Features of the Process of Surface Preparation of Products Using Glow Discharge Plasma During the Deposition of Modifying Coatings
Abstract
1. Introduction
2. Materials and Methods
- Hollow Cathode Spaces (HCSs).
- Ion Bombardment (IB).
2.1. Hollow Cathode Spaces (HCSs)
2.2. Ion Bombardment (IB)
3. Results
3.1. Comparison of the Effectiveness of Using HCS and IB Methods
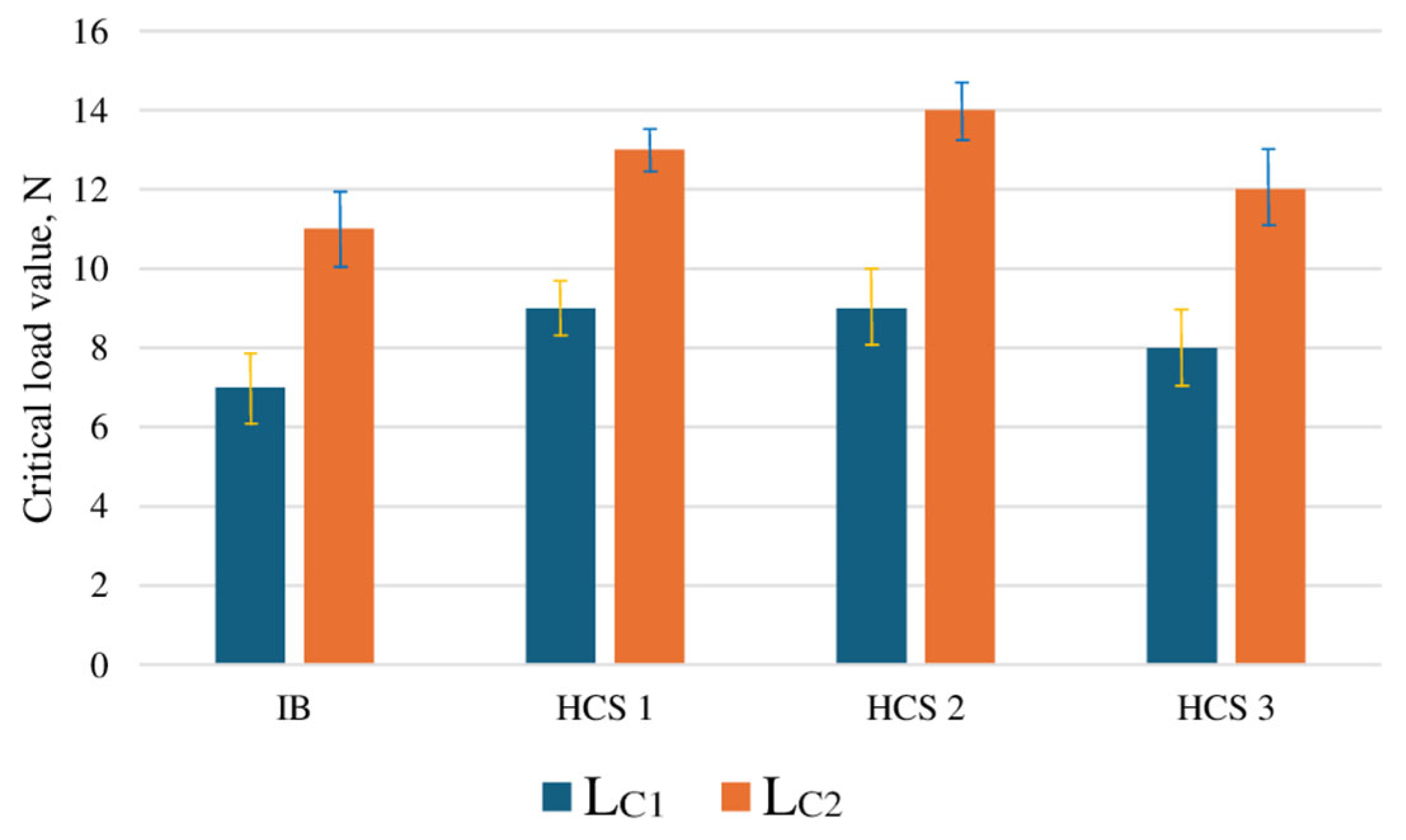
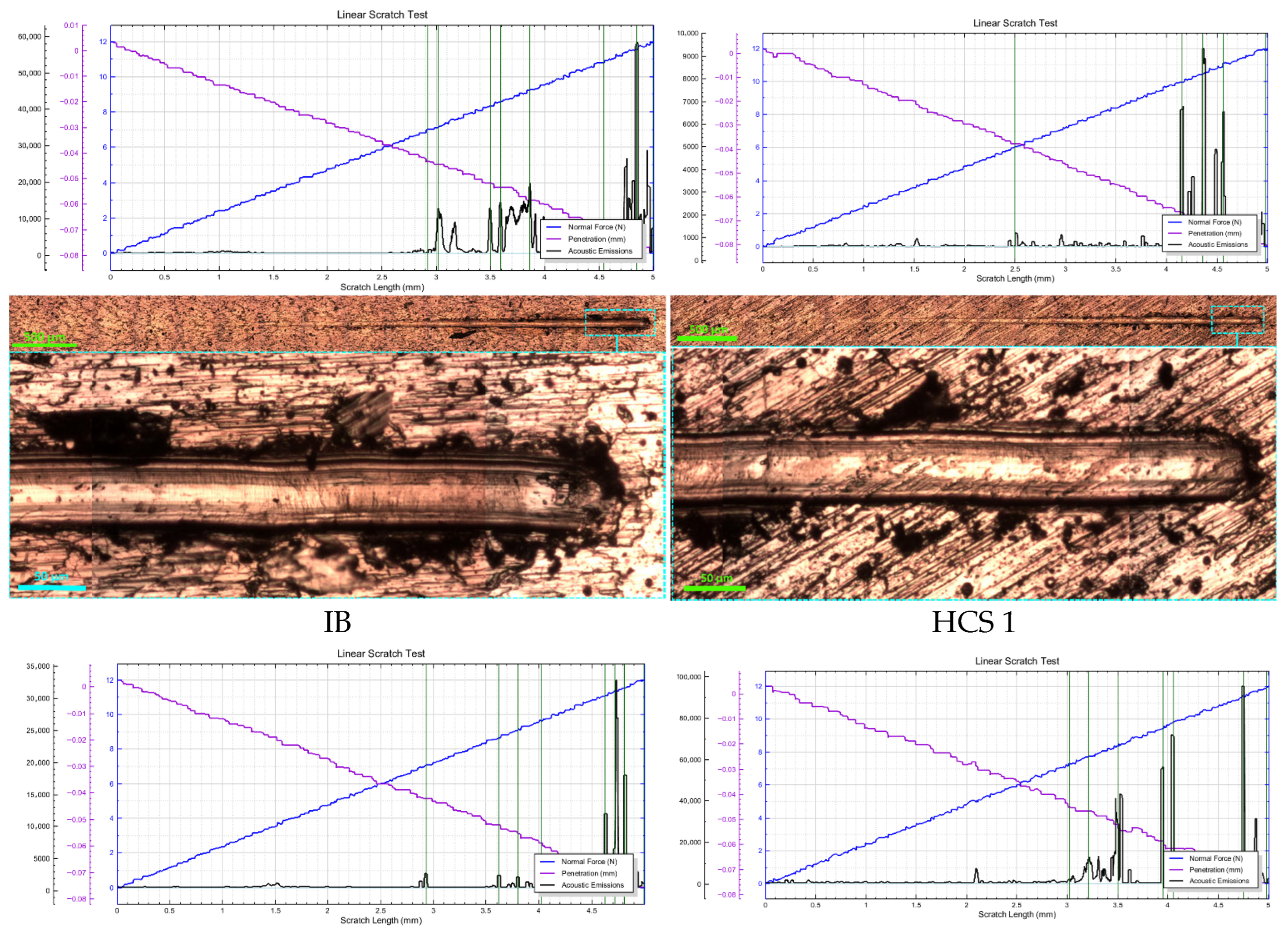
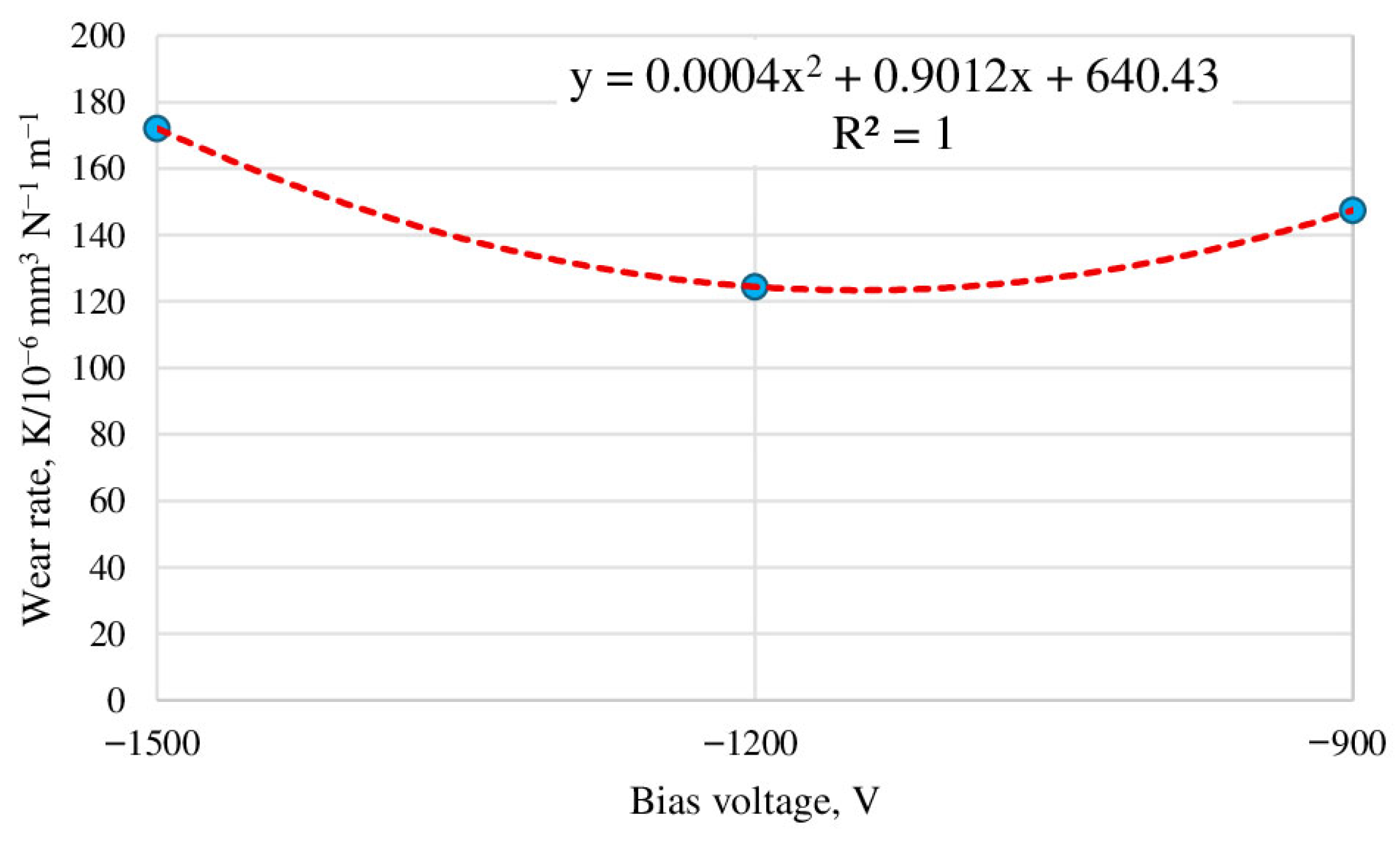
3.2. Determination of the Optimal Bias Voltage of the HCS Process Substrate
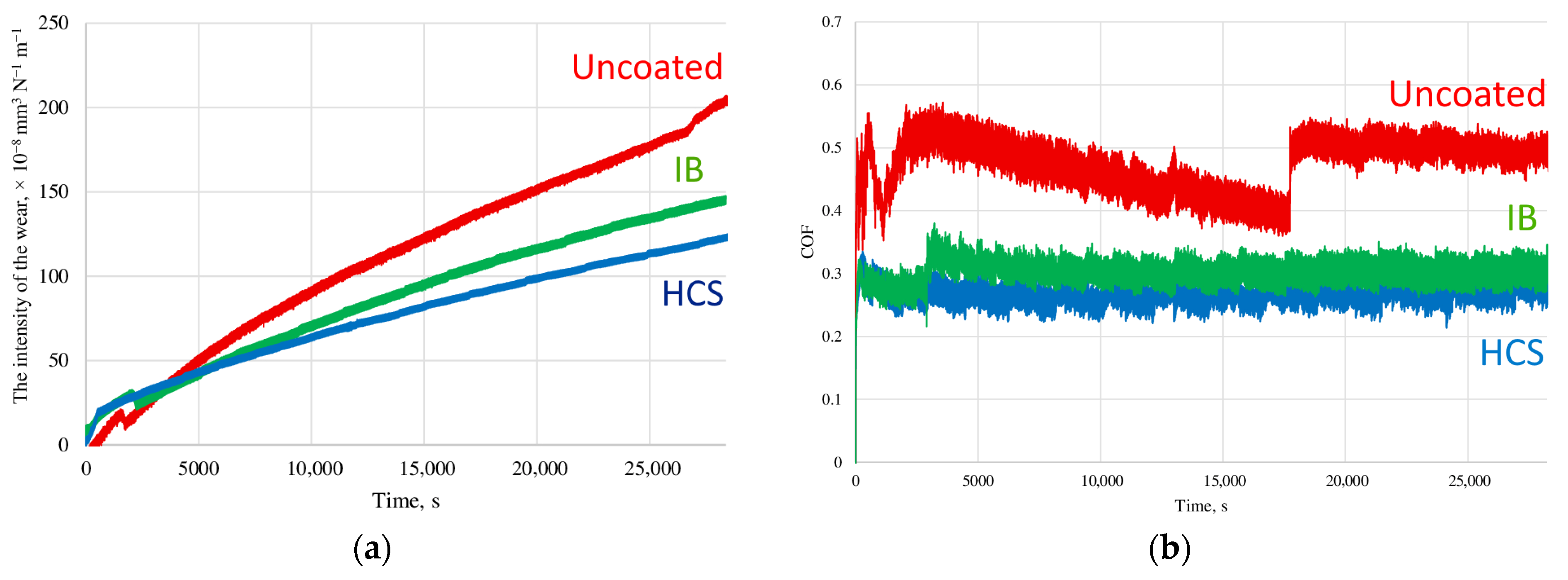
