Role of Surface Charge in the Speciation and Photocatalytic Degradation of 4-Nitrophenol Using ZnO–CeO2–WO3 Photocatalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixture Design of Experiments
2.3. Synthesis of the Materials
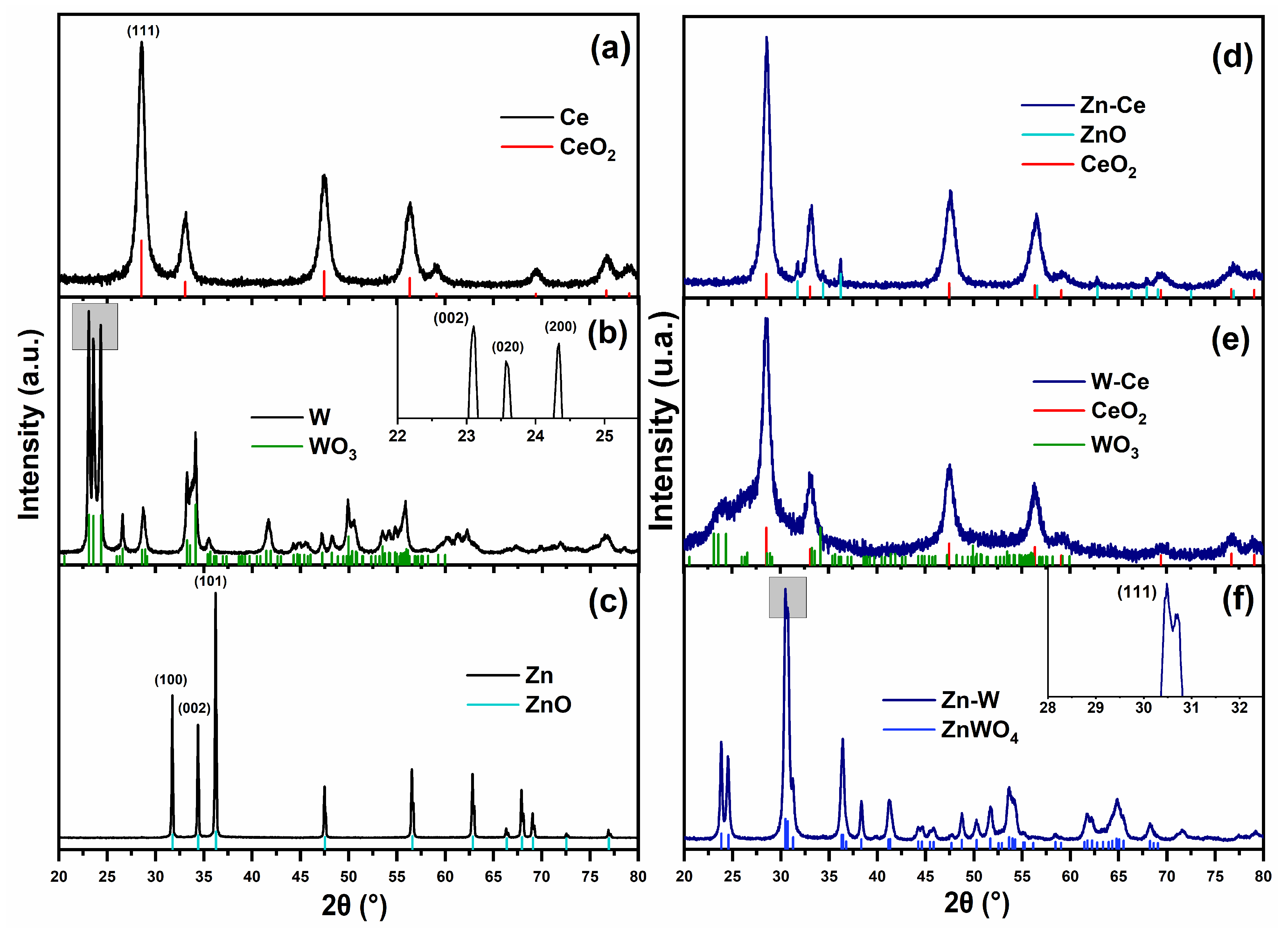
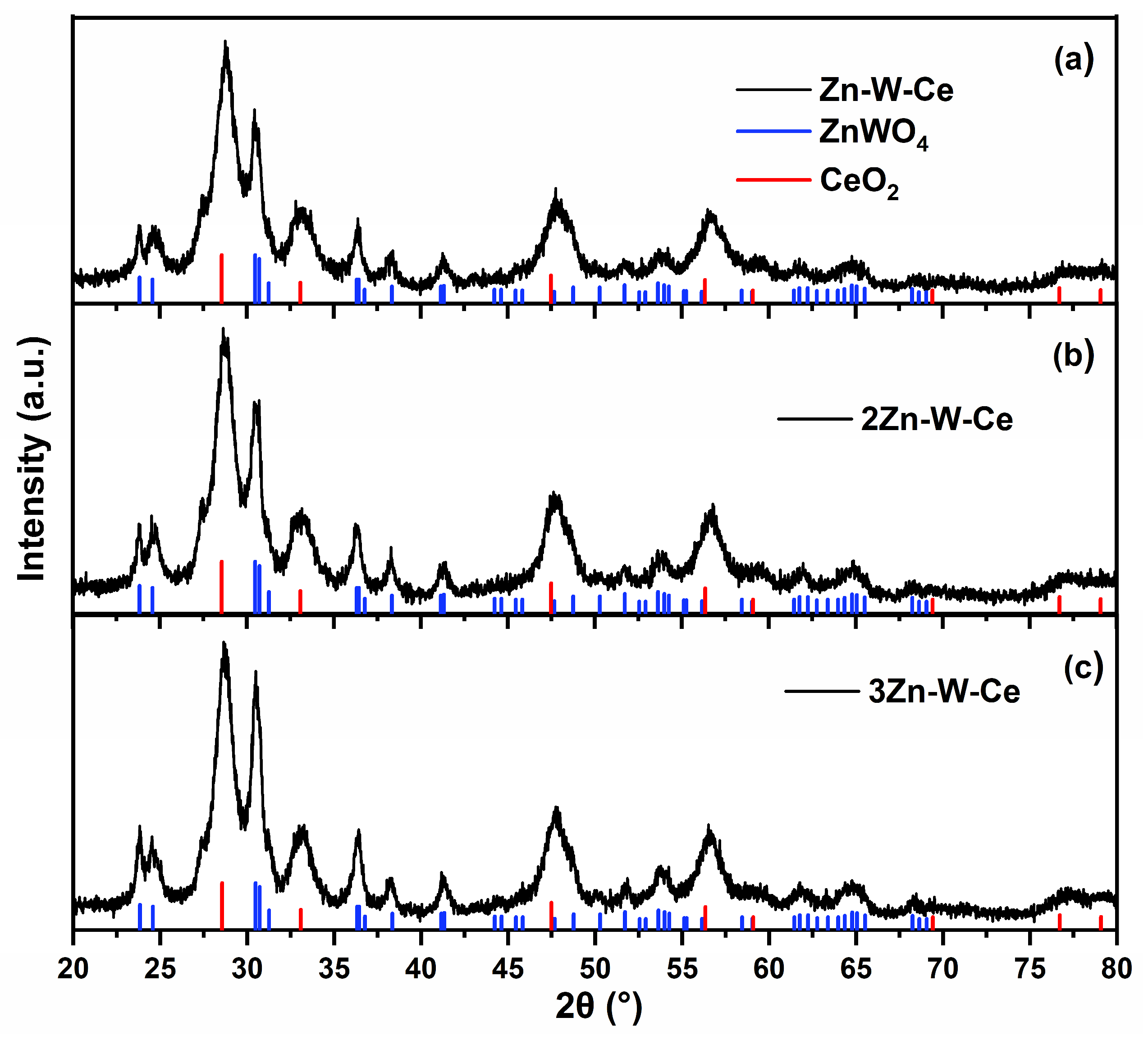
2.4. X-Ray Diffraction (XRD)
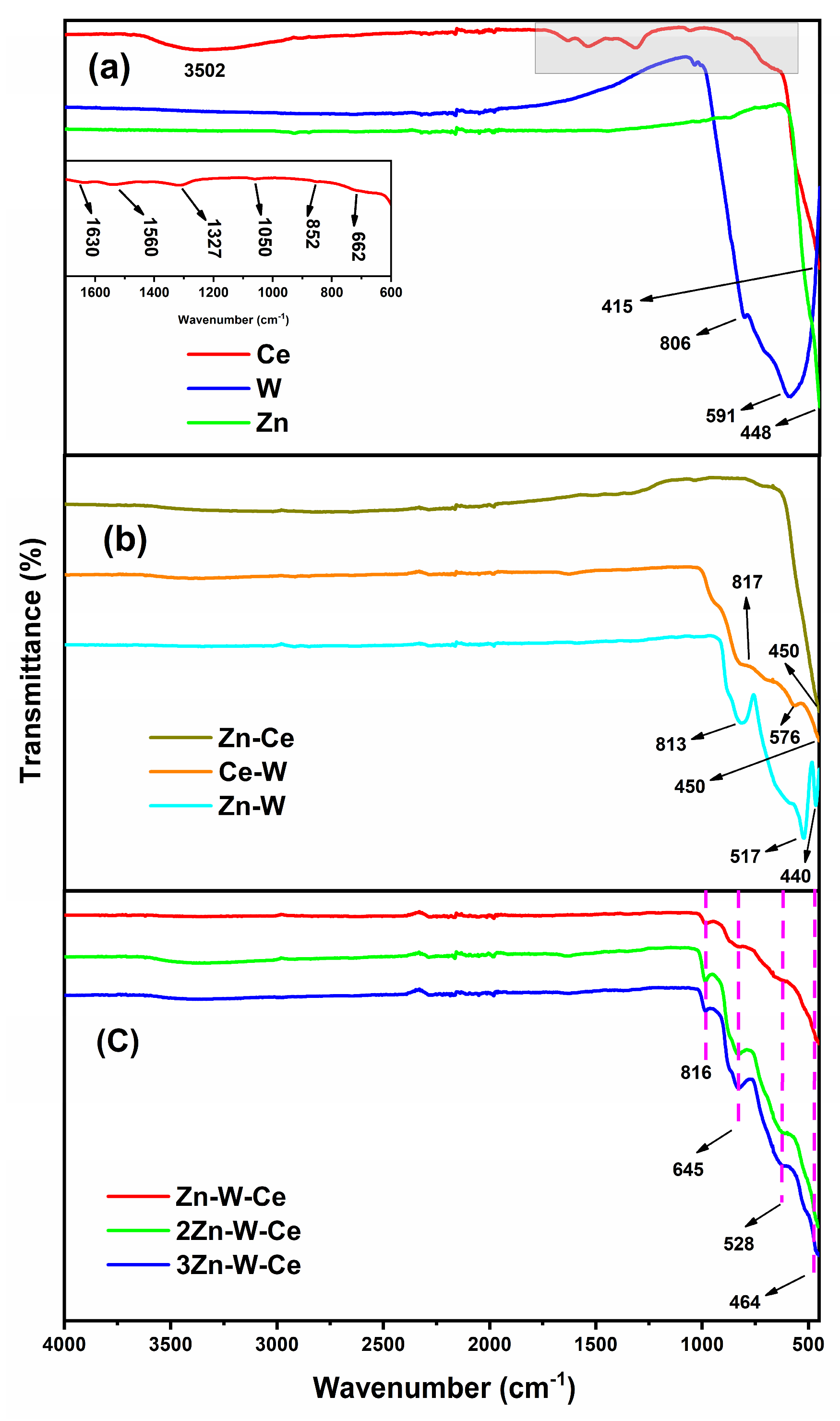
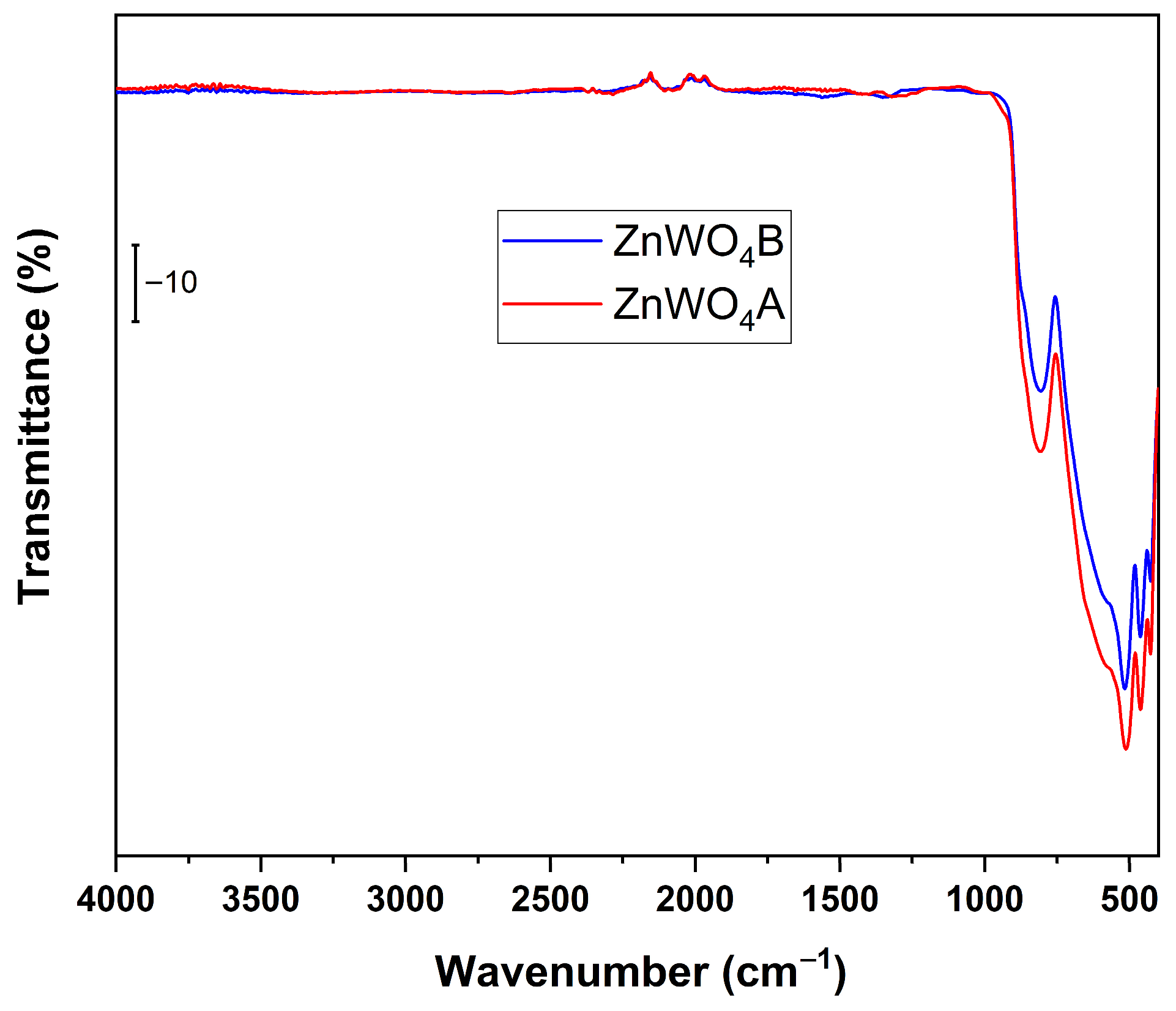
2.5. Fourier Transform Infrared Spectroscopy (FT-IR)
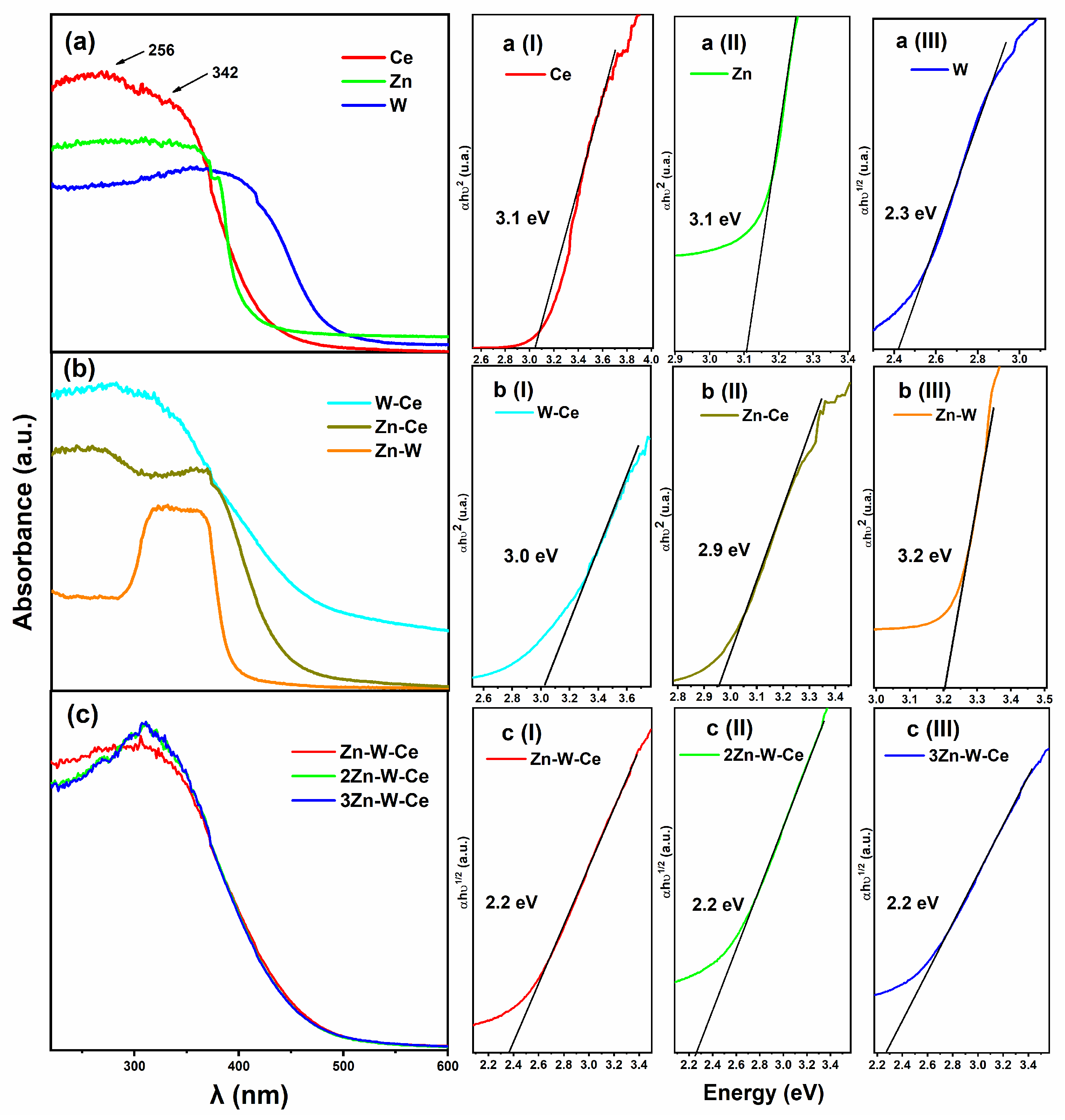
2.6. UV–Vis Diffuse Reflectance Spectroscopy (DRS)
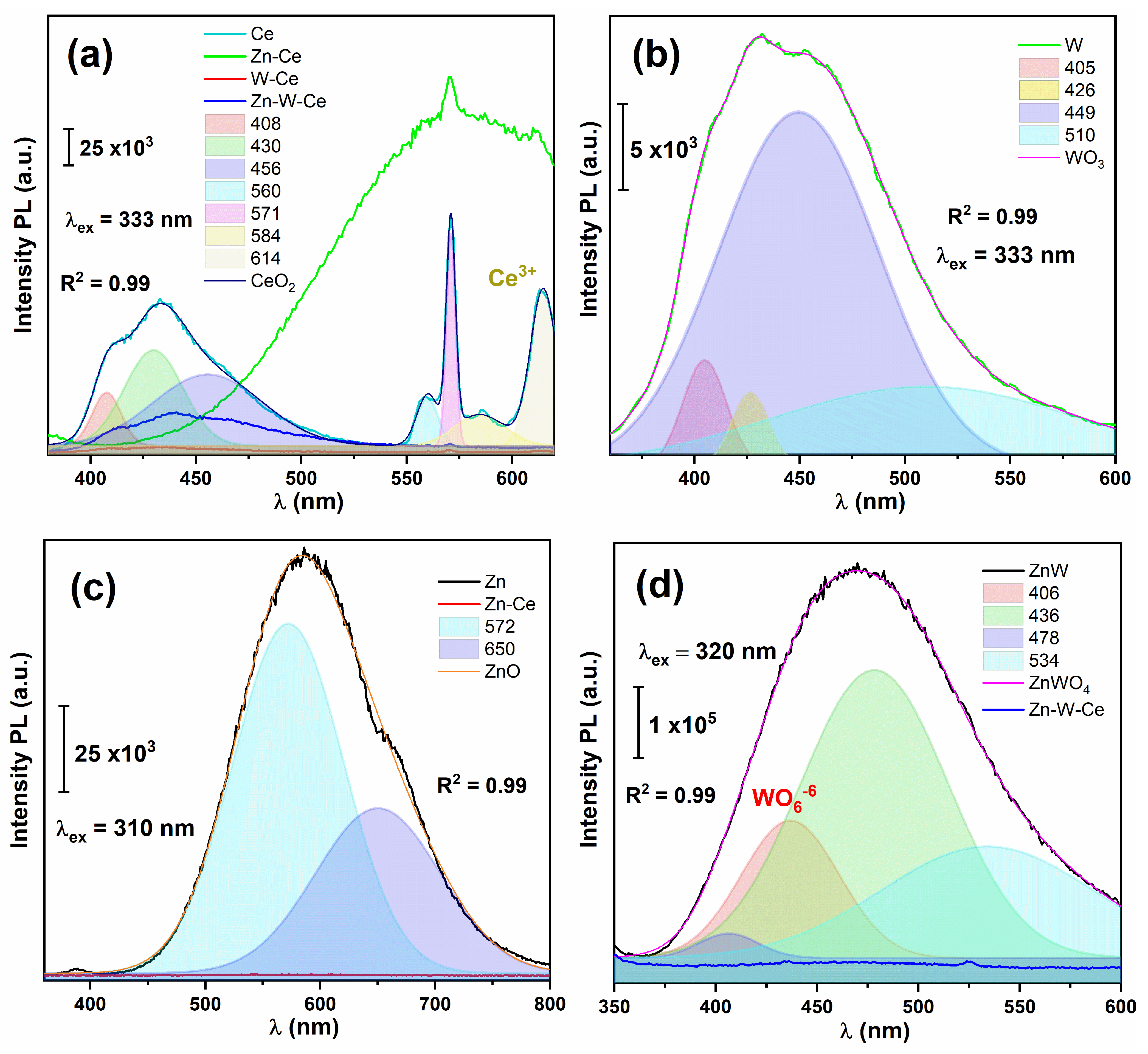
2.7. Photoluminescence (PL) Spectroscopy
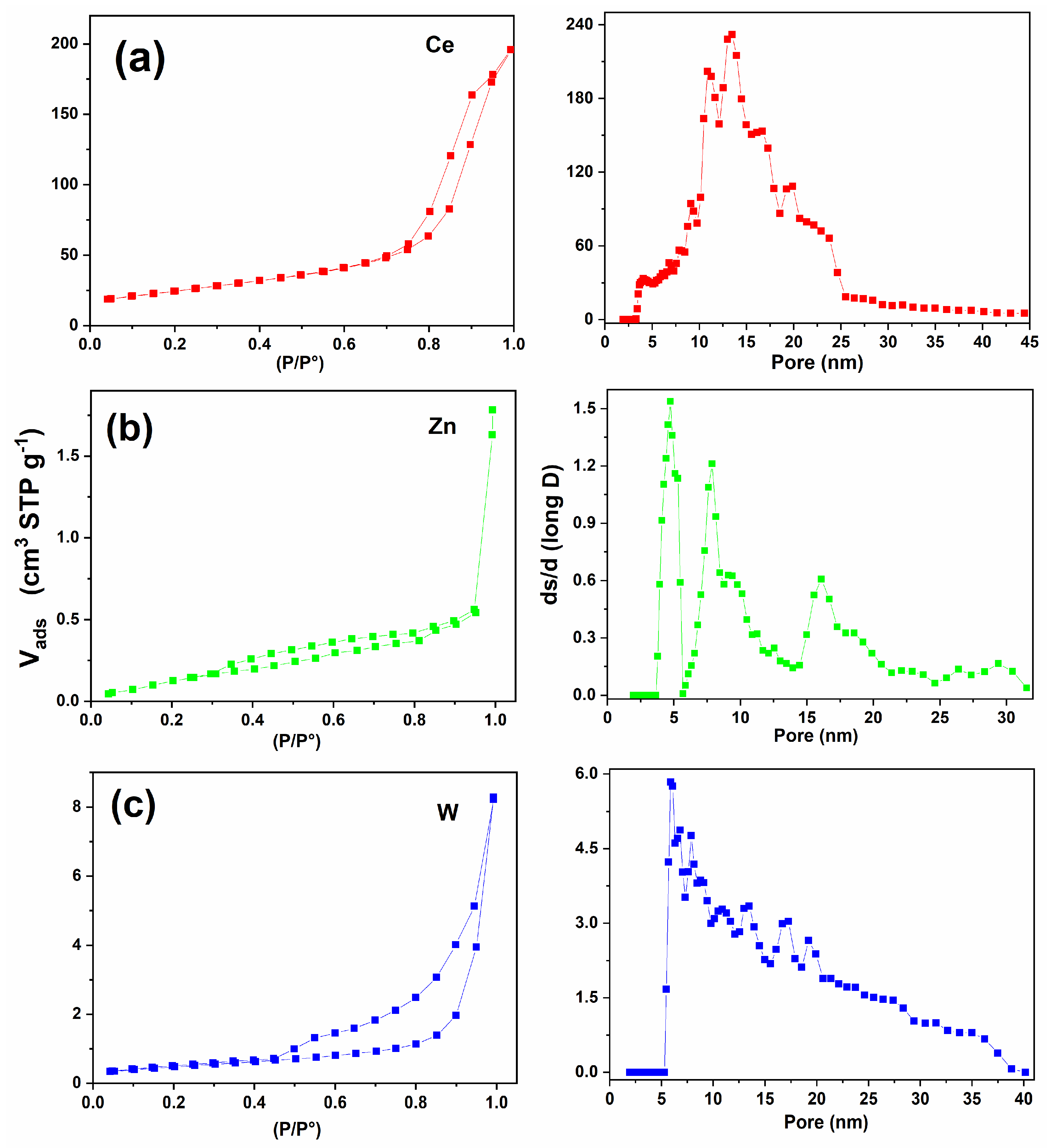
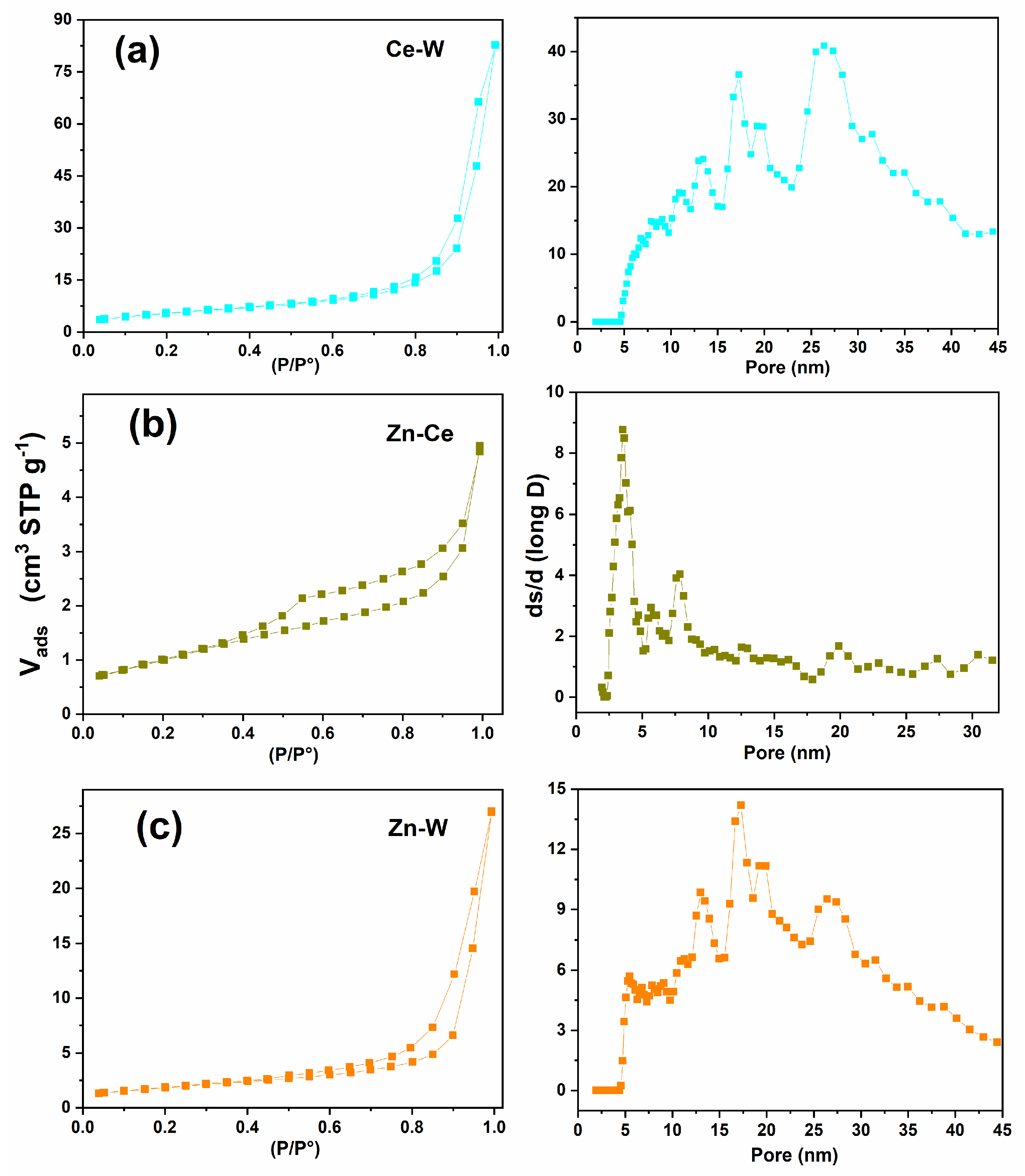
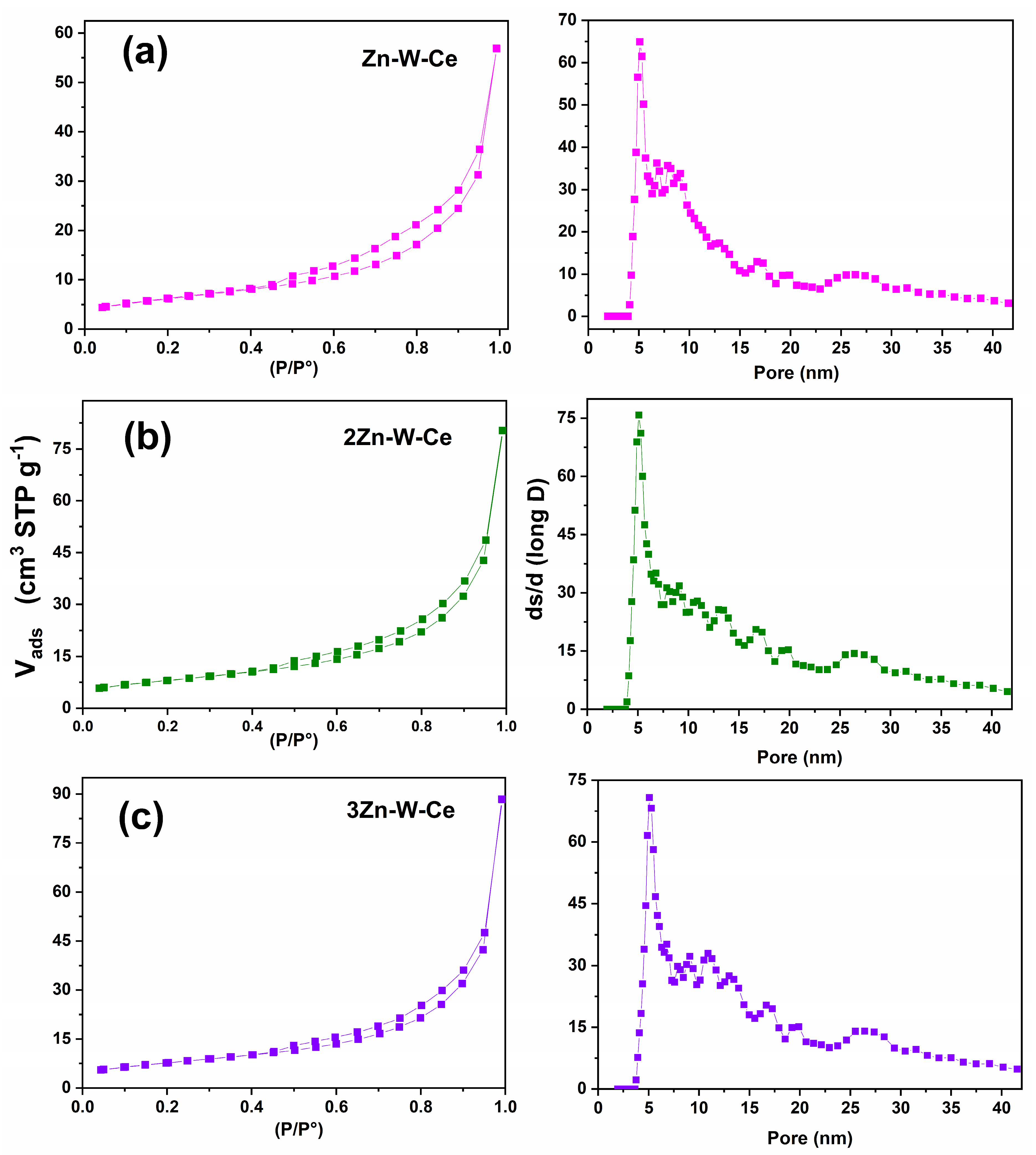
2.8. Nitrogen Adsorption–Desorption Isotherms
2.9. Scanning Electron Microscopy (SEM)
2.10. Photocatalytic Activity Test
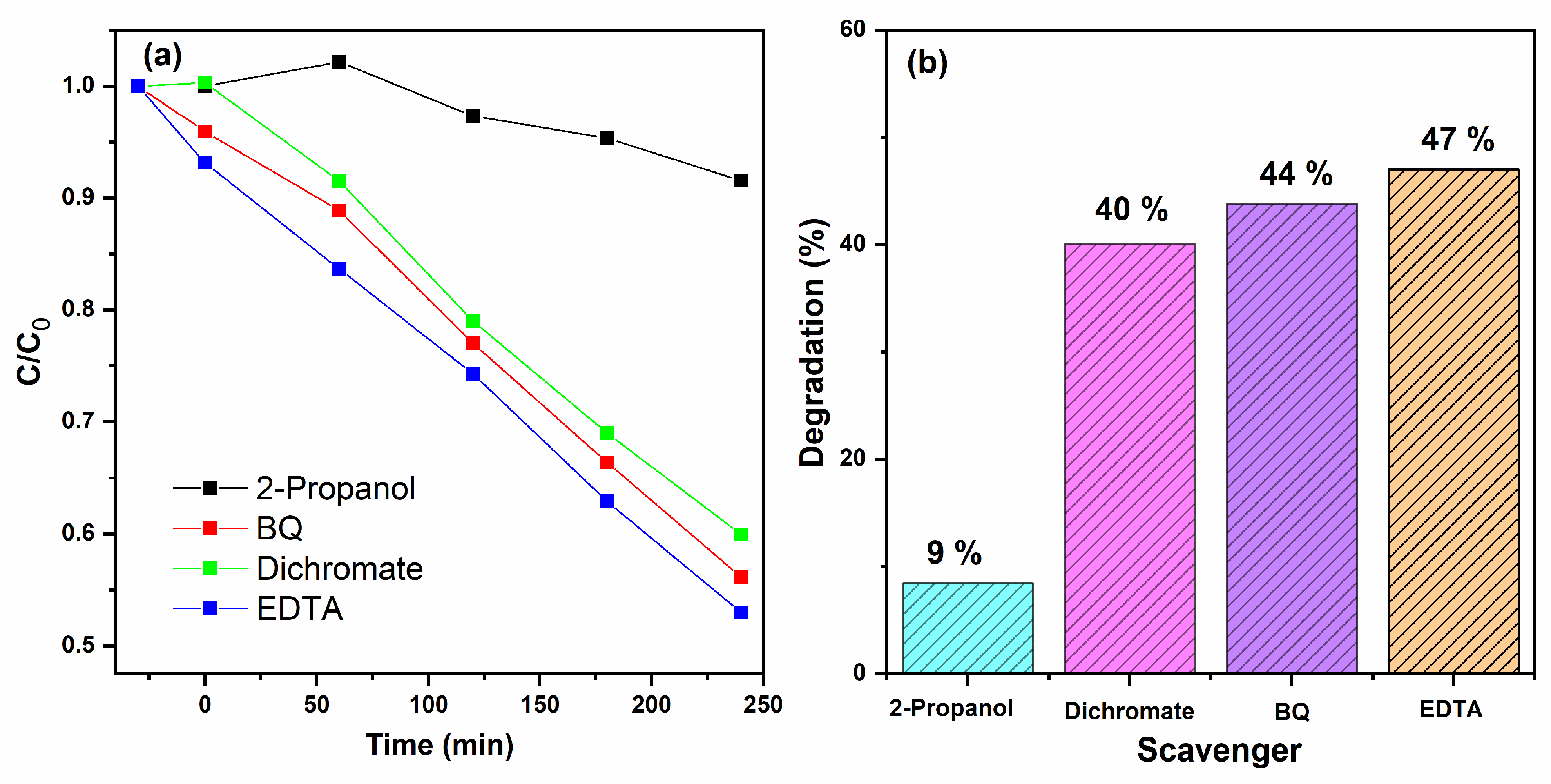
2.11. Scavenger Tests
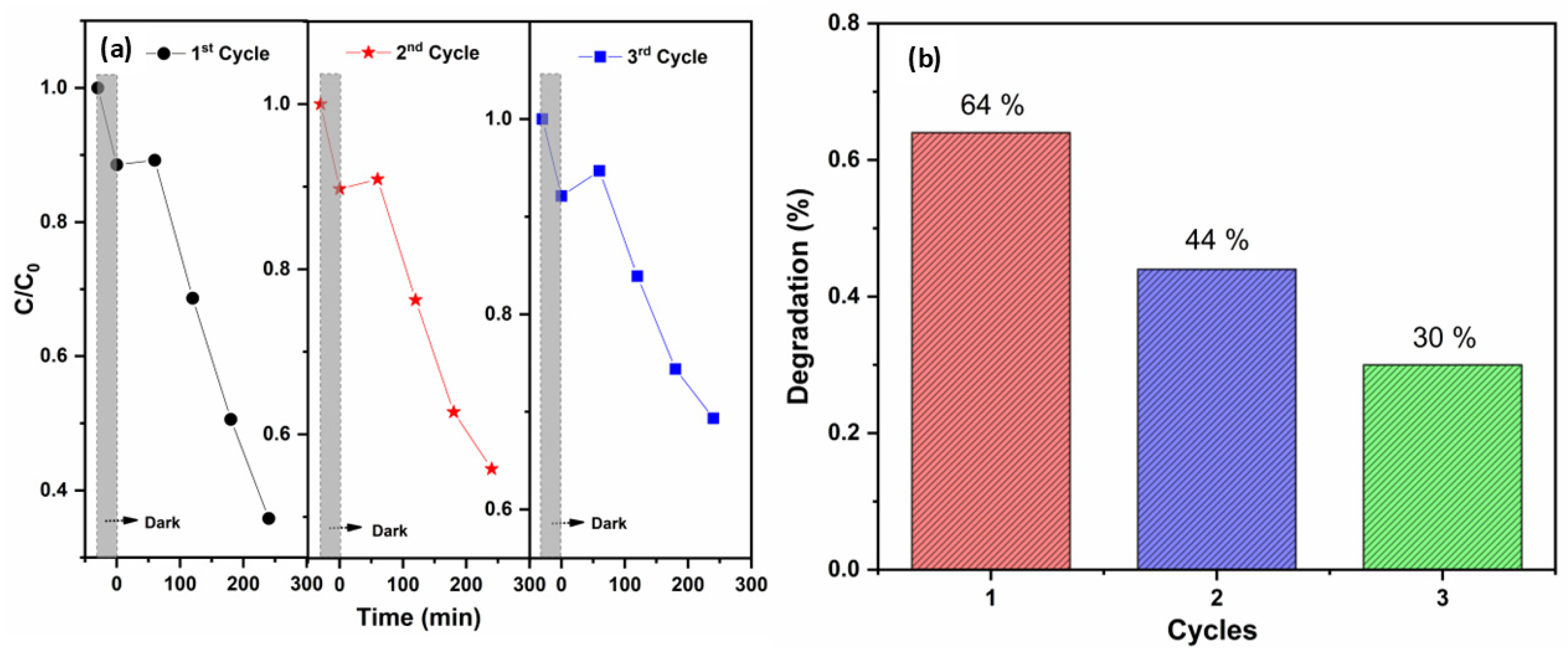
2.12. Reusability Cycles
3. Results and Discussion
3.1. XRD Analysis
3.2. FT-IR Analysis
3.3. UV–Vis Analysis
3.4. Photoluminescence (PL) Analysis
3.5. Nitrogen Adsorption–Desorption Isotherms Analysis
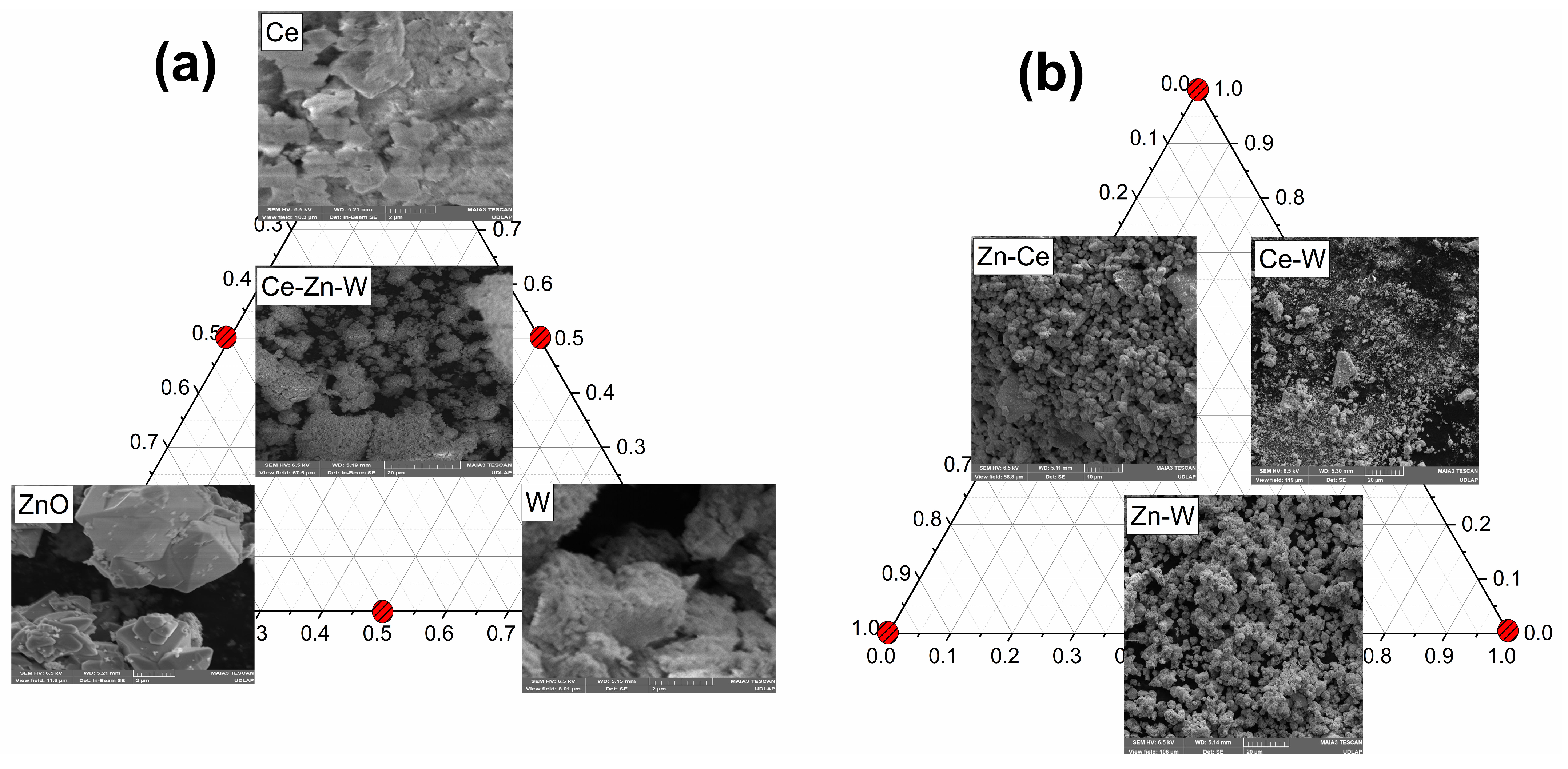
3.6. SEM Analysis
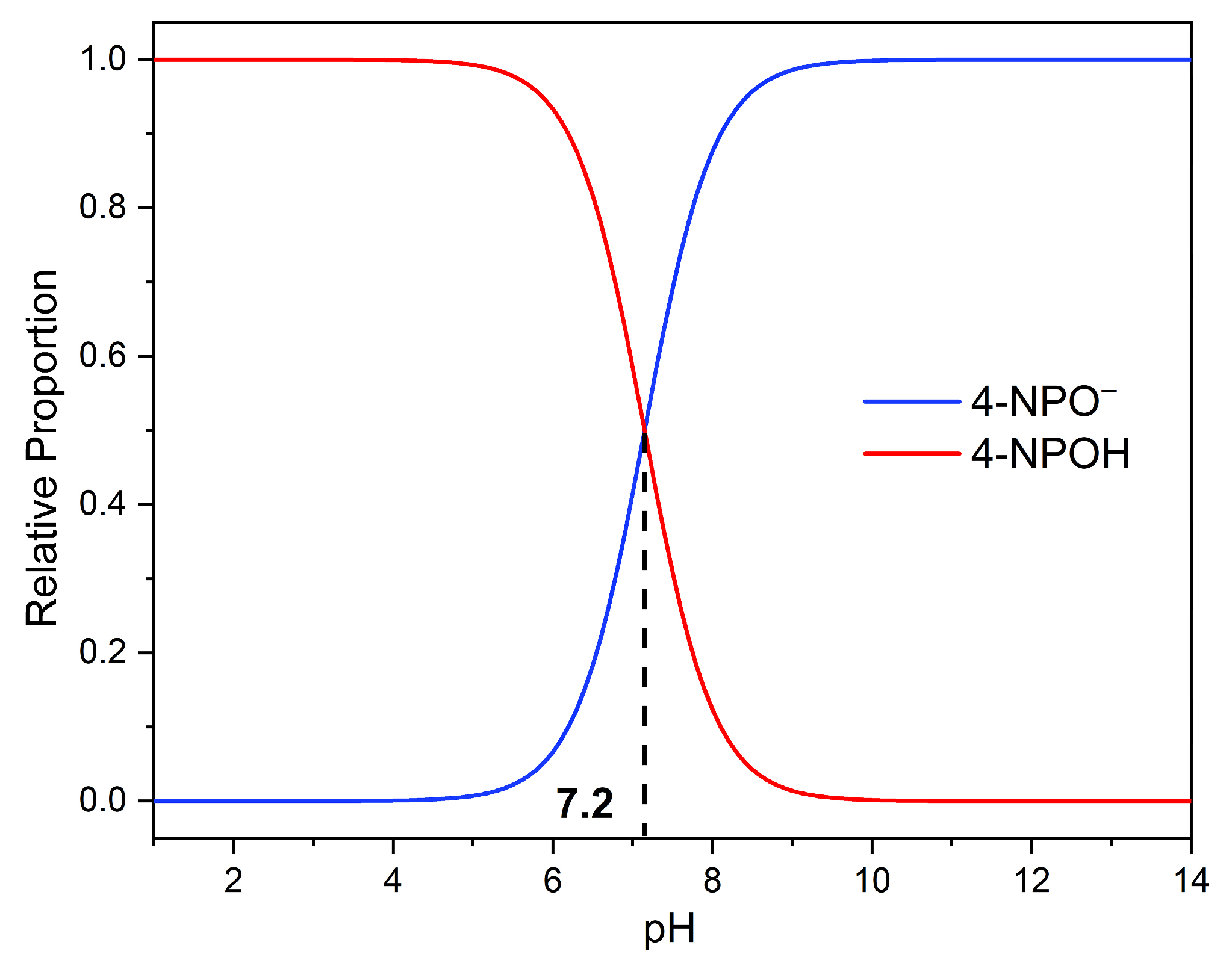
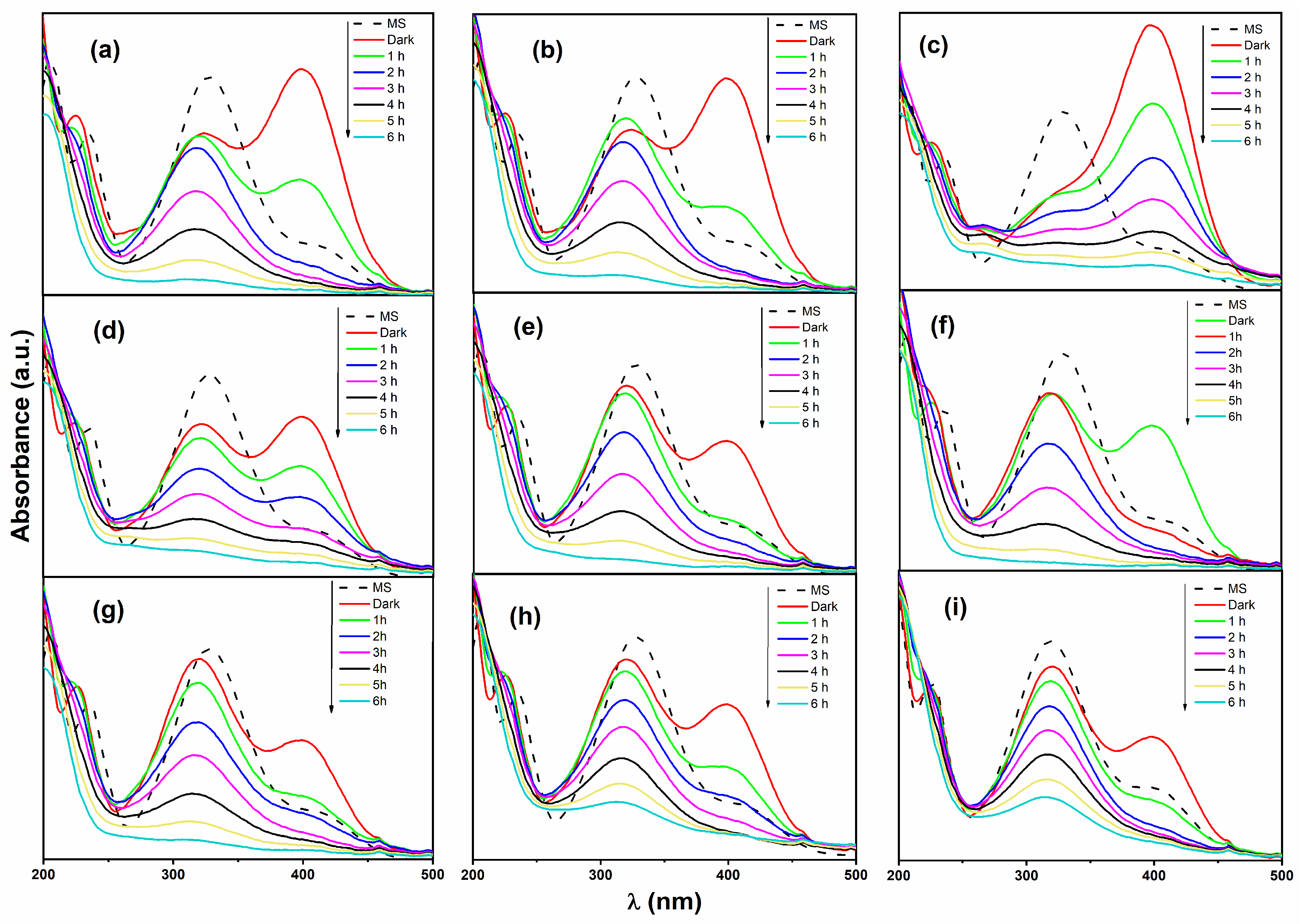
3.7. Photodegradation of 4-Nitrophenol (4-NPOH)
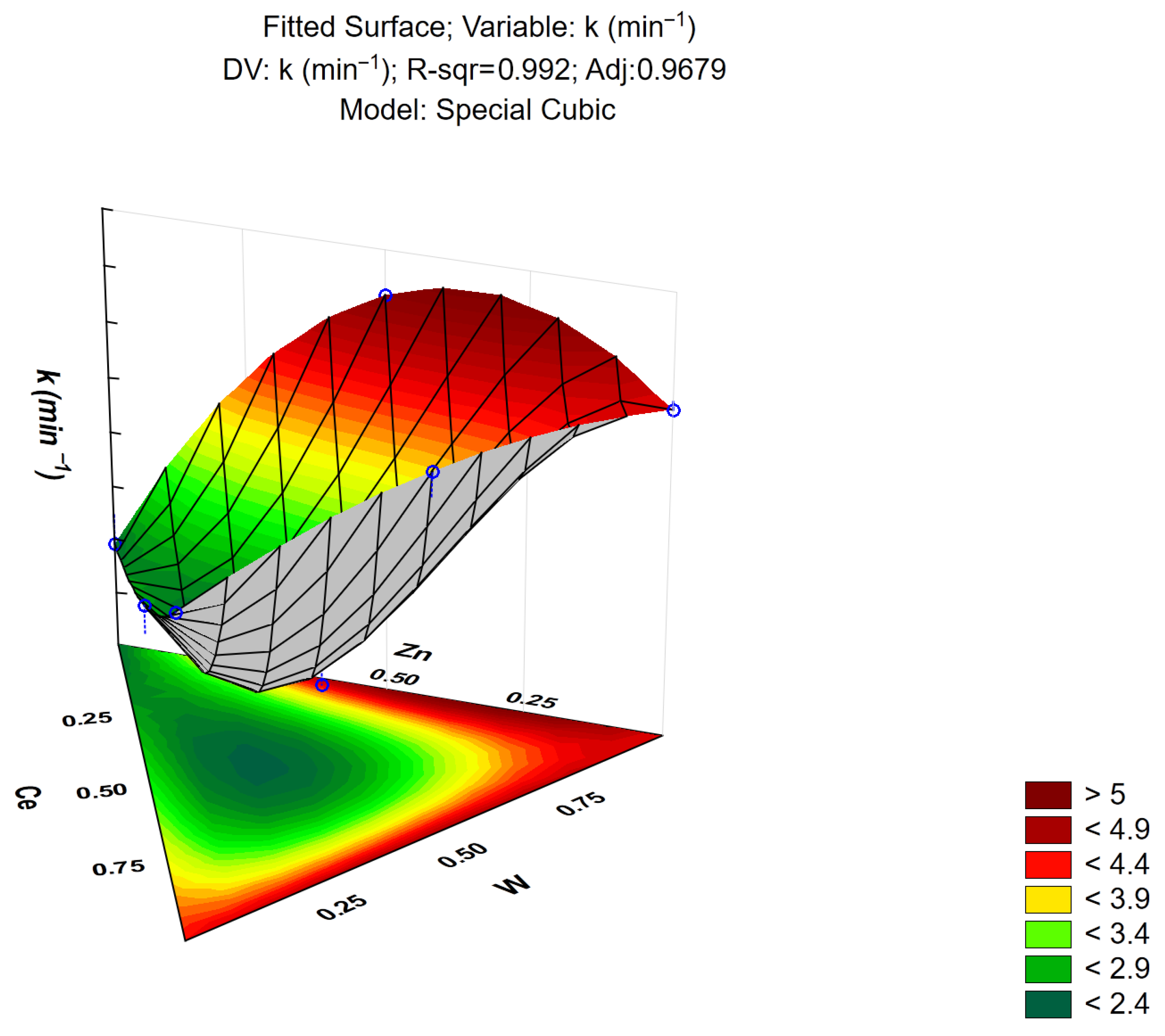
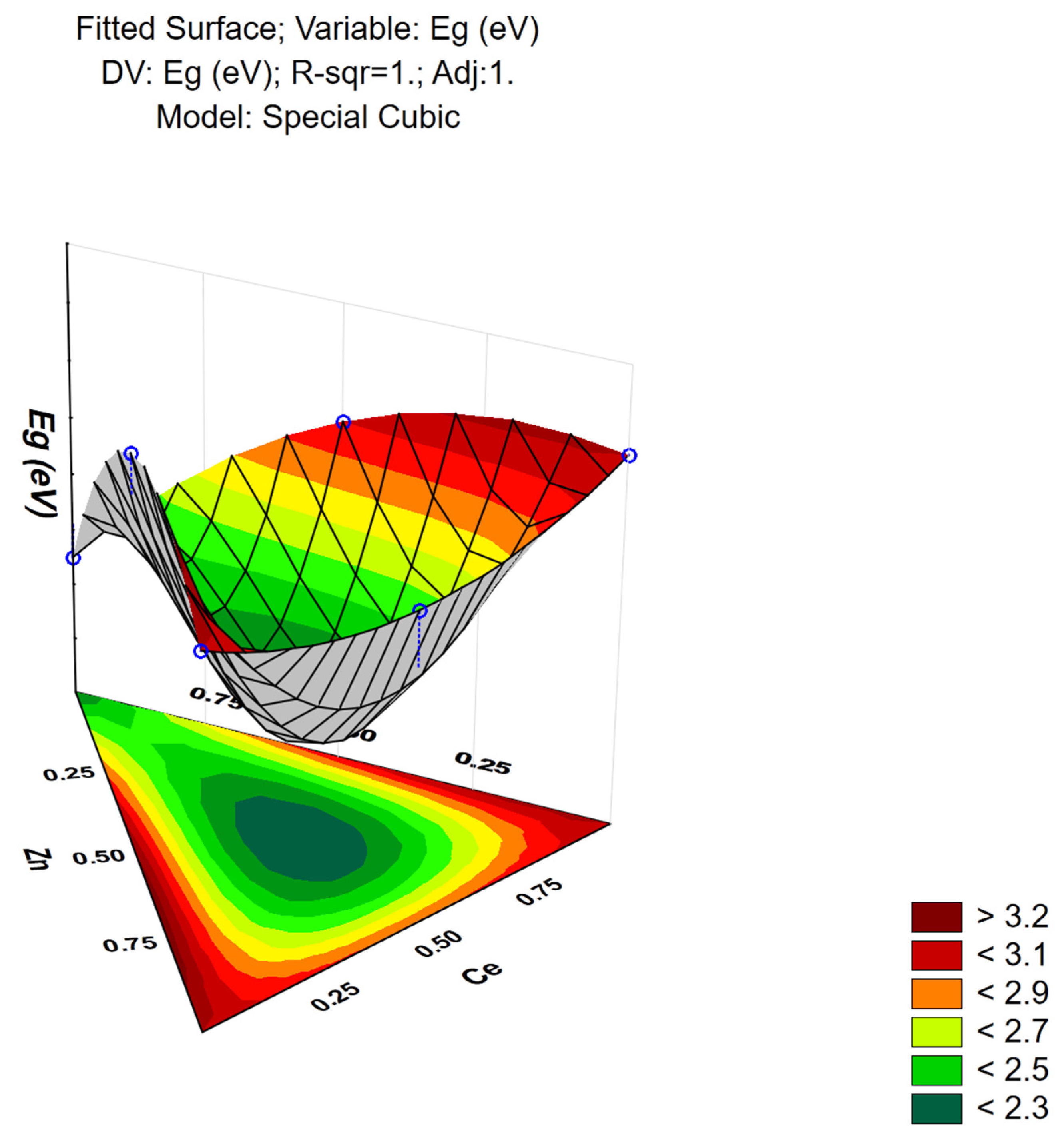
3.8. Response Surface Modeling
3.9. Scavenger Tests and Reusability Cycles Analysis
3.10. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkar, P.; Dey, A. 4-Nitrophenol Biodegradation by an Isolated and Characterized Microbial Consortium and Statistical Optimization of Physicochemical Parameters by Taguchi Methodology. J. Environ. Chem. Eng. 2020, 8, 104347. [Google Scholar] [CrossRef]
- Okoro, H.K.; Orosun, M.M.; Victor, A.; Zvinowanda, C. Health Risk Assessment, Chemical Monitoring and Spatio-Temporal Variations in Concentration Levels of Phenolic Compounds in Surface Water Collected from River Oyun, Republic of Nigeria. Sustain. Water Resour. Manag. 2022, 8, 189. [Google Scholar] [CrossRef]
- Lee, S.J.; Yu, Y.; Jung, H.J.; Naik, S.S.; Yeon, S.; Choi, M.Y. Efficient Recovery of Palladium Nanoparticles from Industrial Wastewater and Their Catalytic Activity toward Reduction of 4-Nitrophenol. Chemosphere 2021, 262, 128358. [Google Scholar] [CrossRef] [PubMed]
- Iben Ayad, A.; Luart, D.; Ould Dris, A.; Guénin, E. Kinetic Analysis of 4-Nitrophenol Reduction by “Water-Soluble” Palladium Nanoparticles. Nanomaterials 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, L.; Liu, S.; Wang, D.; Qin, Y.; Chen, Y.; Dai, W.; Wang, Y.; Xing, Q.; Zou, J. Degradation of 4-Nitrophenol by Electrocatalysis and Advanced Oxidation Processes Using Co3O4@C Anode Coupled with Simultaneous CO2 Reduction via SnO2/CC Cathode. Chin. Chem. Lett. 2020, 31, 1961–1965. [Google Scholar] [CrossRef]
- Sun, Z.; Ni, Y.; Wu, Y.; Yue, W.; Zhang, G.; Bai, J. Electrocatalytic Degradation of Methyl Orange and 4-Nitrophenol on a Ti/TiO2-NTA/La-PbO2 Electrode: Electrode Characterization and Operating Parameters. Environ. Sci. Pollut. Res. 2023, 30, 6262–6274. [Google Scholar] [CrossRef]
