Impact of Cement Storage Temperature on the Mechanical, Microstructural, and Chemical Properties of Sustainable Mortars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Production of Mortars
2.3. X-Ray Diffraction (XRD)
2.4. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
2.5. Compressive Strength Measurements
3. Results and Discussion
3.1. The Impact of the Temperature Storage on the Mechanical Behavior of the Mortars
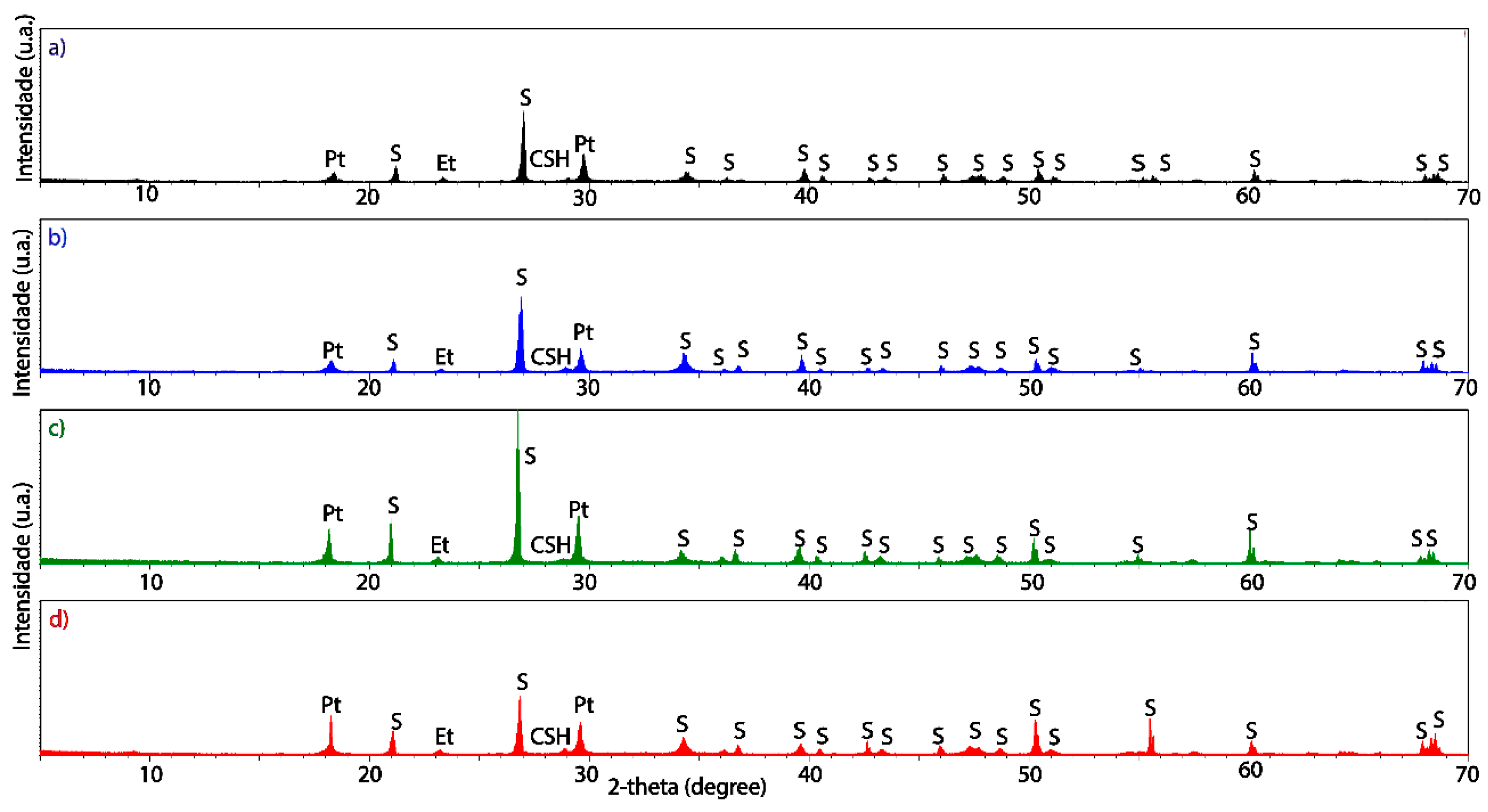
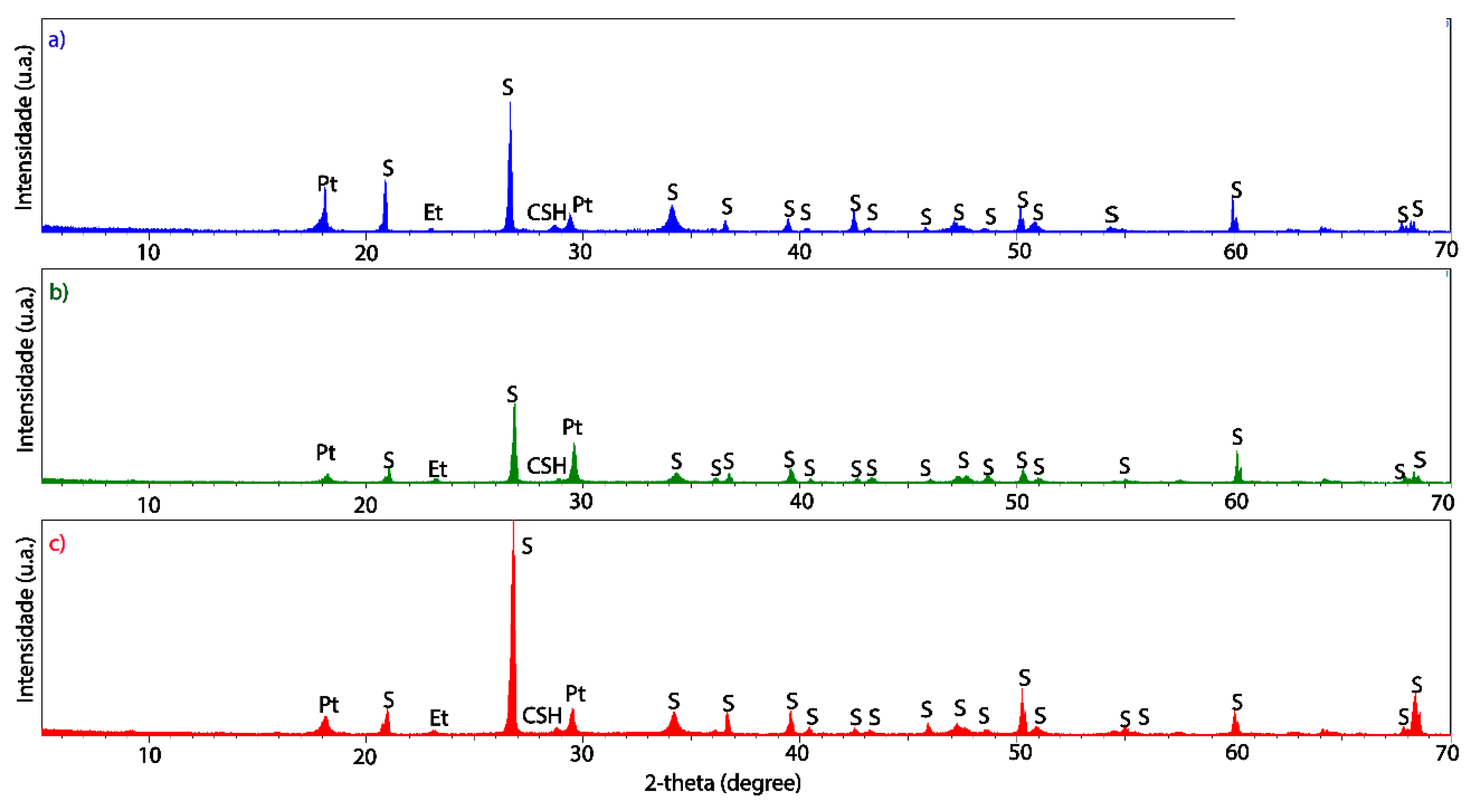
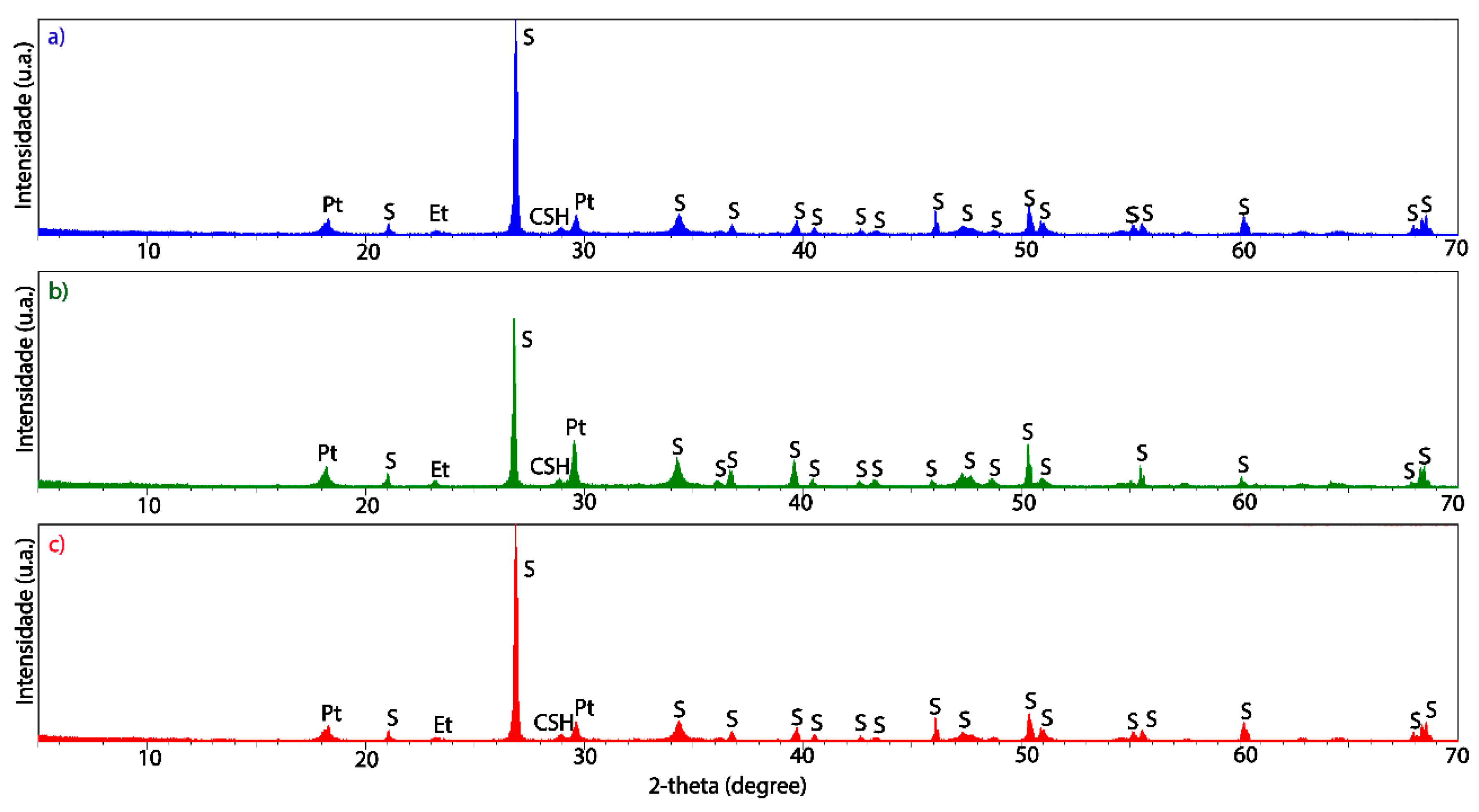
3.2. Structural and Microstructural Characteristics
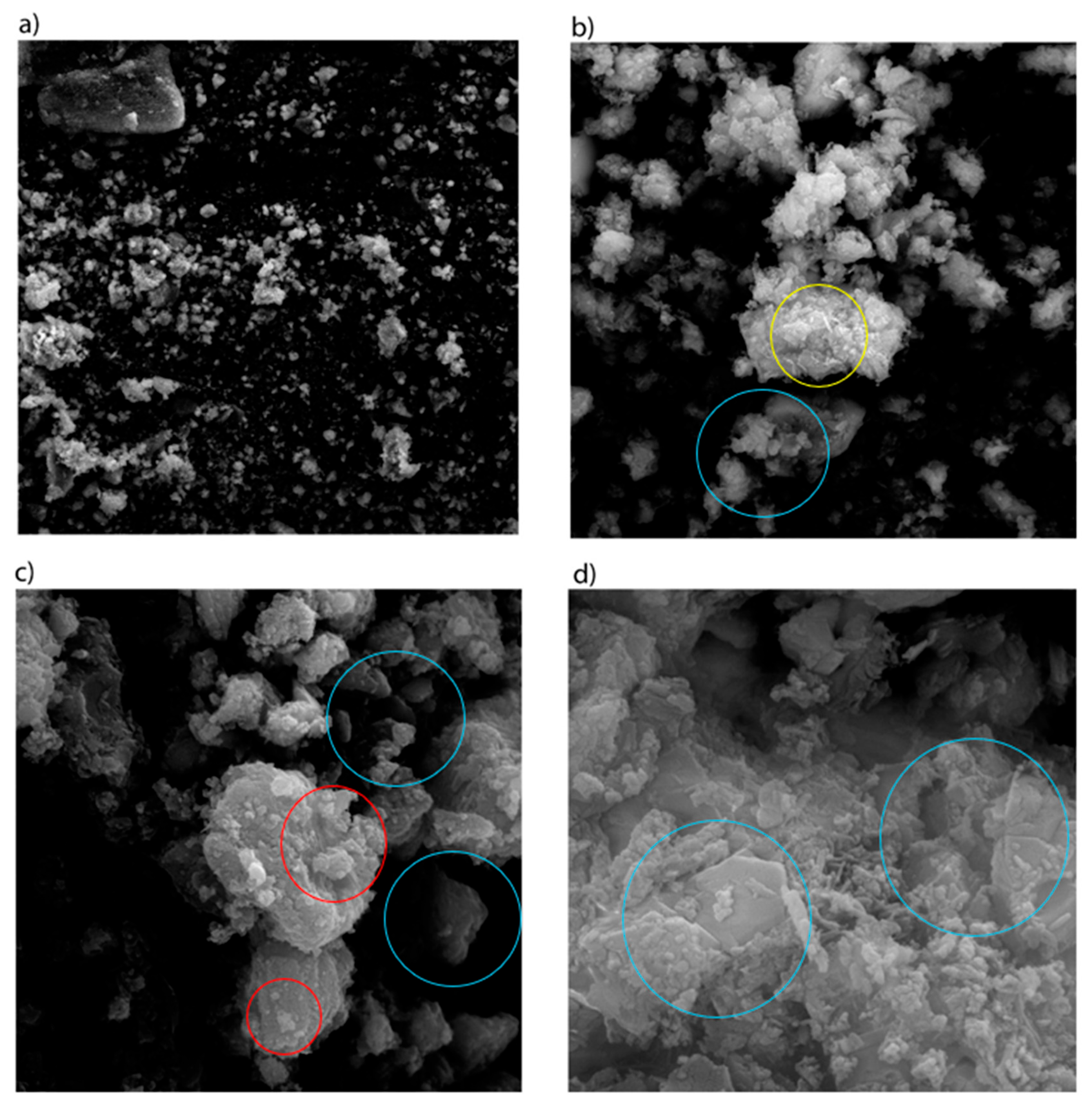
3.3. Morphology Characteristics
3.4. Chemical Aspects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, C.R.; Yadav, D.; Sharma, P.S.; Alli, M.M. Production of Ordinary Portland Cement (OPC) from NALCO Red Mud. In Light Metals 2011; Lindsay, S.J., Ed.; TMS (The Minerals, Metals & Materials Society): Pittsburgh, PA, USA, 2011; pp. 97–100. [Google Scholar] [CrossRef]
- Neto, J.S.A.; De la Torre, A.G.; Kirchheim, A.P. Effects of sulfates on the hydration of Portland cement—A review. Constr. Build. Mater. 2021, 279, 122428. [Google Scholar] [CrossRef]
- Lv, M.; Yang, L.; Wang, F.; Hu, S.; Zhong, H.; Zhang, M.; He, J. Effect of gypsum on hydration properties, composition and kinetics of low carbon belite-ye’elimite-Q phase-ferrite clinker. Cem. Concr. Res. 2024, 178, 107466. [Google Scholar] [CrossRef]
- Huang, T.; Yuan, Q.; Zuo, S.; Xie, Y.; Shi, C. New insights into the effect of gypsum on hydration and elasticity development of C3S paste during setting. Cem. Concr. Res. 2022, 159, 106860. [Google Scholar] [CrossRef]
- Juilland, P.; Nicoleau, L.; Arvidson, R.S.; Gallucci, E. Advances in dissolution understanding and their implications for cement hydration. RILEM Tech. Lett. 2017, 2, 90–98. [Google Scholar] [CrossRef]
- John, E.; Lothenbach, B. Cement hydration mechanisms through time—A review. J. Mater. Sci. 2023, 58, 9805–9833. [Google Scholar] [CrossRef]
