Transfer the Sulfate Environment into a Beneficial Factor: Performance Enhancement and Mechanism of Electrolytic Manganese Residue-Based Mine Filling Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Perparation
2.2. Experiment Methods
2.2.1. Basic Performance
2.2.2. Sulfate Resistance
2.3. Microscopic Testing
3. Results and Discussion
3.1. Basic Performance
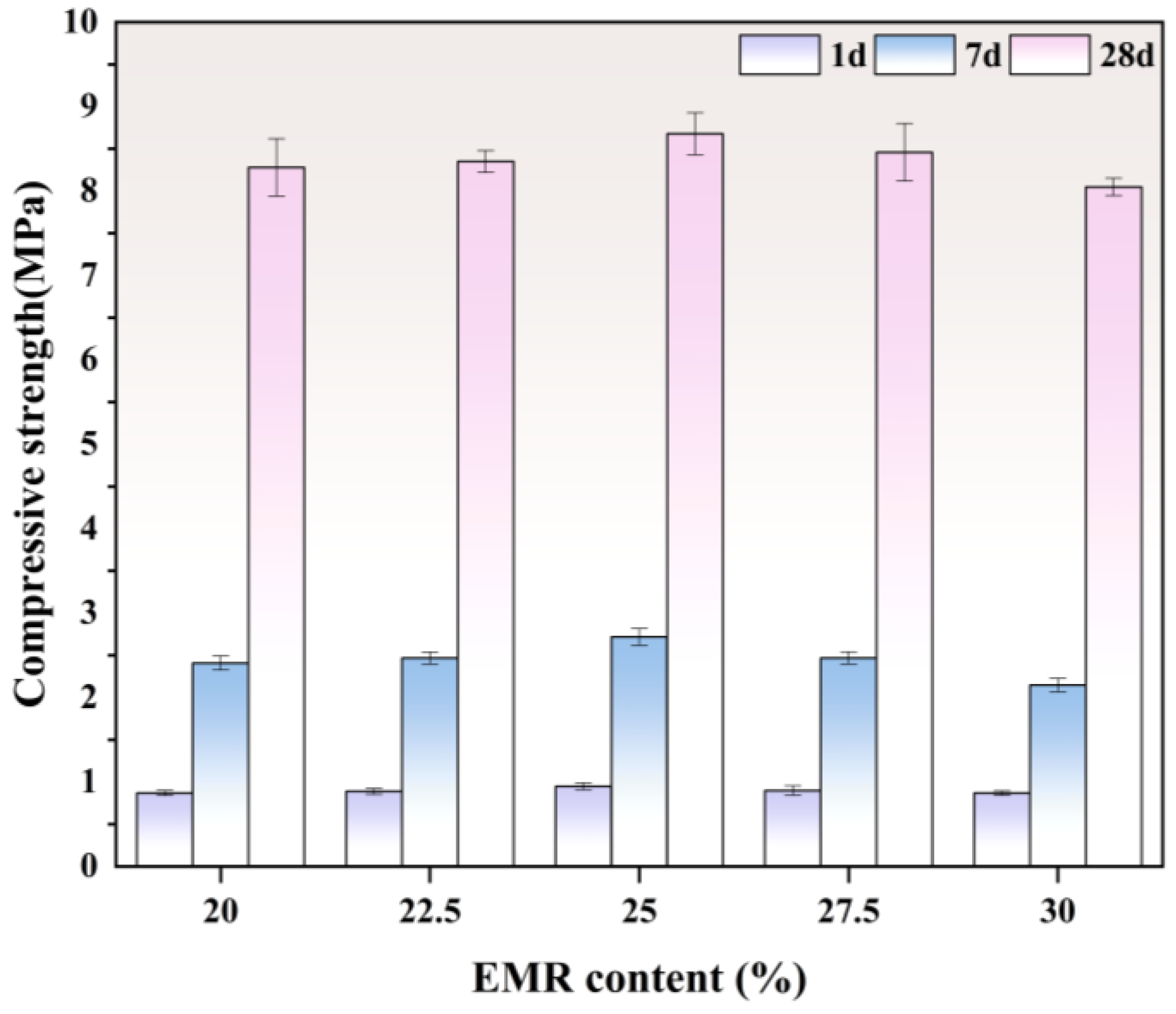
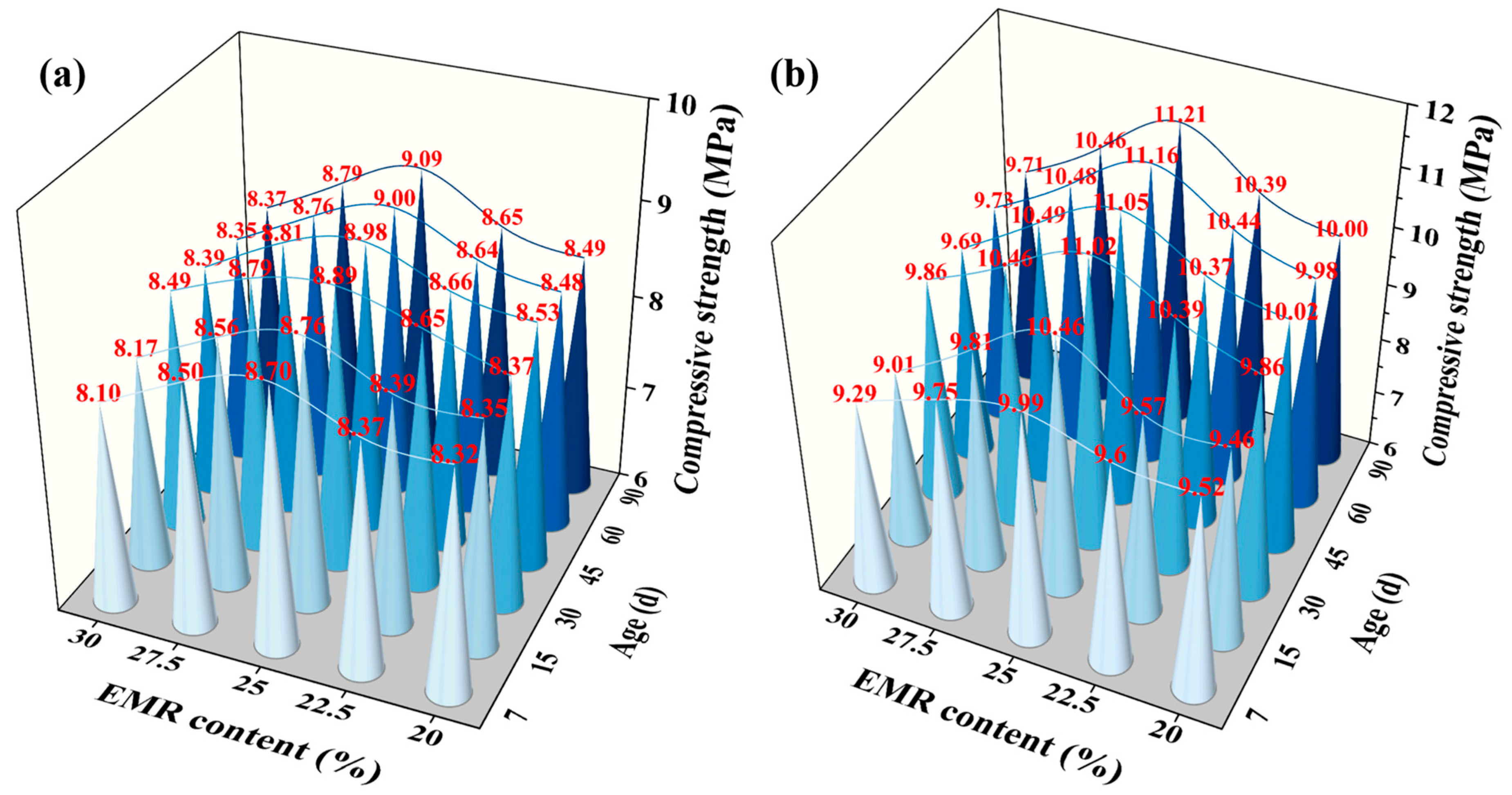
3.1.1. Compressive Strength
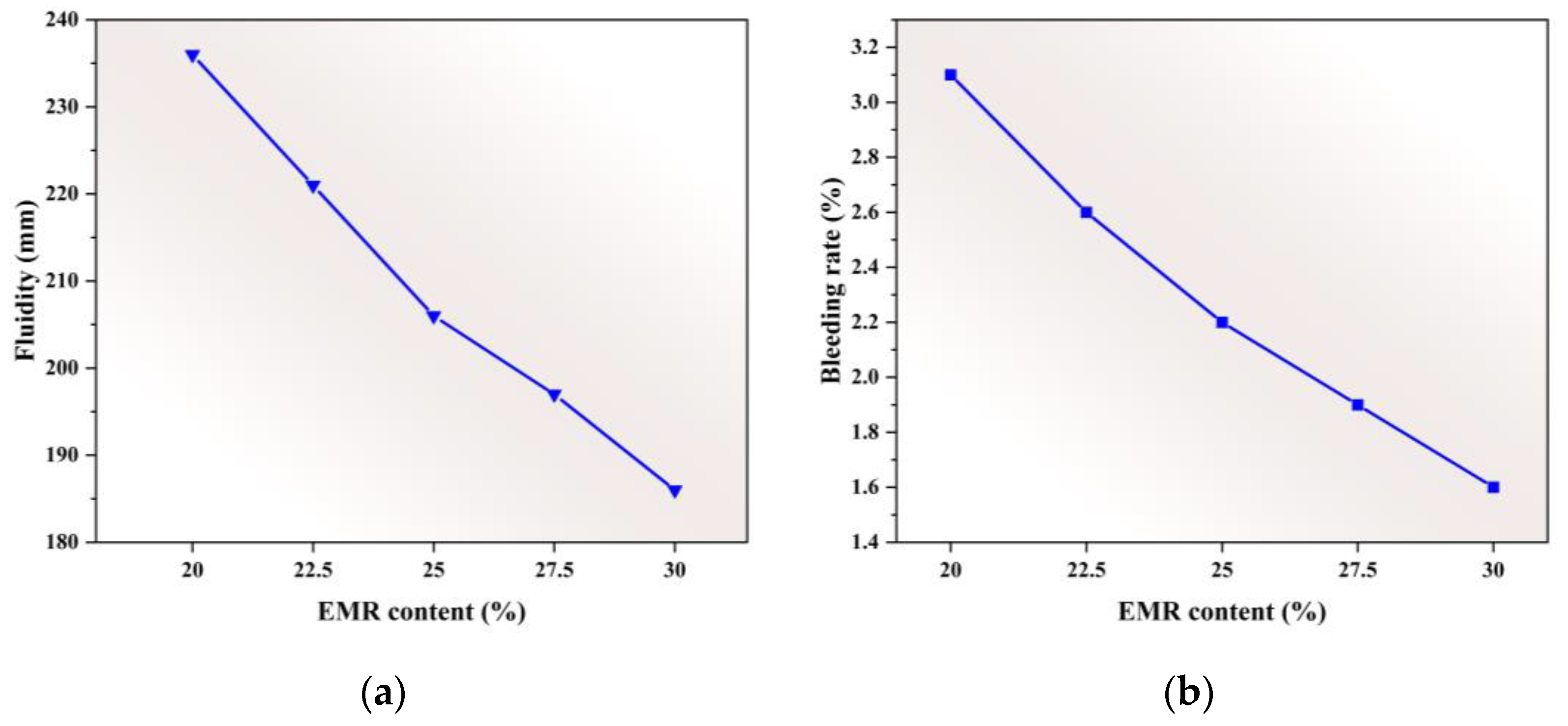
3.1.2. Fluidity and Bleeding Rate
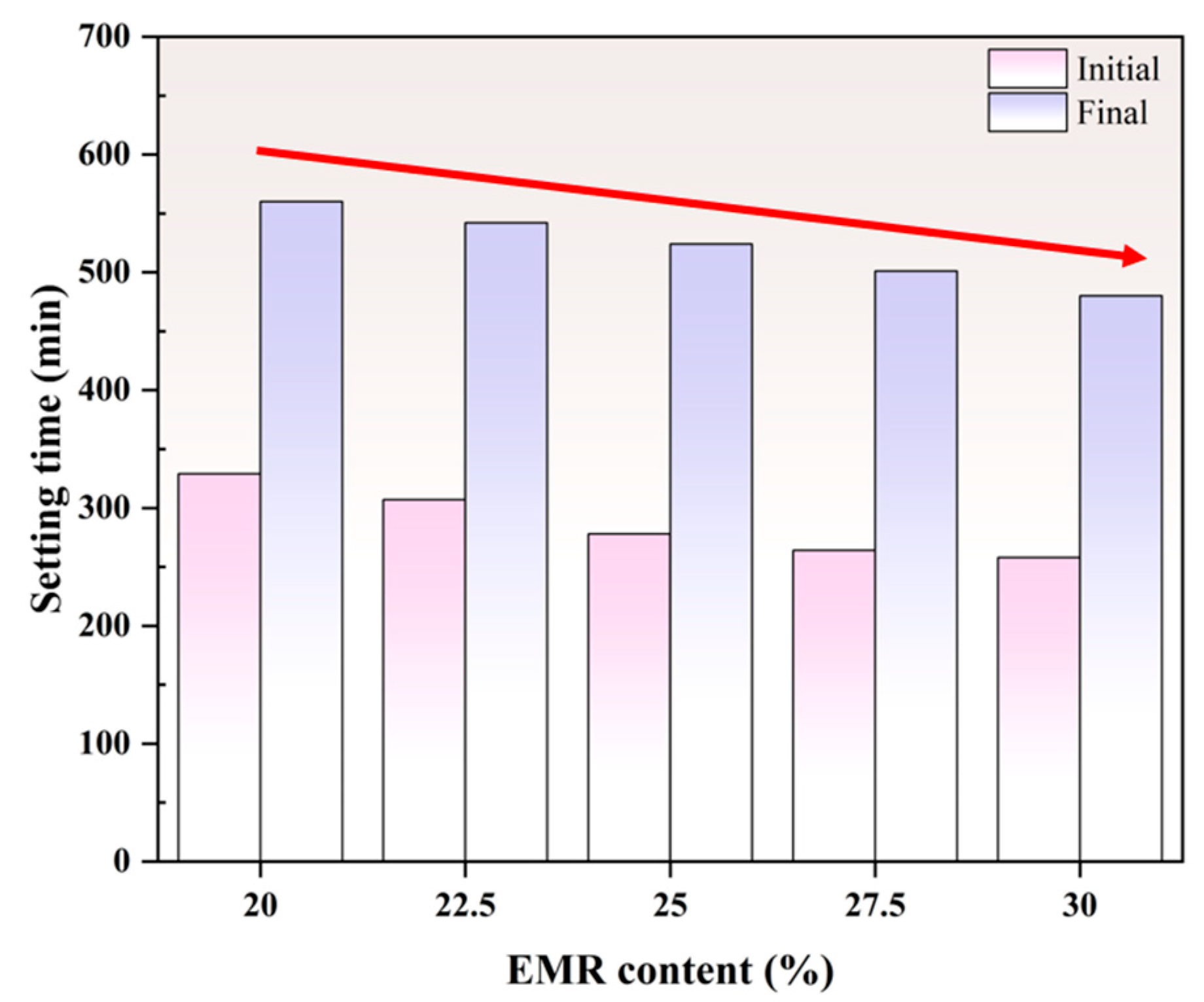
3.1.3. Setting Time
3.2. Sulfate Resistance
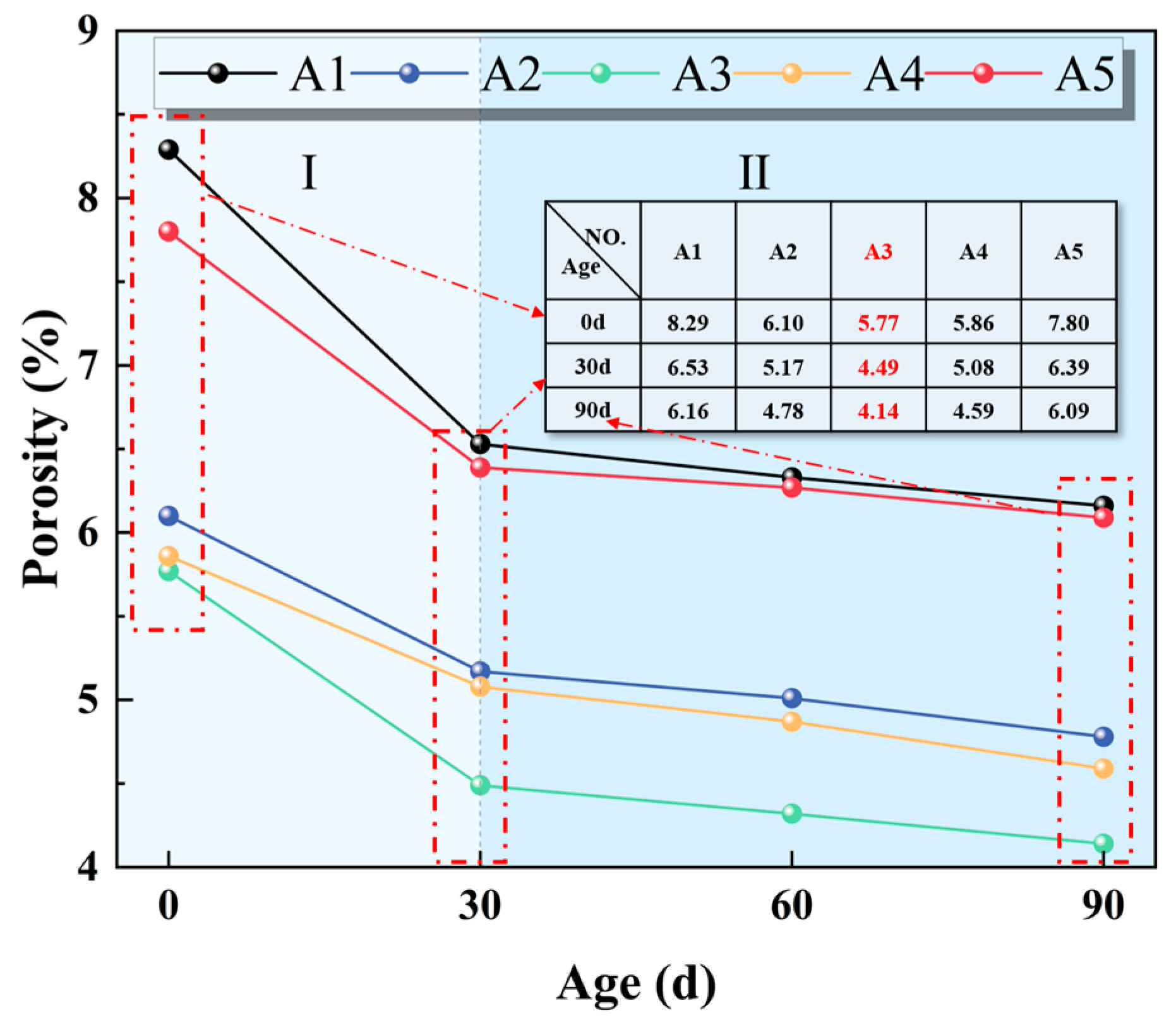
3.2.1. Porosity
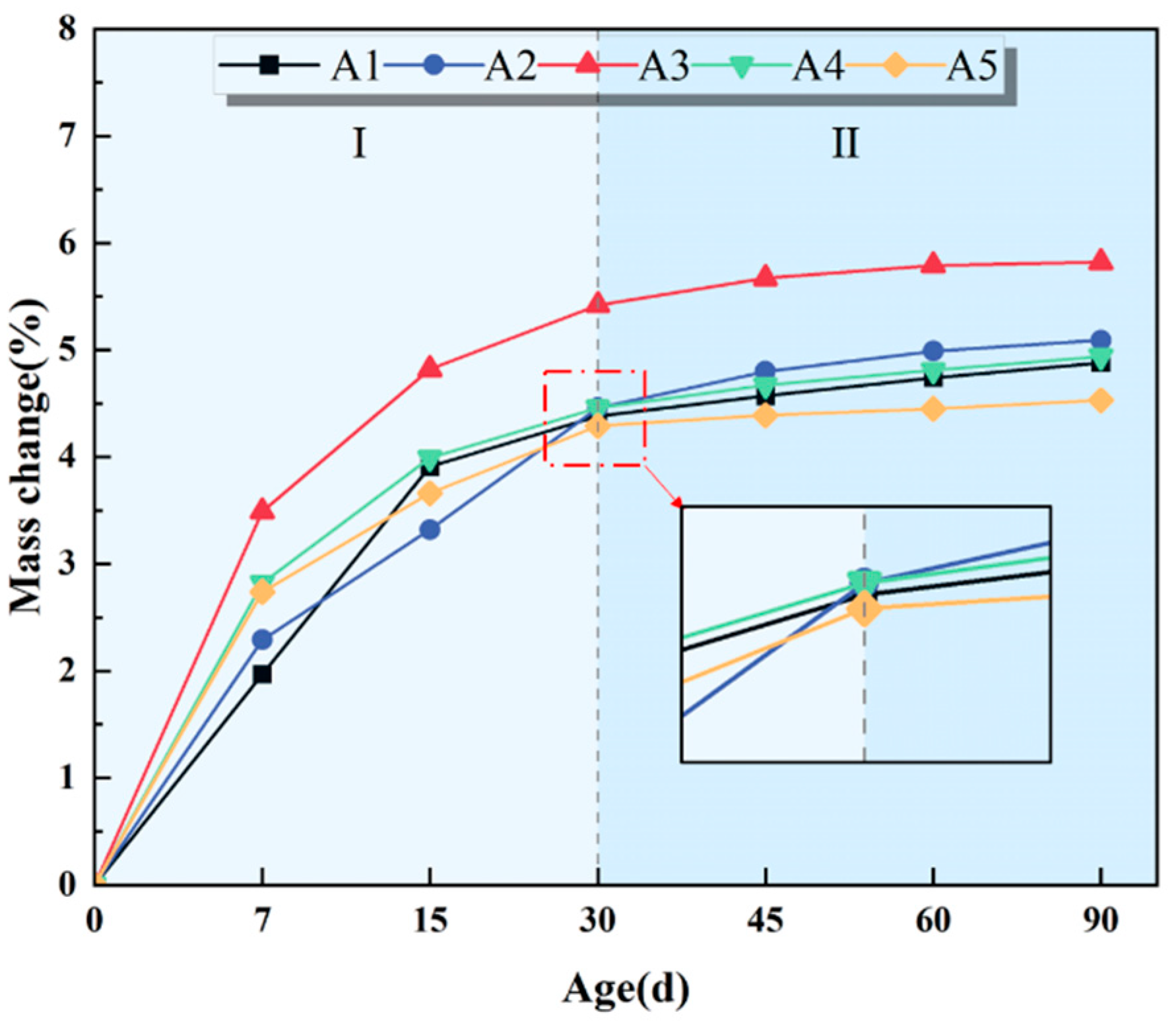
3.2.2. Mass Change
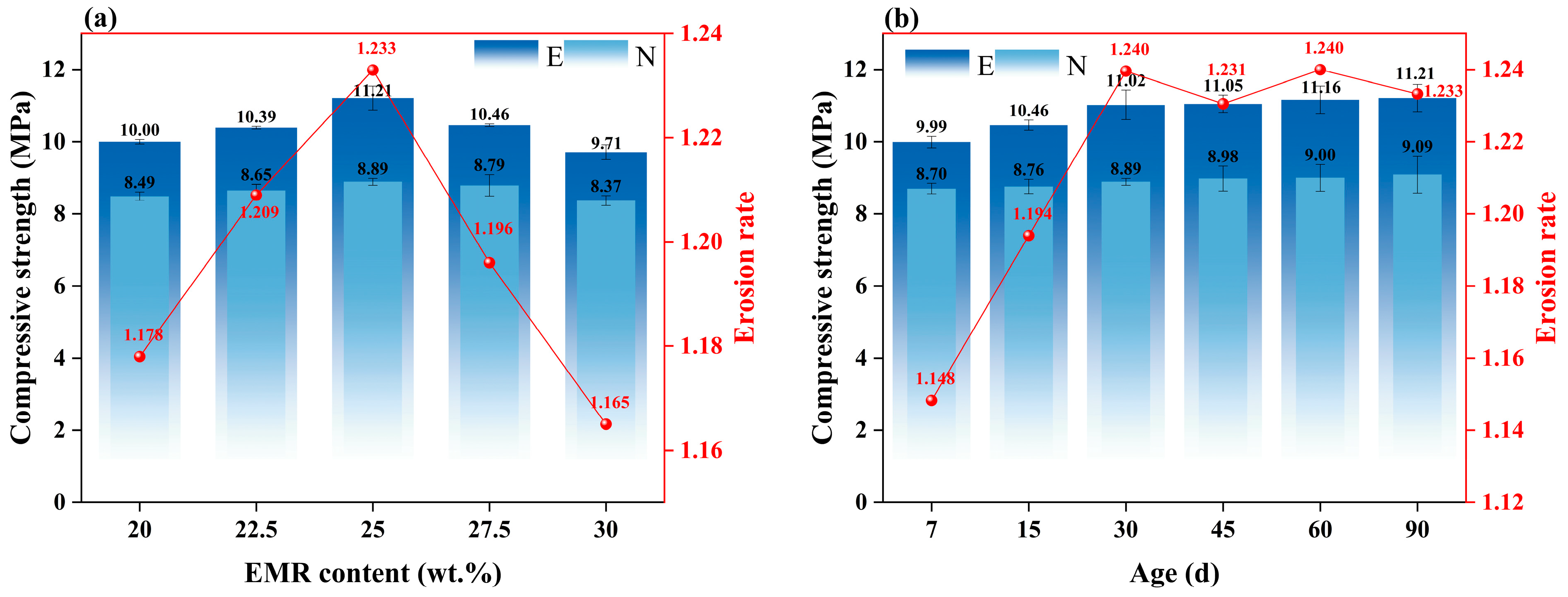
3.2.3. Corrosion Resistance Coefficient
3.3. Microscope Analysis
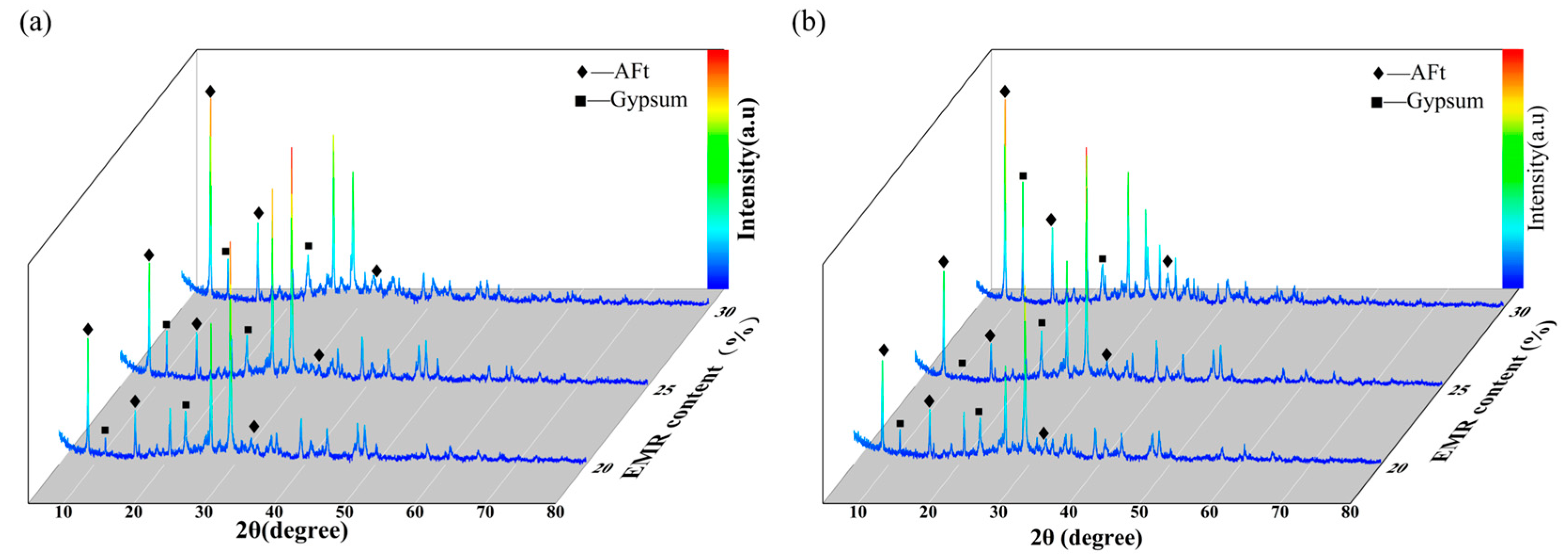
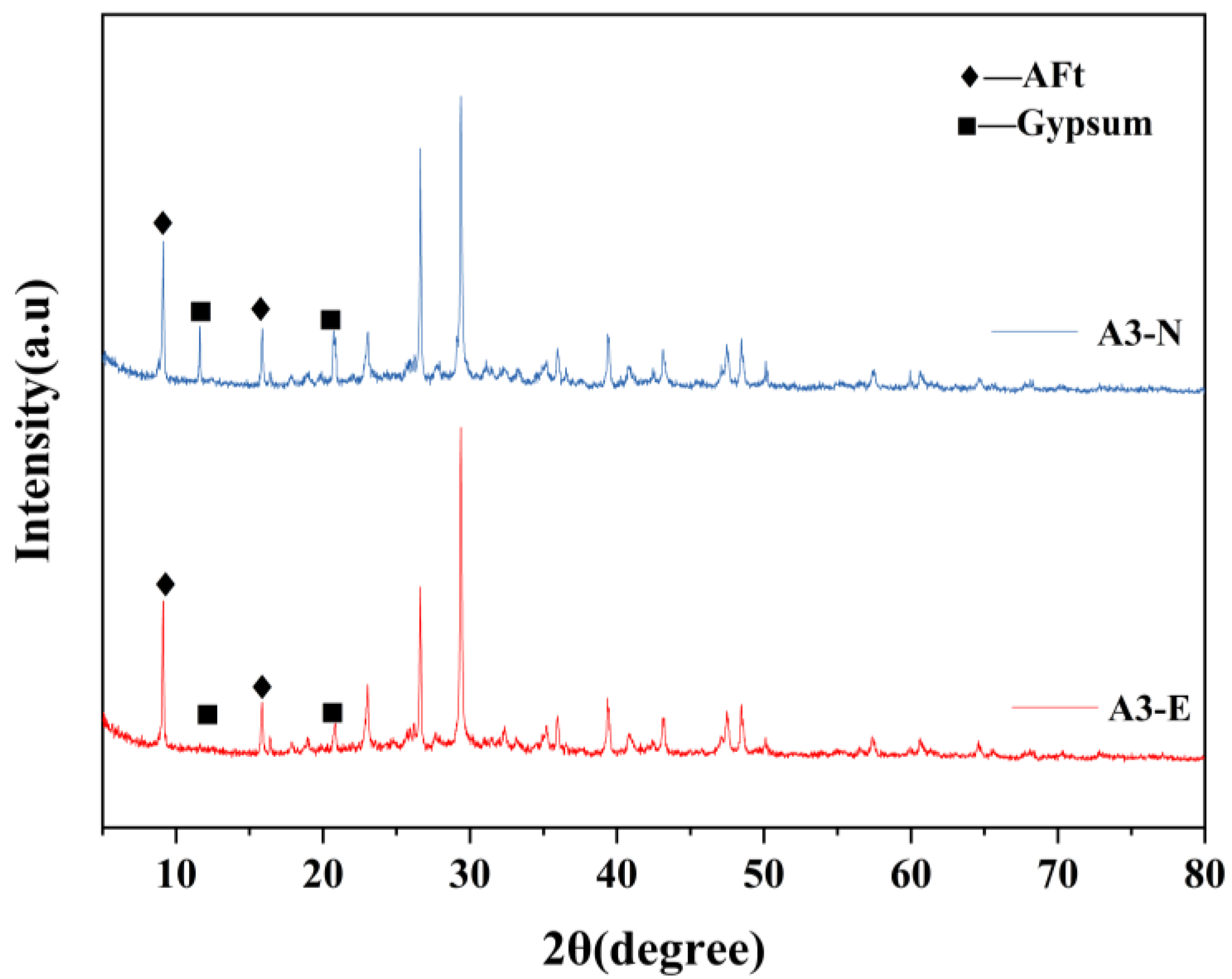
3.3.1. XRD
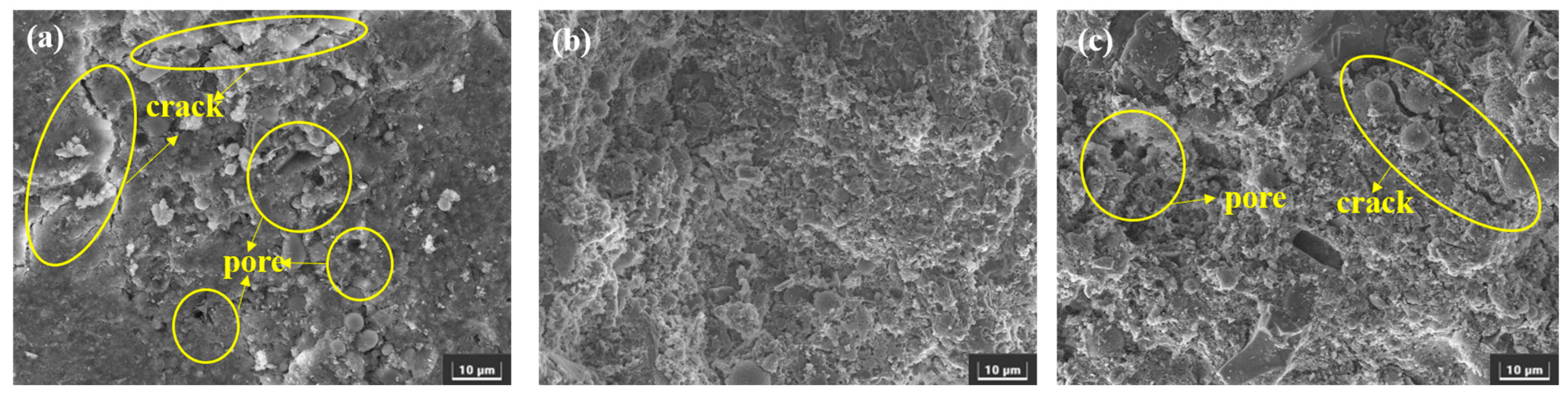
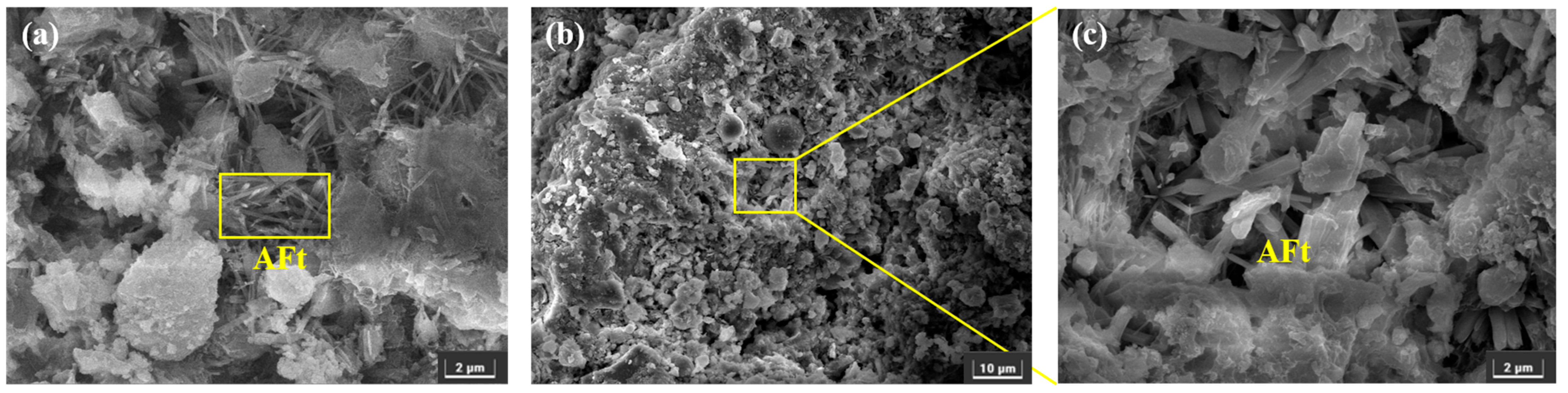
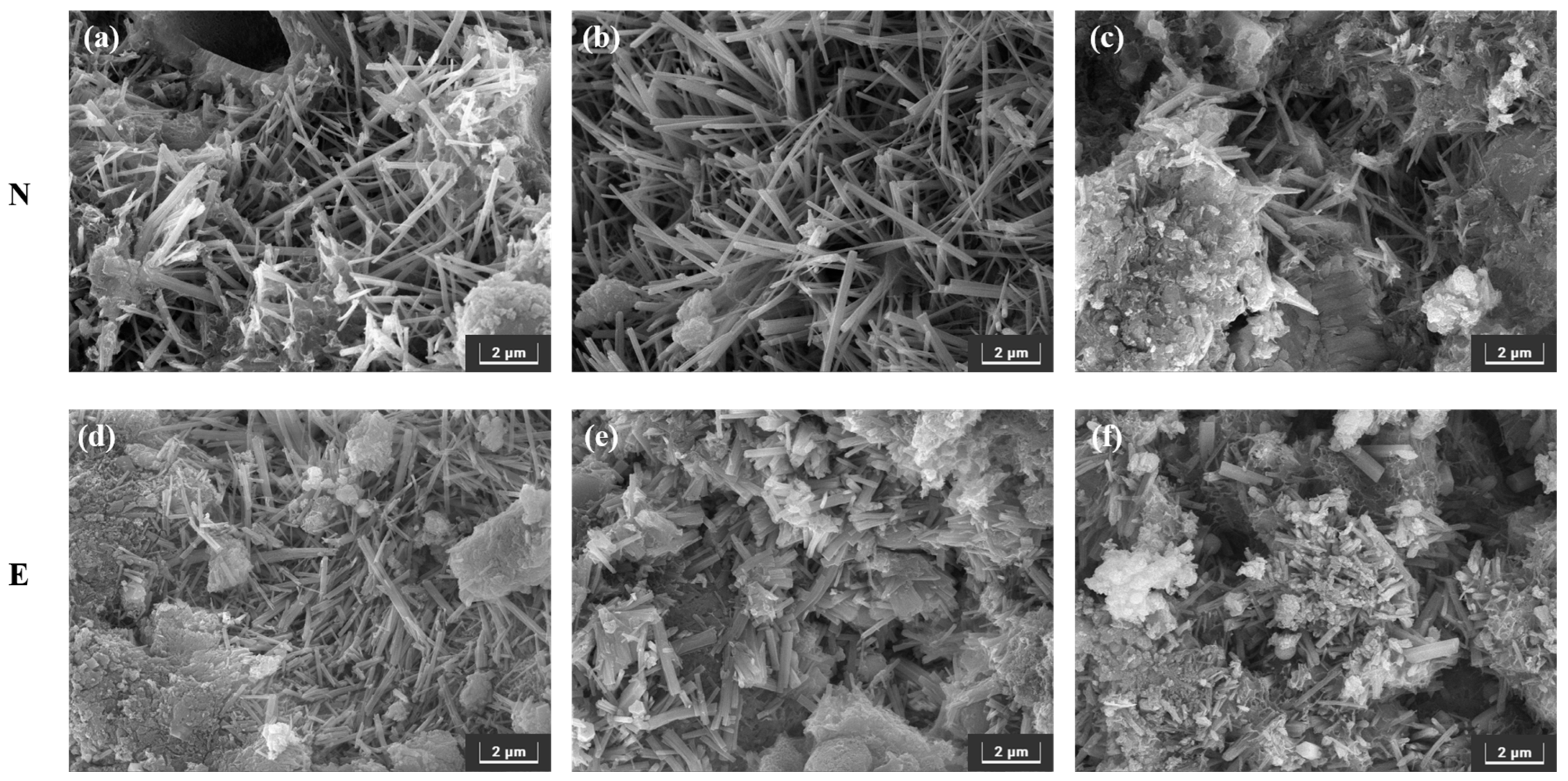
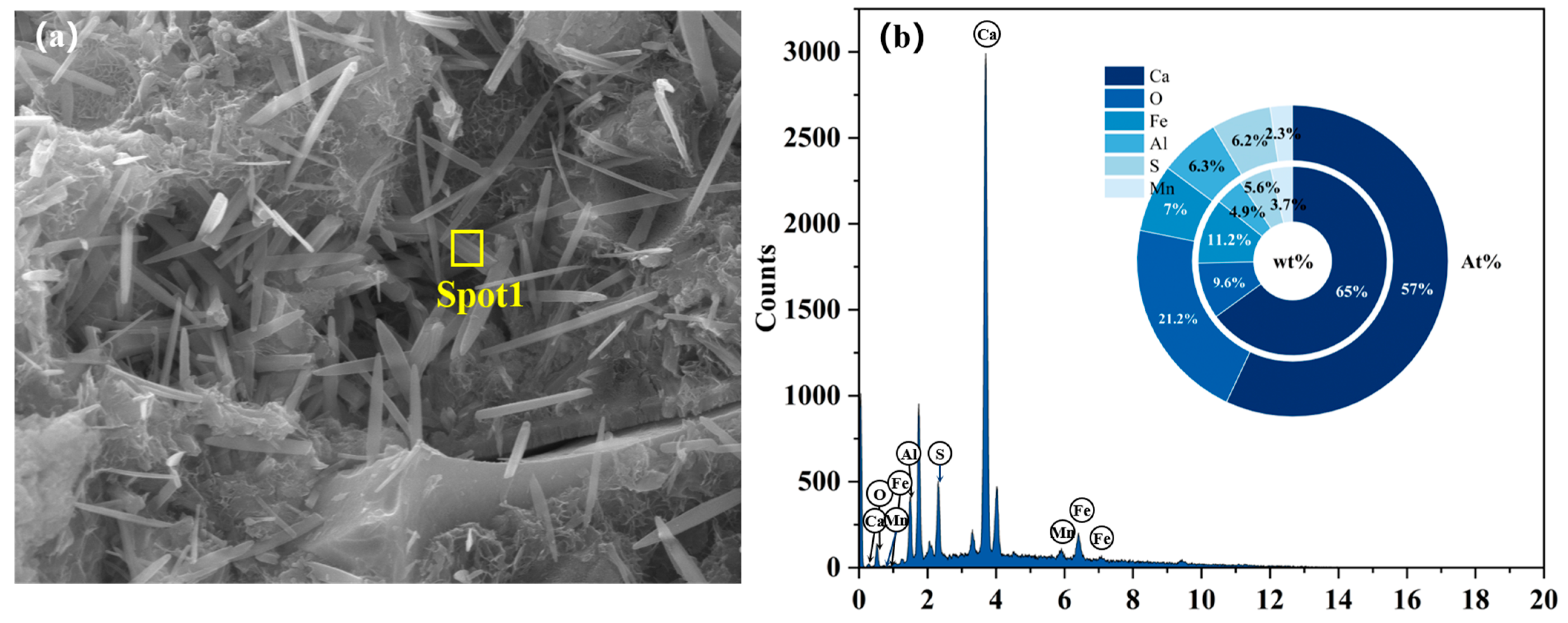
3.3.2. SEM
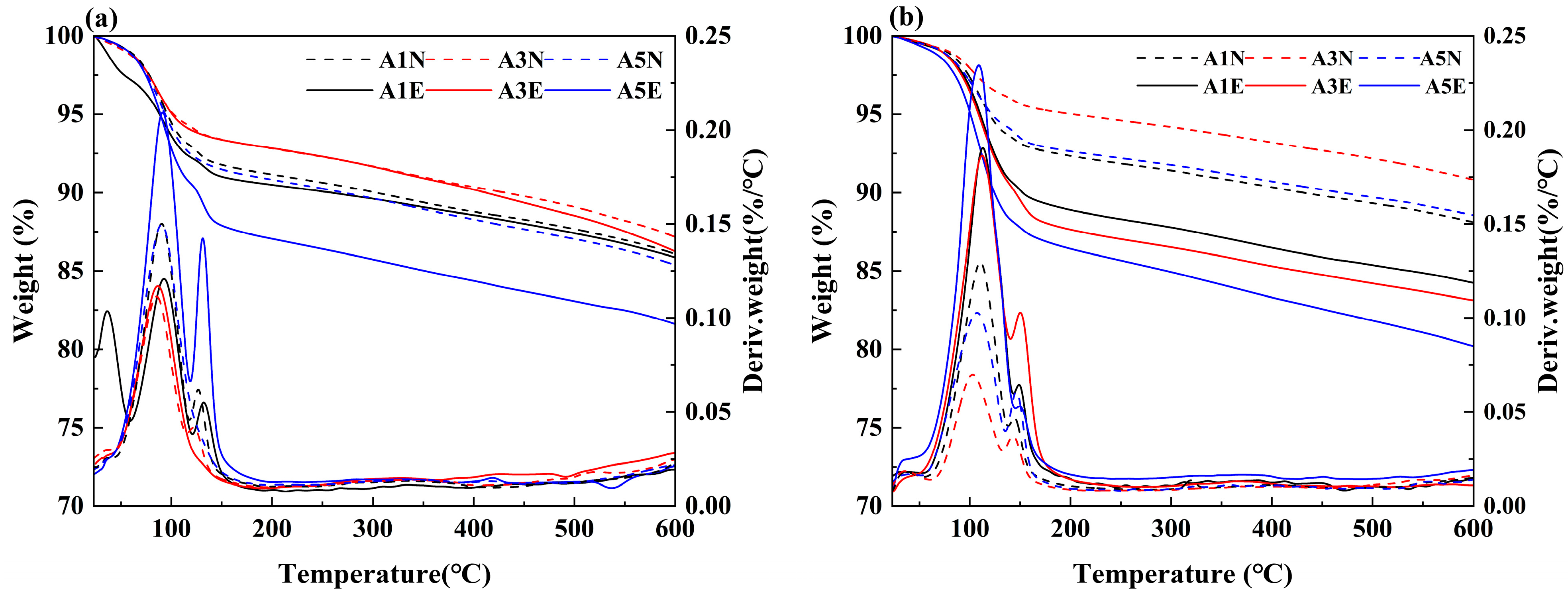
3.3.3. TG-DTG
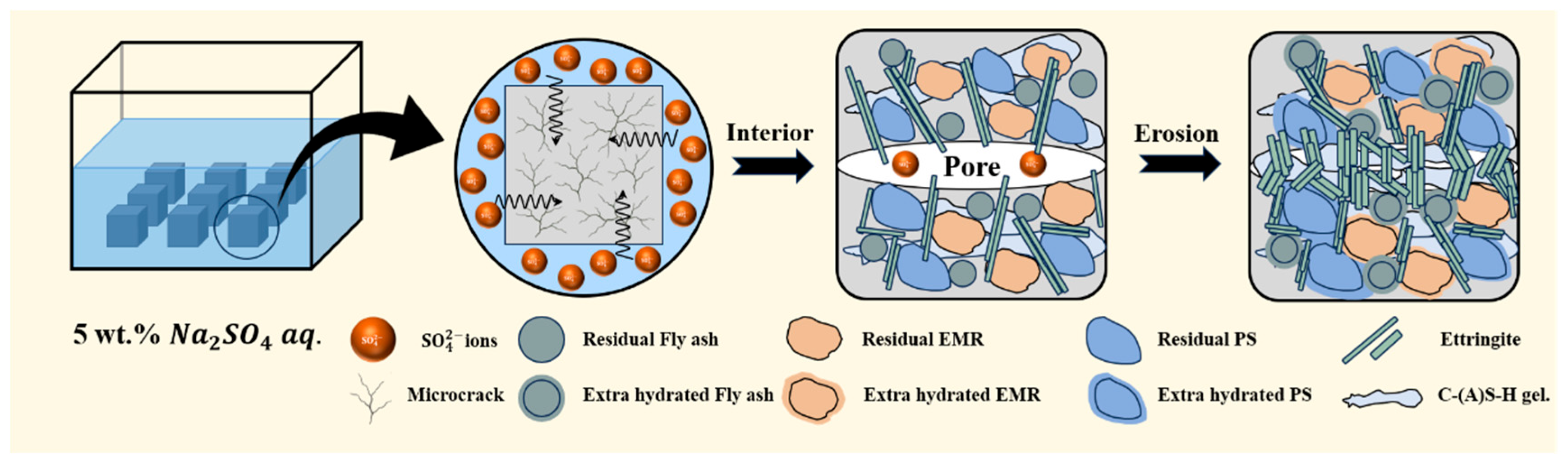
3.4. Mechanism of Sulfate Resistance
4. Conclusions
- (1)
- The mechanical performance of EBFM is optimal when the EMR content is 25 wt.%. The highest compressive strength of 8.68 MPa at 28 d is achieved at a 25 wt.% EMR content. Moreover, an increase in EMR content leads to a decrease in fluidity and bleeding rate of the slurry, and the setting time is progressively shortened.