- Kalaimurugan, D.; Sivasankar, P.; Durairaj, K.; Lakshmanamoorthy, M.; Ali Alharbi, S.; Al Yousef, S.A.; Chinnathambi, A.; Venkatesan, S. Novel Strategy for Biodegradation of 4-Nitrophenol by the Immobilized Cells of Pseudomonas sp. YPS3 with Acacia Gum. Saudi J. Biol. Sci. 2021, 28, 833–839. [Google Scholar] [CrossRef]
- Sudha, M.; Renu, G.; Sangeeta, G. Mineralization and Degradation of 4-Nitrophenol Using Homogeneous Fenton Oxidation Process. Environ. Eng. Res. 2020, 26, 190145. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Akram, K.; Aslam, Z.; Ihsanullah, I.; Baig, N.; Bello, M.M. Photocatalytic Degradation of P-nitrophenol in Wastewater by Heterogeneous Cobalt Supported ZnO Nanoparticles: Modeling and Optimization Using Response Surface Methodology. Environ. Prog. Sustain. Energy 2023, 42, e13984. [Google Scholar] [CrossRef]
- Yahya, A.A.; Rashid, K.T.; Ghadhban, M.Y.; Mousa, N.E.; Majdi, H.S.; Salih, I.K.; Alsalhy, Q.F. Removal of 4-Nitrophenol from Aqueous Solution by Using Polyphenylsulfone-Based Blend Membranes: Characterization and Performance. Membranes 2021, 11, 171. [Google Scholar] [CrossRef]
- Ahn, W.-Y.; Sheeley, S.A.; Rajh, T.; Cropek, D.M. Photocatalytic Reduction of 4-Nitrophenol with Arginine-Modified Titanium Dioxide Nanoparticles. Appl. Catal. B Environ. 2007, 74, 103–110. [Google Scholar] [CrossRef]
- Chen, D.; Ray, A.K. Photodegradation Kinetics of 4-Nitrophenol in TiO2 Suspension. Water Res. 1998, 32, 3223–3234. [Google Scholar] [CrossRef]
- Shokri, A.; Mahanpoor, K.; Soodbar, D. Evaluation of a Modified TiO2 (GO–B–TiO2) Photocatalyst for Degradation of 4-Nitrophenol in Petrochemical Wastewater by Response Surface Methodology Based on the Central Composite Design. J. Environ. Chem. Eng. 2016, 4, 585–598. [Google Scholar] [CrossRef]
- Das, A.; Patra, M.; Kumar, P.; Bhagavathiachari, M.; Nair, R.G. Defect-Induced Visible-Light-Driven Photocatalytic and Photoelectrochemical Performance of ZnO–CeO2 Nanoheterojunctions. J. Alloys Compd. 2021, 858, 157730. [Google Scholar] [CrossRef]
- Haleem, A.; Shafiq, A.; Chen, S.-Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Castro, L.V.; Alcántar-Vázquez, B.; Manríquez, M.E.; Albiter, E.; Ortiz-Islas, E. Photodegradation of Emerging Pollutants Using a Quaternary Mixed Oxide Catalyst Derived from Its Corresponding Hydrotalcite. Catalysts 2025, 15, 173. [Google Scholar] [CrossRef]
- Thai, N.-T.-P.; Nguyen, B.-N.; Luan, V.-H.; Son, L.V.T.; Dang, B.-T.; Le, M.-V. Synthesis and Photocatalytic Activity of BiOI Particles: An Efficient Visible-Light-Driven Degradation of Organic Pollutants in Aqueous Environments. IOP Conf. Ser. Earth Environ. Sci. 2024, 1340, 012009. [Google Scholar] [CrossRef]
- Zyoud, A.H.; Zubi, A.; Zyoud, S.H.; Hilal, M.H.; Zyoud, S.; Qamhieh, N.; Hajamohideen, A.; Hilal, H.S. Kaolin-Supported ZnO Nanoparticle Catalysts in Self-Sensitized Tetracycline Photodegradation: Zero-Point Charge and pH Effects. Appl. Clay Sci. 2019, 182, 105294. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, J.; Sun, Z.; Zhai, X. Mechanism of Influence of Initial pH on the Degradation of Nitrobenzene in Aqueous Solution by Ceramic Honeycomb Catalytic Ozonation. Environ. Sci. Technol. 2008, 42, 4002–4007. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.; Naguib, E.M.; Mohamed, M.M. Enhancement of Photocatalytic and Sonophotocatalytic Degradation of 4-Nitrophenol by ZnO/Graphene Oxide and ZnO/Carbon Nanotube Nanocomposites. J. Photochem. Photobiol. A Chem. 2020, 396, 112507. [Google Scholar] [CrossRef]