- Shu, X.; Huang, B. Recycling of waste tire rubber in asphalt and portland cement concrete: An overview. Constr. Build. Mater. 2014, 67, 217–224. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Karpova, E.; Skripkiūnas, G.; Barauskas, I.; Barauskienė, I.; Hodul, J. Influence of carbon nanotubes and polycarboxylate superplasticiser on the Portland cement hydration process. Constr. Build. Mater. 2021, 304, 124648. [Google Scholar] [CrossRef]
- Krstulović, R.; Dabić, P. A conceptual model of the cement hydration process. Cem. Concr. Res. 2000, 30, 693–698. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Zhang, G.; Wang, Y.; Wang, Y. Effect of aluminum sulfate alkali-free liquid accelerator with compound alkanol lamine on the hydration processes of Portland cement. Constr. Build. Mater. 2021, 308, 125101. [Google Scholar] [CrossRef]
- Liu, Z.; Sha, A.; Hu, L.; Lu, Y.; Jiao, W.; Tong, Z.; Gao, J. Kinetic and thermodynamic modeling of Portland cement hydration at low temperatures. Chem. Pap. 2017, 71, 741–751. [Google Scholar] [CrossRef]
- Soriano, L.; Monzó, J.; Bonilla, M.; Tashima, M.M.; Payá, J.; Borrachero, M.V. Effect of pozzolans on the hydration process of Portland cement cured at low temperatures. Cem. Concr. Compos. 2013, 42, 41–48. [Google Scholar] [CrossRef]
- Land, G.; Stephan, D. The influence of nano-silica on the hydration of ordinary Portland cement. J. Mater. Sci. 2012, 47, 1011–1017. [Google Scholar] [CrossRef]
- Chen, W.; Brouwers, H.J.H. Mitigating the effects of system resolution on computer simulation of Portland cement hydration. Cem. Concr. Compos. 2008, 30, 779–787. [Google Scholar] [CrossRef]
- Ye, Q.; Yu, K.; Zhang, Z. Expansion of ordinary Portland cement paste varied with nano-MgO. Constr. Build. Mater. 2015, 78, 189–193. [Google Scholar] [CrossRef]
