Laser-Induced Synthesis of Crystalline Silicon Compounds from Aluminum–Silica–Carbon Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Research Methods
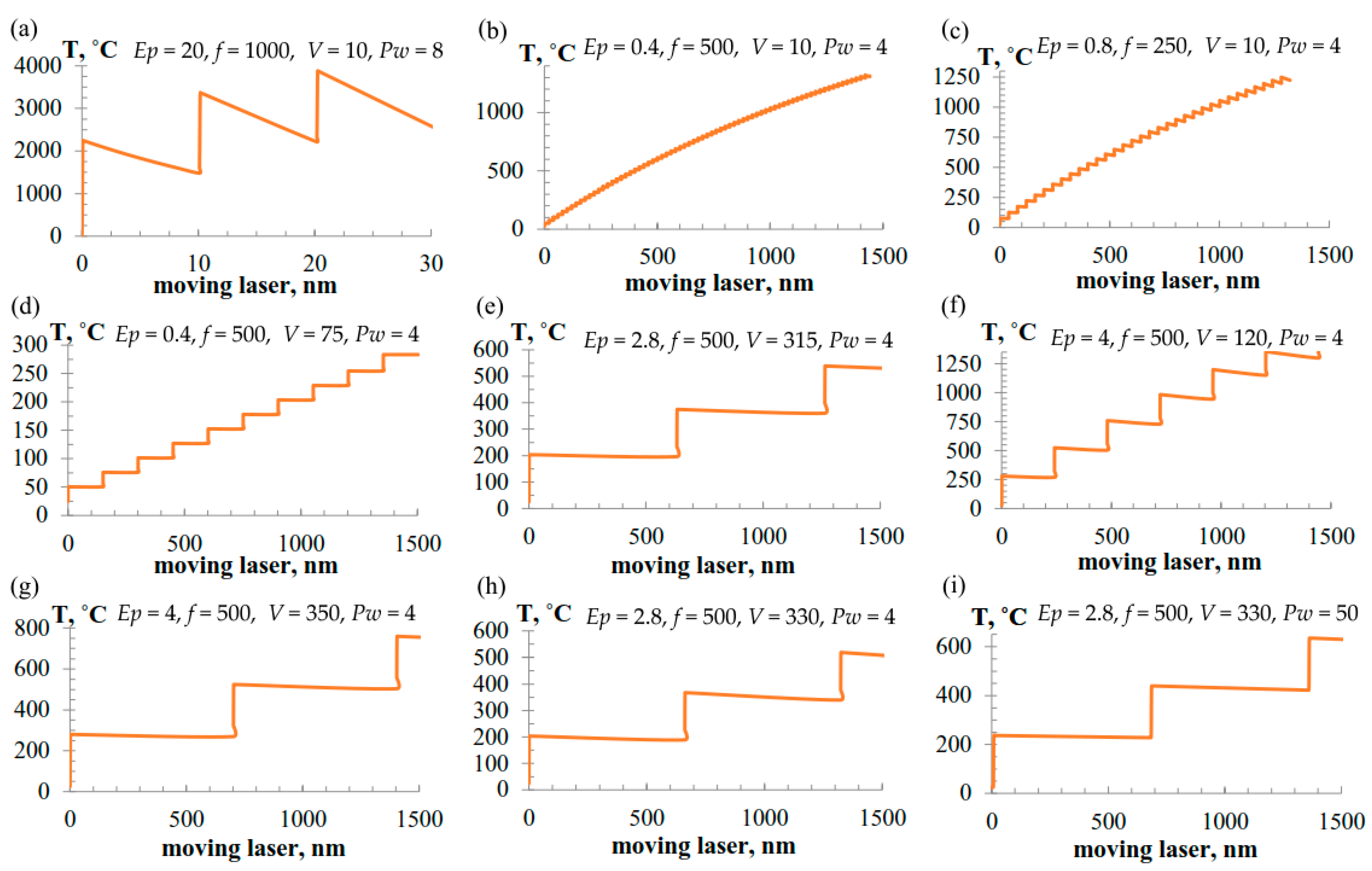
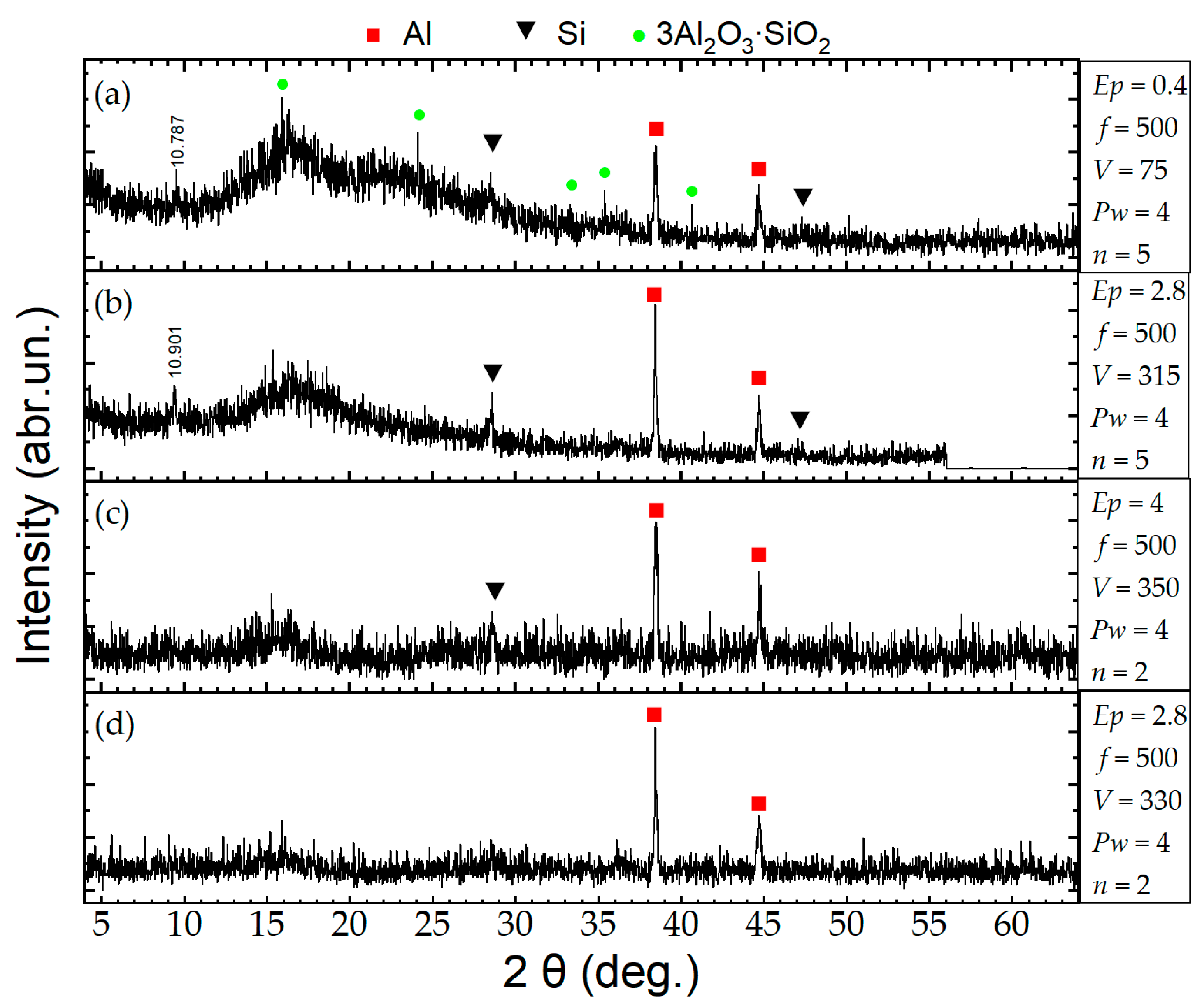
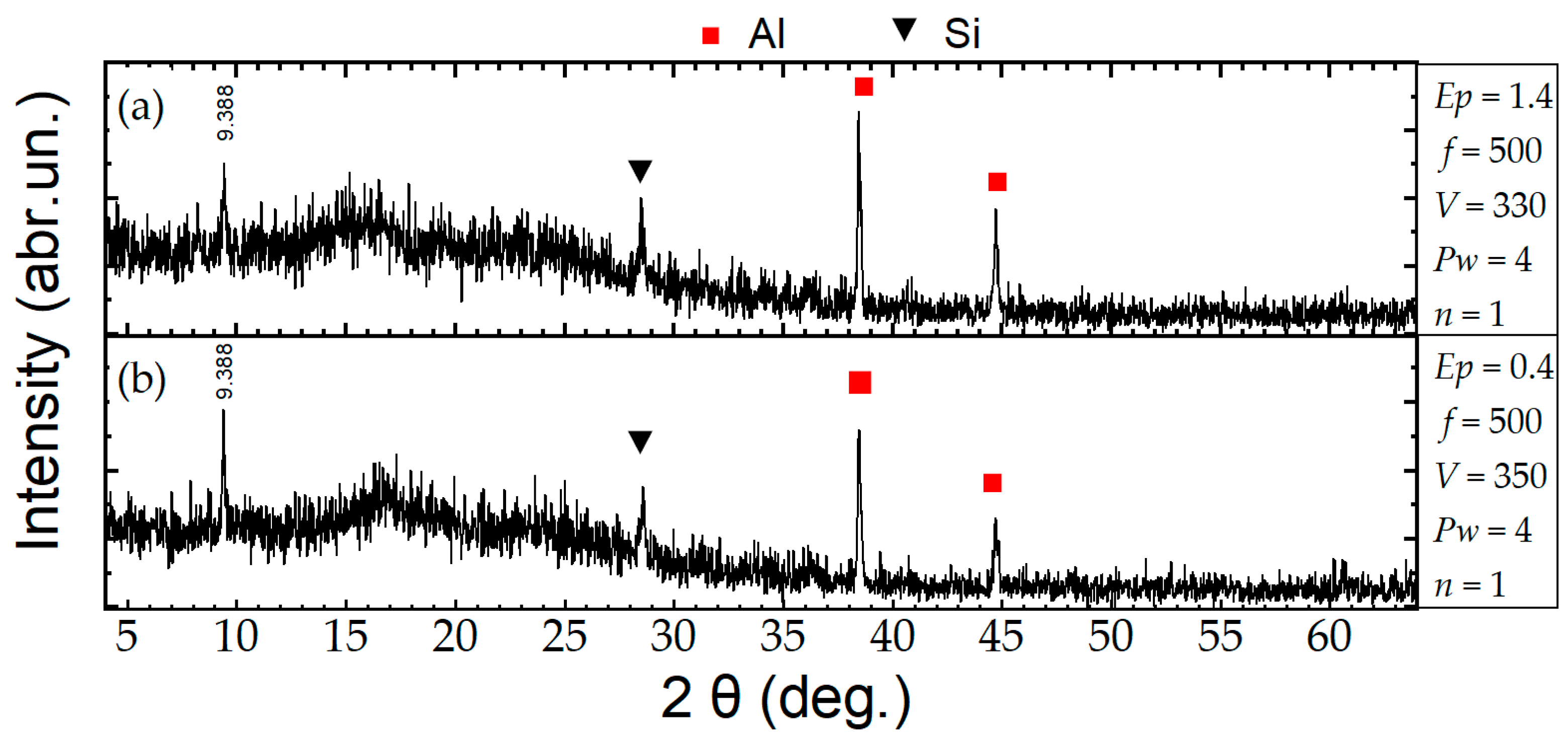
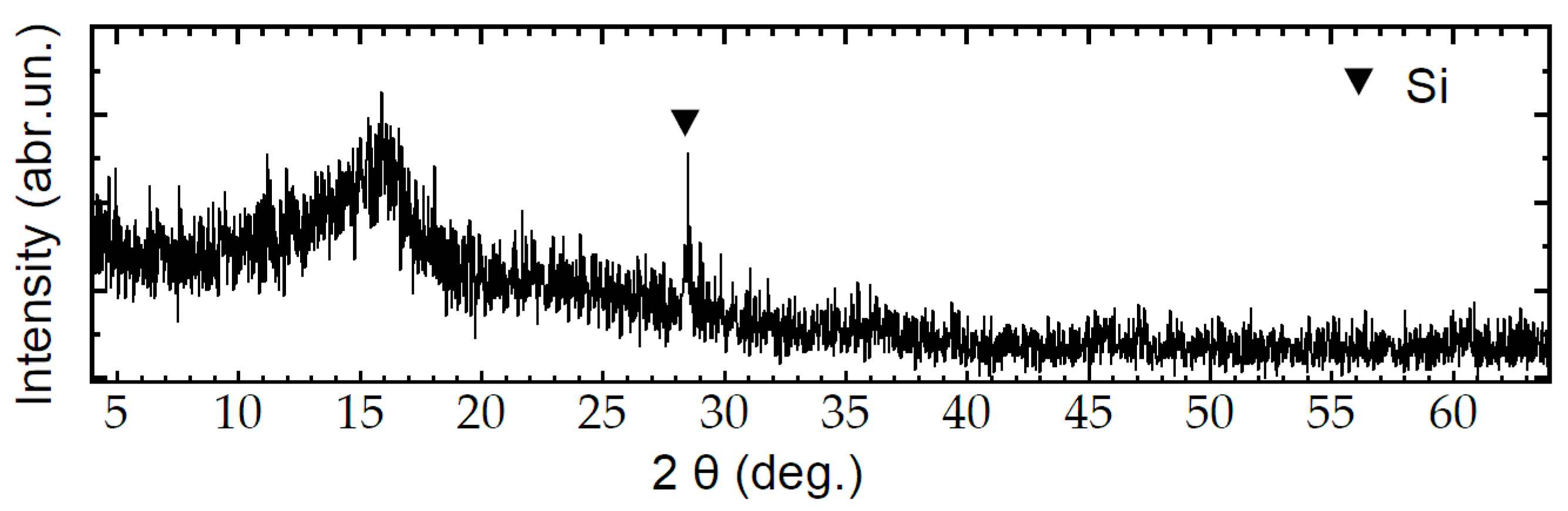
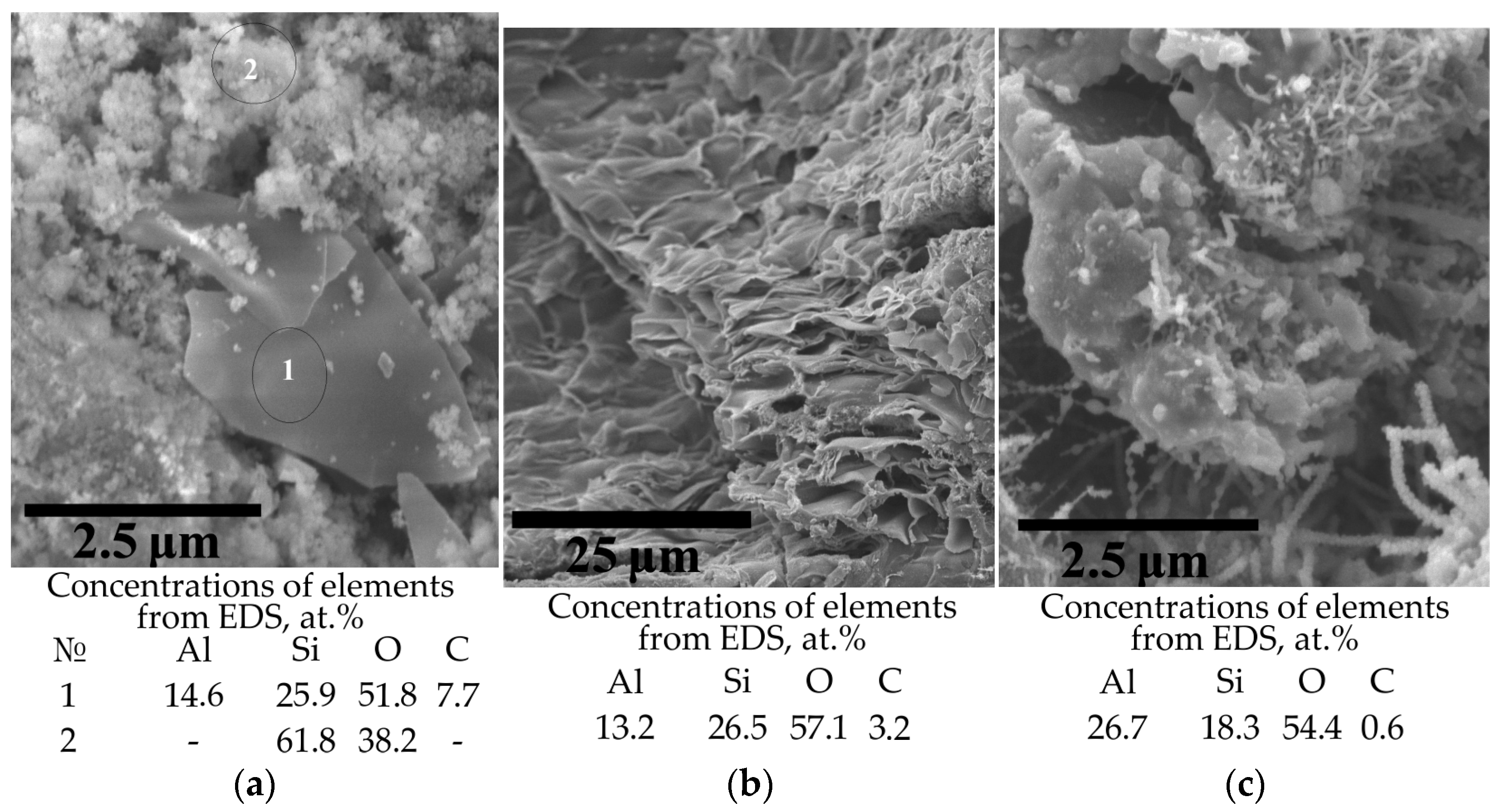
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Belaforte, D.A. The global market for industrial laser processing. Photonics Views 2020, 17, 35–37. [Google Scholar] [CrossRef]
- Bonse, J.; Lasagni, A.F. Laser micro- and nano-material processing—Part 1. Adv. Opt. Technol. 2020, 9, 7–9. [Google Scholar] [CrossRef]
- Liu, X.; Du, D.; Mourou, G. Laser ablation and micromachining with ultrashort laser pulses. IEEE J. Quantum Electron. 1997, 33, 1706. [Google Scholar] [CrossRef]
- Gattass, R.R.; Mazur, E. Femtosecond laser micromachining in transparent materials. Nat. Photonics 2008, 2, 219. [Google Scholar] [CrossRef]
- Bäuerle, D. Laser Processing and Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Bonse, J.; Sturm, H.; Schmidt, D.; Kautek, W. Chemical, morphological and accumulation phenomena in ultrashort-pulse laser ablation of TiN in air. Appl. Phys. A 2000, 71, 657–665. [Google Scholar] [CrossRef]