- Kiwaan, H.A.; Atwee, T.M.; Azab, E.A.; El-Bindary, A.A. Photocatalytic Degradation of Organic Dyes in the Presence of Nanostructured Titanium Dioxide. J. Mol. Struct. 2020, 1200, 127115. [Google Scholar] [CrossRef]
- Mirikaram, N.; Pérez-Molina, Á.; Morales-Torres, S.; Salemi, A.; Maldonado-Hódar, F.J.; Pastrana-Martínez, L.M. Photocatalytic Performance of ZnO-Graphene Oxide Composites towards the Degradation of Vanillic Acid under Solar Radiation and Visible-LED. Nanomaterials 2021, 11, 1576. [Google Scholar] [CrossRef]
- Muhammad, W.; Khan, A.; Hussain, S.; Khan, H.; Abumousa, R.A.; Bououdina, M.; Khan, I.; Iqbal, S.; Humayun, M. Enhanced Light Absorption and Charge Carrier’s Separation in g-C3N4-Based Double Z-Scheme Heterostructure Photocatalyst for Efficient Degradation of Navy-Blue Dye. Green Chem. Lett. Rev. 2024, 17, 2381591. [Google Scholar] [CrossRef]
- Shah, B.R.; Patel, U.D. Reductive Transformation of Aqueous Pollutants Using Heterogeneous Photocatalysis: A Review. J. Inst. Eng. India Ser. A 2022, 103, 305–318. [Google Scholar] [CrossRef]
- Nugroho, D.; Thinthasit, A.; Wannakan, K.; Surya, R.; Nanan, S.; Benchawattananon, R. Well-Synthesized Carbon Dots from Flower of Cassia Fistula and Its Hydrothermally Grown Heterojunction Photocatalyst with Zinc Oxide (CDs@ZnO-H400) for Photocatalytic Degradation of Ciprofloxacin and Paracetamol. Arab. J. Chem. 2024, 17, 105931. [Google Scholar] [CrossRef]
- Jin, S.; Bang, G.; Liu, L.; Lee, C.-H. Synthesis of Mesoporous MgO–CeO2 Composites with Enhanced CO2 Capture Rate via Controlled Combustion. Microporous Mesoporous Mater. 2019, 288, 109587. [Google Scholar] [CrossRef]
- Peng, C.; Liu, H.C.; Wu, M.; Han, L.; Wang, Z. A Sensitive Electrochemical Sensor for Detection of Methyltestosterone as a Doping Agent in Sports by CeO2/CNTs Nanocomposite. Int. J. Electrochem. Sci. 2023, 18, 25–30. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Selvapandiyan, M.; Sasikumar, P.; Parthibavaraman, M.; Nithiyanantham, S.; Srisuvetha, V.T. Investigation on the Properties of Vanadium Doping WO3 Nanostructures by Hydrothermal Method. Mater. Sci. Energy Technol. 2022, 5, 411–415. [Google Scholar] [CrossRef]
- Pathak, T.K.; Coetsee-Hugo, E.; Swart, H.C.; Swart, C.W.; Kroon, R.E. Preparation and Characterization of Ce Doped ZnO Nanomaterial for Photocatalytic and Biological Applications. Mater. Sci. Eng. B 2020, 261, 114780. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, T. Development of Nanostructured Based ZnO@WO3 Photocatalyst and Its Photocatalytic and Electrochemical Properties: Degradation of Rhodamine B. Int. J. Electrochem. Sci. 2023, 18, 100055. [Google Scholar] [CrossRef]
- Meng, Q.; Cui, J.; Tang, Y.; Han, Z.; Zhao, K.; Zhang, G.; Diao, Q. Solvothermal Synthesis of Dual-Porous CeO2-ZnO Composite and Its Enhanced Acetone Sensing Performance. Ceram. Int. 2019, 45, 4103–4107. [Google Scholar] [CrossRef]