- Clark, W.E. The Isolation of Radioiodine with Portland Cement. Part I: Scoping Leach Studies. Nucl. Technol. 1977, 36, 215–221. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Lotfi, M.; Joupari, M.D.; Aeinehchi, M.; Saghiri, A.M. Effects of Storage Temperature on Surface Hardness, Microstructure, and Phase Formation of White Mineral Trioxide Aggregate. J. Endod. 2010, 36, 1414–1418. [Google Scholar] [CrossRef]
- Sun, H.; Jain, R.; Nguyen, K.; Zuckerman, J. Sialite technology—Sustainable alternative to portland cement. Clean Technol. Environ. Policy 2010, 12, 503–516. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Huang, Y.; Jiang, Z.; Yang, X.; Yang, Z.; Chen, Q. Investigation on the potential of waste cooking oil as a grinding aid in Portland cement. J. Environ. Manag. 2016, 184, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Meireles, P.D.S.; Pereira, D.S.S.; Melo, M.A.F.; Braga, R.M.; Freitas, J.C.O.; Melo, D.M.A.; Silvestre, F.R.S. Technical evaluation of calcium sulphate α-hemihydrate in oilwell application: An alternative to reduce the environmental impacts of Portland cement. J. Clean. Prod. 2019, 220, 1215–1221. [Google Scholar] [CrossRef]
- Kryvenko, P.; Rudenko, I.; Sikora, P.; Sanytsky, M.; Konstantynovskyi, O.; Kropyvnytska, T. Alkali-activated cements as sustainable materials for repairing building construction: A review. J. Build. Eng. 2024, 90, 109399. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Ahmed, M.; Bashar, I.; Alam, S.T.; Wasi, A.I.; Jerin, I.; Khatun, S.; Rahman, M. An overview of Asian cement industry: Environmental impacts, research methodologies and mitigation measures. Sustain. Prod. Consum. 2021, 28, 1018–1039. [Google Scholar] [CrossRef]
- Li, C.; Cui, S.; Nie, Z.; Gong, X.; Wang, Z.; Itsubo, N. The LCA of portland cement production in China. Int. J. Life Cycle Assess. 2015, 20, 117–127. [Google Scholar] [CrossRef]
