Characterization and Antibacterial Properties of Centrifugally Spun Polyvinylpyrrolidone/Copper(II) Acetate Composite Fibers
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of CuAc/PVP Composite Fibers
2.3. Characterization
2.4. Antibacterial Testing
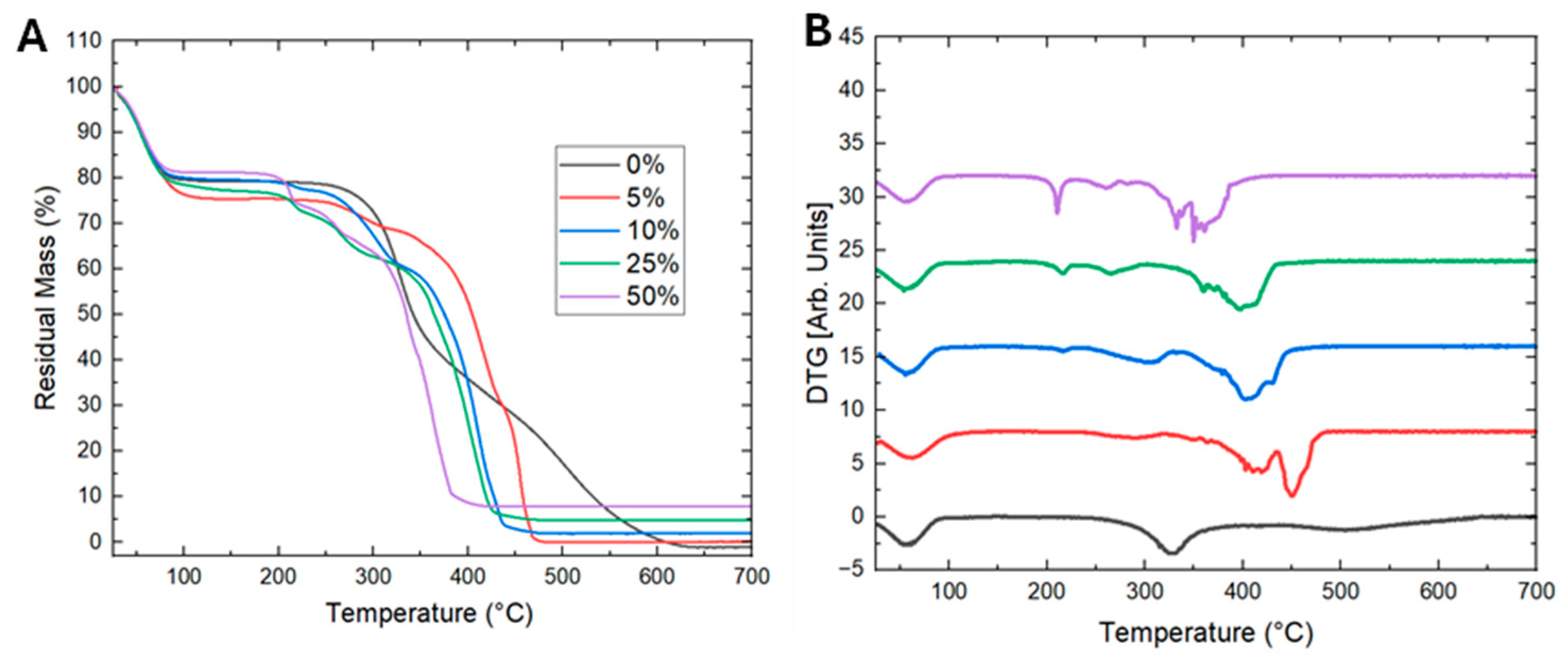
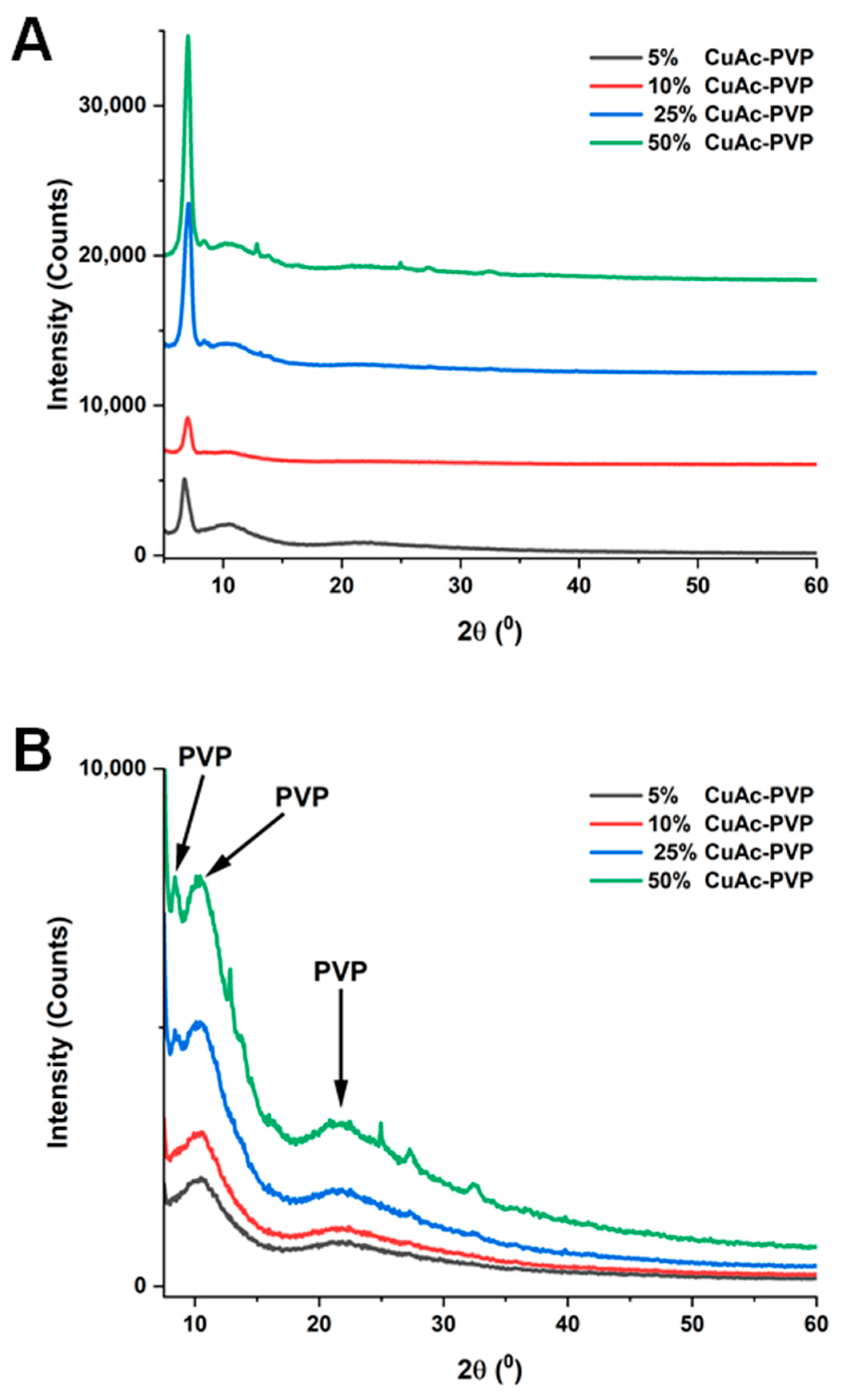
3. Results and Discussion
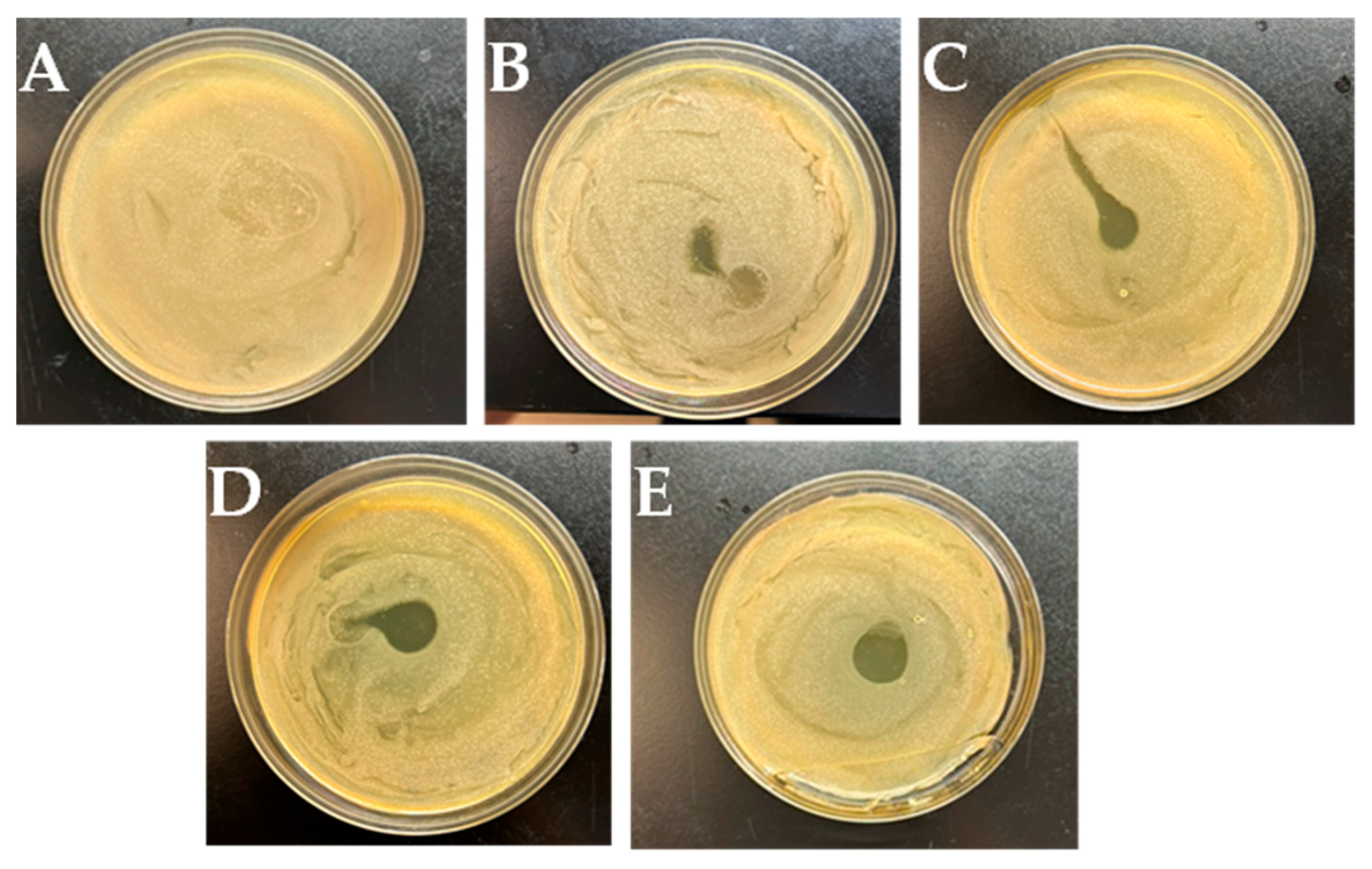
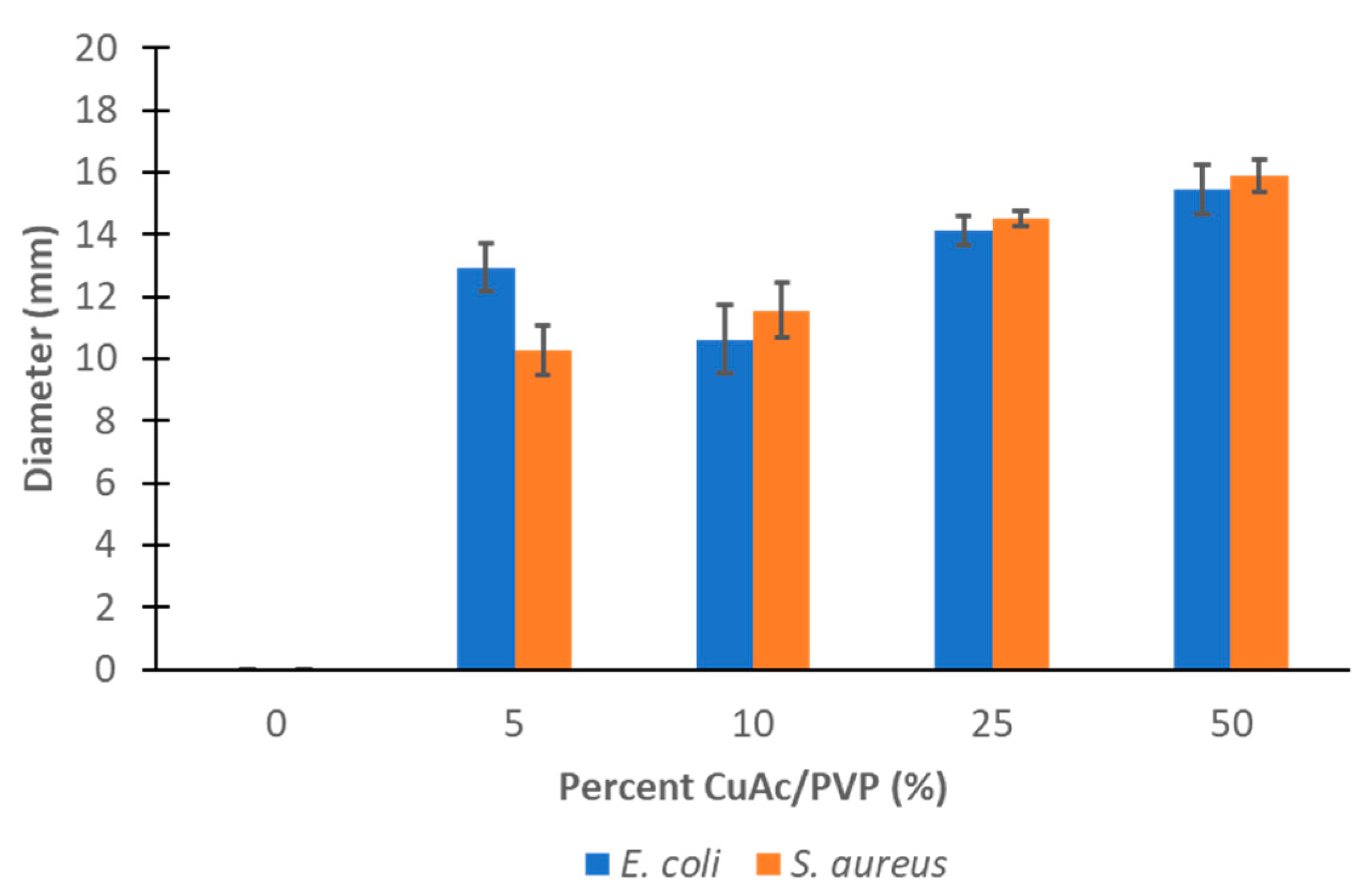
Antibacterial Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Zhao, X. Antibacterial bioactive materials. In Bioactive Materials in Medicine; Woodhead Publishing: Cambridge, UK, 2011; pp. 97–123. [Google Scholar]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, D.; Zhang, H.; Wang, Z.; Liu, Y. Evolutionary trajectory of bacterial resistance to antibiotics and antimicrobial peptides in Escherichia coli. mSystems 2025, 10, e0170024. [Google Scholar] [CrossRef] [PubMed]
- Giner-Lamia, J.; Lopez-Maury, L.; Florencio, F.J. Global transcriptional profiles of the copper responses in the cyanobacterium Synechocystis sp. PCC 6803. PLoS ONE 2014, 9, e108912. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.A.; Materon, L.; Parsons, J.G.; Alcoutlabi, M. Synthesis, Characterization, and Antibacterial Activity of Graphene Oxide/Zinc Hydroxide Nanocomposites. Appl. Sci. 2024, 14, 6274. [Google Scholar] [CrossRef]
- Chemler, S.R. Copper catalysis in organic synthesis. Beilstein J. Org. Chem. 2015, 11, 2252–2253. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.S.; Nguyen, N.C.; Nguyen, H.T. Electrospinning: A Versatile Fabrication Technique for Nanofibrous Membranes for Use in Desalination. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–273. [Google Scholar]
- Wentworth, B.J.; Stotts, M. Wilson’s Disease: The Copper Connection. Pract. Gastroenterol. 2020, 44, 10–20. [Google Scholar]