- (2)
- The EBFM exhibits excellent sulfate resistance. It contributes to compressive strength enhancement, porosity reduction, and mass increase of EBFM in a sulfate environment. The compressive strength of EBFM in sulfate solution is significantly higher than that of those cured in deionized water, while the porosity is notably reduced. Corrosion resistance coefficients of all groups exceed 1.0. In the same erosion age, the A3 group with a 25 wt.% EMR content has the highest corrosion resistance coefficient. After 30 days of erosion, the corrosion resistance coefficient stabilized between 1.23 and 1.24.
- (3)
- After sulfate erosion, there are more hydration products generated in the EBFM, which makes microstructure denser. In sulfate-rich environments, sulfate ions penetrated the interior of the material through pore channels, promoting the dissolution of residual particles. These active components further participate in the hydration to generate additional hydration products such as C–(A)S–H and AFt. The morphology of ettringite crystals transforms from needle-like to columnar structure, while the gels encapsulate the ettringite crystals, effectively inhibiting the expansion of Al-AFt. In addition, the Fe-AFt formed during hydration demonstrates superior stability. These hydration products fill the internal pores and cracks, interlocking within the matrix, which results in a denser microstructure and enhancement of mechanical strength.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luan, H.J.; Jiang, Y.J.; Lin, H.L.; Wang, Y.H. A New Thin Seam Backfill Mining Technology and Its Application. Energies 2017, 10, 2023. [Google Scholar] [CrossRef]