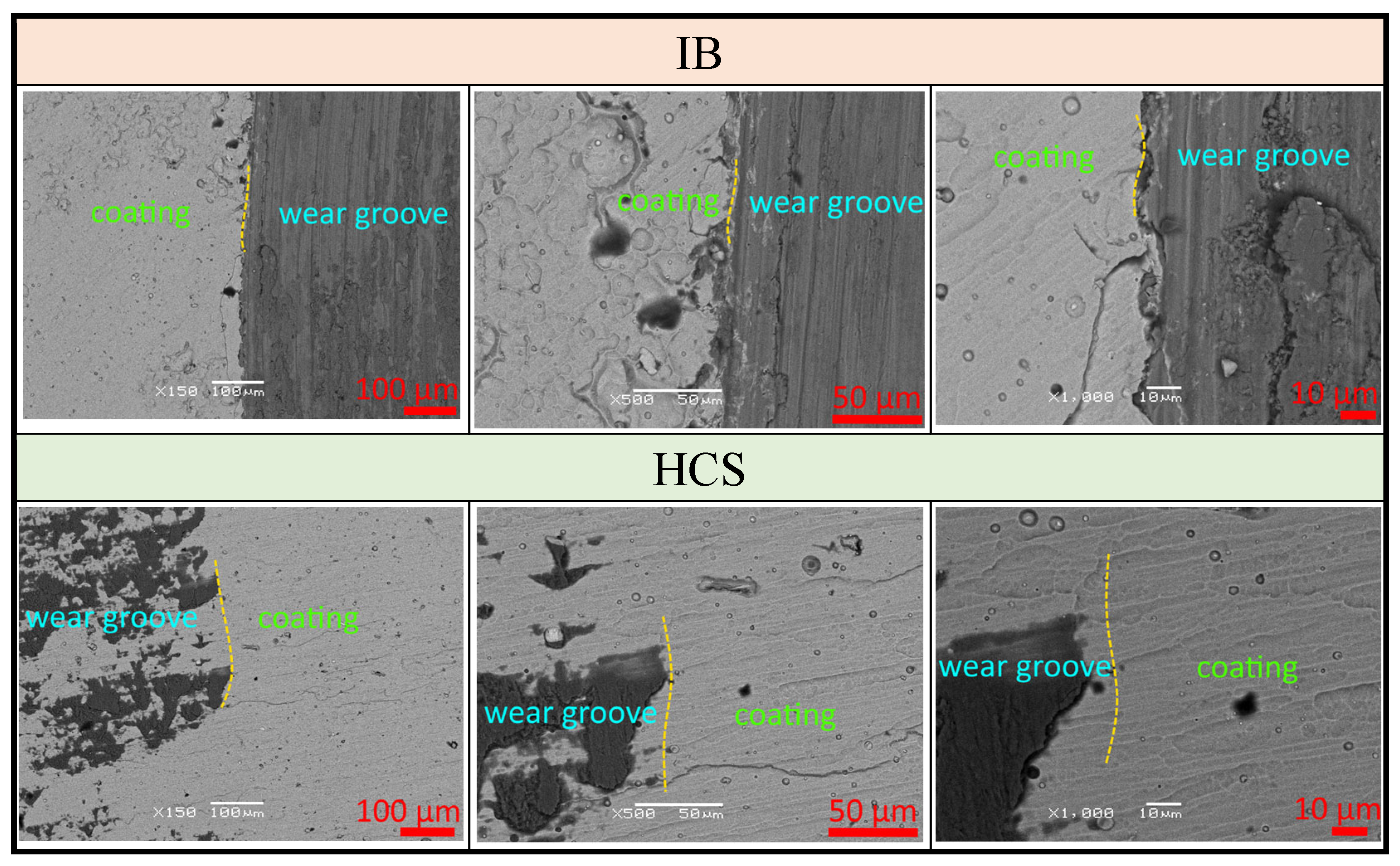
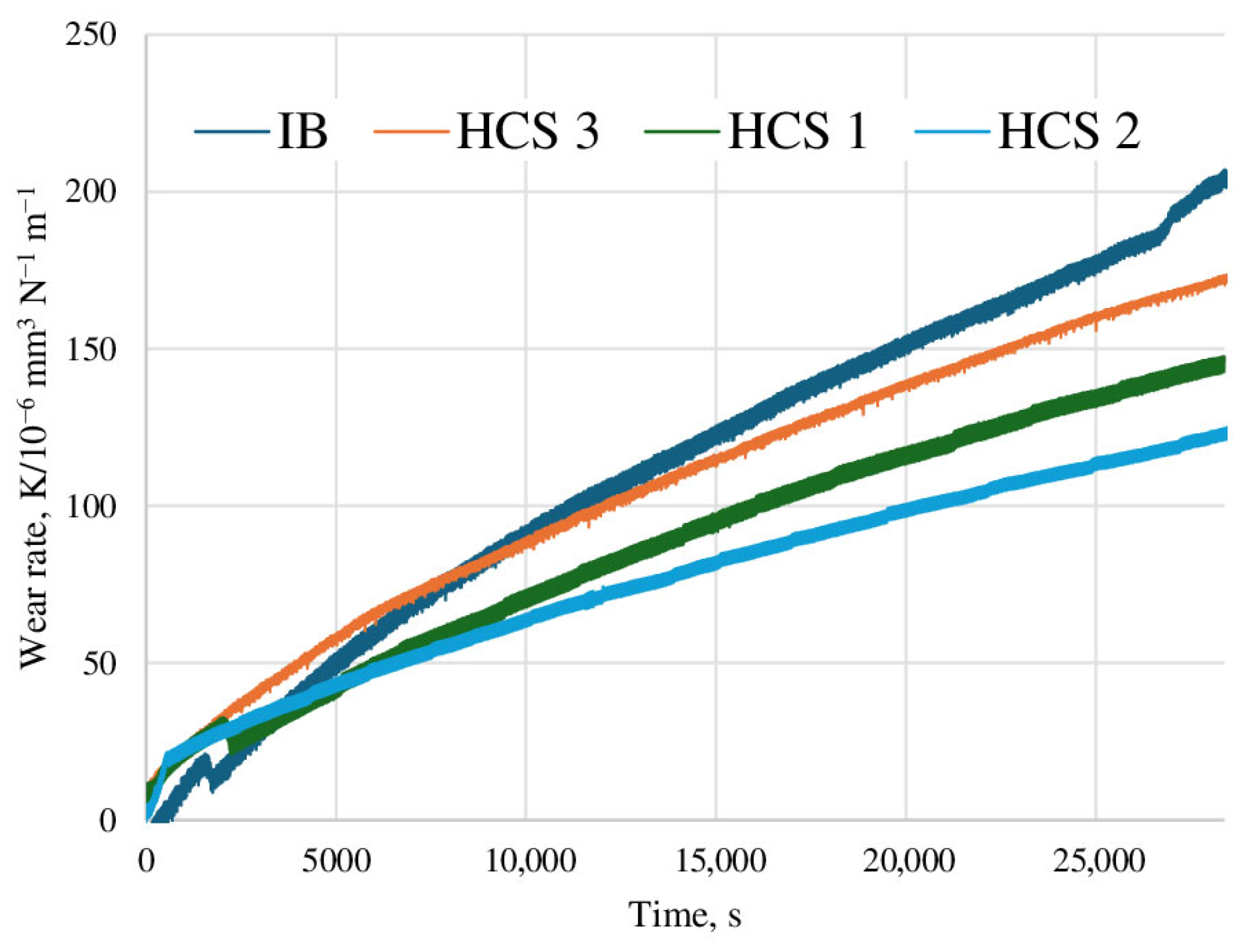
3.3. Wear Behavior of Samples After Pin-on-Disk Testing
4. Conclusions
- After deposition of the coatings, the wear resistance of the samples increases by 50–70% compared to samples made of uncoated 6Al-4V titanium alloy, while the use of the HCS method provides wear resistance that is 30% higher than the IB method.
- Studies of the friction coefficient show that the deposition of coatings provides a reduction in this parameter from 0.5 to 0.3 (by 65%). At the same time, samples with coatings deposited using the HCS and IB methods demonstrate very close values of the friction coefficient, with an insignificant (10–15%) reduction in this indicator for HCS samples.
- The use of Ion Bombardment (IB) or glow discharge (HCS) methods significantly affects the wear resistance of the coating, but only slightly affects the coefficient of friction.
- Changing the bias voltage on the substrate (−900, −1200, and −1500 V) has a significant effect on wear resistance. The best wear resistance is observed for the HCS sample with a bias voltage of −1200 V. The calculated optimum value of the bias voltage is −1126 V.