- Flores-Rábago, K.M.; Rivera-Mendoza, D.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Castro-Longoria, E. Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile. Antibiotics 2023, 12, 1251. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Vasiliev, G.; Kubo, A.L.; Vija, H.; Kahru, A.; Bondar, D.; Karpichev, Y.; Bondarenko, O. Synergistic antibacterial effect of copper and silver nanoparticles and their mechanism of action. Sci. Rep. 2023, 13, 9202. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Lubasova, D. Quantitative evaluation of antibacterial activities of nanoparticles (ZnO, TiO2, ZnO/TiO2, SnO2, CuO, ZrO2, and AgNO3) incorporated into polyvinyl butyral nanofibers. Polym. Adv. Technol. 2017, 28, 137–140. [Google Scholar] [CrossRef]
- Akhidime, I.D.; Saubade, F.; Benson, P.S.; Butler, J.A.; Olivier, S.; Kelly, P.; Verran, J.; Whitehead, K.A. The antimicrobial effect of metal substrates on far food pathogens. Food Bioprod. Process 2019, 113, 68–76. [Google Scholar] [CrossRef]
- Phan, D.N.; Dorjjugder, N.; Saito, Y.; Khan, M.Q.; Ullah, A.; Bie, X.Y.; Taguchi, G.; Kim, I.S. Antibacterial mechanisms of various copper species incorporated in polymeric nanofibers against bacteria. Mater. Today Commun. 2020, 25, 101377. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Maghsoodlou, M.T.; Heydari, R.; Lashkari, M. Copper(II) acetate monohydrate: An efficient and eco-friendly catalyst for the one-pot multi-component synthesis of biologically active spiropyrans and 1-pyrazolo[1,2-]phthalazine-5,10-dione derivatives under solvent-free conditions. Res. Chem. Intermed. 2016, 42, 7841–7853. [Google Scholar] [CrossRef]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.W.A.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. [Google Scholar] [CrossRef]
- Lee, H.; Alcoutlabi, M.; Watson, J.V.; Zhang, X.W. Electrospun nanofiber-coated separator membranes for lithium-ion rechargeable batteries. J. Appl. Polym. Sci. 2013, 129, 1939–1951. [Google Scholar] [CrossRef]
- Anusiya, G.; Jaiganesh, R. A review on fabrication methods of nanofibers and a special focus on application of cellulose nanofibers. Carbohydr. Polym. Technol. Appl. 2022, 4, 100262. [Google Scholar] [CrossRef]