- Harrison, E.; Berenjian, A.; Seifan, M. Recycling of waste glass as aggregate in cement-based materials. Environ. Sci. Ecotechnol. 2020, 4, 100064. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Li, X.; Sun, T.; Chen, Y.; Xu, F.; Yan, G.; Xu, M.; Tian, K. Utilization of waste glass powder as partial replacement of cement for the cementitious grouts with superplasticizer and viscosity modifying agent binary mixtures: Rheological and mechanical performances. Constr. Build. Mater. 2021, 286, 122953. [Google Scholar] [CrossRef]
- Saha, A.K. Effect of class F fly ash on the durability properties of concrete. Sustain. Environ. Res. 2018, 28, 25–31. [Google Scholar] [CrossRef]
- Naresh, B.; Saravanan, M. Experimental study of replacement of cement with ground granulated blast furnace slag. Mater. Today Proc. 2022, 62, 3493–3496. [Google Scholar] [CrossRef]
- Nedeljković, M.; Visser, J.; Šavija, B.; Valcke, S.; Schlangen, E. Use of fine recycled concrete aggregates in concrete: A critical review. J. Build. Eng. 2021, 38, 102196. [Google Scholar] [CrossRef]
- Grigoriadis, K.; Ban, J.; Caverzan, A.; Negro, P.; Senaldi, C.; Ceccone, G. Use of irradiated PET plastic waste for partially replacing cement in concrete. Waste Manag. 2023, 170, 193–203. [Google Scholar] [CrossRef]
- Khankhaje, E.; Kim, T.; Jang, H.; Kim, C.S.; Kim, J.; Rafieizonooz, M. Properties of pervious concrete incorporating fly ash as partial replacement of cement: A review. Dev. Built Environ. 2023, 14, 100130. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.İ. Utilization and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Shahidan, S.; Azmi, M.A.M.; Kupusamy, K.; Zuki, S.S.M.; Alie, N. Utilizing Construction and Demolition (C&D) Waste as Recycled Aggregates (RA) in Concrete. Procedia Eng. 2017, 174, 1028–1035. [Google Scholar] [CrossRef]
- Yao, J.J.; Chu, S.H. Durability of sustainable marine sediment concrete. Dev. Built Environ. 2023, 13, 100118. [Google Scholar] [CrossRef]
- Yu, H.; Da, B.; Ma, H.; Zhu, H.; Yu, Q.; Ye, H.; Jing, X. Durability of concrete structures in tropical atoll environment. Ocean Eng. 2017, 135, 1–10. [Google Scholar] [CrossRef]