- Zhu, W.B.; Xu, J.M.; Xu, J.L.; Chen, D.Y.; Shi, J.X. Pier-column backfill mining technology for controlling surface subsidence. Int. J. Rock Mech. Min. Sci. 2017, 96, 58–65. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Yang, K.; He, X.; Zhao, X.Y.; Wei, Z.; He, S.X. Research status of comprehensive utilization of coal-based solid waste (CSW) and key technologies of filling mining in China: A review. Sci. Total Environ. 2024, 926, 171855. [Google Scholar] [CrossRef]
- Xue, G.L.; Yilmaz, E.; Wang, Y.D. Progress and prospects of mining with backfill in metal mines in China. Int. J. Miner. Metall. Mater. 2023, 30, 1455–1473. [Google Scholar] [CrossRef]
- Cao, H.; Gao, Q.; Zhang, X.Z.; Guo, B. Research Progress and Development Direction of Filling Cementing Materials for Filling Mining in Iron Mines of China. Gels 2022, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.Q.; Cao, G.D.; Liang, Y.H. Experimental Study on Lead-Smelting Slag as Paste Filling Cementing Material. Geofluids 2022, 2022, 6126881. [Google Scholar] [CrossRef]
- Yang, L.; Hu, X.; Liu, Y.; Zhou, D.; Yuan, B.; Liu, S.; Luo, Z.; Li, X.; Jin, D.; Xu, F. Multiscale characterization of geopolymers modified with alkali-catalyzed nano-silica: Effects on dispersion and mechanical properties. Cem. Concr. Compos. 2026, 165, 106324. [Google Scholar] [CrossRef]