- De la Garza, D.; De Santiago, F.; Materon, L.; Chipara, M.; Alcoutlabi, M. Fabrication and characterization of centrifugally spun poly(acrylic acid) nanofibers. J. Appl. Polym. Sci. 2019, 136, 47480. [Google Scholar] [CrossRef]
- Flores, D.; Villarreal, J.; Lopez, J.; Alcoutlabi, M. Production of carbon fibers through Forcespinning® for use as anode materials in sodium ion batteries. Mater. Sci. Eng. B 2018, 236, 70–75. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; De la Garza, D.; Gallegos, L.; Pokhrel, M.; Alcoutlabi, M. Forcespinning: A new method for the mass production of Sn/C composite nanofiber anodes for lithium ion batteries. Solid State Ion. 2016, 286, 72–82. [Google Scholar] [CrossRef]
- Chavez, R.O.; Lodge, T.P.; Alcoutlabi, M. Recent developments in centrifugally spun composite fibers and their performance as anode materials for lithium-ion and sodium-ion batteries. Mater. Sci. Eng. B 2021, 266, 115024. [Google Scholar] [CrossRef]
- Chavez, R.O.; Lodge, T.P.; Huitron, J.; Chipara, M.; Alcoutlabi, M. Centrifugally spun carbon fibers prepared from aqueous poly(vinylpyrrolidone) solutions as binder-free anodes in lithium-ion batteries. J. Appl. Polym. Sci. 2021, 138, e50396. [Google Scholar] [CrossRef]
- Lopez, J.; Gonzalez, R.; Ayala, J.; Cantu, J.; Castillo, A.; Parsons, J.; Myers, J.; Lodge, T.P.; Alcoutlabi, M. Centrifugally spun TiO2/C composite fibers prepared from TiS2/PAN precursor fibers as binder-free anodes for LIBS. J. Phys. Chem. Solids 2021, 149, 109795. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Nanofiber diameter as a critical parameter affecting skin cell response. Eur. J. Pharm. Sci. 2015, 66, 29–35. [Google Scholar] [CrossRef]
- Liu, Y.B.; Chen, X.H.; Gao, Y.H.; Liu, Y.Y.; Yu, D.G.; Liu, P. Electrospun Core-Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance. Biomolecules 2022, 12, 1057. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Ibrahim, A.; Materon, L.; Parsons, J.G.; Alcoutlabi, M. Centrifugally spun PVP/ZnO composite fibers with different surfactants and their use as antibacterial agents. J. Appl. Polym. Sci. 2023, 140, e54314. [Google Scholar] [CrossRef]