- Chu, T.; Zheng, J.; Chen, D.; Nguyen, T.; Elbashiry, E.; Tang, V. Utilization of Industrial Waste in Cement in a Marine Environment with a Tropical Climate. J. Mar. Sci. Eng. 2019, 7, 245. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Melo, U.F.C.; Kamseu, E.; Tchamba, A.B. Laterite Based Stabilized Products for Sustainable Building Applications in Tropical Countries: Review and Prospects for the Case of Cameroon. Sustainability 2011, 3, 293–305. [Google Scholar] [CrossRef]
- Nascimento, H.C.B.; Lima, N.B.; Jesus, S.D.; Rocha, D.G.; Cavalcante, H.S.; Teti, B.S.; Manta, R.; Santos, L.B.T.; Campelo, S.; Lima, N.B.D. A molecular formalism of the hydraulic cement deterioration stored at different temperatures and its impact on the mechanical behavior. Cement 2025, 19, 100130. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Elmoaty, A.E.M.A.; Aboshama, A.Y. Utilization of waste glass powder in the production of cement and concrete. Constr. Build. Mater. 2016, 124, 866–877. [Google Scholar] [CrossRef]
- Tkachenko, N.; Tang, K.; McCarten, M.; Reece, S.; Kampmann, D.; Hickey, C.; Bayaraa, M.; Foster, P.; Layman, C.; Rossi, C.; et al. Global database of cement production assets and upstream suppliers. Sci. Data 2023, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mossie, A.T.; Khatiwada, D.; Palm, B.; Bekele, G. Investigating energy saving and climate mitigation potentials in cement production—A case study in Ethiopia. Energy Convers. Manag. 2023, 287, 117111. [Google Scholar] [CrossRef]
- Rihan, M.A.M.; Abdalla, T.A. Factors Influencing Compressive Strength in Fly Ash-Based Geopolymer Concrete: A Comprehensive Review. Iran. J. Sci. Technol. Trans. Civ. Eng. 2024, 48, 3853–3869. [Google Scholar] [CrossRef]
- Navaratnam, S.; Tushar, Q.; Jahan, I.; Zhang, G. Environmental Sustainability of Industrial Waste-Based Cementitious Materials: A Review, Experimental Investigation and Life-Cycle Assessment. Sustainability 2023, 15, 1873. [Google Scholar] [CrossRef]