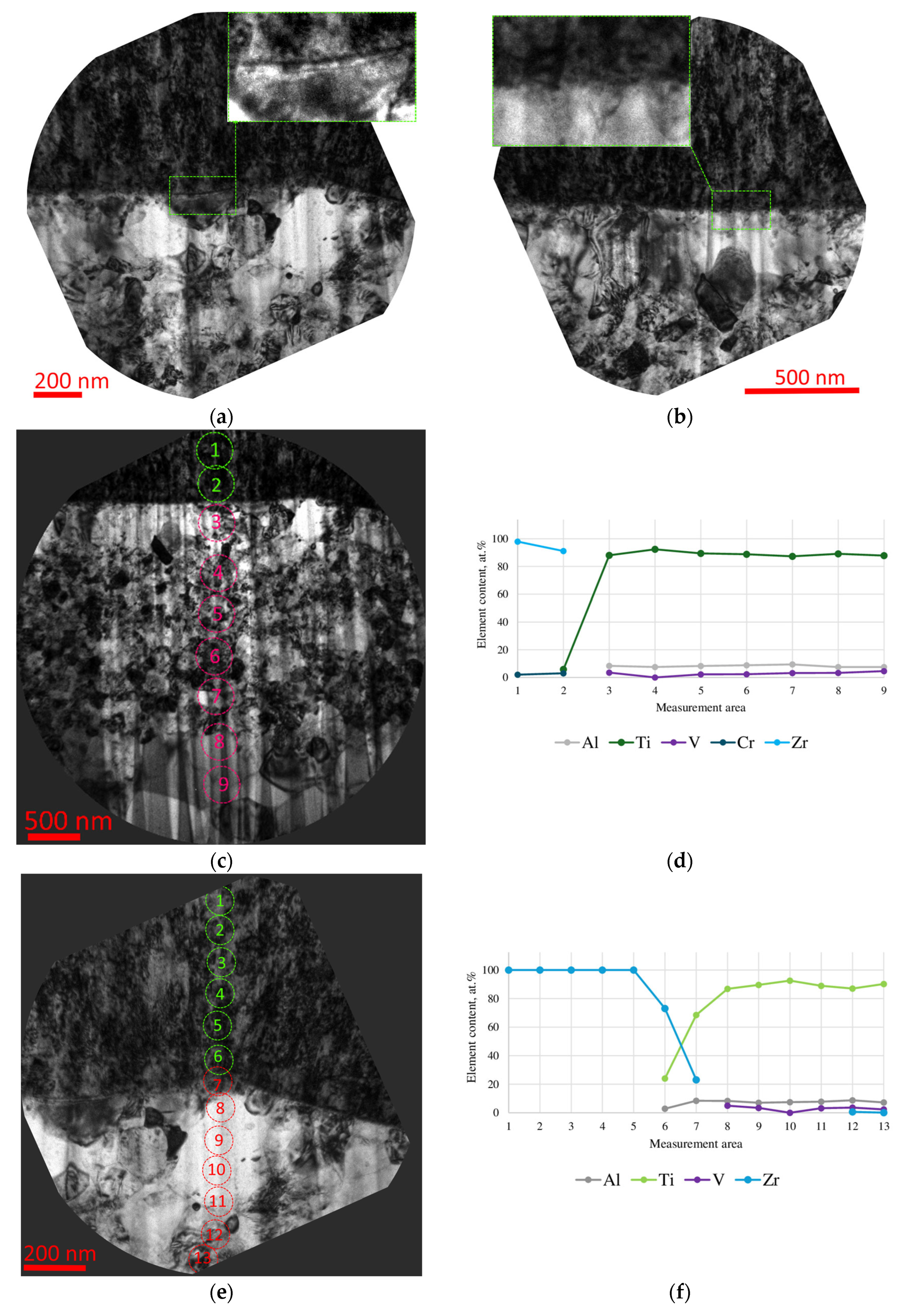
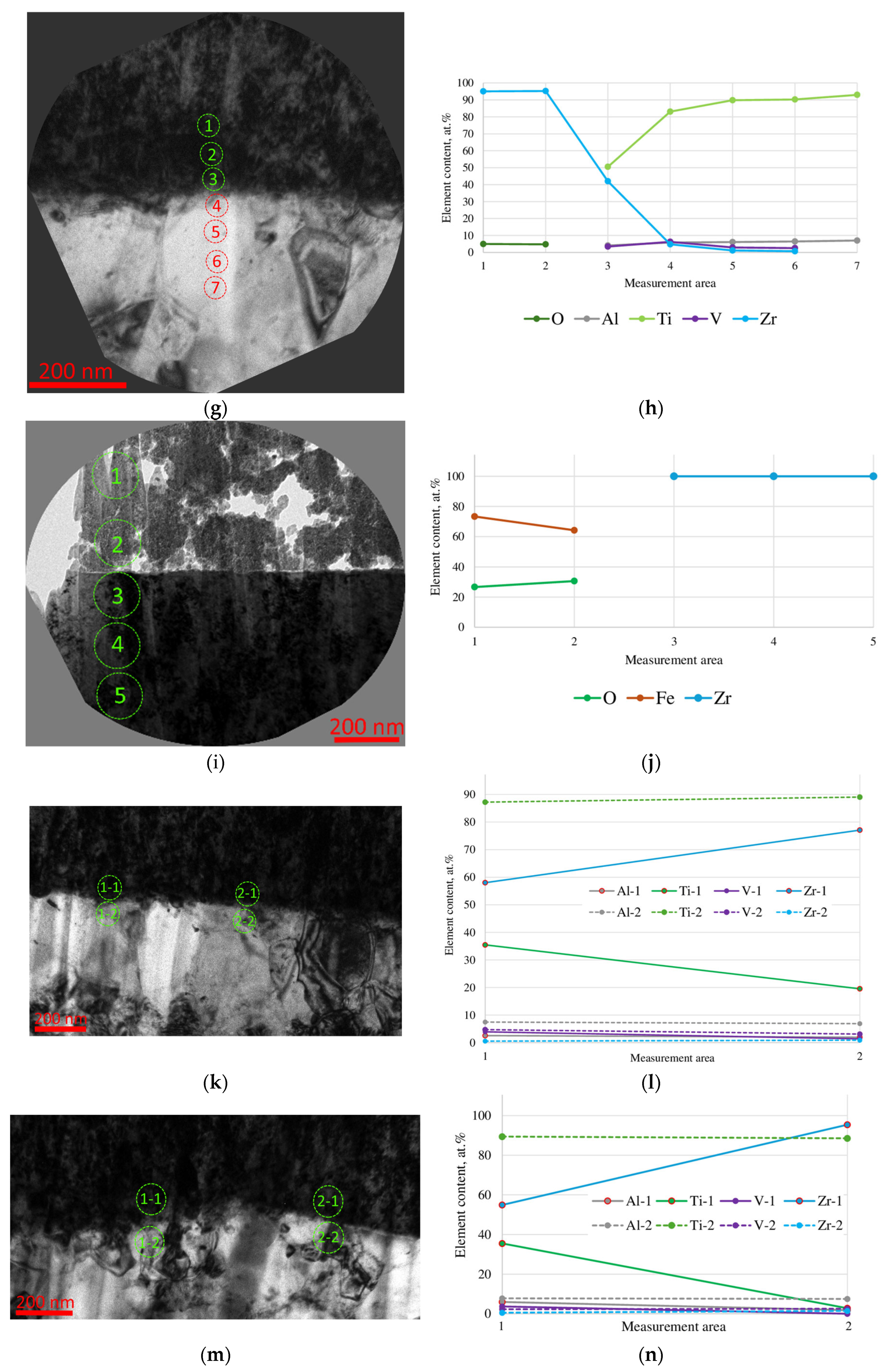
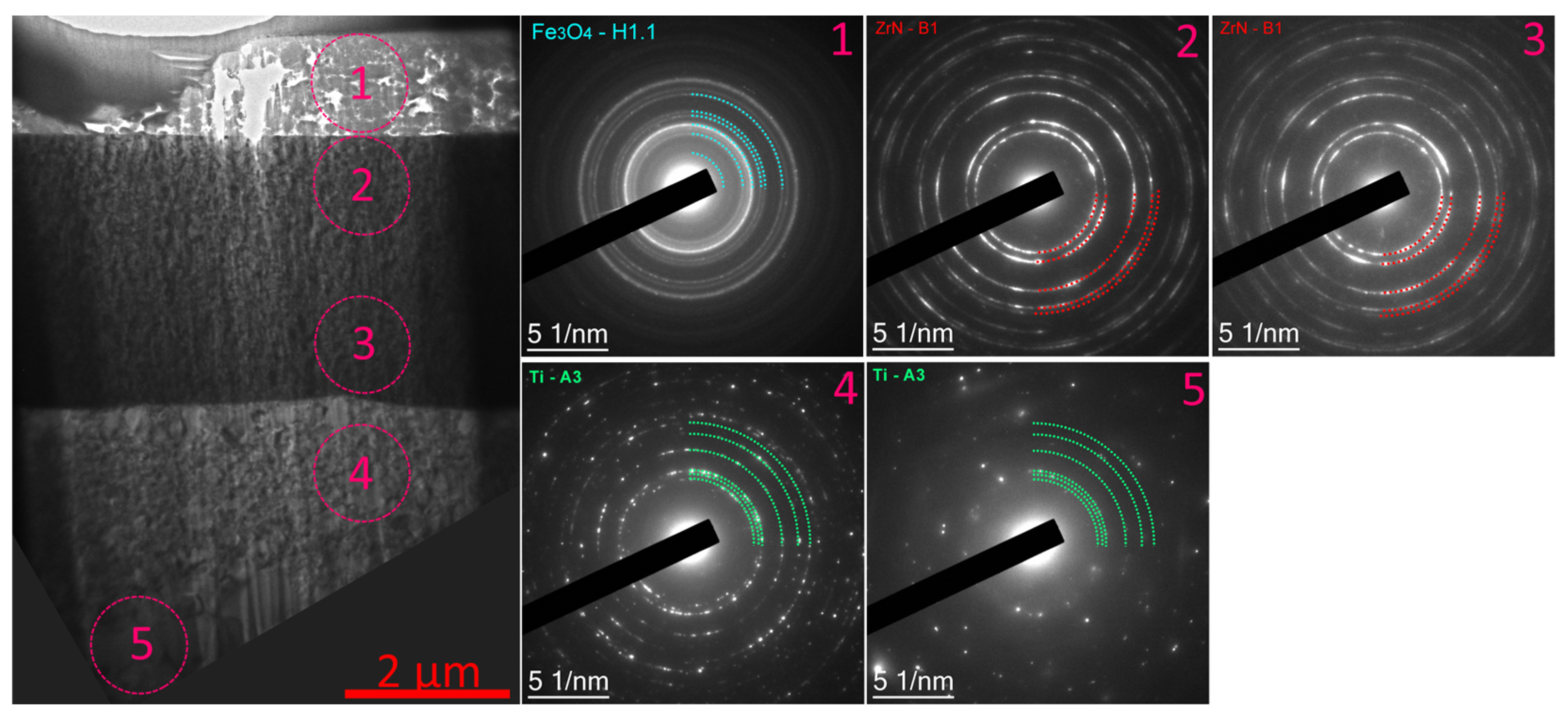
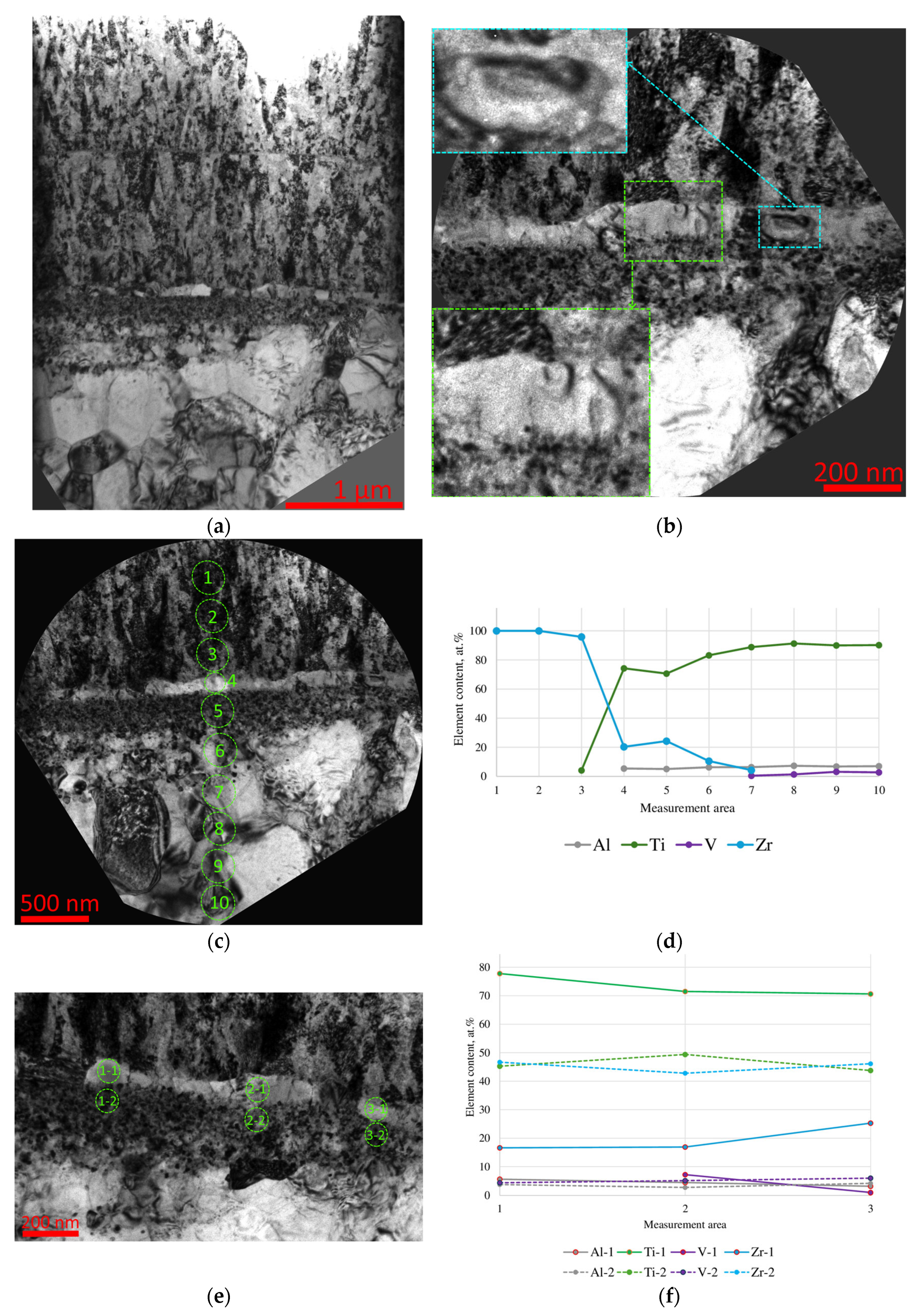
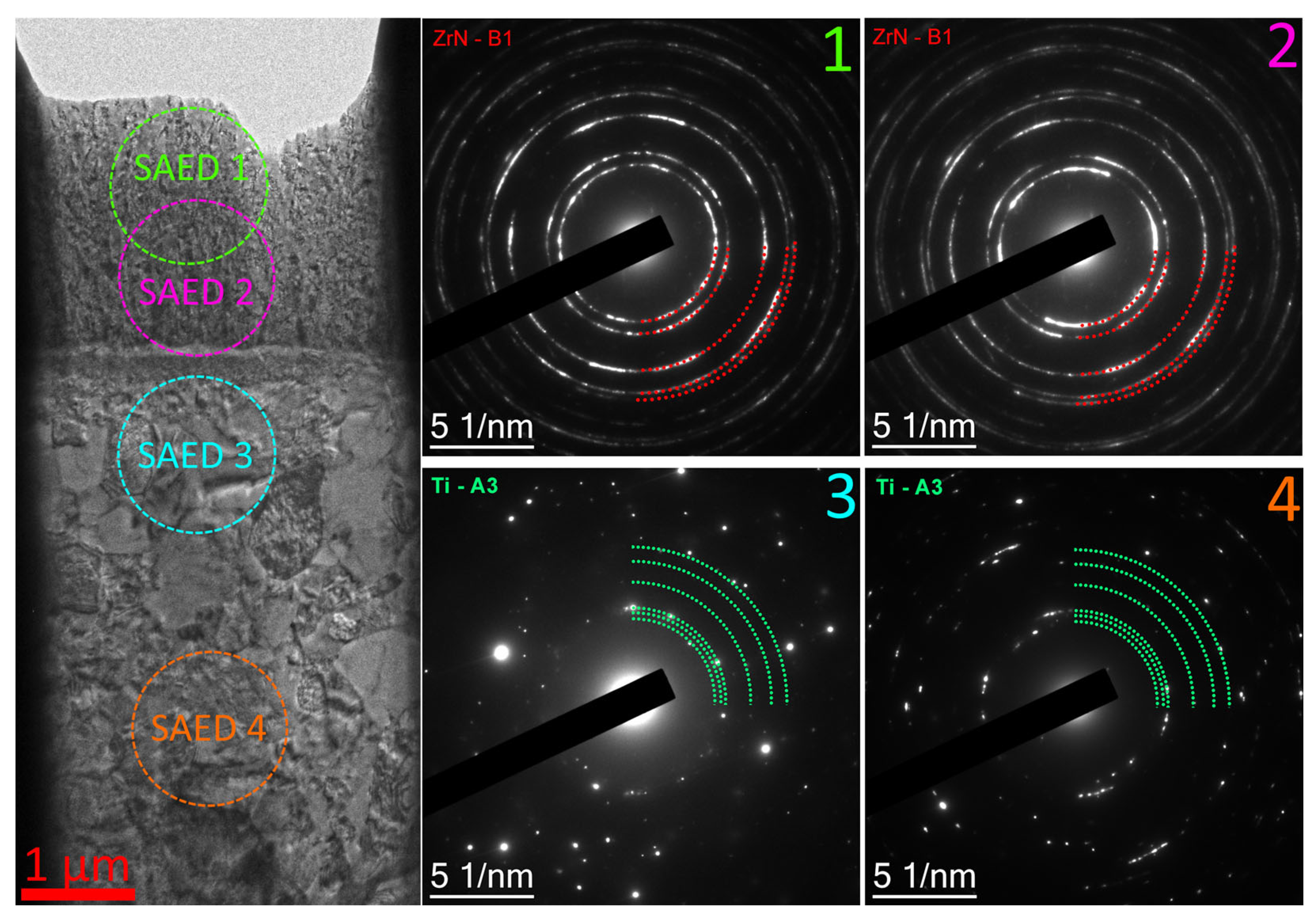
- During the pre-treatment of the substrate using the HCS and IB methods, a transition layer can be formed in the area of the coating–substrate interface; the thickness of this layer varies within the range of 15–400 nm, and the composition is a mixture of coating (zirconium) and substrate (titanium, aluminum, and vanadium) materials.
- When using the HCS method, a clearly defined interface is formed between the coating and the substrate, with preferential diffusion of titanium from the substrate into the adjacent layers of the coating; when using the IB method, the interface has a complex layered shape with noticeable mutual diffusion of titanium and zirconium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frey, H.; Khan, H.R. Handbook of Thin Film Technology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Ilyin, A.A.; Plihunov, V.V.; Petrov, L.M.; Zelenkov, V.V.; Ivanchuk, S.B.; Sokolov, I.V. Cleaning and Activation of the Surface of Products from Structural Metallic Materials in the Process of Vacuum Ion-Plasma Treatment. Technol. Light Alloy. 2009, 2, 111–117. [Google Scholar]
- Luchkin, A.G.; Luchkin, G.S. Cleaning the Surface of Substrates for Coating by Vacuum-Plasma Methods. Bull. Kazan Technol. Univ. 2012, 15, 208–210. [Google Scholar]
- Oura, K.; Lifshitz, V.G.; Saranin, A.A.; Zotov, A.V.; Katayama, M. Introduction to Surface Physics; Nauka: Moscow, Russia, 2006. [Google Scholar]
- Tillmann, W.; Hagen, L.; Stangier, D.; Krabiell, M.; Elbers, M. Influence of etching-pretreatment on nano-grained WC-Co surfaces and properties of PVD/HVOF duplex coatings. Surf. Coat. Technol. 2019, 374, 32–43. [Google Scholar] [CrossRef]
- Pemmasani, S.P.; Valleti, K.; Gundakaram, R.C.; Rajulapati, K.V.; Mantripragada, R.; Koppoju, S.; Joshi, S.V. Effect of microstructure and phase constitution on mechanical properties of Ti1−xAlxN coatings. Appl. Surf. Sci. 2014, 313, 936–946. [Google Scholar] [CrossRef]
- Yang, J.; Odén, M.; Johansson-Jõesaar, M.P.; Esteve, J.; Llanes, L. Mechanical strength of ground WC-Co cemented carbides after coating deposition. Mater. Sci. Eng. A 2017, 689, 72–77. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Ananth, A.; Khan, J.; Srinivas, G. Ar + H2 plasma etching for improved adhesion of PVD coatings on steel substrates. Vacuum 2012, 86, 1165–1173. [Google Scholar] [CrossRef]
- Buchwalder, A.; Zenker, R. Pre- and post-surface treatments using electron beam technology for load-related application of thermochemical and PVD hard coatings on soft substrate materials. Surf. Coat. Technol. 2019, 375, 920–932. [Google Scholar] [CrossRef]
- Guo, X.; Liu, F.; Zhang, K.; Wang, C.; Piao, Z.; Sun, L. Controllable preparation of micro-textures on WC/Co substrate surface by an integrated laser-dry etching process for improving PVD coatings adhesion. Appl. Surf. Sci. 2020, 534, 147580. [Google Scholar] [CrossRef]