- Dan, H.C.; Ma, Z.M.; Li, M.J.; Ma, S.L.; Tan, J.W. Early performance and bonding mechanism of metakaolin (MK)- ground granulated blast furnace slag (GGBS) based geopolymer road repair mortar. Int. J. Pavement Eng. 2023, 24, 2252156. [Google Scholar] [CrossRef]
- Peng, Y.M.; Ma, K.L.; Long, G.C.; Xie, Y.J.; Yu, L.N.; Xie, Q.Q. Effect of Packing Density According to CPM on the Rheology of Cement-Fly Ash-Slag Paste. J. Mater. Civ. Eng. 2021, 33, 04021209. [Google Scholar] [CrossRef]
- Meng, W.Q.; Ma, K.L.; Long, G.C.; Meng, Q.Y.; Chen, R.J.; Fan, J.Z. Influence of Molybdenum Tailings Powder on the Hydration Characteristics of Cement Paste. J. Mater. Civ. Eng. 2024, 36, 04024115. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, B.; He, Y.Z.; Li, F.; Long, G.C.; Du, Y.F. Preparation and properties of sustainable magnesium phosphate cement composites with recycled tire rubber particles. J. Clean. Prod. 2020, 262, 121253. [Google Scholar] [CrossRef]
- Chu, C.F.; Deng, Y.F.; Zhou, A.N.; Feng, Q.; Ye, H.; Zha, F.S. Backfilling performance of mixtures of dredged river sediment and iron tailing slag stabilized by calcium carbide slag in mine goaf. Constr. Build. Mater. 2018, 189, 849–856. [Google Scholar] [CrossRef]
- Wu, M.G.; Wang, C.; Zuo, Y.J.; Yang, S.; Zhang, J.Z.; Luo, Y. Study on strength prediction and strength change of Phosphogypsum-based composite cementitious backfill based on BP neural network. Mater. Today Commun. 2024, 41, 110331. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.S.; Qi, C.C.; Zhang, Q.L.; Xiao, C.C. Lithium slag and fly ash-based binder for cemented fine tailings backfill. J. Environ. Manag. 2019, 248, 109282. [Google Scholar] [CrossRef]
