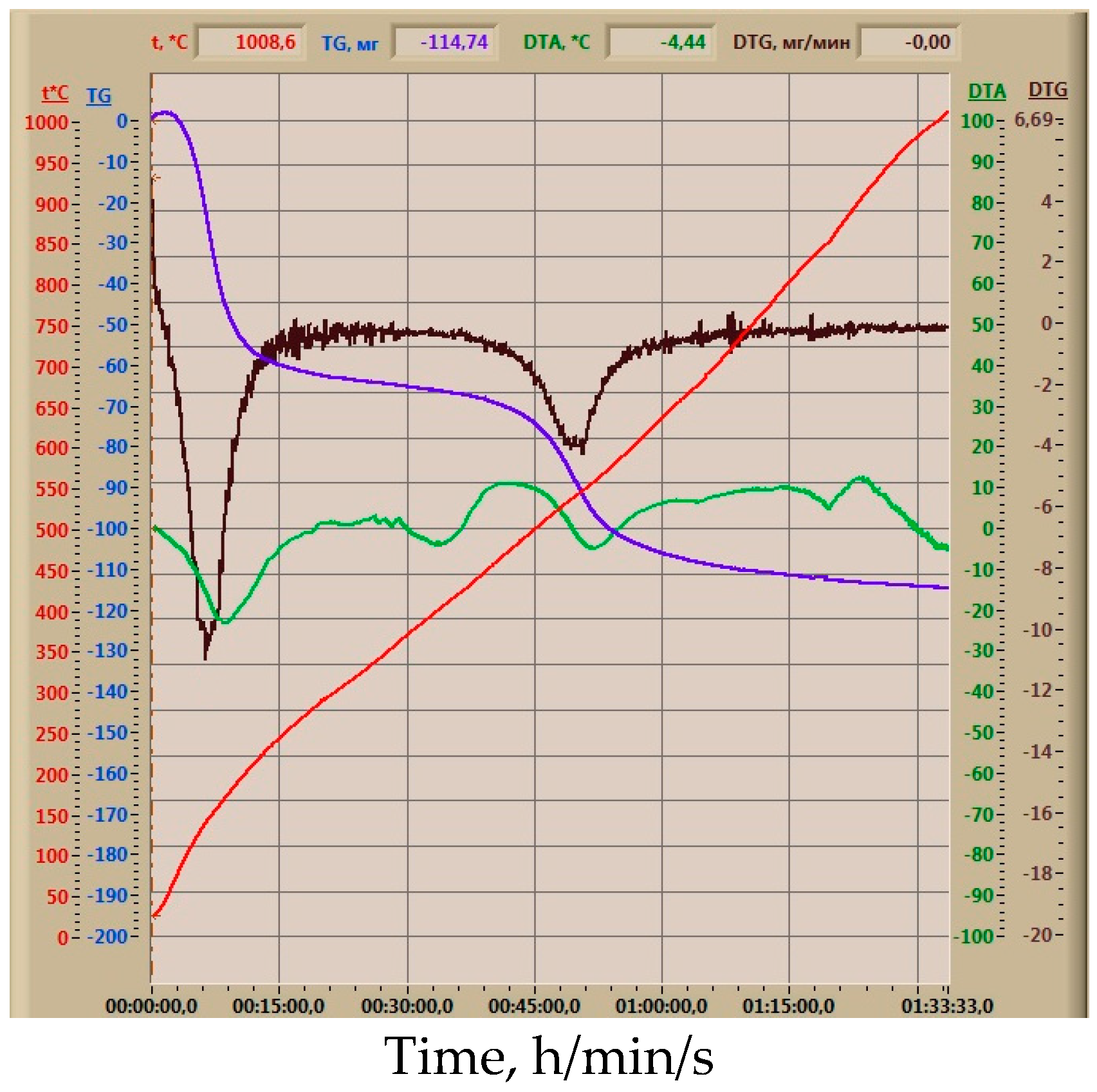

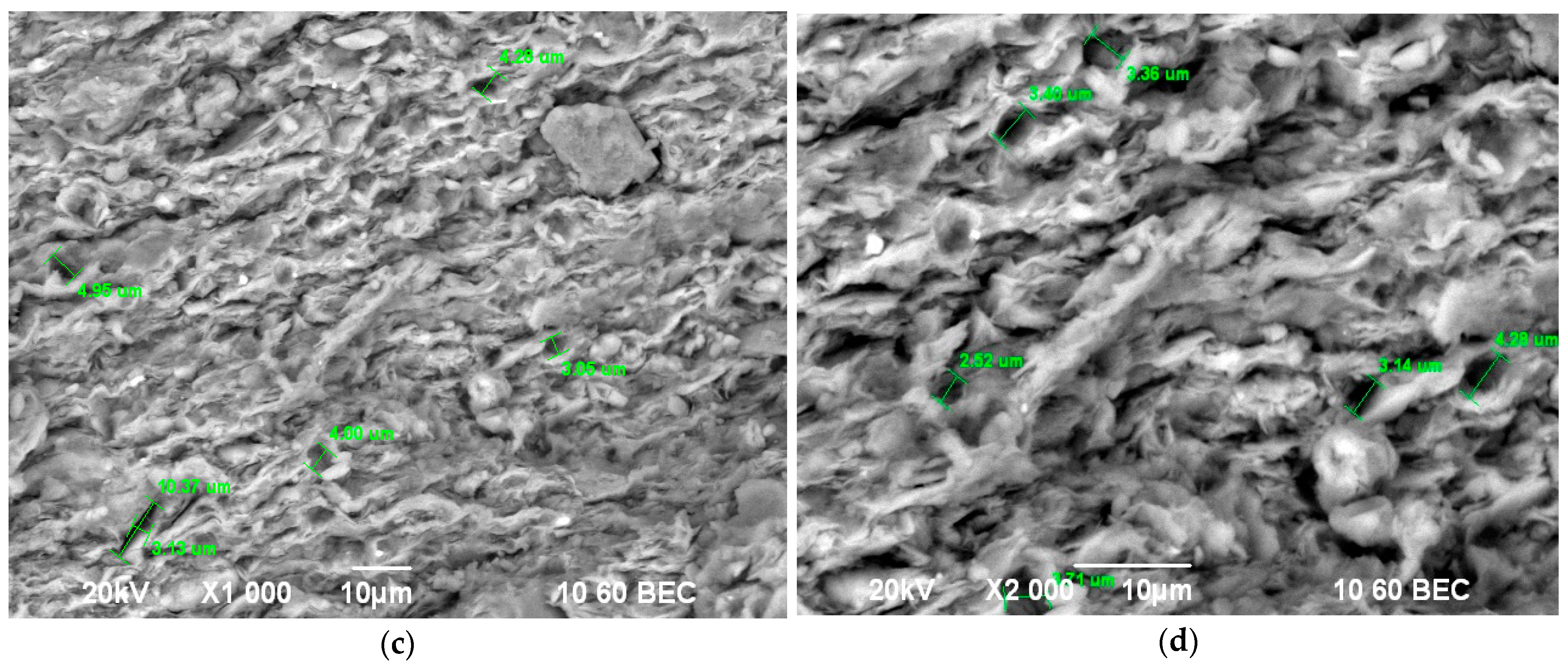
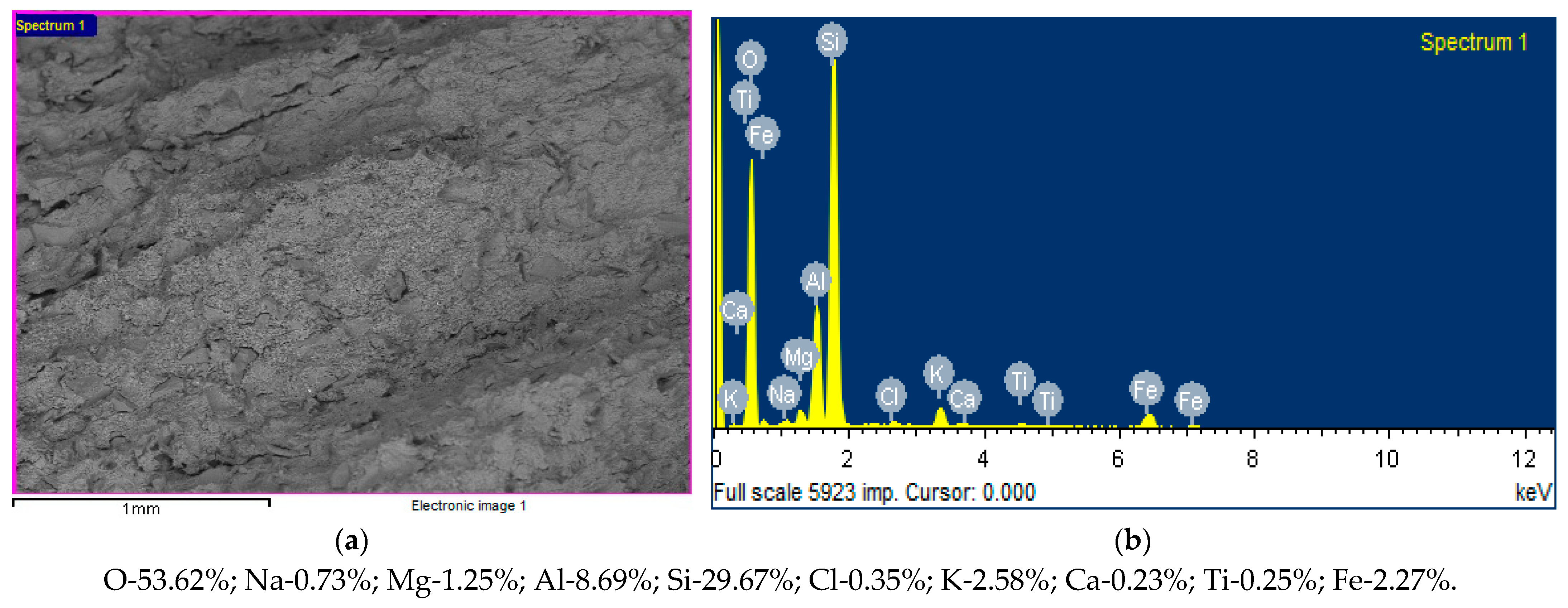
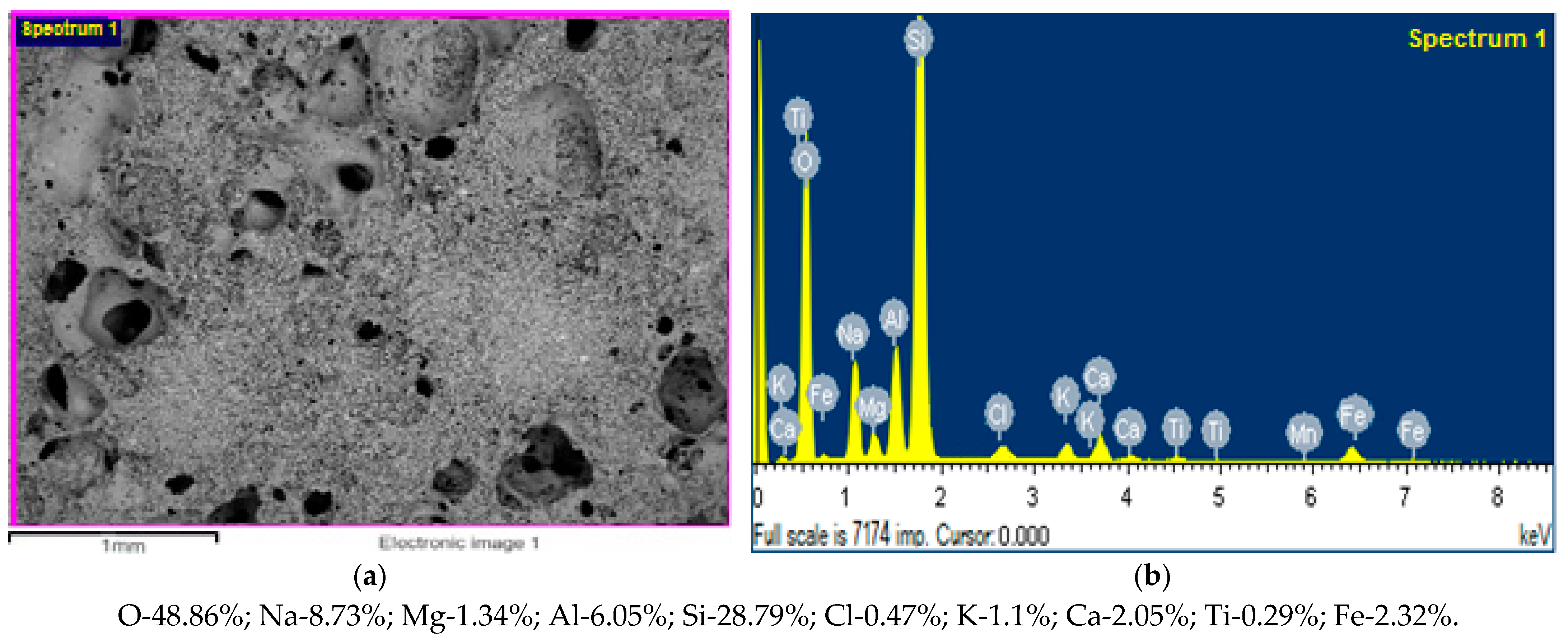
| SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | Na2O | K2O | SO3 | H2O | PPP |
|---|---|---|---|---|---|---|---|---|---|---|
| 68.97–79.57 | 6.11–10.38 | 2.37–3.44 | 0.25–0.45 | 0.3–2.19 | 0.98–1.82 | 0.55–1.07 | 0.69–1.12 | 0.76–2.75 | 3.34–4.39 | 3.97–4.53 |
| Pair | Corner | Square | Intensive | Half-Width | Interpol | % Max | |
|---|---|---|---|---|---|---|---|
| No. | |||||||
| 1 | 6.840 | 469.821 | 367 | 1.2802 | 12.9225 | 11.76 | |