- Zhang, K.D.; Deng, J.X.; Guo, X.H.; Sun, L.N.; Lei, S.T. Study on the adhesion and tribological behavior of PVD TiAlN coatings with a multi-scale textured substrate surface. Int. J. Refract. Met. Hard Mater. 2018, 72, 292–305. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, K.; Guo, X.; Wang, C.; Sun, L. Preparation of micro-textures on cemented carbide substrate surface by plasma-assisted laser machining to enhance the PVD tool coatings adhesion. J. Mater. Process. Technol. 2021, 288, 116870. [Google Scholar] [CrossRef]
- Stepanovsky, A.S. Ionic Processing of Materials. Mech. Eng. Sci. Technol. 2013, 14, 1–3. [Google Scholar]
- Komarovskaya, V.M.; Ivashchenko, S.A. Optimization of modes of ion treatment of the surface of non-metallic materials. Hardening Technol. Coat. 2013, 3, 23–27. [Google Scholar]
- Zabrodin, I.G.; Zorina, M.V.; Kaskov, I.A.; Malyshev, I.V.; Mikhailenko, M.S.; Pestov, A.E.; Salashchenko, N.N.; Chernyshev, A.K.; Chkhalo, N.I. Ion-beam techniques for precision processing of optical surfaces. J. Tech. Phys. 2020, 90, 1922–1930. [Google Scholar]
- Grebenyuk, V.F.; Rudakov, V.I. Ion-plasma technology for hardening a deforming tool. Vestn. OGU 2003, 5, 137–140. [Google Scholar]
- Berlin, E.; Dvinin, S.; Seidman, L. World of Materials and Technologies. In Vacuum Technology and Equipment for Deposition and Etching of Thin Films; Technosphere: Moscow, Russia, 2007. [Google Scholar]
- Hovsepian, P.; Reinhard, C.; Ehiasarian, A.P. CrAlYN/CrN superlattice coatings deposited by the combined high power impulse magnetron sputtering/unbalanced magnetron sputtering technique. Surf. Coat. Technol. 2006, 201, 4105–4110. [Google Scholar] [CrossRef]
- Berish, R. Problems of Applied Physics. In Sputtering of Solids by Ion Bombardment; Springer: Berlin/Heidelberg, Germany, 1986; Volume II, p. 482. [Google Scholar]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Lattemann, M.; Ehiasarian, A.P.; Bohlmark, J.; Persson, P.; Helmersson, U. Investigation of high power impulse magnetron sputtering pretreated interfaces for adhesion enhancement of hard coatings on steel. Surf. Coat. Technol. 2006, 200, 6495–6499. [Google Scholar] [CrossRef]
- Mukha, I.M.; Shcherbakova, L.I. Effect of Heating by a Glow Discharge on the Surface State of Transition-Metal Carbides. Elektron. Obrab. Mater. 1976, 3, 53–56. [Google Scholar]
- Betiuk, M.; Michalski, J.; Burdyński, K.; Wach, P.; Nakonieczny, A. Influence of ion etching in low pressure arc discharge in plasma on duplex coat adhesion produced by gas nitriding and PA-PVD-Arc processes. Mater. Manuf. Process. 2009, 24, 859–862. [Google Scholar] [CrossRef]
- Breidenstein, B.; Denkena, B. Significance of residual stress in PVD-coated carbide cutting tools. CIRP Ann. Manuf. Technol. 2013, 62, 67–70. [Google Scholar] [CrossRef]
- Denkena, B.; Breidenstein, B. Residual stress gradients in PVD-coated carbide cutting tools. Mater. Sci. Forum 2006, 524–525, 607–612. [Google Scholar]
- Gassner, M.; Schalk, N.; Sartory, B.; Pohler, M.; Czettl, C.; Mitterer, C. Influence of Ar ion etching on the surface topography of cemented carbide cutting inserts. Int. J. Refract. Met. Hard. Mater. 2017, 69, 234–239. [Google Scholar] [CrossRef]