- Mishra, B.G.; Rao, G.R. Promoting Effect of Ceria on the Physicochemical and Catalytic Properties of CeO2–ZnO Composite Oxide Catalysts. J. Mol. Catal. A Chem. 2006, 243, 204–213. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Wang, Y.; Liang, Y.; Cao, W.; Su, Y. Ionic Liquid-Templated Synthesis of Mesoporous CeO2–TiO2 Nanoparticles and Their Enhanced Photocatalytic Activities under UV or Visible Light. J. Photochem. Photobiol. A Chem. 2011, 223, 157–164. [Google Scholar] [CrossRef]
- Bahadoran, A.; Ramakrishna, S.; Masudy-Panah, S.; Roshan De Lile, J.; Sadeghi, B.; Li, J.; Gu, J.; Liu, Q. Novel S-Scheme WO3/CeO2 Heterojunction with Enhanced Photocatalytic Degradation of Sulfamerazine under Visible Light Irradiation. Appl. Surf. Sci. 2021, 568, 150957. [Google Scholar] [CrossRef]
- Kumar, P.; Verma, S.; Korošin, N.Č.; Žener, B.; Štangar, U.L. Increasing the Photocatalytic Efficiency of ZnWO4 by Synthesizing a Bi2WO6/ZnWO4 Composite Photocatalyst. Catal. Today 2022, 397, 278–285. [Google Scholar] [CrossRef]
- Bonanni, M.; Spanhel, L.; Lerch, M.; Füglein, E.; Müller, G.; Jermann, F. Conversion of Colloidal ZnO−WO3 Heteroaggregates into Strongly Blue Luminescing ZnWO 4 Xerogels and Films. Chem. Mater. 1998, 10, 304–310. [Google Scholar] [CrossRef]
- Castaño, L.I.; Doria Herrera, G.M.; Grisales Castañeda, D.S. Wastewater Treatment by Heterogeneous Photocatalysis: A Systematic Review. Rev. Fac. De Cienc. Básicas 2021, 16, 51–64. [Google Scholar] [CrossRef]
- Wang, G.; Mu, Q.; Chen, T.; Wang, Y. Synthesis, Characterization and Photoluminescence of CeO2 Nanoparticles by a Facile Method at Room Temperature. J. Alloys Compd. 2010, 493, 202–207. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. Investigation on the Synthesis and Photocatalytic Activity of Activated Carbon–Cerium Oxide (AC–CeO2) Nanocomposite. Appl. Phys. A 2019, 125, 742. [Google Scholar] [CrossRef]
- Balogun, S.W.; James, O.O.; Sanusi, Y.K.; Olayinka, O.H. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Bashful (Mimosa Pudica), Leaf Extract: A Precursor for Organic Electronics Applications. SN Appl. Sci. 2020, 2, 504. [Google Scholar] [CrossRef]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Ahamed, M. Bi2O3 -Doped WO3 Nanoparticles Decorated on rGO Sheets: Simple Synthesis, Characterization, Photocatalytic Performance, and Selective Cytotoxicity toward Human Cancer Cells. ACS Omega 2023, 8, 25020–25033. [Google Scholar] [CrossRef]
- Zhao, X.; Yao, W.; Wu, Y.; Zhang, S.; Yang, H.; Zhu, Y. Fabrication and Photoelectrochemical Properties of Porous ZnWO4 Film. J. Solid State Chem. 2006, 179, 2562–2570. [Google Scholar] [CrossRef]
- Aboukaïs, A.; Skaf, M.; Hany, S.; Cousin, R.; Aouad, S.; Labaki, M.; Abi-Aad, E. A Comparative Study of Cu, Ag and Au Doped CeO2 in the Total Oxidation of Volatile Organic Compounds (VOCs). Mater. Chem. Phys. 2016, 177, 570–576. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, L.; Tu, X.; Hu, F. Hydrothermal Synthesis of ZnO Crystals: Diverse Morphologies and Characterization of the Photocatalytic Properties. Polyhedron 2023, 246, 116668. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Liu, X.; Yang, X.; Yang, Y. A Ternary Magnetic Recyclable ZnO/Fe3O4/g-C3N4 Composite Photocatalyst for Efficient Photodegradation of Monoazo Dye. Nanoscale Res. Lett. 2019, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, I.M.; Fórizs, B.; Rosseler, O.; Szegedi, Á.; Németh, P.; Király, P.; Tárkányi, G.; Vajna, B.; Varga-Josepovits, K.; László, K.; et al. WO3 Photocatalysts: Influence of Structure and Composition. J. Catal. 2012, 294, 119–127. [Google Scholar] [CrossRef]