- Im, J.S.; Kim, H.J.; Thompson, M.O. Phase transformation mechanisms involved in excimer laser crystallization of amorphous silicon films. Appl. Phys. Lett. 1993, 63, 1969–1971. [Google Scholar] [CrossRef]
- Miyasaka, M.; Stoemenos, J. Excimer laser annealing of amorphous and solid-phase-crystallized silicon films. J. Appl. Phys. 1999, 86, 5556–5565. [Google Scholar] [CrossRef]
- Smith, P.M.; Carey, P.G.; Sigmon, T.W. Excimer laser crystallization and doping of silicon films on plastic substrates. Appl. Phys. Lett. 1997, 70, 342–344. [Google Scholar] [CrossRef]
- Song, D.; Inns, D.; Straub, A.; Terry, M.L.; Campbell, P.; Aberle, A.G. Solid phase crystallized polycrystalline thin-films on glass from evaporated silicon for photovoltaic applications. Thin Solid Film. 2006, 513, 356–363. [Google Scholar] [CrossRef]
- Sameshima, T.; Usui, S.; Sekiya, M. XeCl Excimer laser annealing used in the fabrication of poly-Si TFT’s. IEEE Electron Device Lett. 1986, 7, 276–278. [Google Scholar] [CrossRef]
- Marqués, L.A.; Pelaz, L.; Aboy, M.A.; Barbolla, J. The laser annealing induced phase transition in silicon: A molecular dynamics study. Nucl. Instrum. Methods Phys. Res. Sect. B 2004, 216, 57–61. [Google Scholar] [CrossRef]
- Goto, T.; Saito, K.; Imaizumi, F.; Hatanaka, M.; Takimoto, M.; Mizumura, M.; Gotoh, J.; Ikenoue, H.; Sugawa, S. LTPS Thin-Film Transistors Fabricated Using New Selective Laser Annealing System. IEEE Trans. Electron Devices 2018, 65, 3250–3256. [Google Scholar] [CrossRef]