- Yang, J.; Odén, M.; Johansson-Jõesaar, M.P.; Llanes, L. Grinding effects on surface integrity and mechanical strength of WC-Co cemented carbides. Procedia CIRP 2014, 13, 257–263. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Zelenkov, V.; Sitnikov, N.; Bublikov, J.; Milovich, F.; Andreev, N.; Sotova, C. Investigation of the influence of the features of the deposition process on the structural features of microparticles in PVD coatings. Vacuum 2022, 202, 111144. [Google Scholar] [CrossRef]
- Kablov, E.N.; Muboyadzhyan, S.A. Ion etching and surface modification of critical machine parts in vacuum-arc plasma. Bull. MSTU Im. N.E. Bauman 2011, 2, 149–162. [Google Scholar]
- Mattox, M.D. Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; William Andrew: Norwich, NY, USA, 2010. [Google Scholar]
- Broitman, E.; Hultman, L. Adhesion improvement of carbon-based coatings through a high ionization deposition technique. J. Phys. Conf. Ser. 2012, 370, 012009. [Google Scholar] [CrossRef]
- Panjan, M. Influence of substrate rotation and target arrangement on the periodicity and uniformity of layered coatings. Surf. Coat. Technol. 2013, 235, 32–44. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Mahne, N.; Čekada, M.; Panjan, M. Surface Topography of PVD Hard Coatings. Coatings 2021, 11, 1387. [Google Scholar] [CrossRef]
- Schönjahn, C.; Lewis, D.B.; Münz, W.-D.; Petrov, I. Shortlisted substrate ion etching in combined steered cathodic arc–ubm deposition system: Effects on interface architecture, adhesion, and tool performance. Surf. Eng. 2000, 16, 176–180. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Čekada, M.; Panjan, M. Contamination of Substrate-Coating Interface Caused by Ion Etching. Coatings 2022, 12, 846. [Google Scholar] [CrossRef]
- Zadorozhnyi, V.G.; Rafalovich, D.M.; Roikh, I.L. Effect of Surface Treatment of Steel with a Glow Discharge on the Adhesion and Porosity of Vacuum Coatings of Polyfluorotrichloroethylene. Elektron. Obrab. Mater. 1977, 1, 43–45. [Google Scholar]
- Vetter, J.; Burgmer, W.; Perry, A.J. Arc-enhanced glow discharge in vacuum arc machines. Surf. Coat. Technol. 1993, 59, 152–155. [Google Scholar] [CrossRef]
- Wang, Y.H.; Guo, F.; Ren, H.; Hu, S.Y.; Chen, Y.J.; Zhao, Y.H.; Gong, F.; Xie, Z.W. Enhancing wear resistance of TiN coating by gradient bias voltage and arc-enhanced glow discharge. Ceram. Int. 2022, 48, 8746–8750. [Google Scholar] [CrossRef]
- Xue, G.; Wang, Z.; Xiang, L.; Xiao, H.; Zhao, Y.; Wan, Y.; Ning, H.; Xie, Z. Enhancing hot corrosion performance of NiCoCrAlY/AlSiY coating by arc enhanced glow discharge. Mater. Lett. X 2022, 13, 100130. [Google Scholar] [CrossRef]
- Marciniak, A. Non-uniform heating effects during treatment in a glow discharge. Thin Solid Film. 1988, 156, 337–344. [Google Scholar] [CrossRef]
- Samir, T.; Liu, Y.; Zhao, L.-L.; Zhou, Y.-W. Effect of driving frequency on electron heating in capacitively coupled RF argon glow discharges at low pressure. Chin. Phys. B 2017, 26, 115201. [Google Scholar] [CrossRef]
- Grigoriev, S.; Melnik, Y.; Metel, A. Broad fast neutral molecule beam sources for industrial-scale beam-assisted deposition. Surf. Coat. Technol. 2002, 156, 44–49. [Google Scholar] [CrossRef]
- Bublikov, J.; Volosova, M.; Vereschaka, A.; Milovich, F.; Seleznev, A.; Shmakov, I.; Grigoriev, S. Hollow cathode effect in a glow discharge for cleaning and heating substrate surfaces during wear-resistant coating deposition. J. Mater. Res. Technol. 2025, 39, 7657–7670. [Google Scholar] [CrossRef]