- Xie, Y.P.; Liu, G.; Yin, L.; Cheng, H.-M. Crystal Facet-Dependent Photocatalytic Oxidation and Reduction Reactivity of Monoclinic WO3 for Solar Energy Conversion. J. Mater. Chem. 2012, 22, 6746. [Google Scholar] [CrossRef]
- Yadav, V.; Verma, P.; Sharma, H.; Tripathy, S.; Saini, V.K. Photodegradation of 4-Nitrophenol over B-Doped TiO2 Nanostructure: Effect of Dopant Concentration, Kinetics, and Mechanism. Environ. Sci. Pollut. Res. 2020, 27, 10966–10980. [Google Scholar] [CrossRef]
- Shih, C.J.; Chen, Y.J.; Hon, M.H. Synthesis and Crystal Kinetics of Cerium Oxide Nanocrystallites Prepared by Co-Precipitation Process. Mater. Chem. Phys. 2010, 121, 99–102. [Google Scholar] [CrossRef]
- Klubnuan, S.; Suwanboon, S.; Amornpitoksuk, P. Effects of Optical Band Gap Energy, Band Tail Energy and Particle Shape on Photocatalytic Activities of Different ZnO Nanostructures Prepared by a Hydrothermal Method. Opt. Mater. 2016, 53, 134–141. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Han, D.H.; Lee, J.; Cho, M.H. Defect-Induced Band Gap Narrowed CeO2 Nanostructures for Visible Light Activities. Ind. Eng. Chem. Res. 2014, 53, 9754–9763. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Rafique, M.; Khalid, N.R. Nanostructured-Based WO3 Photocatalysts: Recent Development, Activity Enhancement, Perspectives and Applications for Wastewater Treatment. Int. J. Environ. Sci. Technol. 2017, 14, 2519–2542. [Google Scholar] [CrossRef]
- Tseng, C.-F.; Hsu, W.-Y. Sol–Gel Derived ZnO–CeO2 Thin Films on Glass Substrate. Thin Solid Film 2013, 544, 44–47. [Google Scholar] [CrossRef]
- He, D.; Wang, L.; Xu, D.; Zhai, J.; Wang, D.; Xie, T. Investigation of Photocatalytic Activities over Bi2 WO6 /ZnWO4 Composite under UV Light and Its Photoinduced Charge Transfer Properties. ACS Appl. Mater. Interfaces 2011, 3, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, J.; Liang, Q.; Li, Y.; Yu, H. CoS/ZnWO4 Composite with Band Gap Matching: Simple Impregnation Synthesis, Efficient Dye Sensitization System for Hydrogen Production. J. Nanoparticle Res. 2020, 22, 114. [Google Scholar] [CrossRef]
- Jin, Y.; Govoni, M.; Wolfowicz, G.; Sullivan, S.E.; Heremans, F.J.; Awschalom, D.D.; Galli, G. Photoluminescence Spectra of Point Defects in Semiconductors: Validation of First-Principles Calculations. Phys. Rev. Mater. 2021, 5, 084603. [Google Scholar] [CrossRef]
- Babitha, K.K.; Priyanka, K.P.; Sreedevi, A.; Ganesh, S.; Varghese, T. Effect of 8 MeV Electron Beam Irradiation on the Structural and Optical Properties of CeO2 Nanoparticles. Mater. Charact. 2014, 98, 222–227. [Google Scholar] [CrossRef]
- Meng, F.; Wang, L.; Cui, J. Controllable Synthesis and Optical Properties of Nano-CeO2 via a Facile Hydrothermal Route. J. Alloys Compd. 2013, 556, 102–108. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Ce3+-Ion, Surface Oxygen Vacancy, and Visible Light-Induced Photocatalytic Dye Degradation and Photocapacitive Performance of CeO2-Graphene Nanostructures. Sci. Rep. 2017, 7, 5928. [Google Scholar] [CrossRef]