- Mahmood, R.A.; Kockal, N.U. Cementitious materials incorporating waste plastics: A Review. SN Appl. Sci. 2020, 2, 2072. [Google Scholar] [CrossRef]
- Catalin, S.; Daniela, M.L.; Moldovan, M.; Monica, L.M.; Borodi, G.; Petean, I.; Sorin, L. Recycled Aggregates Influence on the Mechanical Properties of Cement Lime-Based Mortars. Materials 2024, 17, 5122. [Google Scholar] [CrossRef] [PubMed]
- Cieri, L.; Cassese, P.; Fabbrocino, G.; Occhiuzzi, A.; Rainieri, C. Experimental Study about the Influence of Storage Conditions of Bulk Cement on the Early-Age Stiffness Evolution of Cementitious Pastes. Appl. Sci. 2023, 13, 11734. [Google Scholar] [CrossRef]
- Golewski, G.L.; Szostak, B. Application of the C-S-H Phase Nucleating Agents to Improve the Performance of Sustainable Concrete Composites Containing Fly Ash for Use in the Precast Concrete Industry. Materials 2021, 14, 6514. [Google Scholar] [CrossRef] [PubMed]
- Maciel, M.H.; Soares, G.S.; Romano, R.C.; Cincotto, M.A. Monitoring of Portland Cement Chemical Reaction and Quantification of the Hydrated Products by XRD and TG in Function of the Stoppage Hydration Technique. J. Therm. Anal. Calorim. 2019, 136, 1269–1284. [Google Scholar] [CrossRef]
- Jiang, J.; Li, M.; Yan, J.; Zhou, M.; Duan, P.; Zhang, Z.; Liu, J. Assessment of Early Hydration and Microstructures of Portland Cement Incorporating Calcined Attapulgite. Arab. J. Sci. Eng. 2023, 48, 12891–12902. [Google Scholar] [CrossRef]
- Kunther, W.; Ferreiro, S.; Skibsted, J. Influence of the Ca/Si Ratio on the Compressive Strength of Cementitious Calcium–Silicate–Hydrate Binders. J. Mater. Chem. A 2017, 5, 17401–17412. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A.; Neves, J. Drying and Carbonation Shrinkage of Cement Paste Containing Alkalis. Mater. Struct. 2017, 50, 132. [Google Scholar] [CrossRef]
- Cheng, D.; Reiner, D.M.; Yang, F.; Cui, C.; Meng, J.; Shan, Y.; Liu, Y.; Tao, S.; Guan, D. Projecting future carbon emissions from cement production in developing countries. Nat. Commun. 2023, 14, 8213. [Google Scholar] [CrossRef] [PubMed]

