- Wei, G.; Tan, M.; Attarilar, S.; Uglov, V.V.; Wang, B.; Liu, J.; Lu, L.; Wang, L. An overview of surface modification, A way toward fabrication of nascent biomedical Ti–6Al–4V alloys. J. Mater. Res. Technol. 2023, 24, 5896–5921. [Google Scholar] [CrossRef]
- Freitas, B.J.M.; Vidilli, A.L.; de Bribean Guerra, A.; Ferreira, D.C.F.; Kasama, A.H.; Paes, M.T.P.; Koga, G.Y.; Bolfarini, C. Ti-6Al-4V and Ti-6Al-4V-0.1Ru alloys in oil and gas exploration: A review of performance and hydrogen embrittlement. J. Alloys Compd. 2025, 1019, 179258. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Z. High-pressure coolant effect on the surface integrity of machining titanium alloy Ti-6Al-4V: A review. Mater. Res. Express 2018, 5, 032001. [Google Scholar] [CrossRef]
- ASTM F1472-20a; Standard Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications (UNS R56400). ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Arias, D.F.; Arango, Y.C.; Devia, A. Study of TiN and ZrN thin films grown by cathodic arc technique. Appl. Surf. Sci. 2006, 253, 1683–1690. [Google Scholar] [CrossRef]
- Harrison, R.W.; Lee, W.E. Processing and properties of ZrC, ZrN and ZrCN ceramics: A review. Adv. Appl. Ceram. 2016, 115, 294–307. [Google Scholar] [CrossRef]
- Patsalas, P. Zirconium nitride: A viable candidate for photonics and plasmonics? Thin Solid Film. 2019, 688, 137438. [Google Scholar] [CrossRef]
- Aristova, N.M.; Onufriev, S.V.; Savvatimskiy, A.I. Thermodynamic properties of zirconium nitride in the condensed state. Phys. Uspekhi 2024, 67, 1022–1033. [Google Scholar] [CrossRef]
- Slaymaker, J.; Rossouw, E.; Michelogiannakis, D. The influence of protective coatings on corrosion resistance in orthodontic magnets: A systematic review of in vitro studies. Int. Orthod. 2026, 24, 101080. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Sitnikov, N.; Andreev, N.; Bublikov, J.; Kutina, N. Investigation of the properties of the Cr,Mo-(Cr,Mo,Zr,Nb)N-(Cr,Mo,Zr,Nb,Al)N multilayer composite multicomponent coating with nanostructured wear-resistant layer. Wear 2021, 468–469, 203597. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Bublikov, J.I.; Sitnikov, N.N.; Oganyan, G.V.; Sotova, C.S. Influence of nanolayer thickness on the perfor-mance properties of multilayer composite nano-structured modified coatings for metal-cutting tools. Int. J. Adv. Manuf. Technol. 2018, 95, 2625–2640. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Migranov, M.; Andreev, N.; Bublikov, J.; Sitnikov, N.; Oganyan, G. Investigation of the tribological properties of Ti-TiN-(Ti,Al,Nb,Zr)N composite coating and its efficiency in increasing wear resistance of metal cutting tools. Tribol. Int. 2021, 164, 107236. [Google Scholar] [CrossRef]
- Vereschaka, A.S.; Grigoriev, S.N.; Sotova, E.S.; Vereschaka, A.A. Improving the efficiency of the cutting tools made of mixed ceramics by applying modifying nano-scale multilayered coatings. Adv. Mater. Res. 2013, 712–715, 391–394. [Google Scholar]
- Metel, A.; Bolbukov, V.; Volosova, M.; Grigoriev, S.; Melnik, Y. Equipment for Deposition of thin metallic films bombarded by fast argon atoms. Instrum. Exp. Tech. 2014, 57, 345–351. [Google Scholar] [CrossRef]