- Ai, X.; Yan, S.; Lin, C.; Lu, K.; Chen, Y.; Ma, L. Facile Fabrication of Highly Active CeO2@ZnO Nanoheterojunction Photocatalysts. Nanomaterials 2023, 13, 1371. [Google Scholar] [CrossRef] [PubMed]
- Petruleviciene, M.; Juodkazyte, J.; Parvin, M.; Tereshchenko, A.; Ramanavicius, S.; Karpicz, R.; Samukaite-Bubniene, U.; Ramanavicius, A. Tuning the Photo-Luminescence Properties of WO3 Layers by the Adjustment of Layer Formation Conditions. Materials 2020, 13, 2814. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, W.; Jin, L.; Cui, F.; Song, Z.; Zhu, C. High Performance Acetylene Sensor with Heterostructure Based on WO3 Nanolamellae/Reduced Graphene Oxide (rGO) Nanosheets Operating at Low Temperature. Nanomaterials 2018, 8, 909. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Krishnan, A.; Beena, S.; Chandran, M. Fabrication and Evaluation of CeO2-Fe2O3 Mixed Oxide for Hydrogen Evolution by Photo Water Splitting Reaction under Visible Light Irradiation. Mater. Today Proc. 2019, 18, 4968–4976. [Google Scholar] [CrossRef]
- Charipar, K.; Kim, H.; Piqué, A.; Charipar, N. ZnO Nanoparticle/Graphene Hybrid Photodetectors via Laser Fragmentation in Liquid. Nanomaterials 2020, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Kaftelen, H.; Ocakoglu, K.; Thomann, R.; Tu, S.; Weber, S.; Erdem, E. EPR and Photoluminescence Spectroscopy Studies on the Defect Structure of ZnO Nanocrystals. Phys. Rev. B 2012, 86, 014113. [Google Scholar] [CrossRef]
- Hussein, H.M. Photosensitive Analysis of Spin Coated Cu Doped ZnO Thin Film Synthesized by Hydrothermal Method. Results Opt. 2023, 13, 100543. [Google Scholar] [CrossRef]
- Sherly, E.D.; Vijaya, J.J.; Kennedy, L.J. Effect of CeO2 Coupling on the Structural, Optical and Photocatalytic Properties of ZnO Nanoparticle. J. Mol. Struct. 2015, 1099, 114–125. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, J.; Liu, J.; Ren, Y.; Lin, B.; Tao, J.; Zhu, X. Synthesis of ZnWO4 Nano-Particles by a Molten Salt Method. Mater. Lett. 2007, 61, 4595–4598. [Google Scholar] [CrossRef]
- Itoh, M.; Katagiri, T.; Aoki, T.; Fujita, M. Photo-Stimulated Luminescence and Photo-Induced Infrared Absorption in ZnWO4. Radiat. Meas. 2007, 42, 545–548. [Google Scholar] [CrossRef]
- Jia, R.-P.; Zhang, G.-X.; Wu, Q.-S.; Ding, Y.-P. ZnWO4–TiO2 Composite Nanofilms: Preparation, Morphology, Structure and Photoluminescent Enhancement. Mater. Lett. 2007, 61, 1793–1797. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Wu, X.; Chen, P.; Deng, N. Photodegradation of Acetaminophen in TiO2 Suspended Solution. J. Hazard. Mater. 2008, 157, 300–307. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Beg, A.E. The Effect of pH and Various Additives on Extinction Coefficients for p-Nitrophenol. J. Chem. Soc. Pak. 1984, 6, 55–61. [Google Scholar]
- Asadzadeh, S.N.; Malakootian, M.; Mehdipoor, M.; Neyestanaki, D.K. The Removal of Tetracycline with Biogenic CeO2 Nanoparticles in Combination with US/PMS Process from Aqueous Solutions: Kinetics and Mechanism. Water Sci. Technol. 2021, 83, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Anik, M.; Cansizoglu, T. Dissolution Kinetics of WO3 in Acidic Solutions. J. Appl. Electrochem. 2006, 36, 603–608. [Google Scholar] [CrossRef]