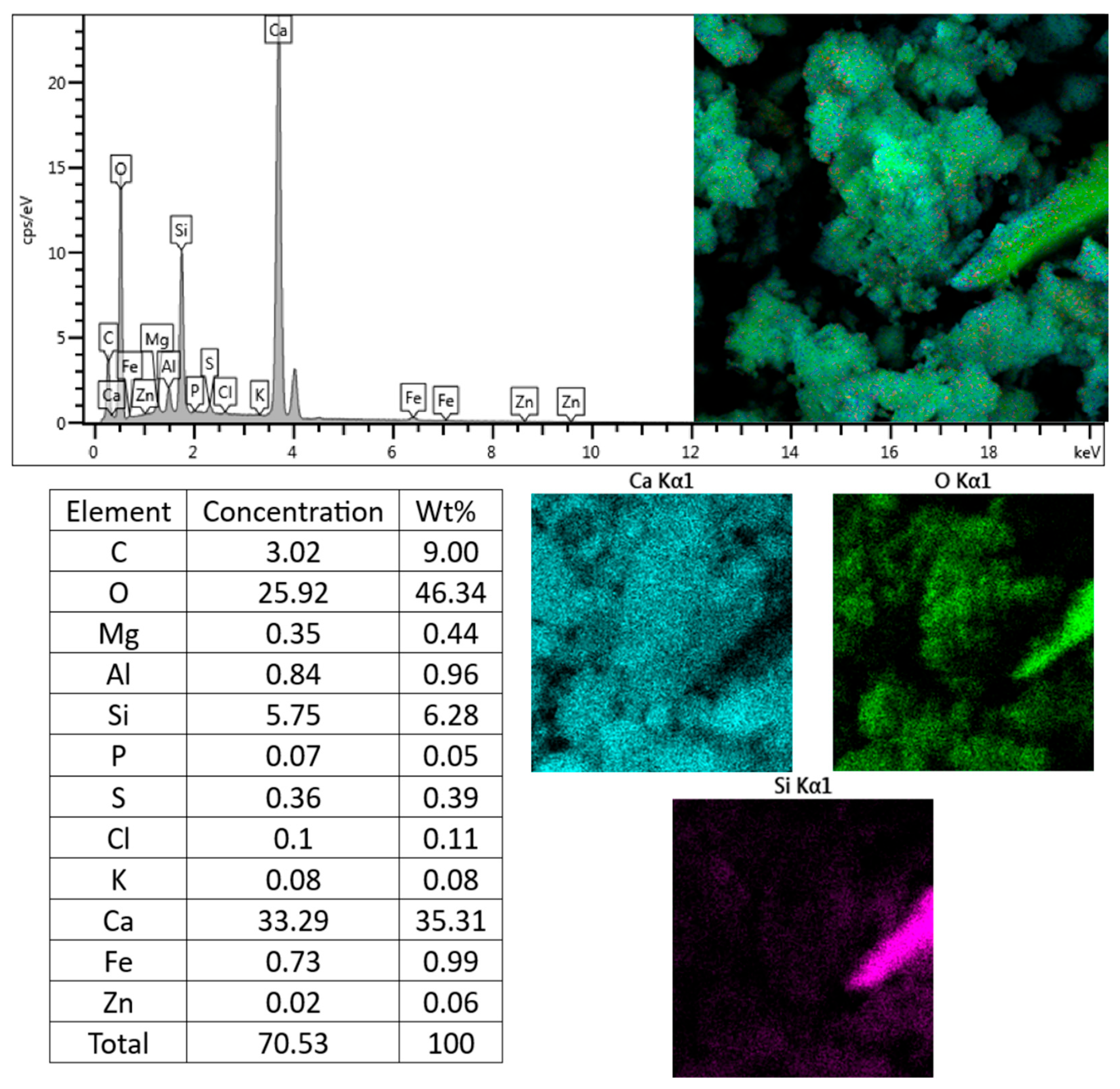


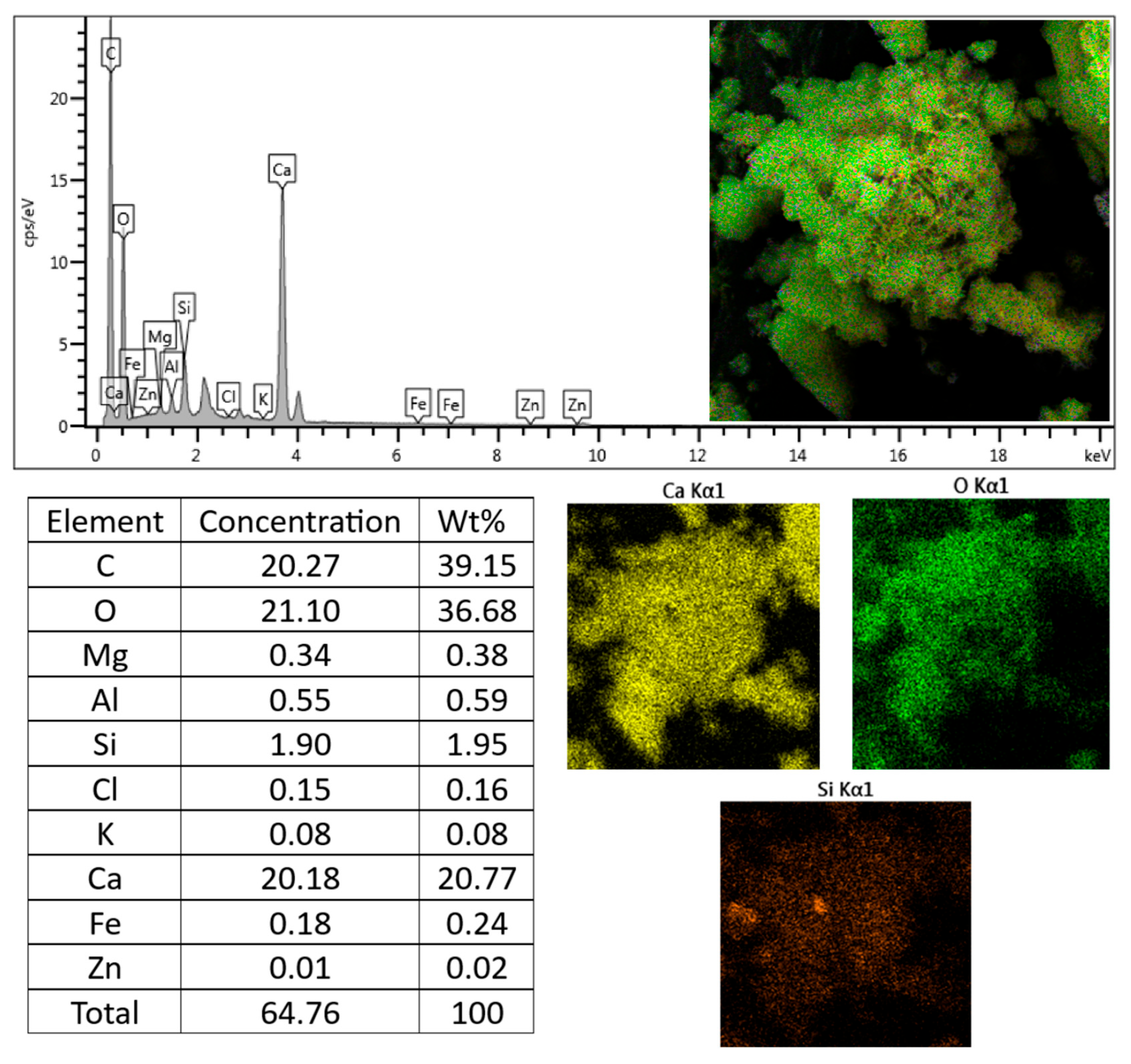

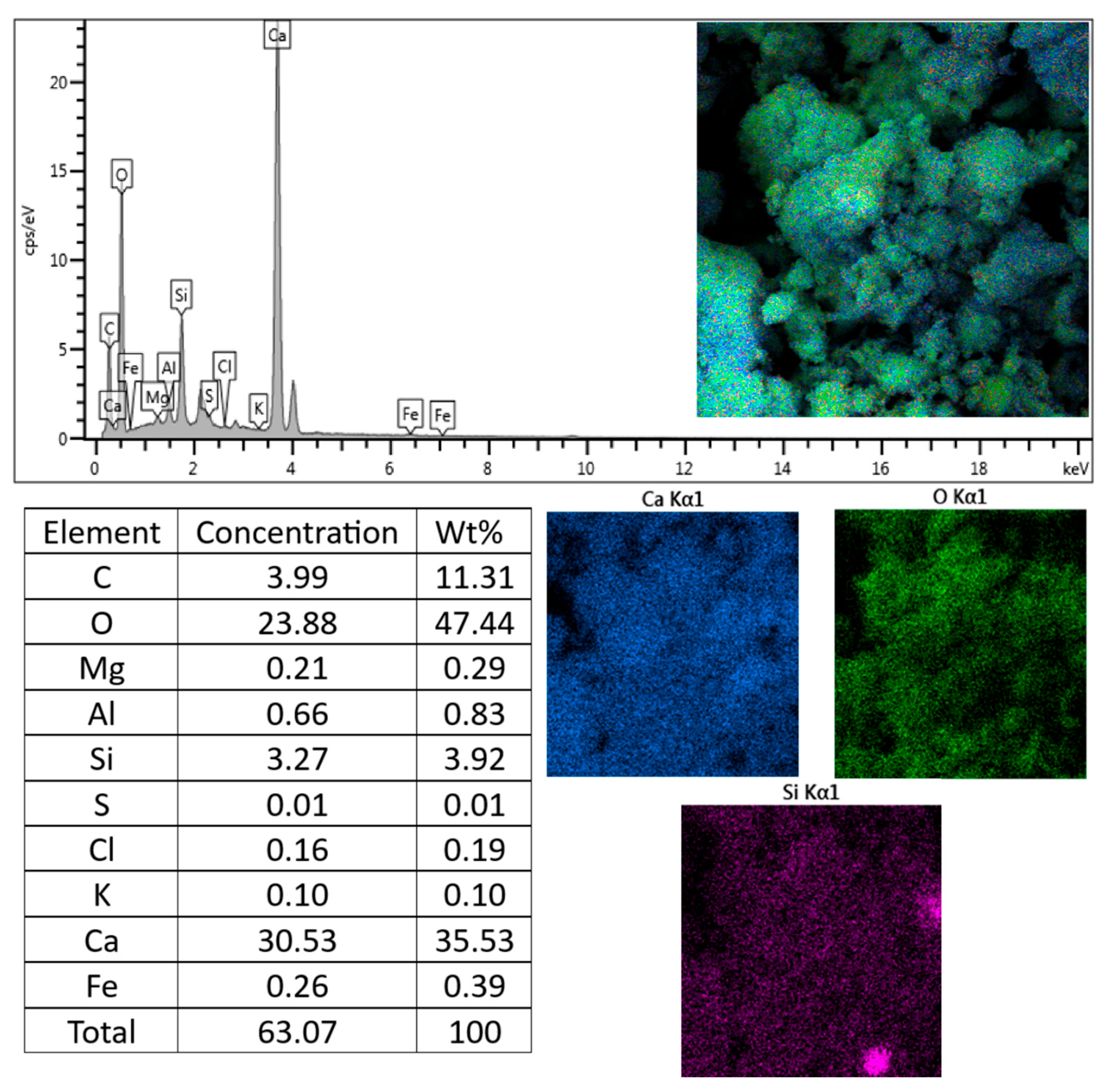
| Cement Type | Components (% by Mass) | ||
|---|---|---|---|
| Clinker + Calcium Sulfate | Pozzolana | Carbonaceous Material | |
| II Z | 71–94 | 6–14 | 0–15 |
| Criteria | Results of the Material Used | Normative Parameters | |
|---|---|---|---|
| Loss to fire (1.000 ± 50 °C) | 0.59% | - | |
| Moisture (110 ± 5 °C) | 22.94% | - | |
| Carbonic Anhydride | 2.01% CO2 | ≤7% | |
| Sulfuric Anhydride | 0.12% SO3 | - | |
| Total Calcium Oxide | 66.90% CaO | - | |
| Magnesium oxide | 2.96% MgO | - | |
| CaO + MgO not hydrated | 4.00% | ≤10% | |
| Total oxides on a non-volatile basis | 90.70% | ≥90% | |
| Fineness (% accumulated retained) | sieve #30 (600 µm) | 0.00% | ≤0.5 |
| sieve #200 (75 µm) | 1.80% | ≤10% | |
| Type of Material | Cement (g) | Lime (g) | Sand (g) | Fine Aggregate (g) | Water/Cement |
|---|---|---|---|---|---|
| Reference—Type A | 468 | 468 | 2106 | 0 | 1.25 |
| Addition—Type B | 468 | 468 | 2106 | 46.8 | 1.25 |
| Replacement—Type C | 421.2 | 468 | 2106 | 46.8 | 1.25 |
| Day | Compressive Strength (MPa) | ||
|---|---|---|---|
| 10 °C | 30 °C | 50 °C | |
| 15 | 8.3 | 9.8 | 9.0 |
| 30 | 6.6 | 7.7 | 6.8 |
| 45 | 8.1 [39] | 8.4 [39] | 7.9 [39] |
| 60 | 6.5 | 7.8 | 7.7 |
| 75 | 6.3 | 7.5 | 8.0 |
| 90 | 6.7 [39] | 7.7 [39] | 7.2 [39] |
| 105 | 7.8 | 8.2 | 8.5 |
| Average | 7.2 | 8.2 | 7.9 |
| Standard deviation | 0.845 | 0.789 | 0.743 |
| Variance | 0.715 | 0.623 | 0.552 |
| Median | 6.7 | 7.8 | 7.9 |
| Day | Compressive Strength (MPa) | ||
|---|---|---|---|
| 10 °C | 30 °C | 50 °C | |
| 15 | 6.7 | 8.6 | 9.2 |
| 30 | 6.6 | 7.7 | 7.4 |
| 45 | 7.9 [39] | 7.7 [39] | 9.2 [39] |
| 60 | 7.2 | 7.2 | 7.7 |
| 75 | 6.5 | 8.0 | 8.4 |
| 90 | 5.1 [39] | 6.0 [39] | 7.5 [39] |
| 105 | 7.8 | 8.3 | 7.1 |
| Average | 6.8 | 7.6 | 8.1 |