- Yin, L.L.; Guo, Q.; Wang, X.; Yuan, J.; Zhang, Q.F. Environmental filling materials based on phosphogypsum powder with municipal solid waste incineration ash. Sci. Rep. 2023, 13, 478. [Google Scholar] [CrossRef]
- Liu, X.M.; Liu, E. The Synergistic Mechanism and Stability Evaluation of Phosphogypsum and Recycled Fine Powder-Based Multi-Source Solid Waste Geopolymer. Polymers 2023, 15, 2696. [Google Scholar] [CrossRef]
- Liu, X.M.; Liu, E.R.; Fu, Y.T. Reduction in Drying Shrinkage and Efflorescence of Recycled Brick and Concrete Fine Powder-Slag-Based Geopolymer. Appl. Sci. 2023, 13, 2997. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.R.; Shen, X.D. Hydration of ternary cement in the presence of triisopropanolamine. Constr. Build. Mater. 2016, 111, 513–521. [Google Scholar] [CrossRef]
- Wang, F.; Long, G.C.; Ma, K.L.; Zeng, X.H.; Tang, Z.; Dong, R.Z.; He, J.H.; Shangguan, M.H.; Hu, Q.C.; Liew, R.K.; et al. Recyling manganese-rich electrolytic residues: A review. Environ. Chem. Lett. 2023, 21, 2251–2284. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Guo, W.C.; Wang, X.L.; Pan, H.M.; Zhao, Q.X.; Wang, D.L. Utilization of municipal solid waste incineration fly ash with red mud-carbide slag for eco-friendly geopolymer preparation. J. Clean. Prod. 2022, 340, 130820. [Google Scholar] [CrossRef]