- Degen, A.; Kosec, M. Efect of pH and Impurities on the Surface Charge of Zinc Oxide in Aqueous Solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- Marcelino, R.B.P.; Amorim, C.C. Towards Visible-Light Photocatalysis for Environmental Applications: Band-Gap Engineering versus Photons Absorption—A Review. Environ. Sci. Pollut. Res. 2019, 26, 4155–4170. [Google Scholar] [CrossRef]
- Huisman, R.; Van Kamp, H.V.; Weyland, J.W.; Doornbos, D.A.; Bolhuis, G.K.; Lerk, C.F. Development and Optimization of Pharmaceutical Formulations Using a Simplex Lattice Design. Pharm. Weekbl. Sci. Ed. 1984, 6, 185–194. [Google Scholar] [CrossRef]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data, 3rd ed.; Wiley Series in Probability and Statistics; Wiley: New York, NY, USA, 2002; ISBN 978-0-471-39367-2. [Google Scholar]
- TAO, X.; Shao, L.; Wang, R.; Xiang, H.; Li, B. Synthesis of BiVO4 Nanoflakes Decorated with AuPd Nanoparticles as Selective Oxidation Photocatalysts. J. Colloid Interface Sci. 2019, 541, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Xu, D.; Shi, W. Synthesis Plasmonic Bi/BiVO4 Photocatalysts with Enhanced Photocatalytic Activity for Degradation of Tetracycline (TC). Chin. J. Chem. Eng. 2019, 27, 3053–3059. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, Y.; Kong, J.; Yuan, J.; Sun, C.; Xian, Q.; Yang, S.; He, H. High Photocatalytic Degradation Efficiency of Oxytetracycline Hydrochloride over Ag/AgCl/BiVO4 Plasmonic Photocatalyst. Solid State Sci. 2019, 96, 105946. [Google Scholar] [CrossRef]
- Gecol, H.; Ergican, E.; Miakatsindila, P. Biosorbent for Tungsten Species Removal from Water: Effects of Co-Occurring Inorganic Species. J. Colloid Interface Sci. 2005, 292, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, V.P.; Matviichuk, A.A. Computer Modeling of Chemical Equilibria in the WO3–H2O System. Energy Technol. Resour. Sav. 2017, 4, 35–48. [Google Scholar] [CrossRef]


| Sample | ZnO (%) | WO3 (%) | CeO2 (%) | Amount (g) | Symbol |
|---|---|---|---|---|---|
| 1 | 100 | - | - | 13.95 | Zn |
| 2 | - | 100 | - | 9.41 | W |
| 3 | - | - | 100 | 4.02 | Ce |
| 4 | 50 | 50 | - | 2.28/407 | Zn-W |
| 5 | - | 50 | 50 | 4.43/6.4 | W-Ce |
| 6 | 50 | - | 50 | 3.65/2.97 | Zn-Ce |
| 7 | 33 | 33 | 33 | 3.12/2.53/4.51 | Zn-W-Ce |
| Sample | CeO2 * (nm) | ZnO * (nm) | WO3 * (nm) | ZnWO4 * (nm) | Eg (eV) | ABET (m2/g) | DPDFT (nm) |
|---|---|---|---|---|---|---|---|
| Zn | - | 70 | - | - | 3.1 | 0.43 | 4.72 |
| W | - | - | 35 | - | 2.3 | 2.3 | 17.28 |
| Ce | 10 | - | - | - | 3.1 | 80 | 13.4 |
| Zn-W | - | - | - | 13 | 3.2 | 6.43 | 27.4 |
| W-Ce | 9 | - | 7 | - | 3.0 | 18.8 | 27.3 |
| Zn-Ce | 10 | 56 | - | - | 2.9 | 2.90 | 3.65 |
| Zn-W-Ce | 7 | - | - | 9 | 2.2 | 24.9 | 30.4 |
| 2Zn-W-Ce | 7 | - | - | 10 | 2.2 | 24.9 | 30.4 |
| 3Zn-W-Ce | 7 | - | - | 10 | 2.2 | 24.9 | 30.4 |
| Sample | -ri * (103) (ppm/min * gcat) | K * (103) (min−1) | t1/2 (h) | x (%) | R2 |
|---|---|---|---|---|---|
| Photolysis | 0.537 | 0.62 | 18.65 | 20 | 0.993 |
| Zn | 11.8 | 2.46 | 4.68 | 72 | 0.999 |
| W | 22.5 | 4.48 | 2.60 | 78 | 0.978 |
| Ce | 20.2 | 4.41 | 2.61 | 78 | 0.979 |
| Zn-Ce | 17.1 | 3.17 | 3.38 | 72 | 0.987 |