- Hasan, M.T.; Gonzalez, R.; Chipara, M.; Materon, L.; Parsons, J.; Alcoutlabi, M. Antibacterial activities of centrifugally spun polyethylene oxide/silver composite nanofibers. Polym. Adv. Technol. 2021, 32, 2327–2338. [Google Scholar] [CrossRef]
- Hasan, M.T.; Gonzalez, R.; Munoz, A.A.; Materon, L.; Parsons, J.G.; Alcoutlabi, M. Forcespun polyvinylpyrrolidone/copper and polyethylene oxide/copper composite fibers and their use as antibacterial agents. J. Appl. Polym. Sci. 2022, 139, e51773. [Google Scholar] [CrossRef]
- Rodriguezcarvajal, J. Recent Advances in Magnetic-Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Allahverdi, Ç. Synthesis of copper nano/microparticles via thermal decomposition and their conversion to copper oxide film. Turk. J. Chem. 2023, 47, 616–632. [Google Scholar] [CrossRef]
- Tamaekong, N.; Liewhiran, C.; Phanichphant, S. Synthesis of Thermally Spherical CuO Nanoparticles. J. Nanomater. 2014, 2014, 507978. [Google Scholar] [CrossRef]
- Naderi-Samani, H.; Razavi, R.S.; Mozaffarinia, R. The effects of complex agent and sintering temperature on conductive copper complex paste. Heliyon 2022, 8, e12624. [Google Scholar] [CrossRef] [PubMed]
- Heyns, A.M. The low-temperature infrared spectra of the copper(II)acetates. J. Mol. Struct. 1972, 11, 93–103. [Google Scholar] [CrossRef]
- Rahma, A.; Munir, M.M.; Khairurrijal; Prasetyo, A.; Suendo, V.; Rachmawati, H. Intermolecular Interactions and the Release Pattern of Electrospun Curcumin-Polyvinyl(pyrrolidone) Fiber. Biol. Pharm. Bull. 2016, 39, 163–173. [Google Scholar] [CrossRef]
- Bette, S.; Costes, A.; Kremer, R.K.; Eggert, G.; Tang, C.C.; Dinnebier, R.E. On Verdigris, Part III: Crystal Structure, Magnetic and Spectral Properties of Anhydrous Copper(II) Acetate, a Paddle Wheel Chain. Z. Für Anorg. Und Allg. Chem. 2019, 645, 988–997. [Google Scholar] [CrossRef]
- Wang, Z.; Li, K.F. Understanding water vapor sorption hysteresis and scanning behaviors of hardened cement pastes: Experiments and modeling. Cem. Concr. Res. 2024, 177, 107435. [Google Scholar] [CrossRef]