- Phillips, K.C.; Gandhi, H.H.; Mazur, E.; Sundaram, S.K. Ultrafast laser processing of materials: A review. Adv. Opt. Photonics 2015, 7, 684–712. [Google Scholar] [CrossRef]
- Arduino, D.; Stassi, S.; Spano, C.; Scaltrito, L.; Ferrero, S.; Bertana, V. Silicon and Silicon Carbide Recrystallization by Laser Annealing: A Review. Materials 2023, 16, 7674. [Google Scholar] [CrossRef] [PubMed]
- Theodorakos, I.; Zergioti, I.; Vamvakas, V.; Tsoukalas, D.; Raptis, Y.S. Picosecond and nanosecond laser annealing and simulation of amorphous silicon thin films for solar cell applications. J. Appl. Phys. 2014, 115, 043108. [Google Scholar] [CrossRef]
- Nayak, B.K.; Gupta, M.C. Femtosecond-laser-induced-crystallization and simultaneous formation of light trapping microstructures in thin a-Si:H films. Appl. Phys. A 2007, 89, 663. [Google Scholar] [CrossRef]
- Wang, X.C.; Zheng, H.Y.; Tan, C.W.; Wang, F.; Yu, H.Y.; Pey, K.L. Femtosecond laser induced surface nanostructuring and simultaneous crystallization of amorphous thin silicon film. Opt. Express 2010, 18, 19379. [Google Scholar] [CrossRef]