| Standard deviation | 0.948 | 0.854 | 0.867 |
| Variance | 0.899 | 0.730 | 0.752 |
| Median | 6.7 | 7.7 | 7.7 |
| Day | Compressive Strength (MPa) | ||
|---|---|---|---|
| 10 °C | 30 °C | 50 °C | |
| 15 | 5.2 | 6.2 | 7.3 |
| 30 | 4.7 | 5.2 | 5.5 |
| 45 | 5.8 [39] | 6.2 [39] | 6.4 [39] |
| 60 | 6.3 | 6.7 | 6.6 |
| 75 | 5.7 | 6.0 | 5.4 |
| 90 | 8.2 [39] | 7.4 [39] | 8.6 [39] |
| 105 | 5.7 | 6.6 | 6.2 |
| Average | 5.9 | 6.3 | 6.6 |
| Standard deviation | 1.115 | 0.680 | 1.106 |
| Variance | 1.243 | 0.462 | 1.222 |
| Median | 5.7 | 6.2 | 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, H.C.B.; Teti, B.S.; Manta, R.C.; Rocha, D.G.; Dantas, J.A.F.; Jesus, S.D.; Souza, P.R.L.; Lima, N.B.; Lima, N.B.D. Impact of Cement Storage Temperature on the Mechanical, Microstructural, and Chemical Properties of Sustainable Mortars. J. Compos. Sci. 2025, 9, 583. https://doi.org/10.3390/jcs9110583
Nascimento HCB, Teti BS, Manta RC, Rocha DG, Dantas JAF, Jesus SD, Souza PRL, Lima NB, Lima NBD. Impact of Cement Storage Temperature on the Mechanical, Microstructural, and Chemical Properties of Sustainable Mortars. Journal of Composites Science. 2025; 9(11):583. https://doi.org/10.3390/jcs9110583
Chicago/Turabian StyleNascimento, Heliana C. B., Bruno S. Teti, Rafael C. Manta, Delma G. Rocha, José Allef F. Dantas, Sanderson D. Jesus, Paulo R. L. Souza, Nathan B. Lima, and Nathalia B. D. Lima. 2025. "Impact of Cement Storage Temperature on the Mechanical, Microstructural, and Chemical Properties of Sustainable Mortars" Journal of Composites Science 9, no. 11: 583. https://doi.org/10.3390/jcs9110583
APA StyleNascimento, H. C. B., Teti, B. S., Manta, R. C., Rocha, D. G., Dantas, J. A. F., Jesus, S. D., Souza, P. R. L., Lima, N. B., & Lima, N. B. D. (2025). Impact of Cement Storage Temperature on the Mechanical, Microstructural, and Chemical Properties of Sustainable Mortars. Journal of Composites Science, 9(11), 583. https://doi.org/10.3390/jcs9110583