- Luo, W.J.; Li, B.; Yang, G.; Xu, M.X.; Pang, C.H.; Kow, K.W.; Wu, T. Utilisation of electrolytic manganese residue as a sulphate activator in producing concrete blocks with high-volume fly ash. J. Clean. Prod. 2024, 434, 139813. [Google Scholar] [CrossRef]
- He, S.C.; Jiang, D.Y.; Hong, M.H.; Liu, Z.H. Hazard-free treatment and resource utilisation of electrolytic manganese residue: A review. J. Clean. Prod. 2021, 306, 127224. [Google Scholar] [CrossRef]
- Wang, D.Q.; Wang, Q.; Xue, J.F. Reuse of hazardous electrolytic manganese residue: Detailed leaching characterization and novel application as a cementitious material. Resour. Conserv. Recycl. 2020, 154, 104645. [Google Scholar] [CrossRef]
- Li, W.L.; Jin, H.X.; Xie, H.Y.; Wang, D.L. Progress in comprehensive utilization of electrolytic manganese residue: A review. Environ. Sci. Pollut. Res. 2023, 30, 48837–48853. [Google Scholar] [CrossRef]
- Wang, G.J.; Sun, Q.; Qi, C.X.; Liu, L.; Tan, Y.; Su, L.J. Mechanical properties and microscopic characterization of cemented paste backfill with electrolytic manganese residue matrix binder. J. Mater. Res. Technol. 2023, 23, 2075–2088. [Google Scholar] [CrossRef]
- Lan, J.R.; Sun, Y.; Tian, H.; Zhan, W.; Du, Y.G.; Ye, H.P.; Du, D.Y.; Zhang, T.C.; Hou, H.B. Electrolytic manganese residue-based cement for manganese ore pit backfilling: Performance and mechanism. J. Hazard. Mater. 2021, 411, 124941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Liu, X.M.; Xu, Y.T.; Tang, B.W.; Wang, Y.G. Preparation of road base material by utilizing electrolytic manganese residue based on Si-Al structure: Mechanical properties and Mn2+ stabilization/solidification characterization. J. Hazard. Mater. 