- Pavlikov, A.V.; Forsh, P.A.; Svyakhovskiy, S.E.; Matsukatova, A.N.; Forsh, E.A.; Kazanskii, A.G.; Kashkarov, P.K. Giant enhancement of free charge carrier concentration in boron-doped amorphous hydrogenated silicon under femtosecond laser crystallization. Appl. Phys. Lett. 2018, 113, 203103. [Google Scholar] [CrossRef]
- Li, D.; Ilyas, N.; Song, Y.; Zhong, H.; Li, W.; Jiang, Y. Inhomogeneous crystallization of a-Si thin films irradiated by femtosecond laser. J. Raman Spectrosc. 2019, 50, 793. [Google Scholar] [CrossRef]
- Torregrosa, F.; Canino, M.; Li, F.; Tamarri, F.; Roux, B.; Morata, S.; La Via, F.; Zielinski, M.; Nipoti, R. Ion implantation and activation of aluminum in bulk 3C-SiC and 3C-SiC on Si. MRS Adv. 2022, 7, 1347–1352. [Google Scholar] [CrossRef]
- Choi, T.Y.; Hwang, D.J.; Grigoropoulos, C.P. Ultrafast laser-induced crystallization of amorphous silicon films. Opt. Eng. 2003, 42, 3383. [Google Scholar] [CrossRef]
- Volodin, V.A.; Efremov, M.D.; Kachurin, G.A.; Cherkov, A.G.; Deutschmann, M.; Baersch, N. Phase transitions in a-Si:H films on a glass irradiated by high-power femtosecond pulses: Manifestation of nonlinear and nonthermal effects. JETP Lett. 2007, 86, 119. [Google Scholar] [CrossRef]
- Izawa, Y.; Tokita, S.; Fujita, M.; Norimatsu, T.; Izawa, Y. Ultra fast melting process in femtosecond laser crystallization of thin a-Si layer. Appl. Surf. Sci. 2009, 255, 9764. [Google Scholar] [CrossRef]