- Wu, K.H.; Wang, Y.R.; Hwu, W.H. FTIR and TGA studies of poly(4-vinylpyridine--divinylbenzene)-Cu(II) complex. Polym. Degrad. Stab. 2003, 79, 195–200. [Google Scholar] [CrossRef]
- Mireles, L.K.; Wu, M.R.; Saadeh, N.; Yahia, L.; Sacher, E. Physicochemical Characterization of Polyvinyl Pyrrolidone: A Tale of Two Polyvinyl Pyrrolidones. ACS Omega 2020, 5, 30461–30467. [Google Scholar] [CrossRef]
- Huang, S.W.; Lin, Y.F.; Li, Y.X.; Hu, C.C.; Chiu, T.C. Synthesis of Fluorescent Carbon Dots as Selective and Sensitive Probes for Cupric Ions and Cell Imaging. Molecules 2019, 24, 1785. [Google Scholar] [CrossRef] [PubMed]
- Zidan, H.M.; Abdelrazek, E.M.; Abdelghany, A.M.; Tarabiah, A.E. Characterization and some physical studies of PVA/PVP filled with MWCNTs. J. Mater. Res. Technol. 2019, 8, 904–913. [Google Scholar] [CrossRef]
- Ito, K.; Bernstein, H.J. The Vibrational Spectra of the Formate, Acetate, and Oxalate Ions. Can. J. Chem. 1956, 34, 170–178. [Google Scholar] [CrossRef]
- Cena, C.R.; Silva, M.J.; Malmonge, L.F.; Malmonge, J.A. Poly(vinyl pyrrolidone) sub-microfibers produced by solution blow spinning. J. Polym. Res. 2018, 25, 238. [Google Scholar] [CrossRef]
- Teng, J.; Bates, S.; Engers, D.A.; Leach, K.; Schields, P.; Yang, Y. Effect of water vapor sorption on local structure of poly(vinylpyrrolidone). J. Pharm. Sci. 2010, 99, 3815–3825. [Google Scholar] [CrossRef]
- Timaeva, O.; Pashkin, I.; Mulakov, S.; Kuzmicheva, G.; Konarev, P.; Terekhova, R.; Sadovskaya, N.; Czakkel, O.; Prevost, S. Synthesis and physico-chemical properties of poly(N-vinyl pyrrolidone)-based hydrogels with titania nanoparticles. J. Mater. Sci. 2020, 55, 3005–3021. [Google Scholar] [CrossRef]
- Khan, M.Q.; Kharaghani, D.; Nishat, N.; Ishikawa, T.; Ullah, S.; Lee, H.; Khatri, Z.; Kim, I.S. The development of nanofiber tubes based on nanocomposites of polyvinylpyrrolidone incorporated gold nanoparticles as scaffolds for neuroscience application in axons. Text. Res. J. 2019, 89, 2713–2720. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Agrafiotis, C.; Roeb, M.; Sattler, C. Applications and limitations of two step metal oxide thermochemical redox cycles; a review. J. Mater. Chem. A 2017, 5, 18951–18966. [Google Scholar] [CrossRef]