2020, 390, 122188. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Fall, M. Sulphate effect on the early age strength and self-desiccation of cemented paste backfill. Constr. Build. Mater. 2016, 106, 296–304. [Google Scholar] [CrossRef]
- Bondar, D.; Nanukuttan, S. External Sulphate Attack on Alkali-Activated Slag and Slag/Fly Ash Concrete. Buildings 2022, 12, 94. [Google Scholar] [CrossRef]
- Aydin, S.; Baradan, B. Sulfate resistance of alkali-activated slag and Portland cement based reactive powder concrete. J. Build. Eng. 2021, 43, 103205. [Google Scholar] [CrossRef]
- Komljenovic, M.; Bascarevic, Z.; Marjanovic, N.; Nikolic, V. External sulfate attack on alkali-activated slag. Constr. Build. Mater. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- GB/T 8077-2023; Test Methods for the Homogeneity of Concrete Admixtures. Standardization Administration of the People’s Republic of China: Beijing, China, 2023.
- GB/T 1346-2024; Test Method for Standard Consistency, Setting Time, and Stability of Cement. Standardization Administration of the People’s Republic of China: Beijing, China, 2024.
- JTG E30-2005; Testing Methods of Cement and Concrete for Highway Engineering. Ministry of Transport of the People’s Republic of China: Wuhan, China, 2005.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Standardization Administration of the People’s Republic of China: Beijing, China, 2009.