- Maeng, S.H.; Lee, H.; Park, M.S.; Park, S.; Jeong, J.; Kim, S. Ultrafast carbothermal reduction of silica to silicon using a CO2 laser beam. Sci. Rep. 2020, 10, 21730. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, D.; Niu, F.; Huang, Y.; Zhu, J.; Ma, G. In situ synthesis of melt-grown mullite ceramics using directed laser deposition. J. Mater. Sci. 2020, 55, 12761–12775. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Pikulin, A.; Sapogova, N.; Bityurin, N. Femtosecond Laser Irradiation of Plasmonic Nanoparticles in Polymer Matrix: Implications for Photothermal and Photochemical Material Alteration. Micromachines 2014, 5, 1202–1218. [Google Scholar] [CrossRef]
- Lavrov, N.N.; Lavrov, B.A.; Serzhanov, G.M. Preparation of polycrystalline silicon by the aluminothermic method. News St. Petersburg State Technol. Inst. Tech. Univ. 2015, 31, 30–32. (In Russian) [Google Scholar]
- Pavlenko, V.I.; Gorodov, A.I.; Cherkashina, N.I.; Ryzhikh, D.A.; Ruchii, A.Y. Influence of Temperature of Silicon Dioxide Powder Annealing on the Aluminum-Induced Crystallization of Polycrystalline Silicon. Russ. J. Appl. Chem. 2023, 96, 838–846. [Google Scholar] [CrossRef]
- Cherkashina, N.I.; Pavlenko, V.I.; Gorodov, A.I.; Ryzhikh, D.A. Preparation of Polycrystalline Silicon by Metal-Induced Crystallization of Silicon–Carbon Powder. Ceramics 2024, 7, 989–1001. [Google Scholar] [CrossRef]