- Morales, H.M.; Vieyra, H.; Sanchez, D.A.; Fletes, E.M.; Odlyzko, M.; Lodge, T.P.; Padilla-Gainza, V.; Alcoutlabi, M.; Parsons, J.G. Synthesis and Characterization of Vanadium Nitride/Carbon Nanocomposites. Int. J. Mol. Sci. 2024, 25, 6952. [Google Scholar] [CrossRef]
- Morales, H.M.; Sanchez, D.A.; Fletes, E.M.; Odlyzko, M.; Padilla-Gainza, V.; Alcoutlabi, M.; Parsons, J.G. Synthesis and Characterization of Titanium and Vanadium Nitride-Carbon Composites. J. Compos. Sci. 2024, 8, 485. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Komarek, M.; Daniela Lubasova, D.; Sanetrnik, F.; Maryska, J. Preparation of Antibacterial Nanofibre/Nanoparticle Covered Composite Yarns. J. Nanomater. 2016, 2016, 7565972. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Kim, I.S. Copper oxide (CuO) loaded polyacrylonitrile (PAN) nanofiber membranes for antimicrobial breath mask applications. Curr. Res. Biotechnol. 2019, 1, 1–10. [Google Scholar] [CrossRef]






| Sample | ||||||||
|---|---|---|---|---|---|---|---|---|
| Element | 5% | SE | 10% | SE | 25% | SE | 50% | SE |
| C | 64.07 | 0.57 | 62.44 | 3.51 | 65.01 | 1.52 | 67.90 | 2.51 |
| N | 18.26 | 0.54 | 17.74 | 1.09 | 15.41 | 2.23 | 23.67 | 1.59 |
| O | 15.64 | 0.19 | 17.70 | 2.43 | 15.57 | 0.69 | 14.32 | 1.37 |
| Cu | 1.91 | 0.15 | 2.10 | 0.29 | 5.25 | 1.29 | 5.10 | 0.34 |
| Sample | CuAc, % | PVP, % |
|---|---|---|
| PVP | 0.00 | 100.00 |
| 5% (w/w) CuAc/PVP | 4.38 | 95.62 |
| 10% (w/w) CuAc/PVP | 7.94 | 92.06 |
| 25% (w/w) CuAc/PVP | 16.41 | 83.59 |
| 50% (w/w) CuAc/PVP | 16.44 | 83.56 |
| FTIR Peaks (cm−1) | ||||||
|---|---|---|---|---|---|---|
| CuAc | 50% (w/w) CuAc/PVP | 25% (w/w) CuAc/PVP | 10% (w/w) CuAc/PVP | 5% (w/w) CuAc/PVP | PVP | Assignment |
| 3462 | O-H | |||||
| 3358 | 3400 | 3389 | 3389 | 3389 | 3389 | OH |
| 3265 | 3265 | 3265 | 3266 | 3266 | OH | |