- Metel, A.S.; Grigoriev, S.N.; Melnik, Y.A.; Bolbukov, V.P. Characteristics of a fast neutral atom source with electrons injected into the source through its emissive grid from the vacuum chamber. Instrum. Exp. Tech. 2012, 55, 288–293. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Sitnikov, N.; Seleznev, A.; Sotova, C.; Bublikov, J. Influence of the yttrium cathode arc current on the yttrium content in the (Ti,Y,Al) N coating and the coating properties. Vacuum 2024, 222, 113028. [Google Scholar] [CrossRef]
- ASTM C1624-05; Standard Test Method for Adhesion Strength and Mechanical Failure Modes of Ceramic Coatings by Quantitative Single Point Scratch Testing. ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- ASTM G99-23; Standard Test Method for Wear and Friction Testing with a Pin-on-Disk or Ball-on-Disk Apparatus. ASTM: West Conshohocken, PA, USA, 2023. Available online: https://store.astm.org/standards/g99 (accessed on 22 June 2025).
- Sotova, C.; Bublikov, Y.; Seleznev, A.; Shmakov, I.; Vereschaka, A. Study of the effect of healing surface defects during deposition of nitride coatings on titanium alloy parts. High Temp. Mater. Process. 2025, 29, 1–8. [Google Scholar] [CrossRef]
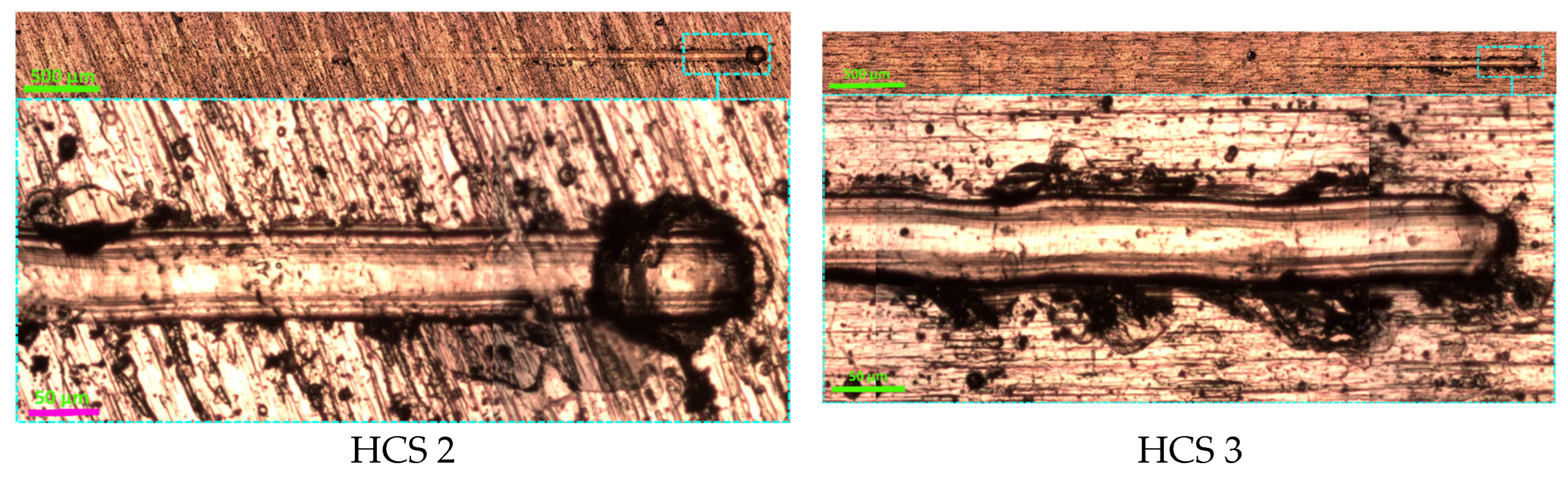

| Ti | Al | V | Fe | Zr | O | C | Si | N | H | Other Impurities |
|---|---|---|---|---|---|---|---|---|---|---|
| 86.45–90.90 | 5.3–6.8 | 3.5–5.3 | max 0.6 | max 0.3 | max 0.2 | max 0.1 | max 0.1 | max 0.05 | max 0.015 | 0.3 |
| Designation of Samples | HCS 1 | HCS 2 | HCS 3 |
|---|---|---|---|
| Substrate bias voltage, V | −900 V | −1200 V | −1500 V |
| Roughness, µm | Uncoated | IB | HCS |
|---|---|---|---|
| Ra | 10.22 | 4.94 | 3.58 |
| Rz | 7.32 | 5.98 | 4.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metel, A.; Bublikov, Y.; Melnik, Y.; Sotova, C.; Milovich, F.; Seleznev, A.; Shmakov, I.; Borovik, T.; Potapov, Y.; Vereschaka, A. Features of the Process of Surface Preparation of Products Using Glow Discharge Plasma During the Deposition of Modifying Coatings. J. Compos. Sci. 2025, 9, 640. https://doi.org/10.3390/jcs9120640
Metel A, Bublikov Y, Melnik Y, Sotova C, Milovich F, Seleznev A, Shmakov I, Borovik T, Potapov Y, Vereschaka A. Features of the Process of Surface Preparation of Products Using Glow Discharge Plasma During the Deposition of Modifying Coatings. Journal of Composites Science. 2025; 9(12):640. https://doi.org/10.3390/jcs9120640
Chicago/Turabian StyleMetel, Alexander, Yuri Bublikov, Yury Melnik, Catherine Sotova, Filipp Milovich, Anton Seleznev, Ilya Shmakov, Tatyana Borovik, Yuri Potapov, and Alexey Vereschaka. 2025. "Features of the Process of Surface Preparation of Products Using Glow Discharge Plasma During the Deposition of Modifying Coatings" Journal of Composites Science 9, no. 12: 640. https://doi.org/10.3390/jcs9120640
APA StyleMetel, A., Bublikov, Y., Melnik, Y., Sotova, C., Milovich, F., Seleznev, A., Shmakov, I., Borovik, T., Potapov, Y., & Vereschaka, A. (2025). Features of the Process of Surface Preparation of Products Using Glow Discharge Plasma During the Deposition of Modifying Coatings. Journal of Composites Science, 9(12), 640. https://doi.org/10.3390/jcs9120640