| W-Ce | 25.3 | 4.76 | 2.43 | 79 | 0.993 |
| Zn-W | 28.6 | 5.11 | 2.26 | 81 | 0.996 |
| Zn-W-Ce | 15.6 | 2.54 | 4.53 | 62 | 0.978 |
| 2Zn-W-Ce | 14.3 | 2.29 | 5.03 | 60 | 0.983 |
| 3Zn-W-Ce | 16.1 | 2.69 | 4.28 | 64 | 0.996 |
| Model | R2 | R2 Adjusted | SS | dF | MS | F | p-Value |
|---|---|---|---|---|---|---|---|
| Lineal | 0.3184 | 0.0912 | 3.241 | 2 | 1.621 | 1.41 | 0.316 |
| Quadratic | 0.5726 | 0 | 5.831 | 5 | 1.166 | 0.803 | 0.613 |
| Cubic | 0.992 | 0.9679 | 10.1 | 6 | 1.683 | 41.22 | 0.023 |
| Factor | Coeff. | Est. Err | Statistical T | p-Value |
|---|---|---|---|---|
| Zinc Nitrate (A) | 2.46 | 0.2021 | 12.17 | 0.006 |
| Cerium Nitrate (B) | 4.41 | 0.0201 | 21.82 | 0.002 |
| Ammonium tungstate (C) | 4.48 | 0.2021 | 22.17 | 0.002 |
| AB | −1.060 | 0.9899 | −1.07 | 0.396 |
| AC | 6.560 | 0.9899 | 6.62 | 0.022 |
| BC | 1.260 | 0.9899 | 1.270 | 0.331 |
| ABC | −54.75 | 5.3597 | −10.22 | 0.009 |
| Model | R2 | R2 Adjusted | SS | dF | MS | F | p-Value |
|---|---|---|---|---|---|---|---|
| Lineal | 0.1649 | 0 | 0.265 | 2 | 0.132 | 0.59 | 0.582 |
| Quadratic | 0.4772 | 0 | 0.767 | 5 | 0.153 | 0.547 | 0.740 |
| Cubic | 1 | 1 | 1.608 | 6 | 0.268 | 3.36 | 0.043 |
| Factor | Coeff. | Est. Err | Statistical T | p-Value |
|---|---|---|---|---|
| Zinc Nitrate (A) | 3.100 | 0 | ||
| Cerium Nitrate (B) | 3.100 | 0 | ||
| Ammonium tungstate (C) | 2.300 | 0 | ||
| AB | −0.800 | 0 | 0 | 0 |
| AC | 2.000 | 0 | 0 | 0 |
| BC | 1.200 | 0 | 0 | 0 |
| ABC | −24.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alejandro-López, A.R.; Serrano de la Rosa, L.E.; Castillejos-Mosqueda, S.L.; Córdova-Pérez, G.E.; Cerna-Cortez, J.R.; M. Gómez, C.; Silahua-Pavón, A.A.; Saavedra-Díaz, R.O.; Godavarthi, S.; Cervantes-Uribe, A. Role of Surface Charge in the Speciation and Photocatalytic Degradation of 4-Nitrophenol Using ZnO–CeO2–WO3 Photocatalysts. J. Compos. Sci. 2025, 9, 646. https://doi.org/10.3390/jcs9120646
Alejandro-López AR, Serrano de la Rosa LE, Castillejos-Mosqueda SL, Córdova-Pérez GE, Cerna-Cortez JR, M. Gómez C, Silahua-Pavón AA, Saavedra-Díaz RO, Godavarthi S, Cervantes-Uribe A. Role of Surface Charge in the Speciation and Photocatalytic Degradation of 4-Nitrophenol Using ZnO–CeO2–WO3 Photocatalysts. Journal of Composites Science. 2025; 9(12):646. https://doi.org/10.3390/jcs9120646
Chicago/Turabian StyleAlejandro-López, Alma Rosa, Laura Elvira Serrano de la Rosa, Sandra Leticia Castillejos-Mosqueda, Gerardo E. Córdova-Pérez, Jorge R. Cerna-Cortez, Claudia M. Gómez, Adib Abiu Silahua-Pavón, Rafael Omar Saavedra-Díaz, Srinivas Godavarthi, and Adrián Cervantes-Uribe. 2025. "Role of Surface Charge in the Speciation and Photocatalytic Degradation of 4-Nitrophenol Using ZnO–CeO2–WO3 Photocatalysts" Journal of Composites Science 9, no. 12: 646. https://doi.org/10.3390/jcs9120646
APA StyleAlejandro-López, A. R., Serrano de la Rosa, L. E., Castillejos-Mosqueda, S. L., Córdova-Pérez, G. E., Cerna-Cortez, J. R., M. Gómez, C., Silahua-Pavón, A. A., Saavedra-Díaz, R. O., Godavarthi, S., & Cervantes-Uribe, A. (2025). Role of Surface Charge in the Speciation and Photocatalytic Degradation of 4-Nitrophenol Using ZnO–CeO2–WO3 Photocatalysts. Journal of Composites Science, 9(12), 646. https://doi.org/10.3390/jcs9120646