| 2986 | 2959 | 2955 | 2955 | 2955 | 2955 | Asymmetric CH2 |
| 2918 | 2918 | 2918 | 2918 | 2918 | 2918 | Sym CH2 |
| 1650 | 1650 | 1643 | 1642 | 1643 | 1637 | C=O/OH (from H2O) |
| 1594 | sym C-O | |||||
| 1546 | 1550 | 1552 | 1556 | Cu2+ = O and Cu2+-N pyridine ring | ||
| 1494 | 1492 | 1491 | 1494 | 1494 | C-H, C-N | |
| 1458 | 1461 | 1465 | 1463 | 1462 | C-N | |
| 1435 | 1438 | 1438 | 1438 | 1438 | 1430 | sym CH2 |
| 1423 | 1423 | 1423 | C-O sym | |||
| 1417 | Sym C-O | |||||
| 1354 | CH3 sym | |||||
| 1374 | 1373 | 1373 | 1371 | 1372 | C-H | |
| 1318 | 1317 | 1317 | 1313 | 1318 | C-H | |
| 1287 | 1287 | 1286 | 1282 | 1287 | CH2 Wag, C-N | |
| 1274 | 1274 | CH2 Wag, C-N | ||||
| 1223 | 1220 | 1225 | 1224 | CH2 Wag, C-N | ||
| 1169 | 1169 | 1169 | 1165 | CH2 twist of the pyrrole | ||
| 1047 | CH rocking | |||||
| 1031 | CH3 Rocking | |||||
| 1018 | 1016 | 1012 | 1012 | 1012 | C-C, CH2 rock | |
| 926 | 926 | 930 | 929 | 937 | C-C | |
| 896 | 891 | 891 | 890 | 888 | Pyrrolidone ring breathing | |
| 842 | 842 | 841 | 842 | 847 | CH2 | |
| 732 | 732 | 734 | 734 | 736 | CH2 rock | |
| 683 | COO-Cu | |||||
| Sample | Space Group | a(Å) | b(Å) | c(Å) | α(°) | β(°) | γ(°) | χ2 | Rp/Rwp |
|---|---|---|---|---|---|---|---|---|---|
| CuAclit | C 2/c | 13.168 | 8.654 | 13.858 | 90.0 | 117.02 | 90.0 | N/A [42] | N/A |
| CuAc | C/2c | 13.151 | 9.595 | 13.852 | 90.0 | 116.9 | 90.0 | 1.3 | 8.0/6.8 |
| Cu3(OH)4(CH3COO)2 lit | Pbca | 20.974 | 7.207 | 13.122 | 90.0 | 90.0 | 90.0 | N/A [39] | N/A |
| 50% (w/w) CuAc/PVP | Pbca | 20.984 | 7.214 | 12.914 | 90.0 | 90.0 | 90.0 | 3.26 | 6.5/8.6 |
| Sample | Peak 1 Position | Peak 2 Position | Peak Position 3 |
|---|---|---|---|
| PVPlit | N/A | 10.50 | 21.0 [33] |
| PVPlit | N/A | 11.25 | 21.21 [38] |
| 5% (w/w) CuAc/PVP | N/A | 10.15 | 21.32 |
| 10% (w/w) CuAc/PVP | 8.52 | 10.28 | 21.80 |
| 25% (w/w) CuAc/PVP | 8.46 | 10.38 | 21.70 |
| 50% (w/w) CuAc/PVP | 8.46 | 10.55 | 21.75 |
| Fibers/Bacteria | Loading (wt%) | Fiber Dia. (μm) | Inhibition Zone Diameter (cm) | Refs. |
|---|---|---|---|---|