- Tang, D.S.; Yang, C.H.; Li, X.Y.; Zhu, X.H.; Yang, K.; Yu, L.W. Mitigation of efflorescence of alkali-activated slag mortars by incorporating calcium hydroxide. Constr. Build. Mater. 2021, 298, 123873. [Google Scholar] [CrossRef]
- Qin, X.T.; Cao, Y.H.; Guan, H.W.; Hu, Q.S.; Liu, Z.H.; Xu, J.; Hu, B.; Zhang, Z.Y.; Luo, R. Resource utilization and development of phosphogypsum-based materials in civil engineering. J. Clean. Prod. 2023, 387, 135858. [Google Scholar] [CrossRef]
- Xu, Y.T.; Liu, X.M.; Zhang, Y.L.; Tang, B.W.; Mukiza, E. Investigation on sulfate activation of electrolytic manganese residue on early activity of blast furnace slag in cement-based cementitious material. Constr. Build. Mater. 2019, 229, 116831. [Google Scholar] [CrossRef]
- Wu, Z.H.; Zhang, H.; Pu, S.Y.; Cai, G.J.; Duan, W.; Song, H.L.; Zeng, C.; Yang, Y.H. Synergistic preparation of geopolymer using electrolytic manganese residue, coal slag and granulated blast furnace slag. J. Build. Eng. 2024, 91, 109609. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, J.X.; Ouyang, S.Y.; Deng, X.J.; Dong, C.W.; Du, E.B. Feasibility study and performance optimization of sand-based cemented paste backfill materials. J. Clean. Prod. 2020, 259, 120798. [Google Scholar] [CrossRef]
- Liu, C.B.; Gao, J.M.; Tang, Y.B.; Zhao, Y.S. Early hydration and microstructure of gypsum plaster revealed by environment scanning electron microscope. Mater. Lett. 2019, 234, 49–52. [Google Scholar] [CrossRef]
- Yin, S.S.; Yang, L.C. α or β?-hemihydrates transformed from dihydrate calcium sulfate in a salt-mediated glycerol-water solution. J. Cryst. Growth 2020, 550, 125885. [Google Scholar] [CrossRef]
- Wang, F.; Long, G.C.; Bai, M.; Wang, J.L.; Zhou, J.L.; Zhou, X. Application of electrolytic manganese residues in cement products through pozzolanic activity motivation and calcination. J. Clean. Prod. 2022, 338, 130629. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Lv, Q.F.; Wang, Z.S.; Gu, L.Y.; Chen, Y.; Shan, X.K. Effect of sodium sulfate on strength and microstructure of alkali-activated fly ash based geopolymer. J. Cent. South Univ. 2020, 27, 1691–1702. [Google Scholar] [CrossRef]
- Chen, F.; Gao, J.M.; Qi, B.; Shen, D.M. Deterioration mechanism of plain and blended cement mortars partially exposed to sulfate attack. Constr. Build. Mater. 2017, 154, 849–856. [Google Scholar] [CrossRef]
- Fall, M.; Pokharel, M. Coupled effects of sulphate and temperature on the strength development of cemented tailings backfills: Portland cement-paste backfill. Cem. Concr. Compos. 2010, 32, 819–828. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, G.J.; Zhao, K.; Wang, M.L.; Wu, B.S.; Li, S.J.; Chen, Q.L.; Geng, J.B. Mechanical properties, permeability and microstructure of steam-cured fly ash mortar mixed with phosphogypsum. Constr. Build. Mater. 2023, 400, 132582. [Google Scholar] [CrossRef]
- Wan, D.W.; Zhang, W.Q.; Tao, Y.; Wan, Z.H.; Wang, F.Z.; Hu, S.G.; He, Y.J. The impact of Fe dosage on the ettringite formation during high ferrite cement hydration. J. Am. Ceram. Soc. 2021, 104, 3652–3664. [Google Scholar] [CrossRef]
- Sha, S.N.; Ma, Y.H.; Lei, L.; Zhang, Y.R.; Liu, Y.; Xiao, Y.C.; Shi, C.J. Effect of polycarboxylate superplasticizers on the growth of ettringite in deionized water and synthetic cement pore solution. Constr. Build. Mater. 2022, 341, 127602. [Google Scholar] [CrossRef]
- Gijbels, K.; Nguyen, H.; Kinnunen, P.; Schroeyers, W.; Pontikes, Y.; Schreurs, S.; Illikainen, M. Feasibility of incorporating phosphogypsum in ettringite-based binder from ladle slag. J. Clean. Prod. 2019, 237, 117793. [Google Scholar] [CrossRef]
- Meskini, S.; Samdi, A.; Ejjaouani, H.; Remmal, T. Valorization of phosphogypsum as a road material: Stabilizing effect of fly ash and lime additives on strength and durability. J. Clean. Prod. 2021, 323, 129161. [Google Scholar] [CrossRef]
- Huang, X.Q.; Jiang, M.M.; Zhao, X.R.; Tang, C.L. Mechanical properties and hydration mechanisms of high-strength fluorogypsum-blast furnace slag-based hydraulic cementitious binder. Constr. Build. Mater. 2016, 127, 137–143. [Google Scholar] [CrossRef]
- Cao, W.X.; Yi, W.; Peng, J.H.; Li, G.G.; Yin, S.H. Preparation of anhydrite from phosphogypsum: Influence of phosphorus and fluorine impurities on the performances. Constr. Build. Mater. 2022, 318, 126021. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, B.; Liang, F.; Xue, Z.; Qian, B.; Wang, J.; Zhou, L.; Zang, J.; Liang, R.; Li, Y.; et al. Use of untreated phosphogypsum as a raw material for autoclaved aerated concrete preparation. J. Build. Eng. 2023, 64, 105607. [Google Scholar] [CrossRef]
- Hirsch, T.; Matschei, T.; Stephan, D. The hydration of tricalcium aluminate (Ca3Al2O6) in Portland cement-related systems: A review. Cem. Concr. Res. 2023, 168, 107150. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms of alkali-activated/geopolymer systems: Effects of chemical additives. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Zhang, K.C.; Lu, Y.; Rao, M.J.; Zhang, W.Q.; Wang, F.Z. Understanding the role of brownmilerite on corrosion resistance. Constr. Build. Mater. 2020, 254, 119262. [Google Scholar] [CrossRef]
- Dilnesa, B.Z.; Wieland, E.; Lothenbach, B.; Dähn, R.; Scrivener, K.L. Fe-containing phases in hydrated cements. Cem. Concr. Res. 2014, 58, 45–55. [Google Scholar] [CrossRef]
- Herrera-Mesen, C.; Salvador, R.P.; Ikumi, T.; Cavalaro, S.H.P.; Aguado, A. External sulphate attack of sprayed mortars with sulphate-resisting cement: Influence of accelerator and age of exposition. Cem. Concr. Compos. 2020, 114, 103614. [Google Scholar] [CrossRef]
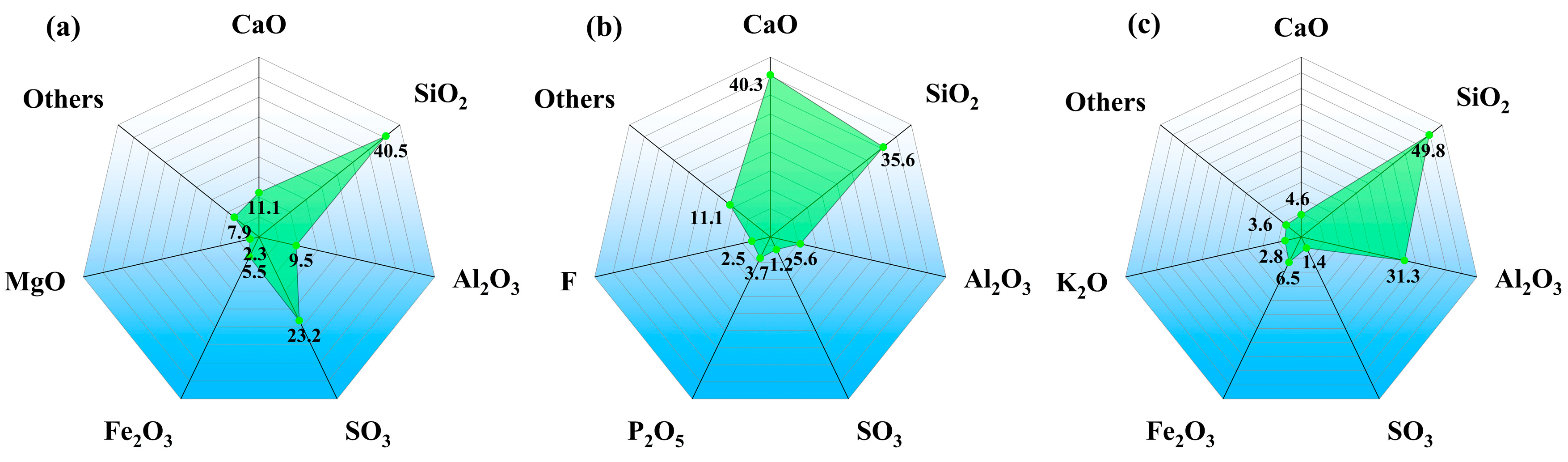

| NO. | Material Composition | |||
|---|---|---|---|---|
| EMR | FA | PS | QL | |
| A1 | 20 | 40 | 20 | 20 |
| A2 | 22.5 | 37.5 | 20 | 20 |
| A3 | 25 | 35 | 20 | 20 |
| A4 | 27.5 | 32.5 | 20 | 20 |
| A5 | 30 | 30 | 20 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, X.; Fu, Z.; Zhai, S.; Liu, X. Transfer the Sulfate Environment into a Beneficial Factor: Performance Enhancement and Mechanism of Electrolytic Manganese Residue-Based Mine Filling Materials. J. Compos. Sci. 2025, 9, 642. https://doi.org/10.3390/jcs9120642
Zhang X, Liu X, Fu Z, Zhai S, Liu X. Transfer the Sulfate Environment into a Beneficial Factor: Performance Enhancement and Mechanism of Electrolytic Manganese Residue-Based Mine Filling Materials. Journal of Composites Science. 2025; 9(12):642. https://doi.org/10.3390/jcs9120642
Chicago/Turabian StyleZhang, Xihe, Xin Liu, Zimeng Fu, Shuchao Zhai, and Xiaoming Liu. 2025. "Transfer the Sulfate Environment into a Beneficial Factor: Performance Enhancement and Mechanism of Electrolytic Manganese Residue-Based Mine Filling Materials" Journal of Composites Science 9, no. 12: 642. https://doi.org/10.3390/jcs9120642
APA StyleZhang, X., Liu, X., Fu, Z., Zhai, S., & Liu, X. (2025). Transfer the Sulfate Environment into a Beneficial Factor: Performance Enhancement and Mechanism of Electrolytic Manganese Residue-Based Mine Filling Materials. Journal of Composites Science, 9(12), 642. https://doi.org/10.3390/jcs9120642