| 2 | 9.840 | 84.770 | 459 | 0.1847 | 8.9884 | 14.70 | |
| 3 | 20.800 | 757.996 | 1079 | 0.7025 | 4.2704 | 34.56 | |
| 4 | 21.500 | 1283.277 | 939 | 1.3666 | 4.1329 | 30.08 | |
| 5 | 26.620 | 548.748 | 3122 | 0.1758 | 3.3485 | 100.00 | |
| 6 | 29.860 | 242.550 | 330 | 0.7350 | 2.9921 | 10.57 | |
| 7 | 32.000 | 110.006 | 414 | 0.2657 | 2.7968 | 13.26 | |
| 8 | 36.520 | 167.571 | 380 | 0.4410 | 2.4603 | 12.17 | |
| 9 | 42.440 | 73.787 | 291 | 0.2536 | 2.1298 | 9.32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhakipbayev, B.; Kolesnikov, A.; Syrlybekkyzy, S.; Seidaliyeva, L.; Koishina, A.; Taizhanova, L. Physicochemical Studies of Opoka as a Raw Material Component of Sodium Silicate Mixture for Subsequent Synthesis of Foam Glass Material Based on It. J. Compos. Sci. 2025, 9, 70. https://doi.org/10.3390/jcs9020070
Zhakipbayev B, Kolesnikov A, Syrlybekkyzy S, Seidaliyeva L, Koishina A, Taizhanova L. Physicochemical Studies of Opoka as a Raw Material Component of Sodium Silicate Mixture for Subsequent Synthesis of Foam Glass Material Based on It. Journal of Composites Science. 2025; 9(2):70. https://doi.org/10.3390/jcs9020070
Chicago/Turabian StyleZhakipbayev, Bibol, Alexandr Kolesnikov, Samal Syrlybekkyzy, Leila Seidaliyeva, Akmaral Koishina, and Lyailim Taizhanova. 2025. "Physicochemical Studies of Opoka as a Raw Material Component of Sodium Silicate Mixture for Subsequent Synthesis of Foam Glass Material Based on It" Journal of Composites Science 9, no. 2: 70. https://doi.org/10.3390/jcs9020070
APA StyleZhakipbayev, B., Kolesnikov, A., Syrlybekkyzy, S., Seidaliyeva, L., Koishina, A., & Taizhanova, L. (2025). Physicochemical Studies of Opoka as a Raw Material Component of Sodium Silicate Mixture for Subsequent Synthesis of Foam Glass Material Based on It. Journal of Composites Science, 9(2), 70. https://doi.org/10.3390/jcs9020070