| PEO/Cu in E. coli | 0–35 | 0.2–0.25 | 1.64 out of 2.26 | [32] |
| PEO/Cu in B. cereus | 0–35 | 0.2–0.25 | 1.68 out of 2.26 | [32] |
| PVP/Cu in E. coli | 0–35 | 4.9–5.5 | 1.56 out of 2.26 | [32] |
| PVP/Cu in B. cereus | 0–35 | 4.9–5.5 | 1.53 out of 2.26 | [32] |
| PVB/CuO in E. coli | 0–10 | >200 | 100% inhibition after 1–4 h | [53] |
| PU/CuO in E. coli | 0–10 | >200 | 70% inhibition after 1–4 h | [53] |
| PAN/CuO in E. coli | 0–100 | 0.14–0.18 | inhibition not observed | [54] |
| PAN/CuO in B. subtilis | 0–100 | 0.14–0.18 | inhibition not observed) | [54] |
| PVP/CuAc in E. coli | 0–50 | 2.26–2.38 | 1.327 out of 1.2 | This work |
| PVP/CuAc in B. cereus | 0–50 | 2.26–2.38 | 1.3 out of 1.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, B.; Curiel, R.; Ibrahim, S.; Materon, L.; Ermolinsky, O.; Morales, H.; Parsons, J.G.; Alcoutlabi, M. Characterization and Antibacterial Properties of Centrifugally Spun Polyvinylpyrrolidone/Copper(II) Acetate Composite Fibers. J. Compos. Sci. 2025, 9, 590. https://doi.org/10.3390/jcs9110590
Ibrahim B, Curiel R, Ibrahim S, Materon L, Ermolinsky O, Morales H, Parsons JG, Alcoutlabi M. Characterization and Antibacterial Properties of Centrifugally Spun Polyvinylpyrrolidone/Copper(II) Acetate Composite Fibers. Journal of Composites Science. 2025; 9(11):590. https://doi.org/10.3390/jcs9110590
Chicago/Turabian StyleIbrahim, Batool, Roberto Curiel, Sara Ibrahim, Luis Materon, Oleg Ermolinsky, Helia Morales, Jason G. Parsons, and Mataz Alcoutlabi. 2025. "Characterization and Antibacterial Properties of Centrifugally Spun Polyvinylpyrrolidone/Copper(II) Acetate Composite Fibers" Journal of Composites Science 9, no. 11: 590. https://doi.org/10.3390/jcs9110590
APA StyleIbrahim, B., Curiel, R., Ibrahim, S., Materon, L., Ermolinsky, O., Morales, H., Parsons, J. G., & Alcoutlabi, M. (2025). Characterization and Antibacterial Properties of Centrifugally Spun Polyvinylpyrrolidone/Copper(II) Acetate Composite Fibers. Journal of Composites Science, 9(11), 590. https://doi.org/10.3390/jcs9110590