- Pavlenko, V.I.; Cherkashina, N.I.; Edamenko, O.D.; Yastrebinsky, R.N.; Noskov, A.V.; Prokhorenkov, D.S.; Gorodov, A.I.; Piskareva, A.O. Synthesis and Characterization of Silicon–Carbon Powder and Its Resistance to Electron Irradiation. J. Compos. Sci. 2023, 7, 340. [Google Scholar] [CrossRef]
- Vora, H.D.; Santhanakrishnan, S.; Harimkar, S.P.; Boetcher, S.K.S.; Dahotre, N.B. One-dimensional multipulse laser machining of structural alumina: Evolution of surface topography. Int. J. Adv. Manuf. Technol. 2013, 68, 69–83. [Google Scholar] [CrossRef]
- Abdullayev, A.; Zemke, F.; Gurlo, A.; Bekheet, M.F. Low-temperature fluoride-assisted synthesis of mullite whiskers. RSC Adv. 2020, 10, 31180–31186. [Google Scholar] [CrossRef]
- Arletti, R.; Fois, E.; Gigli, L.; Vezzalini, G.; Quartieri, S.; Tabacchi, G. Irreversible Conversion of a Water–Ethanol Solution into an Organized Two-Dimensional Network of Alternating Supramolecular Units in a Hydrophobic Zeolite under Pressure. Angew. Chem. Int. Ed. 2017, 56, 2105–2109. [Google Scholar] [CrossRef]
- Rakoczy, R.A.; Traa, Y.; Kortunov, P.; Vasenkov, S.; Kärger, J.; Weitkamp, J. Synthesis of large crystals of all-silica zeolite ferrierite. Microporous Mesoporous Mater. 2007, 104, 179–184. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorodov, A.I.; Pavlenko, V.I.; Sinebok, D.A.; Cherkashina, N.I.; Yastrebinsky, R.N.; Yastrebinskaya, A.V.; Bondarenko, N.I. Laser-Induced Synthesis of Crystalline Silicon Compounds from Aluminum–Silica–Carbon Powder. J. Compos. Sci. 2025, 9, 643. https://doi.org/10.3390/jcs9120643
Gorodov AI, Pavlenko VI, Sinebok DA, Cherkashina NI, Yastrebinsky RN, Yastrebinskaya AV, Bondarenko NI. Laser-Induced Synthesis of Crystalline Silicon Compounds from Aluminum–Silica–Carbon Powder. Journal of Composites Science. 2025; 9(12):643. https://doi.org/10.3390/jcs9120643
Chicago/Turabian StyleGorodov, Andrey Ivanovich, Vyacheslav Ivanovich Pavlenko, Daria Alexandrovna Sinebok, Natalia Igorevna Cherkashina, Roman Nikolaevich Yastrebinsky, Anna Viktorovna Yastrebinskaya, and Nadezhda Ivanovna Bondarenko. 2025. "Laser-Induced Synthesis of Crystalline Silicon Compounds from Aluminum–Silica–Carbon Powder" Journal of Composites Science 9, no. 12: 643. https://doi.org/10.3390/jcs9120643
APA StyleGorodov, A. I., Pavlenko, V. I., Sinebok, D. A., Cherkashina, N. I., Yastrebinsky, R. N., Yastrebinskaya, A. V., & Bondarenko, N. I. (2025). Laser-Induced Synthesis of Crystalline Silicon Compounds from Aluminum–Silica–Carbon Powder. Journal of Composites Science, 9(12), 643. https://doi.org/10.3390/jcs9120643





